Abstract
The innate immune system includes microbial pattern recognition receptors that detect bacteria and viral products at the cell surface, in vesicles, and within the cytoplasm. Transmembrane signaling occurs through Toll-like receptors (TLRs). Cytoplasmic receptors are generally members of the nucleotide-binding domain (NOD)-leucine-rich repeat (LRR) family (CATERPILLER family). They influence the effects of other family members and of TLRs. Most NOD-LRR members enhance signal transduction, but Monarch-1 counterbalances TLR activity. NOD-LRR family members also act within the adaptive immune system. The class II transactivator regulates major histocompatibility complex class II expression. In the intestine, it is developmentally regulated, and its expression depends on weaning and, independently, on age.
Keywords: CATERPILLER, nucleotide-binding domain, leucin-rich repeat, Toll-like receptor, class II transactivator, leucin-rich repeat- and pyrin domain-containing protein 3
Almost uniquely in the history of human warfare, the Knights Templar of the Middle East in the 13th century defended castles entirely surrounded by hostile territory belonging to an enemy as technologically advanced as themselves. Oxford University excavations undertaken 100 years ago by T. E. Lawrence (“Lawrence of Arabia”) discovered a radical departure from traditional defensive construction. Instead of a highly fortified perimeter enclosing a more central living area, the entire interior of the castle contained defensive structures. Much domestic construction was in front of the more internal defenses, and many buildings shared defensive and domestic functions. Excavations unearthed defensive architecture, even at the castles’ centers.
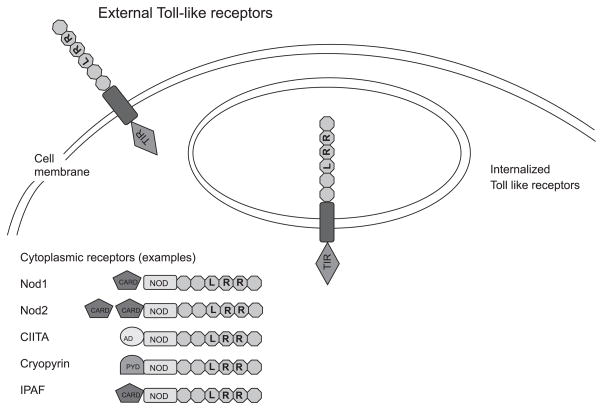
The eukaryotic cell is, similarly, not divided between a defensive exterior and a “housekeeping” interior. This is particularly true of cells of the immune system and gastrointestinal tract. Microbe-associated molecules (sometimes referred to as pathogen-associated molecular patterns) abound in the intestinal lumen. The innate immune system responds to them through pattern recognition receptors (PRRs). The extracellular receptors (Fig. 1) include Toll-like receptors (TLRs), whose expression is tightly controlled in the intestinal epithelium. Certain TLRs, like TLR3 and TLR9, interact with ligands that are topologically external to the cell but are enclosed within an internalized vacuole (Fig. 1). Intracellular pattern recognition occurs in a large family of receptors recently named the caspase recruitment domain (CARD), transcription enhancer, R (purine)-binding, pyrin, lots of leucine repeats (CATERPILLER) family (20) [or nucleotide-binding domain (NOD)-leucine-rich repeat (LRR) proteins (9)]. Members of this ancient family detect microbial products in plants and fungi as well as in animals. The proteins have three distinct functional domains: an amino-terminal effector domain involved in signaling, a centrally placed NOD, and carboxyl-terminal LRRs that act as a ligand recognition domain. In humans, there are over 20 members of this family, including Nod1 (CARD4) and Nod2 (CARD15).
Fig. 1.
