Abstract
Purpose
Glioblastoma multiforme (GBM) is a deadly primary brain tumor. Clinical trials for GBM using dendritic cell (DC) vaccination resulted in anti-tumor immune responses. Herein we tested the hypothesis that combining in situ (intratumoral) Ad-Flt3L/Ad-TK-mediated gene therapy with DC vaccination would increase therapeutic efficacy and anti-tumor immunity.
Experimental Design
We first assessed the immunogenicity of tumor lysates generated by Ad-TK (+GCV), temozolomide (TMZ) or freeze/thawing cycles (FTC) in a syngeneic brain tumor model. We also assessed phenotypic markers, cytokine release, and phagocytosis of bone marrow derived DCs generated by fms-like tyrosine kinase 3 ligand (Flt3L) + IL-6 or by granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4. Inhibition of tumor progression and production of anti-GBM antibodies was assessed following vaccination with (i) tumor cell lysates, (ii) DCs generated with either Flt3L/IL6 or GM-CSF/IL4 loaded with either Ad-TK/GCV, TMZ, or FTC generated tumor lysates, or (iii) DCs in combination with in situ Ad-Flt3L/Ad-TK gene therapy.
Results
DCs loaded with tumor cell lysates generated with either Ad-TK/GCV or TMZ led to increased levels of phagocytosis, therapeutic efficacy and humoral immune response. In situ immunogene therapy in combination with DC vaccination led to brain tumor regression and long-term survival in ~90% of animals, a significant increase when compared to either therapy alone.
Conclusions
Our results indicate that modifying the tumor microenvironment using intra-tumoral Ad-Flt3L/Ad-TK-mediated gene therapy potentiates therapeutic efficacy and anti-tumor immunity induced by DC vaccination. These data support novel Phase I clinical trials to assess the safety and efficacy of this combined approach.
Keywords: glioblastoma, immunotherapy, adenoviral vectors, Flt3 ligand, gene therapy
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults and exhibits a dismal prognosis. Despite therapeutic advances, i.e., surgical resection followed by radiotherapy and chemotherapy, the prognosis for GBM is still poor with a median survival of 15–18 months post-diagnoses (1). Because GBM diffusely infiltrates into the surrounding brain parenchyma, relapse is almost inevitable even after surgical resection.
Immunotherapy strategies, including vaccination with either tumor associated peptides, autologous tumor cells or dendritic cells (DCs) have been intensively studied in human clinical trials for cancer (2–12). One of the most widely used approaches in cancer immunotherapy is DC vaccination, in which patients’ DCs are cultured and expanded ex vivo, loaded with specific tumor antigens or autologous tumor lysates and then systemically administered to the patient. Recently, Sipuleucel-T (Provenge) became the first antigen-specific, cell-based, immunotherapy approach to receive Food and Drug Administration (FDA) approval (13). Sipuleucel-T demonstrated an overall survival benefit to men with castration-resistant prostate cancer in three double-blind, placebo-controlled, multicenter trials (14, 15).
Several clinical trials for GBM using DC vaccination approaches have been implemented including vaccination with DCs pulsed with EGFRvIII, a tumor antigen which is expressed in ~30% of human GBM patients (2, 11), or vaccination with DCs loaded ex vivo with autologous tumor lysates (5–10, 12). These therapeutic approaches are designed to facilitate the presentation of brain tumor antigens to naïve T cells, thereby inducing the proliferation of brain tumor antigen–specific cytotoxic T cells. In these studies, DC vaccination induced increased anti-tumor cellular and humoral immune responses against brain tumors, exhibiting a high safety profile (3, 5–10, 12).
We have developed a combined cytotoxic/immunostimulatory gene therapy using intratumoral injection of adenoviral vectors expressing fms-like tyrosine kinase 3 ligand (Ad-Flt3L) and thymidine kinase (Ad-TK) followed by systemic administration of ganciclovir (GCV) (16). Intratumoral expression of Flt3L induces the migration, differentiation and expansion of antigen presenting cells, i.e., bone marrow-derived DCs (BMDCs), within the tumor microenvironment in mice (17, 18) and rats (16, 19, 20). Ad-TK is a conditional cytotoxic strategy which induces the death of actively dividing tumor cells in the presence of GCV, releasing endogenous brain tumor antigens. Ad-TK/GCV treated tumor cells also release a potent innate adjuvant, i.e., high-mobility group B1 protein (HMGB1), a DNA binding protein constitutively expressed in nucleus of eukaryotic cells (18, 21). We demonstrated that HMGB1 is released from dying tumor cells acting as an endogenous adjuvant to stimulate Toll-like receptor 2 (TLR2) signaling on BMDCs (18). In a large, intracranial GBM model, Ad-Flt3L + Ad-TK immunogene therapy induced long term survival and immunological memory that can eliminate recurrent and multifocal brain tumors (19, 22, 23). We also recently demonstrated that Ad-Flt3L + Ad-TK-mediated gene therapy induces memory T cells capable of recognizing brain tumor neo-antigens (24). Based on these results, gene therapy using Ad-Flt3L and Ad-TK/GCV was recently cleared by the FDA for an upcoming Phase I clinical trial for GBM (BB-IND 14574; NIH/OBA Protocol # 0907-990; OSU Protocol 10089).
Herein, we aimed to test the hypothesis that in situ (intratumoral) gene therapy mediated by expression of Flt3L and HSV1-TK would enhance the therapeutic efficacy of systemic DC vaccination, leading to the design of novel Phase I trials for GBM. Recent reports indicate that vaccination efficacy depends on the type of cell death used to prepare the tumor lysates, i.e., apoptotic tumor lysates are more immunogenic and elicit greater anti-tumor effects than necrotic tumor lysates (25, 26). As such, we compared the immunogenicity of tumor lysates prepared by treatment with Ad-TK/GCV, the chemotherapeutic agent temozolomide (TMZ) or freeze/thaw cycles (FTC). Our results demonstrate that vaccination with cell lysates prepared using Ad-TK/GCV or TMZ displayed equivalent inhibition of brain tumor progression and were more efficacious than necrotic lysates (FTC). We also compared the in vitro and in vivo phenotypic characteristics of DCs, generated by Flt3L + IL-6 treatment or by granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 treatment, i.e., activation markers, cytokine release, and phagocytic ability. We demonstrated the therapeutic efficacy of Flt3L + IL-6 induced DCs loaded with Ad-TK/GCV-generated tumor lysates, in rats bearing established brain tumors. Flt3L is a powerful cytokine that is required for normal development and expansion of DCs in vitro and in vivo (27). Our results show that therapeutic efficacy and induction of anti-tumor immunity in Lewis rats bearing orthotopic, syngeneic brain tumors was significantly enhanced when DC vaccination was combined with Ad-TK/GCV+Ad-Flt3L gene therapy. These data support the implementation of novel Phase I clinical trials for GBM to assess the safety and efficacy of this novel dual therapeutic modality.
MATERIALS AND METHODS
Adenoviral vectors
We used first-generation, E1/E3 deleted adenoviral vectors expressing human fms-like tyrosine kinase 3 ligand (Ad-Flt3L) or Herpes Simplex Virus Type I- thymidine kinase (Ad-TK); described in Supplemental Materials and Methods.
Tumor lysate preparation
CNS1 rat glioma cells were treated with either Ad-TK plus ganciclovir (TK/GCV), temozolomide (TMZ) or freeze-thaw cycles (FTC); described in Supplemental Materials and Methods.
Bone marrow-derived DC preparation
Bone marrow was isolated from the femurs of naïve, adult Lewis rats as described previously and in Supplemental Materials and Methods. Bone marrow precursors were cultured with either Flt3L and IL-6, or GM-CSF and IL-4. Loosely adherent cells were used for subsequent subcutaneous injections and functional assays as described in Supplemental Materials and Methods.
