Abstract
Indigenous intestinal microbes have co-evolved with the intestinal immune system to form a symbiotic ecosystem. In the postnatal period, intestinal microbes provide the developing gut with stimuli that are necessary for healthy maturation of the intestinal immune system. Cross talk between the host and commensal microbes is an essential component of gut homeostasis mechanisms also in later life. During recent years, innovative research has shed light on the molecular mechanisms of these interactions.
Introduction
The human gut harbors an enormous quantity of commensal bacteria that have developed a symbiotic relationship with the host. The complex and dynamic ecosystem of indigenous microbes residing in the intestine may collectively be referred to as the intestinal microbiota. Our knowledge of the interactions between indigenous intestinal microbes and ourselves, the host, is fragmentary at best. Accumulating data indicate, however, that commensal microbes and the host immune system live in a dynamic state of equilibrium. Disruption of this homeostasis is known to contribute to the pathogenesis of intestinal inflammatory conditions, such as inflammatory bowel disease (IBD) and necrotizing enterocolitis (NEC). Intestinal bacteria also contribute to healthy morphologic and functional maturation of the intestinal immune system in the postnatal period, and alterations in gut microbiota composition in infancy have been associated with subsequent development of immune-mediated disease in epidemiologic studies [1]. The clinical implications of understanding host-microbe cross talk are significant, and a considerable amount of research has therefore focused on understanding the molecular basis of these interactions. The purpose of the present paper is to critically review the current body of knowledge on the molecular mechanisms of cross talk between commensal microbes and the gut epithelium.
The Intestinal Immune System
The intestinal epithelium is in constant contact with a flux of enormous amounts of dietary and microbial antigens. The intestinal immune system has therefore developed sophisticated means to monitor gut contents and recognize potential pathogens in order to be able to launch protective immune responses and avoid infection without inappropriate inflammatory responses directed toward harmless dietary compounds or indigenous intestinal microbes. The epithelial layer in the small intestine and in the colon consists of a single layer of cells, the majority of which are polarized absorptive cells. In addition, areas of specialized follicle-associated epithelium are characterized by the presence of microfold (M) cells. The principal function of M cells is the uptake and transfer of luminal antigens to their basolateral surface, which has pockets enfolding lymphocytes, dendritic cells (DCs), and macrophages. Peyer’s patches (PPs) are organized lymphoid structures located in the small intestinal submucosa under follicle-associated epithelium that function as the principal induction site of intestinal adaptive immunity.
Recognition of Microbes by the Host
The host is able to recognize microorganism-associated molecular patterns (MAMPs) through specific pattern-recognition receptors (PRRs) and thus to identify pathogens and initiate immune defense reactions. PRRs are a heterogeneous group of membrane-bound or cytosolic molecules that recognize bacterial MAMPs, such as unmethylated CpG (cytosine-guanine) motifs characteristic of bacterial DNA, flagellin, and the bacterial cell wall components lipoteichoic acid, muramyl dipeptide, and peptidoglycan on gram-positive organisms, and lipopolysaccharide (LPS) on gram-negative bacteria [2–4]. In addition, protozoan, fungal, and viral structures, such as single-stranded RNA, bind to certain PRRs [5]. The human Toll-like receptor (TLR) family of PRRs consists of 11 TLRs (TLR1–11). In the intestinal epithelium, TLRs are expressed on both intestinal epithelial cells (IECs) and immune cells, such as DCs and macrophages [6–8].
TLRs are proteins with a short transmembrane region and an extracellular domain with a leucin-rich repeat region exhibiting divergent ligand-binding properties [4,6]. Recognition of microbial ligands by TLRs leads to the induction of a variety of immune response genes, including cytokines, chemokines, and costimulatory molecules. The precise nature of this response is dependent on the ligand and TLR (or heterodimer of TLRs) in question as well as cofactors and adaptor proteins involved in signal transduction (reviewed recently by Kawai and Akira [9]). In addition, a number of intracellular proteins modulate and downregulate TLR signaling pathways.
Presumably the most important proinflammatory pathway activated by TLR signaling is the myeloid differentiation primary-response protein 88 (MyD88)–dependent pathway, which is shared by all known TLRs with the exception of TLR3. Antigen binding by TLRs activates MyD88 and leads to the activation of two major downstream pathways: the IKK complex and MAP kinases (MAPKs), such as ERK, JNK, and p38 [9]. The IKK complex consists of a regulatory subunit nuclear factor kappa B essential modulator (NEMO) and two catalytic subunits, IKK1 and IKK2, which catalyze phosphorylation of IκB proteins [10]. Phosphorylated IκB proteins are degraded by ubiquitination and detach from the transcription factor nuclear factor kappa B (NF-κB). NF-κB then translocates to the nucleus and controls the expression of a variety of inflammatory genes [6,9,10]. In addition to the MyD88-dependent pathway, a number of alternate TLR signaling pathways may be activated [9,11]. As opposed to inflammatory immune responses directed against pathogens recognized by TLRs, inflammatory responsiveness against harmless indigenous microbes binding to TLRs may be inhibited by a number of mechanisms involving the IRAK activation suppressor molecule Tollip, NF-κB agonist peroxisome proliferator activated receptor gamma (PPARγ), single immunoglobulin interleukin (IL)-1R-related protein (SIGIRR), suppressor of cytokine signaling (SOCS), the zinc finger protein A20, or the cytokines transforming growth factor (TGF)-β and IL-10 [12,13]. The most important molecules involved in epithelial-commensal cross talk are presented in Table 1.
Table 1.