Pattern recognition receptors recognize microbial products on the eukaryotic cell exterior, within vesicles, and in the cytoplasm. In general, Toll-like receptor (TLR)1, TLR2, TLR4, TLR5, and TLR6 act from the cell surface to detect peptide- and carbohydrate-based structures from bacteria. Microbial nucleic acid is internalized into vesicles and recognized by TLR3, TLR7, TLR8, and TLR9. Nucleotide-binding domain (NOD)-leucine-rich repeat (LRR) family (CATERPILLER family) members recognize microbial products in the cytoplasm. Each receptor, when ligated, stimulates one or more signal transduction pathways like NF-κB and JNK (but includes a wide range of processes including interferon regulatory factor). TIR, Toll-IL-1 receptor; CARD, caspase recruitment domain; CIITA, class II transactivator; IPAF, IL-1β-converting enzyme-protease-activating factor; PYD, pyrin domain; AD, activation domain.
This review highlights recent areas of research where NOD-LRR proteins act as PRRs or where they interact in cooperation (positively or negatively) with other NOD-LRRs or TLRs. It assumes a knowledge of the ligands for TLRs, which have been well described (3). The article describes instances where the expressions of proteins involved in these pathways are developmentally regulated. Finally, we allude to their importance in disorders of the gastrointestinal tract in addition to the well-recognized association of NOD2 with small intestinal Crohn’s disease. A major difficulty in the field is the number of different nomenclatures for the individual proteins in the NOD-LRR (or CATERPILLER) family. The variety extends to the name of the family itself, which, like many of its members, has three different designations. Recent attempts to standardize the nomenclature have merely added a further set of names. The review does not attempt to cover each of the possible names for each protein, which are listed in Table 1. However, the reader is referred to recent excellent articles that tabulate the family members and their differing nomenclatures more fully (9, 20). Because of space restrictions, this article does not include all relevant investigators or their work.
Table 1.
Glossary of alternative names for members of the NOD-LRR (CATERPILLER) family
| Name | Alternative Names |
|---|---|
| Class II transactivator | MHC2TA |
| Cryopyrin | CRL1.1, PYPAF1, NALP3, CIAS1 |
| IL-1β-converting enzyme-protease-activating factor | CLR2.1, CARD12, CLAN |
| Monarch-1 | CLR19.3, PYPAF7, NALP12, RNO2, PAN6 |
| Nod1 | CLR7.1, CARD4 |
| Nod2 | CLR16.3, CARD15 |
NOD1 AND NOD2 AS BACTERIAL DETECTORS
Because Nod1 is expressed in the cytoplasm (Fig. 1), original observations of its function as a bacterial detection system focused on the invasive bacteria Shigella flexneri (8) and enteroinvasive bacteria Esherichia coli (11). Both bacteria cause gastroenteritis characterized by blood in the stools. In experiments using epithelial cells that express functional Nod1, bacterial invasion caused an increase in signal transduction to NF-κB. Bacterial invasion induced oligomerization of Nod1. Such binding of molecules containing interacting domains is a central feature of members of the NDD-LRR family, which has been recapitulated in later discoveries in the study of other family members. Oligomerization, in turn, resulted in the expression of a series of NF-κB- and JNK-dependent genes. Introduction of dominant negative Nod1 blocked the effect of Shigella bacteria on NF-κB and JNK signal transcription. Listeria bacteria have similarly been shown to invade intestinal epithelial cells, stimulating Nod2.
The observation that bacteria stimulate internal bacterial receptors resulted in a search for the bacterial moieties that PRRs responded to. The peptidoglycan component of cell walls consists of a backbone of alternating residues of N-acetyl-glucosamine and N-acetyl-muramic acid (Fig. 2). N-acetyl-muramic acid is linked to side chains whose exact composition depends on the bacterial species. Ultimately, these side chains bind one string of N-acetyl-glucosamine/N-acetyl-muramic acid residues to another. Various synthetic or purified subunits of the of peptidoglycan moiety were given to eukaryotic cells transfected with Nod1 (or Nod2). The moiety that stimulated Nod1 muramyl dipeptide (MDP) contained the N-acetyl-muramic acid residue and is present in most bacteria. However, the Nod1 ligand γ-D-glutamyl-meso-diaminopimelic (iE-DAP) is found in most gram-negative bacteria and only in a few gram-positive ones. Macrophages from Nod1-deficient mice responded to MDP and double-stranded RNA but not to iE-DAP.