CNS1 rat glioma model and Ad delivery
All procedures involving live animals were performed in accordance with protocols approved by the Cedars-Sinai Medical Center’s Institutional Animal Care and Use Committee (IACUC). 5,000 CNS1 glioma cells in 3 μL DMEM were stereotactically implanted in the right striatum of syngeneic Lewis rats as described in Supplemental Materials and Methods. Four or 10 days after tumor implantation, rats received a stereotactic, intratumoral injection of Ad vectors; described in Supplemental Materials and Methods. For survival studies, animals were treated with saline (as a control), 3×108 pfu of Ad-Flt3L, 1×108 pfu of Ad-TK or in combination with Ad-Flt3L and Ad-TK.
DC vaccination
Lysates prepared from 6×106 tumor cells were mixed with CpG2006 and injected subcutaneously in the flank three times at 7-day intervals as indicated and described in Supplemental Materials and Methods.
Electron microscopy
CNS1 cells treated with either Ad-TK/GCV, TMZ or FTC were fixed with 2.5% glutaraldehyde, dehydrated through graded acetone and embedded in Eponate. 60 nm sections were counterstained with uranyl acetate in methanol and in Reynold’s lead citrate and viewed with a Jeol 100CX transmission electron microscope; described in Supplemental Materials and Methods.
Acridine orange staining
Acridine orange was used to detect and quantify the development of acidic vesicular organelles. Briefly, acridine orange moves freely across biological membranes and fluoresces green when uncharged, whereas its protonated form accumulates in acidic compartments and fluoresces bright red. The intensity of red fluorescence is proportional to the degree of acidity. CNS1 tumor cells were stained with acridine orange (1 μg/mL) and analyzed by fluorescence microscopy or flow cytometry as described in Supplemental Materials and Methods. Bafilomycin A1, an inhibitor of vacuolar H+-ATPase (V-ATPase) that interferes with the fusion of autophagosome and lysosome, was added to the cells 30 min before addition of acridine orange.
Western blotting
Analysis of cell lysates by Western Blotting was performed as described in Supplemental Materials and Methods. Labeling was performed using anti-LC-3B antibody, or α-tubulin as a control, followed by HRP-conjugated secondary antibody and visualization with ECL.
Quantification of apoptosis and necrosis
CNS1 tumor cell lysates were stained with FITC-conjugated Annexin V and propidium iodide and analyzed by flow cytometry as described by us previously (18, 21) and in Supplemental Materials and Methods.
Quantification of DC maturation status: measuring expression of cell surface markers and cytokine production
DCs were incubated with CpG2006 or LPS and then assessed for either (i) cytokine production by ELISAs specific for IL-10, IFN-γ, TNF-α and IL-12, or (ii) expression of cell surface markers OX-62, CD161a, CD3, CD4, CD45, CD45R, CD11c, CD80, CD86 and MHC-II by flow cyotmetry; described in Supplemental Materials and Methods.
Phagocytic activity of DC
CNS1 tumor cells stained with Qtracker 655 were treated with the above killing treatments (Ad-TK/GCV, TMZ or FTC). Dying, labeled tumor cells were incubated Flt3L+IL6 conditioned DCs labeled with a CD161a-PE antibody. Phagocytosis of tumor cells was assessed by flow cytometry as the percentage of Qtracker 655/CD161a-PE labeled cells compared to CD161a-PE labeled cells. Alternatively microscopy analysis was used to assess the phagocytosis of tumor cell remnants; Qtracker 655 stained tumor cells stained were cultured with DCs, stained with CD161a followed by an Alexa 488 tagged secondary antibody. Nuclei were stained with DAPI and samples were analyzed by confocal microscopy, see Supplemental Materials and Methods.
CTL assay
Cytotoxicity of lymphocytes against tumor cells was assessed by flow cytometry as previously described (28) and in Supplemental Materials and Methods
Detection of anti-CNS1 antibodies
Serum levels of anti-CNS1 antibodies were measured by flow cytometry, described previously (29) and in Supplemental Materials and Methods.
Statistical analyses
Kaplan Meier survival curves were analyzed using the Mantel log-rank test using Prism GraphPad software (version 3.03). The flow cytometry and ELISA data were analyzed by one-way ANOVA followed by Tukey’s post-test using NCSS statistical and power analysis software. Differences between groups were considered significant at p < 0.05. All experiments described in our manuscript were performed twice to confirm the findings.
RESULTS
In vitro characterization of tumor lysates to be used for vaccination strategies
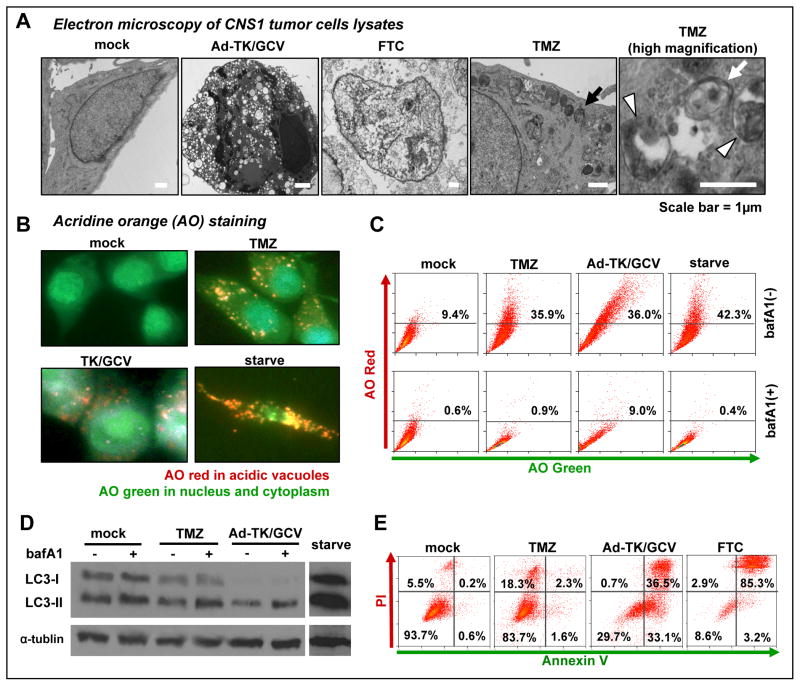
Tumor cell death mechanisms have been implicated in the degree of immunogenicity induced by tumor lysates (25, 26). Thus, we characterized the mechanisms of tumor cell death induced by Ad-TK/GCV, TMZ and freeze-thawing cycles (FTC) in vitro, which we used to prepare tumor cell lysates for vaccination. Electron microscopy analysis indicated that CNS1 cells treated with Ad-TK/GCV exhibited chromatin condensation and cytoplasmic vacuolization, which are typical features of apoptotic cell death (Fig. 1A). FTC-treated CNS1 cells showed typical features of necrosis including cytoplasmic swelling, plasma membrane and cytoplasmic organelle destruction (Fig. 1A). TMZ induces autophagy in CNS1 rat brain tumor cells, displaying an increased number of autophagic vacuoles in the cytoplasm (black arrow), including autophagosomes, double membrane vacuoles containing organelles (Fig 1A; white arrow) and autolysosomes vesicular organelles containing digested residual materials (Fig 1A; white arrowheads). Autophagy was also observed in Ad-TK/GCV treated tumor cells (Suppl Fig. 1).
Figure 1. Characterization of whole cell tumor lysate to be used in vaccination.