Fig. 2.
Nod1 and Nod2 recognize two closely related subunits of bacterial peptidoglycan. The Nod1 ligand γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) is found in gram-negative bacteria and in some gram-positive bacteria. When this includes the D-alanine residue from the peptidoglycan, the ligand is termed TriDAP. Neither the double residue (iE-DAP) of the triple residue (TriDAP) is part of the peptidogly-can backbone, which consists of alternating N-acetyl-glucosamine (GlcNAc)/N-acetyl-muramic acid residues (MurNAc). Nod2, however, recognizes MurNAc when linked to its adjacent side chain residues, muramyl dipeptide (MDP), which is present in both gram-positive and gram-negative bacteria. Regulation of the mechanisms that hydrolyze the cell wall and process the residues for Nod1 and Nod2 recognition is an area of active investigation. mDAP, meso-diaminopimelic acid.
It is easy to comprehend how bacterial invasion and MDP transport stimulates NOD-LRR intracellular receptors. Furthermore, the intestinal epithelial cell has a transporter, the H+-dependent gastrointestinal peptide transporter, that facilitates the entry of MDP into epithelial cell (22), and this may explain how external bacteria stimulate Nod2. However, bacteria that are not intracellular pathogens can also stimulate Nod1 cascades. This is because bacteria inject bacterial products into epithelial cells through type III secretion systems (as in enter-opathogenic E. coli bacteria, which inject proteins important for pedestal formation through a proboscis) or type IV secretion systems (where there is a merging of bacterial and eukaryotic membranes). Helicobacter pylori employ this second method.
The H. pylori proteins necessary for type IV secretion are encoded on the cag pathogenicity island (cagPAI), a clinically recognized determinant of bacterial virulence. In model cells of the gastric epithelium that express Nod1 (23), bacteria that had a functional cagPAI elicited NF-κB activation and IL-8 expression. This did not occur if Nod1 was absent or mutated. Furthermore, transfection with dominant negative Nod1 or with an inhibitory RNA (short interfering RNA) abrogated the effect of cag+ bacteria. Bacterial internalization was not necessary for NF-κB activation. Bacteria with a functional cagPAI delivered labeled peptidoglycan to model gastric cells.
Nod1-deficient mice infected with H. pylori exhibited significantly greater bacterial loads than wild-type mice. They also exhibited a weaker epithelial cell chemokine response, as measured by macrophage inflammatory protein (MIP)-2-CXCL2. To what extent increased MIP-2 was responsible for increased protection is difficult to judge. MIP-2 expression in the gastrointestinal epithelium, even in the absence of any infection, enhances the recruitment of neutrophils and lymphocytes (16). However, other protective mechanisms are possible. For example, lack of Nod1 critically affected β-defensin (BD) expression (particularly human BD2 in humans and murine BD4 in mice) after H. pylori infection (2).
COOPERATION AMONG NOD-LRR AND TLR PROTEINS
The presence of different types of molecular pattern receptors on both the surface and in the cytoplasm raises the question as to whether distinct recognition receptors interact after microbial stimulation. The evidence for this in the intestine still requires further elucidation, but synergic responses are now well established in the systemic immune system. Two models are possible. In the first model, distinct molecules carried by a particular microbe separately activate different receptors, which then cooperate. In the second model, a single microbial pattern molecule activates more than one PRR within the cell.
Nod1 stimulation not only causes JNK and NF-κB activation, as described above, but it also synergizes with a wide range of TLRs. Peripheral blood mononuclear cells from human volunteers, when stimulated with nanomolar concentrations of the Nod1 ligand TriDAP (Fig. 2), induced only minimal cytokine secretion compared with TLR stimulants. However, TriDAP at the same concentrations markedly increased cytokine responses induced by those same TLR ligands. Interestingly, Nod1 did not synergize with Nod2 ligands (21).
When a signal pattern recognition molecule activates two (or more) cooperating PRRs, the downstream signaling effects have been termed the inflammasome. In addition to Nod2, MDP activates another intracellular protein, cryopyrin [also named LRR- and pyrin domain (PYD)-containing protein (NALP)3]. Cryopyrin was known to mediate the activation of caspase-1, which processes IL-1β from its precursor, pro-IL-1β, to its active form. Purified MDP, but not purified E. coli LPS, induced caspase activity in normal human macrophages (13). Cryopyrin is required for the ATP-triggered release of IL-1β from stimulated macrophages, indicating that the protein not only is involved with downstream signaling from receptors to MDP itself (13) but to other stimuli as well, like IL-1β and TLRs. Microbial RNA also stimulates cryopyrin to activate caspase-1 (10).
The cryopyrin activity (1) assembles a complex that includes not only caspase-1 but also a third (non-NOD-LRR) protein, apotosis-associated speck-like protein containing a CARD (ASC). [Mice lacking ASC have a severe defect in caspase-1 activity and IL-1 secretion (12).] Another protein, pyrin, is not a NOD-LRR but resembles cryopyrin by having a specific domain, called PYD. PYD links proteins to other proteins containing this motif. It is not altogether clear whether pyrin binding to ASC inhibits inflammation by reducing cryopyrin-ASC interactions [the sequestration model (20)] or whether pyrin-ASC has proinflammatory consequences of its own. Both hypotheses are possible if one assumes that pyrin acts in a similar way to a partial agonist in receptor theory. Pyrin does not have an LRR domain, and, whatever its role, it is not likely be due to direct microbial action. However, the functions of these proteins are important because of their relationship to human disease (see below).
A further NOD-LRR molecule, IL-1β-converting enzyme-protease-activating factor (IPAF), also activates caspase-1, seemingly independently of ASC. Unlike ASC, IPAF has a LRR domain, which has recently been shown to recognize flagellin, independently of TLR5 (6). Thus, the inflammasome has various ways of being built up, sometimes with intracellular pattern receptors and sometimes with other molecules. The exact relationship between a non-NOD-LRR protein that shares activity with a NOD-LRR and that NOD-LRR [for example, cryopyrin (NOD-LRR) and pyrin (non-NOD-LRR), or IPAF (NOD-LRR) and ASC (non-NOD-LRR)] still requires further elucidation.
Members of the NOD-LRR family, however, do not only interact to increase the inflammatory response. Certain members also counteract. Inhibition of TLR signals by a variety of proteins is well established. Examples include single immunoglobulin and Toll-IL-1 receptor (SIGIRR), Tollip, A20, IL-1 receptor-associated kinase M (IRAK-M), etc, whose actions counterbalance proinflammatory pathways. None of these proteins is a member of the NOD-LRR family. The pattern of their response is generally to increase slowly after stimulation and limit the duration of a response. However, a NOD-LRR member, Monarch-1, decreases, at least in cells of the myeloid lineage, after TLR stimulation (24). It also downregulates signal transduction from TLRs. It is likely, therefore, that the molecules act as a block on inappropriate initiation of the proinflammatory cascade and that their effects are removed by substantive TLR ligation. Although Monarch-1 has convincingly inhibited both TLR and TNF-α responses, no microbial peptide has to date been shown to bind to it. Monarch-1-peptide binding is a matter of pure speculation, but one would predict that if such an event occurred, it would inhibit Monarch-1 activity. By this means, bacterial stimulation of the molecules cooperates by reducing a negative signal to full microbial stimulation.