A, Electron micrographs showing the ultrastructure of CNS1 cells treated with either Ad-TK plus GCV (Ad-TK/GCV) for 72h, three cycles of freeze-thawing (FTC), or 200μM temozolomide (TMZ) for 72h. A black arrow highlights an autophagosome in CNS1 cells treated with TMZ. A white arrow indicates an autophagosome with a characteristic double membrane structure. A white arrowhead identifies an autophagolysosome with characteristic residual digested materials. B, Acridine orange (AO) staining of CNS1 cells treated with TMZ, Ad-TK/GCV or serum-starvation. Red staining reveals acidic vacuoles corresponding to autophagolysosomes, green indicates non-acidic compartments. C, CNS1 cells were treated with TMZ, Ad-TK/GCV or starvation in the presence or absence of bafilomycin A1 (bafA1). Cells were stained with AO and analyzed by flow cytometry. The proportions of cells with acidic vacuoles (AO red positive) are shown in representative dot plots. D, CNS1 cells were treated with TMZ, Ad-TK/GCV or starvation in the presence or absence of bafA1 and analyzed by Western blotting using a primary antibody for LC-3B; α-tubulin was used as a loading control. E, Cell death in CNS1 cells treated with TMZ, Ad-TK/GCV or FTC was assessed by flow cytometry following staining with Annexin V-FITC for apoptosis and propidium iodide (PI) for necrosis. The proportion of cells in each quadrant are shown in representative samples.
Autophagy is an evolutionarily conserved intracellular degradative process, where cytoplasmic organelles are sequestered into lytic compartments; characterized by the formation of acidic vesicular organelles called autolysosomes (30). To quantify the extent of autophagy induction in CNS1 brain tumor cells, we determined the percentage of cells with accumulated acidic vesicular organelles using acridine orange staining (Fig 1B–C). Acridine orange moves freely across biological membranes and fluoresces green when in an uncharged environment, whereas its protonated form accumulates in acidic compartments and fluoresces bright red (31, 32). The intensity of red fluorescence is proportional to the degree of acidity. As one of the most efficient triggers of autophagy is nutrient starvation (30, 32), serum-starved cells were used as a positive control. Fluorescence microscopy analysis shows accumulation of red fluorescence in the cytoplasm in CNS1 tumor cells treated either with TMZ or Ad-TK/GCV, indicating the presence of acidic compartments (Fig. 1B). Flow cytometry analysis demonstrates that both TMZ and Ad-TK/GCV increased red fluorescence intensity from 9.4% (basal levels) to 35.9% and 36.0%, respectively, comparable with serum-starved cells (42.3%). Increased red florescence in CNS1 cells treated with TMZ or Ad-TK/GCV was abolished by treatment with bafilomycin A1, an inhibitor of the fusion of autophagosomes and acidic lysosomes (33) (Fig. 1C). Freeze-thaw lysates did not acquire acridine orange staining. These findings indicate that CNS1 cells treated with TMZ or Ad-TK/GCV exhibit acidic vesicular organelle formation associated with autophagy. Autophagy induction was also assessed by Western blotting of LC3, a specific marker for autophagy (32) (Fig 1D). When cells undergo autophagy, LC3-I is incorporated into autophagosomes and converted to LC3-II (lapidated form). However, the increase in LC3-II expression can be associated with increased formation of autophagosomes, or an impaired autophagic degradation (34). To differentiate these two possibilities, LC3-II expression was assessed in the presence of bafilomycin A1, which blocks autophagic degradation (33). As shown in Fig. 1D, CNS1 cells treated with TMZ or Ad-TK/GCV exhibited further accumulation of LC3-II in the presence of bafilomycin A1, thus supporting that the increased LC3-II expression is a consequence of increased levels of autophagosome formation rather than impaired degradation.
The extent of apoptosis and necrosis in CNS1 brain tumor cells was assessed by flow cytometry following Annexin V and propidium iodide (PI) staining (Fig. 1E). Our results indicate that Ad-TK/GCV induced mainly apoptosis (69.6%), TMZ induced a small population of necrotic cells (18.3%) and minimal apoptosis (3.9%), and FTC induced mostly late apoptosis/necrosis (88.2%) (Fig 1E). Taken together, these data demonstrate that Ad-TK/GCV induces apoptosis that is accompanied with autophagy, TMZ induces mainly autophagy, and FTC induces necrosis in CNS1 brain tumor cells.
Vaccination using Ad-TK/GCV or TMZ generated tumor lysates induced enhanced anti-tumor immunity than necrotic tumor cell’s lysates
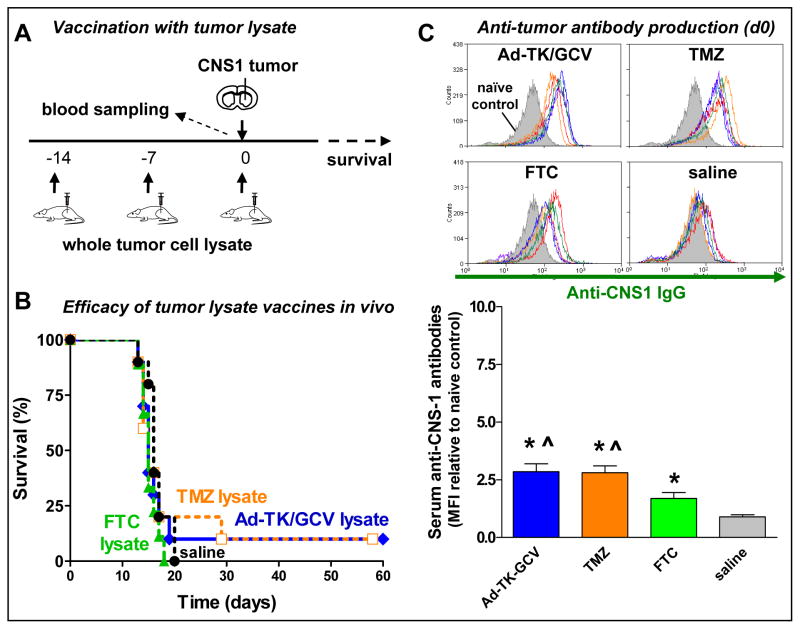
To assess whether immunogenicity of tumor cell lysates is dependent on the mechanism of tumor cell death in our model, we compared the therapeutic efficacy of whole tumor cell lysate vaccines generated by treatment of CNS1 cells with Ad-TK/GCV, TMZ or FTC. In the first paradigm, the animals were vaccinated before tumor cell implantation (Fig 2A). Lysates were administered subcutaneously three times before CNS1 tumor cell implantation into the striatum (Fig 2A). Vaccination with tumor lysates prepared from any of the three methods tested, failed to significantly prolong median survival (Fig 2B). All tumor lysate vaccines increased the levels of circulating anti-CNS1 antibodies in the serum when compared with saline controls (Fig 2C). Levels of circulating anti-CNS1 antibodies were significantly higher in rats vaccinated with Ad-TK/GCV- or TMZ-generated cell lysates than in rats treated with FTC-generated cell lysates (Fig 2C). These findings demonstrate that Ad-TK/GCV-treated (apoptotic and autophagic) or TMZ-treated (autophagic) tumor lysates induce more potent anti-tumor immune responses than FTC lysates (necrotic).
Figure 2. Therapeutic efficacy of vaccination using whole tumor cell lysates.
A, Lewis rats were implanted with 5,000 CNS1 glioma cells in the striatum. Animals were systemically vaccinated (s.c.) with whole tumor cell lysates three times before tumor cell implantation (day −14, −7 and 0). B, Kaplan Meier survival curves of rats treated with Ad-TK/GCV lysate, TMZ lysate, FTC lysate or saline. Vaccination with tumor lysates prepared from any of the three methods tested, failed to significantly prolong median survival. C, Serum levels of anti-CNS1 cell antibodies at the time of tumor inoculation were measured by flow cytometry. Overlays show the fluorescence intensity of CNS-1 cells labelled with serum from non-tumor bearing rats (naïve control; filled, grey histogram) or with serum from rats from each experimental group (unfilled histograms). * p < 0.05 vs. saline, ^ p < 0.05 vs. FTC lysate; one-way ANOVA followed by Tukey’s test.