NOD-LRRS AND ADAPTIVE IMMUNITY
Interestingly, the effects of cryopyrin (19) go beyond the intracellular mechanisms of innate immunity. Mice defective in the cold autoinflammatory syndrome 1 gene (CIAS-1) (which encodes cryopyrin) did not display skin contact hypersensitization. This classical model of T cell function consists of two phases: a sensitization phase, induced by applying the hapten trinitrophenylchloride (TNP-Cl) to abdominal skin; and a localized response, induced by applying TNP-Cl to the ear 4 days later. Mice lacking cyropyrin showed an abrogated second response compared with wild-type controls. The authors demonstrated that this was due to cellular immunity by transferring splenocytes from sensitized mice to mice that had not previously been exposed to TNP-Cl. Wild-type mice that received the splenocytes displayed a brisk ear swelling response on receiving localized TNP-Cl. However, TNP-Cl applied to the ear of splenocyte recipient mice defective in cryopyrin induced very little swelling. Thus, a protein that is a recognized receptor for innate immune signals also plays a role in adaptive immunity.
The role of NOD-LRR in adaptive immunity is even more evident in another member of the NDD-LRR family, the class II transactivator (CIITA), whose role as a PRR or as a component of the innate immune response has yet to be demonstrated. A central feature of adaptive immunity is the presentation of the antigen to the T lymphocyte. The major histocompatibility complex (MHC) class II presents the foreign antigen, initiating T cell activation. CIITA (17) is a non-DNA-binding transcriptional coactivator that functions as a highly specific and essential transactivator of class II MHC genes. It has the essential components of a NOD-LRR protein (Fig. 1). CIITA was first identified because it is defective in one form of bare lymphocyte syndrome, a primary immunodeficiency disease resulting from complete loss of constitutive and inducible class II MHC expression in all cell types. The expression pattern of the gene encoding CIITA (called Mhc2ta in the mouse) is the primary determinant dictating the cell type specificity and induction of class II MHC expression (17). CIITA mRNA directly correlates with CIITA activity. A complex regulatory region (Fig. 1) containing at least three independent promoters (pI, pIII, and pIV) regulates expression of the Mhc2ta gene. Each promoter is linked to a distinct first exon, with the more distal exons being common to all. Although CIITA contains LRRs with high homology to other members of the family, there are no published data examining the microbial patterns that it recognises. Nevertheless, luminal bacteria are central to the expression of class II MHC in the intestinal epithelium, because it is not expressed in the epithelium of mice raised in germ-free conditions. CIITA expression in the intestinal epithelium responds to changes in diet with consequent effects on MHC class II (18). Mice weaned onto a normal diet express CIITA (type IV). But, when mice are weaned onto an elemental diet (consisting only of amino acids, simple sugars, and fats), CIITA type IV is not induced. However, there is a slow developmentally regulated induction of CIITA type III independent of diet.
DISEASES OF THE GASTROINTESTINAL TRACT ASSOCIATED WITH NOD-LRR GENES
Readers are probably familiar with the relationship of Nod2 to Crohn’s disease. However, variants of other NOD-LRR proteins affect the gastrointestinal tract in a number of ways. As already mentioned, bare lymphocyte syndrome is an immune deficiency that, like other immune deficiencies, results in increased opportunistic infections. Variations of the cryopyrin gene result in a series of systemic inflammatory conditions including Muckle-Wells syndrome, chronic infantile neurological, cutaneous, and articular syndrome (CINCA), and familial cold urticaria, which often present as arthritides of the small joints, particularly in children (20). Indeed, it has recently been demonstrated that the uric acid crystals that initiate gout arthropathy stimulate cryopyrin (14). Although primary changes in mucosal inflammatory processes do not, to our knowledge, occur in these conditions, they are often characterized by amyloid deposition in the rectum. Variants of the pyrin gene are the cause of familial Mediterranean fever (FMF) (5), which also manifests with gastrointestinal complications. Peritonitis is common, and the consequent adhesions can cause intestinal obstruction (4). Furthermore, it is likely that future studies will identify further relationships between NOD-LRR genes and inflammatory bowel disease. Recent observations have shown that variations in the gene that encodes pyrin (responsible for FMF) are also found more commonly in ulcerative colitis than in normal controls (7) and that Nod1 variants may be increased in Crohn’s disease (15). Recently, we have done DNA microarrays and RT-PCR on TLR receptors, signaling molecules, and negative regulators. We have shown that the negative regulators are underexpressed in the fetal intestine (N. Nanthakumar and W. A. Walker, unpublished observations). We believe that this helps explain the extensive inflammatory response seen in necrotizing enterocolitis.
In summary, this review described how certain PRRs interact to detect bacteria and maintain homeostasis. They come to light from three separate fields of study: 1) mutations in genes associated with disease, 2) regulatory molecules that control adaptive and immune responses, and 3) receptors that recognize microbial patterns. Not all NOD-LRR members have been found to involved in all three, but future research may extend our understanding of how proteins first discovered by one route also act in other areas.
Acknowledgments
The authors thank Mrs. Nici Kingston for the assistance with the manuscript and figures.
References
- 1.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 2.Boughan PK, Argent RH, Body-Malapel M, Park JH, Ewings KE, Bowie AG, Ong SJ, Cook SJ, Sorensen OE, Manzo BA, Inohara N, Klein NJ, Nunez G, Atherton JC, Bajaj-Elliott M. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: critical regulators of beta-defensins during Helicobacter pylori infection. J Biol Chem. 2006;281:11637–11648. doi: 10.1074/jbc.M510275200. [DOI] [PubMed] [Google Scholar]
- 3.Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182–1193. doi: 10.1136/gut.2004.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciftci AO, Tanyel FC, Buyukpamukcu N, Hicsonmez A. Adhesive small bowel obstruction caused by familial Mediterranean fever: the incidence and outcome. J Pediatr Surg. 1995;30:577–579. doi: 10.1016/0022-3468(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 5.El-Shanti H, Majeed HA, El-Khateeb M. Familial mediterranean fever in Arabs. Lancet. 2006;367:1016–1024. doi: 10.1016/S0140-6736(06)68430-4. [DOI] [PubMed] [Google Scholar]
- 6.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immun. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 7.Giaglis S, Mimidis K, Papadopoulos V, Thomopoulos K, Sidiropoulos P, Rafail S, Nikolopoulou V, Fragouli E, Kartalis G, Tzioufas A, Boumpas D, Ritis K. Increased frequency of mutations in the gene responsible for familial Mediterranean fever (MEFV) in a cohort of patients with ulcerative colitis: evidence for a potential disease-modifying effect? Dig Dis Sci. 2006;51:687–692. doi: 10.1007/s10620-006-3192-1. [DOI] [PubMed] [Google Scholar]
- 8.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 10.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 11.Kim JG, Lee SJ, Kagnoff MF. Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by toll-like receptors. Infect Immun. 2004;72:1487–1495. doi: 10.1128/IAI.72.3.1487-1495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 13.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 15.McGovern DP, Hysi P, Ahmad T, van Heel DA, Moffatt MF, Carey A, Cookson WO, Jewell DP. Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum Mol Genet. 2005;14:1245–1250. doi: 10.1093/hmg/ddi135. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuka Y, Lee J, Stamm DS, Sanderson IR. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49:526–533. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immun. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson IR, Bustin SA, Dziennis S, Paraszczuk J, Stamm DS. Age and diet act through distinct isoforms of the class II transactivator gene in mouse intestinal epithelium. Gastroenterology. 2004;127:203–212. doi: 10.1053/j.gastro.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immun. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 21.Van Heel DA, Ghosh S, Butler M, Hunt K, Foxwell BM, Mengin-Lecreulx D, Playford RJ. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur J Immunol. 2005;35:2471–2476. doi: 10.1002/eji.200526296. [DOI] [PubMed] [Google Scholar]
- 22.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127:1401–1409. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immun. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 24.Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, Vogel SN, Braunstein M, Ting JP. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;280:39914–39924. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J, Fernandes-Alnemri T, Alnemri ES. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]