Flt3L-expanded DCs secrete high levels of type 1 cytokines and elicit potent anti-tumor immunity
DC differentiation and proliferation can be elicited by either Flt3L or GM-CSF (35–37). Also, recent studies have shown that IL6 improves expansion of Flt3L-induced DCs (35, 38). To determine whether vaccination with Flt3L-induced DCs elicits anti-tumor immunity, we compared in vitro and in vivo the characteristics of BMDCs induced by GM-CSF+IL4 or Flt3L+IL6 (Fig 3). Consistent with previous reports (35, 38), expansion of DCs was higher in Flt3L-treated than GM-CSF+IL4-treated BM cultures; the addition of IL-6 greatly enhanced the proliferation of Flt3L-induced DCs (Suppl. Fig 2A). Microscopic analysis showed that under both conditions, DCs exhibited long cytoplasmic processes, a typical feature of DCs (Suppl. Fig 2B). Loosely attached cells conditioned with Flt3L+IL6 were analyzed by flow cytometery; ~33% of the cells displayed elevated levels OX-62, expressed by rat conventional DCs (cDCs; CD11c+ CD45high MHCII+) but not plasmacytoid DCs (pDCs; CD3- CD4+ CD45R+), and ~94% displayed elevated levels of the NK marker CD161a on their cell surface, a phenotype characteristic of all rat DC subtypes (39). Flow cytometric analysis of cell surface markers revealed that a majority of Flt3L+IL6 conditioned DCs displayed a characteristic phenotype of cDCs; a very small population of DCs expressed cell surface markers characteristic of pDCs (39) (Suppl. Figure 3).
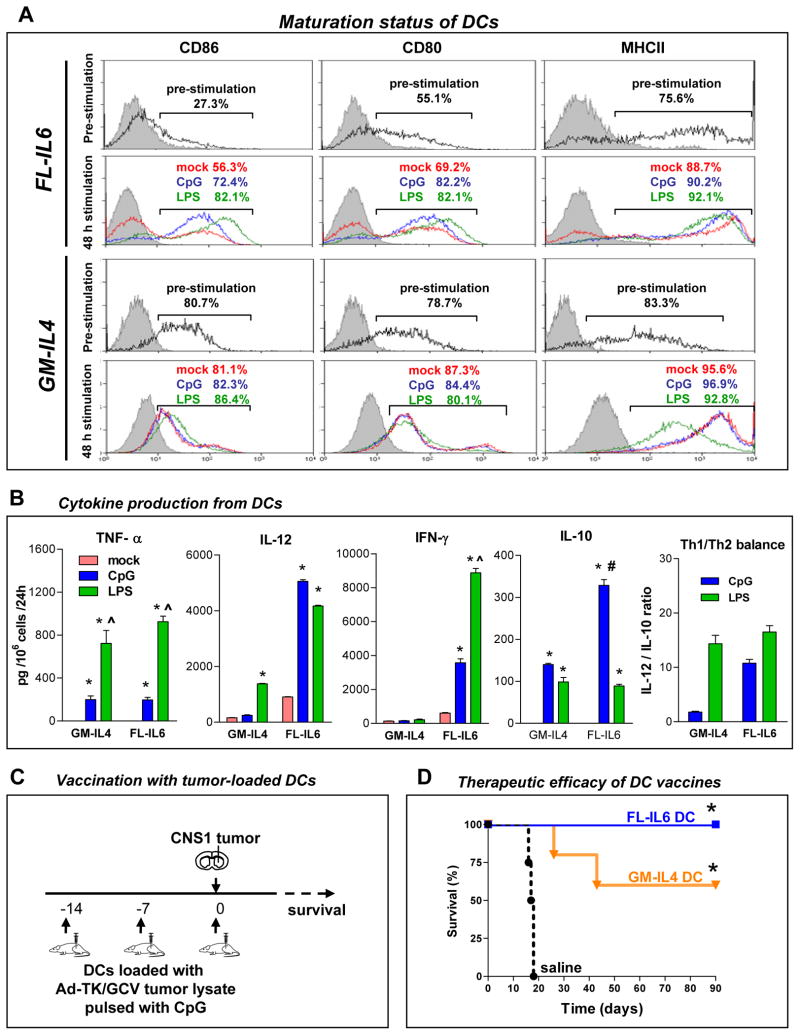
Figure 3. Characterization of bone marrow-derived dendritic cells (DCs) induced by Flt3L+IL-6 or GM-CSF+IL-4.
Ten million bone marrow cells were cultured in RPMI conditioned media supplemented with 100 μg/ml Flt3L + 50 μg/ml IL-6 (FL-IL6), 100 μg/mL Flt3L (FL) or 10 μg/mL GM-CSF + 10 μg/mL IL-4 (GM-IL4) every 2–3 days. Loosely attached cells were harvested at day 7–8. A, Surface molecules of FL-IL6-induced and GM-IL-4-induced DCs were stained after 7 days culture (pre-stimulation) or after stimulation with either 100 ng/mL CpG2006, 50ng/ml LPS, or mock (48h stimulation) and then measured by flow cytometry. Overlays from one representative sample are depicted. The proportion of cells positive for each surface marker are shown in representative samples. B, Cytokine production from FL-IL6 -induced and GM-IL4 induced DCs. Immature DCs harvested after 7 days in culture were treated with either CpG, LPS or mock for 48h. Supernatant was collected and cytokines were measured by ELISA. C, Experimental design to assess therapeutic efficacy of FL-IL6 or GM-IL4 generated DC vaccines in rats challenged with brain tumors. Lewis rats were systemically vaccinated three times (day −14, −7 and 0) with either FL-IL6 -induced or GM-IL4 induced DC vaccines (s.c.) loaded with Ad-TK/GCV treated whole tumor lysate, or saline as a control, before CNS1 tumor cell implantation (5,000 cells) in the striatum. D, Kaplan Meier survival curves of rats treated with FL-IL6 induced DC vaccine (n = 5), GM-IL4-induced DC vaccine (n = 5) and saline (n = 5) are shown. * p < 0.05 vs. saline; Mantel log rank test.
Maturation of DCs, with up-regulation of MHCII or co-stimulatory molecules such as CD86 and CD80, is critical to induce the clonal expansion of antigen-specific T cell populations (40). Thus, we evaluated the maturation status of BMDCs incubated with Flt3L+IL6 or GM-CSF+IL4. Flt3L+IL6 generated DCs expressed CD86 and CD80 (27.3% and 55.1%, respectively); GM-CSF+IL4 generated DCs expressed higher levels of CD86 and CD80 (80.7% and 78.7%, respectively; Fig 3A). MHCII expression was similar in both Flt3L+IL6- and GM-CSF+IL4 cultured DCs (75.6% and 83.3%, respectively; Fig 3A). In order to induce further maturation of DCs, cells were further incubated for 48 h without cytokines in the presence or absence of activation agents CpG oligodendronucleotide (CpG-ODN) or lipopolysaccharide (LPS). Deprivation of cytokines induced up-regulation of CD86, CD80 and MHCII (Fig 3A). Stimulation by CpG-ODN or LPS further increased the expression of those surface molecules in Flt3L+IL6 cultured DCs. Treatment with CpG-ODN or LPS had little effects on the expression of maturation markers on GM-CSF+IL4 induced DCs (Fig 3A). These findings indicate that Flt3L+IL6 conditioning maintains BMDCs in a more immature state (lower expression of maturation makers) when compared to GM-CSF+IL4. The balance between the induction of T helper cell type 1 (Th1) and Th2 CD4+ T cells determines the efficiency and the type of immune response. Secretion of Th1-polarizing cytokines such as IL-12 and IFN-γ produced by DCs, which promote a shift toward Th1 dominant balance, has been implicated in potent anti-tumor immunity (40). Thus, we compared the cytokine secretion profile between GM-CSF+IL4- and Flt3L+IL6-conditioned DCs by ELISA (Fig 3B). Activation of DCs by CpG-ODN or LPS promoted production of both Th1-polarizing cytokines, TNF-α, IL-12 and IFN- γ, and the Th2-polarizing cytokine, IL-10 (40) (Fig 3B). Although there was no difference in the secretion pattern of TNF-α, both IL-12 and IFN-γ were more robustly produced by Flt3L+IL6-generated DCs as compared with GM-CSF+IL4-generated DCs (Fig 3B). Despite evidence of up-regulated production of Th1-polarizing cytokines IL-12 and IFN-γ, we also observed elevated levels of IL-10 production in Flt3L+IL6-generated DCs. The ratio between IL-12 and IL-10 represents the balance between Th1 and Th2 polarizing cytokine production by DCs. (20). Flt3L+IL6 generated DCs displayed a high Th1 balance when stimulated with either CpG or LPS, however, GM-CSF+IL4 generated DCs only displayed a high Th1 balance when stimulated LPS, but not CpG (Fig 3B).
Functionally, DCs are characterized by their capacity to present antigen to T cells and induce clonal expansion of antigen-specific T cells. Thus, we assessed the capacity of Flt3L+IL6 conditioned DCs loaded with Ad-TK/GCV derived tumor lysates to induce T cell proliferation. Splenocytes from tumor bearing rats were co-cultured with tumor-loaded BMDCs, unloaded BMDCs or no BMDCs (Suppl Fig 4A). T cell proliferation was only induced when T lymphocytes were co-cultured with tumor-loaded DCs (Suppl Fig 4B). T cells stimulated by CNS1-loaded DCs effectively killed CNS1 tumor cells in vitro in a dose dependent manner, when compared to lymphocytes from a naïve rat as a control (Suppl Fig 4C). Thus, these data demonstrate that tumor loaded BMDCs have the capacity to induce the proliferation of tumor specific cytotoxic T cells.
While most DC vaccination clinical trials rely on GM-CSF to induce the proliferation and conditioning of DCs, recent studies have shown that Flt3L and IL-6 improves expansion of DCs (38). As such, we assessed the in vivo therapeutic efficacy of systemic vaccination with DCs generated using Flt3L+IL6 or GM-CSF+IL4 loaded with Ad-TK/GCV derived tumor lysates. DCs were delivered three times at 7-day intervals before tumor implantation (days −14, −7 and 0). Vaccinated rats were then challenged with CNS1 cells in the striatum at day 0 (Fig 3C). Rats vaccinated with Flt3L+IL6-generated DCs completely inhibited tumor growth and exhibited 100% long-term survival, whereas only 60% of rats vaccinated with DCs generated by GM-CSF+IL4 exhibited long term survival (Fig 3D). These data suggest that DCs generated with Flt3L+IL6 result in vaccines that induce more robust anti-tumor immune responses than DC vaccines prepared with GM-CSF+IL4.
Mechanism of cell death used to generate tumor lysates determines efficacy of DC vaccination
Uptake of tumor cell remnants followed by processing and presentation of tumor antigens to naïve T cells by DCs is critical for the induction of adaptive immunity. Thus, we tested whether tumor uptake by DCs depends on the method used for tumor cell killing, i.e., autophagy, apoptosis and necrosis. As shown in Fig 4A, DCs displayed higher levels of phagocytic activity when co-cultured with dying tumor cells, as compared to mock treated cells. Furthermore, Ad-TK/GCV or TMZ treatment increased tumor uptake by DCs when compared with FTC treatment.
Figure 4. Comparative efficacy of Flt3L + IL-6 induced DC vaccines loaded with Ad-TK/GCV, TMZ or FTC tumor lysates.
A, Phagocytic activity of FL-IL6-induced DCs. DCs labeled with CD161a-PE were co-cultured for 5h with Qtracker-655 labeled CNS1 tumor cells that were treated with either Ad-TK/GCV, TMZ or FTC. Phagocytosis of tumor cells by DCs was assessed by flow cytometry; representative dot plots and corresponding percentage of double labeled cells are shown. Data are represented as the percentage of double-stained versus CD161a-PE positive cells. * p < 0.05 vs mock, ^ p < 0.05 vs F/T one-way ANOVA followed by Tukey’s test. B, Confocal micrograph shows a representative DC (green, CD161a) that engulfed tumor cell remnants (red, Qtracker 655); nuclei were labeled with DAPI (blue). The large square in the top left is a maximum projection of the confocal Z stack. The images to the right and below are reconstructed vertical sections along the fine green lines shown on the maximum projection. C, Lewis rats were systemically vaccinated with DC vaccines (s.c.) loaded with tumor lysates prepared by Ad-TK/GCV, TMZ, or FTC treatment, or saline as a control, three times before tumor cell implantation (day −14, −7 and 0) with 5,000 CNS1 glioma cells in the striatum. D, Kaplan Meier survival curves of rats treated with FL-IL6-induced DC vaccines loaded with either TK/GCV lysate (DC-TK/GCV, n = 5), TMZ lysate (DC-TMZ, n = 5) or F/T lysate (DC-F/T, n = 5), or unloaded DCs or saline as controls, are shown. * p < 0.05 vs. saline; Mantel log rank test. E, Serum levels of anti-CNS1 cell antibodies at the time of tumor implantation were measured by flow cytometry. * p < 0.05 vs. saline, ^ p < 0.05 vs. DC loaded with FTC lysate; one-way ANOVA followed by Tukey’s test.
We next tested whether the mechanism of tumor cell killing used to prepare the tumor lysates influences the therapeutic efficacy of DC vaccination. Tumor lysate loaded DCs were administered s.c. into Lewis rats three times at 7-day intervals before tumor implantation (days −14, −7 and 0). Vaccinated rats were then challenged with CNS1 cells in the striatum at day 0 (Fig 4C). Rats receiving DCs loaded with TMZ- or Ad-TK/GCV-generated tumor lysates completely inhibited tumor development and animals exhibited 100% long term survival, whereas only 60% of rats vaccinated with DCs loaded with FTC tumor lysates survived long term (Fig 4D). Levels of circulating anti-CNS1 antibodies were significantly higher in rats treated with DCs loaded with either Ad-TK/GCV or TMZ-generated tumor lysates than in rats treated with DCs loaded with FTC tumor lysates (Fig 4E).
Combining in situ immunogene therapy and DC vaccination significantly improves anti-tumor therapeutic efficacy
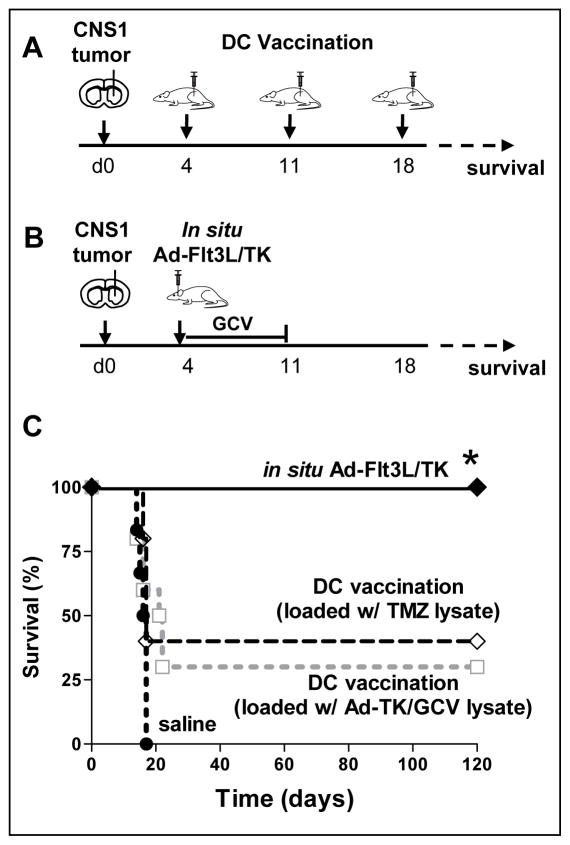
DC vaccination strategies have been shown to induce anti-tumor immune responses in patients with GBM undergoing clinical trials (3, 5–10). In this report, we wished to test the hypothesis that manipulating the tumor microenvironment via in situ immunogene therapy would enhance therapeutic efficacy of DC vaccination. First, we assessed whether DCs loaded with either Ad-TK/GCV- or TMZ-generated tumor lysates in combination with CpG as an adjuvant could elicit regression of an established intracranial tumor mass in the Lewis rat model. We compared therapeutic efficacy of in situ immunogene therapy to DC vaccination in a small tumor model, where tumor bearing rats were treated 4 days after tumor implantation (Fig 5A). In the small brain tumor model, Ad-Flt3L + Ad-TK immunogene therapy induced 100% survival, whereas we observed comparable therapeutic efficacy with either the DC vaccine loaded with TMZ-derived tumor lysates or the DC vaccine loaded with TK/GCV-derived tumor lystaes in the small tumor model (Fig 5B).
Figure 5. Treatment efficacy of in situ immunogene therapy and DC vaccination in a small GBM model.
Lewis rats were implanted with 5,000 CNS1 GBM cells. After 4 days, rats were treated with either (A) serial s.c. injections of Flt3L+IL6-induced DC vaccine loaded with either Ad-TK/GCV treated tumor lysate (DC-TK/GCV), or TMZ treated tumor lysate (DC-TMZ) every 7 days, or with (B) intratumoral administration of Ad-TK+Ad-Flt3L followed by GCV administration for 7 days. C, Kaplan-Meier survival curve of rats treated with in situ Ad-TK+Ad-Flt3L (n = 5), DC-TK/GCV vaccine (n = 10), DC-TMZ vaccine (n = 5), or saline (n = 6). * p < 0.05 vs. saline; Mantel log rank test.
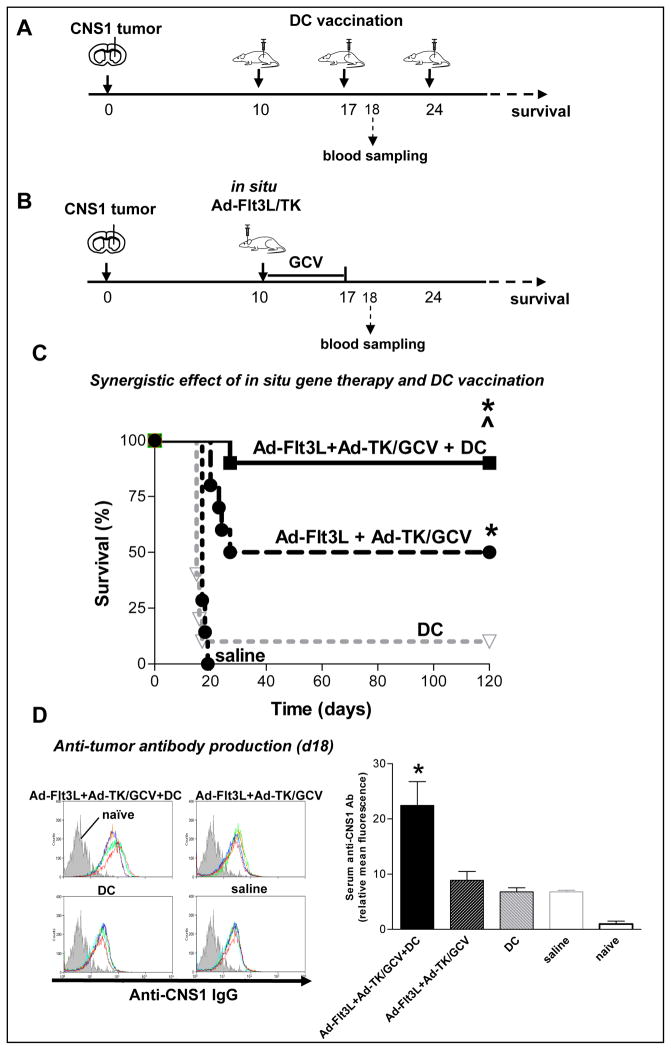
We next tested whether in situ immunogene therapy and DC vaccination could be used in combination to potentiate therapeutic efficacy in a rigorous, large tumor model, where tumor bearing rats were treated 10 days after tumor implantation (Fig 6A). The combination treatment of in situ immunogene therapy with DC vaccination led to long-term survival in ~90% of tumor bearing animals. These data demonstrate a 40% increase in levels of long-survival when compared to in situ immunogene therapy alone, and an 80% increase in levels of long-survival when compared to DC vaccination alone. The therapeutic efficacy data are corroborated by serum levels of anti-CNS1 antibodies in animals treated with combination therapy, which were increased by ~2.5 fold when compared to either arm of the therapy alone (Fig 6C).
Figure 6. Therapeutic efficacy of in situ immunogene therapy and DC vaccination in a large intracranial GBM model.
A–B, Lewis rats were implanted with 5,000 CNS1 GBM cells. After 10 days, rats were treated either with (A) in situ (intratumoral) Ad-TK, in situ Ad-TK+Ad-Flt3L, (B) subcutaneous Flt3L+IL-6-induced DC vaccine loaded with Ad-TK/GCV tumor lysate, or a combination of in situ Ad-TK+Ad-Flt3L gene therapy plus DC vaccination. Anti-CNS1 antibodies were measured 1 day after the second DC vaccine administration (day 18). C, Kaplan-Meier survival curve of rats treated with in situ Ad-TK+Ad-Flt3L combined with DC vaccination (n = 10), in situ Ad-TK+Ad-Flt3L (n = 10), in situ Ad-TK combined with DC vaccine (n = 10), in situ Ad-TK (n = 10), DC vaccine (n = 10), or saline (n = 7). * p < 0.05 vs. saline, ^ p < 0.05 vs. Ad-TK, # p < 0.05 vs. Ad-TK+Ad-Flt3L; Mantel log rank test. D, Serum levels of anti-CNS1 antibodies measured by flow cytometry. Histograms show the fluorescence intensity of CNS1 cells labeled with serum from non–mtumor bearing rats (isotype control; gray histogram) or with serum from rats from each of the experimental groups (colored lines) * p < 0.05 vs. saline; one-way ANOVA followed by Tukey’s test.
DISCUSSION
DC vaccination approaches for brain tumors have been implemented in human clinical trials (5–10). Several clinical trials for GMB using DC vaccines have demonstrated cellular and humoral anti-tumor immune responses; and albeit safe, the clinical efficacy of DC vaccination for GBM remains limited (3, 5–10). Our group has shown that in situ Ad-Flt3L/TK-mediated immunogene therapy elicits an influx of DCs, macrophages, CD4+ T cells, and CD8+ T cells into the brain tumor microenvironment (17) and stimulates effective anti-GBM immune response resulting in tumor regression and long-lasting CD8+ T cell mediated anti-tumor immunological memory in several mouse and rat orthotopic brain tumor models (16, 18, 22–24, 29). Based on these data, a Phase I clinical trial for GBM was recently cleared by the FDA and is slated to commence in 2011.
The combined conditional cytotoxic/immuno-stimulatory gene therapy approach, utilizes HSV1-TK to kill actively dividing brain tumor cells in the presence of GCV, thereby releasing endogenous brain tumor antigens and also innate immune adjuvants, such as high-mobility group protein B1 (HMGB1) (18, 21). HMGB1 belongs to a class of innate immune adjuvants called damage associated molecular pattern molecules (DAMPs) which mediate signaling by binding to a family of receptors called pattern recognition receptors (PPRs), thereby promoting innate and adaptive immune responses (41). DCs express a large repertoire of PRRs and several studies have shown that signaling through of PPRs leads to DC activation, which is characterized by high levels of MHC-antigen complexes on the DC cell surface, upregulation of co-stimulatory molecules such as CD80 and CD86, and the production of cytokines such as IL-12 and IFNα. The production of these cytokines is directly involved in priming Th1 based immune responses (40). We have previously shown that HMGB1 is released from dying brain tumor cells in response to treatment with Ad-TK/GCV or TMZ, and acts as an endogenous TLR2 agonist to activate bone-marrow derived, brain tumor-infiltrating DCs (18). The other arm of our therapeutic strategy involves expression of Flt3L within the tumor microenvironment. Flt3L recreates a missing immune circuit from the brain, by inducing the expansion and migration of DCs into the brain tumor milieu where they encounter and phagocytose newly released endogenous brain tumor antigens (16, 18, 22–24, 29).
Herein we tested the hypothesis that combining Ad-Flt3L/TK in situ immunogene therapy with peripheral DC vaccination, would lead to enhanced therapeutic efficacy in a syngeneic brain tumor model. Our data demonstrate that the therapeutic combination, i.e., in situ immunogene therapy with DC vaccination led to long term survival in 90% of rats bearing large, syngeneic brain tumors; showing a ~40% increase in survival compared to Ad-Flt3L/TK immunogene therapy alone and ~80% increase in survival compared to DC vaccination alone. Our results also showed enhanced anti-tumor humoral immune response, i.e., ~2.5 fold increase in the levels of circulating anti-tumor antibodies compared to either treatment alone.
The enhanced therapeutic efficacy observed could result from expression of Flt3L within the brain tumor microenvironment which could enhance the trafficking of systemically delivered DC vaccines to the draining lymph nodes, where they present tumor antigens to naïve T cells and induce the clonal expansion of tumor-specific CTLs. To this effect, it has been previously shown that Flt3L elicits recruitment of DC populations, including fully differentiated DCs in lymphoid organs (40) and traffic of subcutaneously delivered DC vaccines to the inguinal draining lymph nodes in mice (42). We demonstrated that intracranial delivery of Ad-Flt3L leads to circulating Flt3L in rat serum (19), thus supporting hypothesis that Flt3L could also act systemically and enhance the trafficking of DCs to draining lymph nodes. Increased trafficking of systemically delivered DCs to draining lymph nodes could lead to enhanced priming of anti-tumor immune responses, inducing increased levels of tumor regression and long-term survival as observed in this study.
An additional explanation for the increased therapeutic efficacy of in situ immunogene therapy in combination with DC vaccination is that DAMPs, i.e. HMGB1, are known to enhance the activation status of DCs and facilitate Th1 immune responses (40). We have previously shown that treatment of intracranial brain tumors with TK/GCV results in increased levels of circulating HMGB1 in the sera of mice (18) and rats (21). Therefore HMGB1 released into the systemic circulation could further activate systemically delivered DCs, thus enhancing their ability to prime an adaptive, anti-tumor immune response.
When used to treat small, established intracranial tumors, DC vaccination exhibited higher levels of long-term survival (~30%) when compared to treatment of large intracranial tumors (~10% long-term survival). These data suggest that the efficiency of a DC vaccination approach is associated with the degree of tumor burden in the host. Furthermore, our data demonstrate that DC vaccination is highly efficacious (100% long-term survival) when administered before tumor implantation, suggesting that DC vaccines would be more effective at preventing tumor recurrences after initial surgical debulking, chemotherapy, and radiotherapy.
Effective uptake and loading of tumor-associated antigens onto DCs’ MHC complexes and expansion of DC subgroups that can efficiently prime naïve T cells play a critical role in the effectiveness of DC vaccination. Therefore, it is critically important to optimize the preparation of tumor cell antigens and dendritic cells. In this study, we compared the immunogenicity and levels of phagocytosis of apoptotic, autophagic, and necrotic tumor cells lysates. In line with previous evidence (26, 43, 44), our data indicate that triggering autophagy and/or apoptosis to generate tumor cell lysates increases the immunogenicity of tumor cells and enhances the delivery of tumor-associated antigens to DCs. Furthermore, we have previously reported that treatment of GBM cells with Ad-TK/GCV or TMZ results in the release of the endogenous TLR2 ligand HMBG1 from GBM cells (18). Autophagic GBM cells have also been shown to release HMGB1, without causing lysis of the cell membrane and classical necrosis (45). Previous reports demonstrated that DCs loaded with purified autophagosomes from autophagic tumor cells induced tumor-specific immune responses (26), suggesting that autophagosomes contain a wide range of tumor-associated antigens and immune adjuvants, i.e., HMGB1 (46). There is experimental evidence that suggests that intratumoral delivery of a recombinant cytotoxin composed of Pseudomonas exotoxin fused to IL-13 into mice bearing human xenografts causes cell death not only by apoptosis, but also by necrosis. Furthermore, in this paper, Kawakami et al. demonstrate that delivery of the IL13-PE cytotoxin induced phagocytes which may play a role in cytotoxin mediated tumor regression (47). Additionally, it has been previously demonstrated that the fusion of the recombinant Pseudomonas exotoxin to a model tumor antigen may enhance vaccine potency (48).
GM-CSF combined with IL-4 has been previously used to generate DCs from both murine and human bone marrow progenitor cells (30). Flt3L has been introduced as an alternative means to generate DCs (35, 37, 49) and recent reports demonstrated that Flt3L combined with IL-6 enhances the expansion of Th1-polarizing DCs, a requirement for the efficient induction of anti-tumor immune responses (50). Thus, we compared the characteristics of GM-CSF+IL4- and Flt3L+IL6-generated DCs to establish the optimal parameters for ex vivo expansion of DCs to be used in the vaccination paradigms described. Our results showed that ex vivo conditioning with Flt3L resulted in higher levels of DCs when compared to GM-CSF+IL4; these results are in line with results previously obtained with canine DC cultures (49). Levels of Th1 polarizing cytokines such as IL-12 and IFN-γ were higher when DCs were cultured with Flt3L+IL6. These data are in line with evidence that Flt3L induces DCs that preferentially secrete Th1-polarizing cytokines, when compared with GM-CSF cultured DCs (35). Consistent with these results, inhibition of tumor progression and therapeutic efficacy of DC vaccination was higher when we used Flt3L+IL6-generated DCs. Addition of IL-6 in combination with Flt3L further enhanced the proliferation of BMDCs. Flt3L+IL6 induced DCs displayed decreased levels of cell surface markers CD80, CD86, MHCII and exhibited high phagocytic capacity consistent with an immature phenotype.
The advantage of combining immunogene therapy (Ad-Flt3L/Ad-TK) with DC vaccination is that gene therapy can be administered into the tumor bed/cavity at the time of surgical resection to immediately initiate anti-brain tumor immune responses whilst the autologous DC vaccine with autologous tumor lysate is being prepared ex vivo. After the DC vaccine preparation is completed, it can be administered using the appropriate vaccination regime to enhance the anti-tumor immunity and therapeutic efficacy. In summary, the data presented demonstrate that immunogene therapy not only elicits tumor regression and anti-tumor immunity, it also potentiates the therapeutic efficacy elicited by DC vaccination and support the implementation of novel Phase I clinical trials to assess the safety and efficacy of this combined therapeutic strategy.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVENCE.
Dendritic cell (DC) vaccination strategies have been shown to induce anti-tumor immune responses in human patients undergoing clinical trials for glioblastoma multiforme (GBM). Reports suggest the immunogenicity of DC vaccination strategies depends on the methodologies used to generate tumor cell lysates, and to condition DCs. We tested the hypothesis that modifying the tumor microenvironment using in situ Ad-Flt3L/Ad-TK-mediated gene therapy, which involves expression of Flt3L and TK within the tumor mass with concomitant release of tumor antigens and HMGB1, would potentiate the therapeutic efficacy and anti-tumor immunity induced by DC vaccination. In situ immunogene therapy in combination with DC vaccination led to long-term survival in ~90% of animals, a significant increase when compared to either therapy alone, indicating that immunogene therapy potentiates DC vaccination induced therapeutic efficacy and anti-tumor immune responses. These data support novel Phase I clinical trials to assess the safety and efficacy of this combined approach.
Acknowledgments
Funding: Our work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grant 1R21-NS054143; 1UO1 NS052465 and 1RO1-NS057711 to M.G.C.; NIH/NINDS Grants 1RO1-NS 054193; and 1RO1-NS061107 to P.R.L; F32NS0503034 to GDK. The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics to PRL and MGC, respectively, The Linda Tallen & David Paul Kane Foundation Annual Fellowship, The Drown Foundation and the Board of Governors at CSMC.
References
- 1.Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmittling RJ, Archer GE, Mitchell DA, Heimberger A, Pegram C, Herndon JE, 2nd, et al. Detection of humoral response in patients with glioblastoma receiving EGFRvIII-KLH vaccines. J Immunol Methods. 2008;339:74–81. doi: 10.1016/j.jim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Heimberger AB, Sun W, Hussain SF, Dey M, Crutcher L, Aldape K, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20:267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 6.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 7.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 8.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T cell infiltration and survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2010 Dec 6; doi: 10.1158/1078-0432.CCR-10-2563. (Epub ahead of full print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15:114–121. doi: 10.1016/j.jocn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 11.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA Vaccines, Blood & Biologics. Cellular & Gene Therapy Products; Approved Products: PROVENGER (sipuleucel-T) Vol. 2011. 2010. [Google Scholar]
- 14.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 15.Kantoff P, Higano CS, Shore ND, Berge ER, Eric JSmall MD, Penson DF, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M, et al. Treg Depletion Inhibits Efficacy of Cancer Immunotherapy: Implications for Clinical Trials. PLoS ONE. 2008;3:e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtin J, Liu N, Candolfi M, Xiong W, Assi A, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Medicine. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Candolfi M, Yagiz K, Foulad D, Alzadeh GE, Tesarfreund M, Muhammad AK, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res. 2009;15:4401–4414. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King GD, Muhammad AKM, Curtin JF, Barcia C, Puntel M, Liu C, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King GD, Kroeger KM, Bresee CJ, Candolfi M, Liu C, Manalo CM, et al. Flt3L in combination with HSV1-TK mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol Ther. 2008;16:682–690. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King G, Muhammad AKMG, Larocque D, Kelson K, Xiong W, Liu C, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neo-antigen. Molecular Therapy. 2011 doi: 10.1038/mt.2011.77. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starck L, Scholz C, Blankenstein T, Dorken B, Daniel PT. Necrotic death but not irradiation abolishes costimulation of T-cell effector functions and survival by CD80-expressing tumor cells. Int J Cancer. 2005;116:78–86. doi: 10.1002/ijc.20792. [DOI] [PubMed] [Google Scholar]
- 26.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer. 2003;103:205–211. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 27.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2011;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 28.Marcusson-Stahl M, Cederbrant K. A flow-cytometric NK-cytotoxicity assay adapted for use in rat repeated dose toxicity studies. Toxicology. 2003;193:269–279. doi: 10.1016/s0300-483x(03)00302-0. [DOI] [PubMed] [Google Scholar]
- 29.Ghulam Muhammad AK, Candolfi M, King GD, Yagiz K, Foulad D, Mineharu Y, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 32.Tasdemir E, Galluzzi L, Maiuri MC, Criollo A, Vitale I, Hangen E, et al. Methods for assessing autophagy and autophagic cell death. Methods Mol Biol. 2008;445:29–76. doi: 10.1007/978-1-59745-157-4_3. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 34.Mizushima N. Methods for monitoring autophagy using GFP-LC3 transgenic mice. Methods Enzymol. 2009;452:13–23. doi: 10.1016/S0076-6879(08)03602-1. [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Miyagi T, Satoh E, Sugiura W, Yamamoto N, Kimura H. Phenotype and function of GM-CSF independent dendritic cells generated by long-term propagation of rat bone marrow cells. Cell Immunol. 2004;229:117–129. doi: 10.1016/j.cellimm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Brissette-Storkus CS, Kettel JC, Whitham TF, Giezeman-Smits KM, Villa LA, Potter DM, et al. Flt-3 ligand (FL) drives differentiation of rat bone marrow-derived dendritic cells expressing OX62 and/or CD161 (NKR-P1) J Leukoc Biol. 2002;71:941–949. [PubMed] [Google Scholar]
- 37.Weigel BJ, Nath N, Taylor PA, Panoskaltsis-Mortari A, Chen W, Krieg AM, et al. Comparative analysis of murine marrow-derived dendritic cells generated by Flt3L or GM-CSF/IL-4 and matured with immune stimulatory agents on the in vivo induction of antileukemia responses. Blood. 2002;100:4169–4176. doi: 10.1182/blood-2002-04-1063. [DOI] [PubMed] [Google Scholar]
- 38.Cohen PA, Koski GK, Czerniecki BJ, Bunting KD, Fu XY, Wang Z, et al. STAT3- and STAT5-dependent pathways competitively regulate the pan-differentiation of CD34pos cells into tumor-competent dendritic cells. Blood. 2008;112:1832–1843. doi: 10.1182/blood-2007-12-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubert FX, Voisine C, Louvet C, Heslan M, Josien R. Rat plasmacytoid dendritic cells are an abundant subset of MHC class II+ CD4+CD11b-OX62- and type I IFN-producing cells that exhibit selective expression of Toll-like receptors 7 and 9 and strong responsiveness to CpG. J Immunol. 2004;172:7485–7494. doi: 10.4049/jimmunol.172.12.7485. [DOI] [PubMed] [Google Scholar]
- 40.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 41.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 43.Brusa D, Garetto S, Chiorino G, Scatolini M, Migliore E, Camussi G, et al. Post-apoptotic tumors are more palatable to dendritic cells and enhance their antigen cross-presentation activity. Vaccine. 2008;26:6422–6432. doi: 10.1016/j.vaccine.2008.08.063. [DOI] [PubMed] [Google Scholar]
- 44.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, et al. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 45.Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol Pharm Bull. 2007;30:859–864. doi: 10.1248/bpb.30.859. [DOI] [PubMed] [Google Scholar]
- 46.Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16:175–183. doi: 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawakami M, Kawakami K, Puri RK. Intratumor administration of interleukin 13 receptor-targeted cytotoxin induces apoptotic cell death in human malignant glioma tumor xenografts. Mol Cancer Ther. 2002;1:999–1007. [PubMed] [Google Scholar]
- 48.Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;61:3698–3703. [PubMed] [Google Scholar]
- 49.Xiong W, Candolfi M, Liu C, Muhammad AK, Yagiz K, Puntel M, et al. Human Flt3L generates dendritic cells from canine peripheral blood precursors: implications for a dog glioma clinical trial. PLoS One. 2010;5:e11074. doi: 10.1371/journal.pone.0011074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parajuli P, Mosley RL, Pisarev V, Chavez J, Ulrich A, Varney M, et al. Flt3 ligand and granulocyte-macrophage colony-stimulating factor preferentially expand and stimulate different dendritic and T-cell subsets. Exp Hematol. 2001;29:1185–1193. doi: 10.1016/s0301-472x(01)00722-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.