Key molecules and their functions in epithelial-commensal cross talk
| MAMP | Microbe-associated molecular pattern | Conserved molecular structures characteristic to microbes but not eukaryotes (eg, bacterial CpG DNA, LPS) |
| PRR | Pattern-recognition receptor | Innate immune molecules that recognize MAMPS and initiate immune responses |
| TLR | Toll-like receptor | Group of PRRs with a central role in the intestinal epithelium |
| NF-κB | Nuclear factor κB | Transcription factor controlling expression of a wide variety of proinflammatory genes activated by TLR signaling |
| IκB | Inhibitor of κB | Inhibitory molecule attached to NF-κB phosphorylation and ubiquitination of IκB leads to nuclear translocation and activation of NF-κB |
| NEMO | Nuclear factor κB essential modulator | Regulatory molecule controlling IκB degradation |
| TOLLIP | Toll inhibitory protein | Inhibits inflammatory responses resulting from TLR activation |
CpG—cytosine-guanide; LPS—lipopolysaccharide.
In addition to membrane-bound TLRs, a number of cytosolic PRRs, including nucleotide-binding oligomerization domain-like receptors (NLRs), RIG-like helicases (RLHs) and dectins, have been identified. NLR1 and NLR2 (also known as NOD1 and NOD2 or CARD4 and CARD15, respectively) are involved in regulation of intestinal immune responses. NLR1 or NLR2 binding by their respective ligands, γ-D-glutamyl-meso-diamino-pimelic acid and muramyl dipeptide, both components of bacterial peptidoglycans, leads to NF-κB activation through Rip2/RICK/CARDIAK serine-threonine kinase [14–16]. Epidemiologic data indicating an association between single nucleotide polymorphisms in the NLD2 gene and Crohn’s disease [14] or the TLR2 gene and allergic disease [17] demonstrate the potential clinical relevance of PRRs.
The Indigenous Intestinal Microbiota
Accumulating data indicate that the indigenous intestinal microbiota are an essential component of human physiology. Bacteria are present throughout the human gastrointestinal tract, but the number and spectrum of microbes vary considerably due to differences in pH, presence of immune factors and digestive enzymes, and transit time in different parts of the intestine. The quantity of microbes residing in the human gut is enormous: the number of bacteria (estimated at 1014) exceeds the number of human cells in the body approximately by a factor of 10, and the collective genome of the intestinal microbiota is estimated to be 100 times larger in size than that of the host [18]. Based on bacterial culture, the number of bacterial species residing in the human intestine has been estimated to be 400, but culture-independent molecular identification techniques reveal that only about half of microscopically detected bacteria can be cultured [19].
The gastrointestinal tract of the fetus is sterile, and microbial colonization of the infant begins at birth. Gram-positive cocci (staphylococci, streptococci, and enterococci), lactobacilli, and enterobacteria from the maternal intestinal and vaginal microbiota are the first bacteria colonizing the neonatal gut. Colonization by anaerobic microbes commences during the second day of life, and lactobacilli from other sources eventually replace maternal vaginal lactobacilli in the infant’s gut microbiota [20]. Such environmental factors as mode of birth, early nutrition, and treatment with antibiotics have an impact on the establishment of gut microbiota, in addition to genetic factors of the host [21]. Bifidobacteria are the predominant microbiota, representing 60% to 90% of fecal bacteria in breastfed infants as a consequence of bifidogenic factors in breast milk [22]. After weaning, the gut microbiota composition of infants becomes more complex, resembling that of adults with variance estimated as high as 30% between individuals.
Bacterial colonization patterns in early life have been linked to development of disease during the neonatal period and later in life. Newborns treated in a neonatal intensive care unit reportedly exhibit aberrant fecal microbiota composition [23]. This phenomenon may play a role in the development of NEC, the pathogenesis of which involves bacterial translocation as a result of compromised intestinal epithelial barrier. Indeed, colonization with Clostridium perfringens has been associated with subsequent development of NEC in a case-control study of premature newborns [24]. The role of intestinal bacteria in the pathogenesis of NEC is further underscored by promising data from clinical studies using probiotic lactobacilli or bifidobacteria to reduce the incidence of NEC in premature neonates (reviewed by Rautava [25]).
Host-Commensal Cross Talk
Indigenous intestinal microbes and the host share a long evolutionary history during which complex symbiotic relationships have developed. It may therefore be suggested that the intestinal microbiota should be considered an essential part of homeostasis mechanisms of the intestine. Cross talk between the host and commensal microbes has been an area of intense investigation during the past decade, but the mechanisms by which bacteria in the gut influence host physiology and by which host physiology influences bacteria in the gut remain largely unknown.
The present body of knowledge regarding host-commensal cross talk has been derived mainly from studies conducted using germ-free or gnotobiotic animals. In addition, the promising potential of using selected strains of intestinal microbes as probiotics has spawned research elucidating the effects of specific probiotic bacteria in vitro and in vivo [1]. However, data from these studies should be interpreted cautiously for a number of reasons. Commensal microbes constitute a dynamic ecosystem characterized by interaction between members of the microbiota and the host. Thus, the effects of selected commensal microbes to host physiology may in part depend on microbe-microbe interactions, which are difficult to recapitulate and investigate in an experimental setting. Initial microbial colonization in the neonatal period coincides with structural and functional maturation of the intestinal immune system, and the expression of many of the immune molecules involved in recognition of microbial structures is developmentally regulated. In addition to their homeostatic function in later life, indigenous microbes appear to play an important developmental role in early infancy. After the intestinal microbiota has been established, its composition remains relatively stable. The host is tolerant toward its indigenous microbiota, that is, immune responses toward commensal bacteria are local and noninflammatory in nature. It is likely that host responses to resident commensal bacteria differ from those elicited toward initial colonizers in early life or toward nonpathogenic microbes that do not belong to the indigenous microbiota, such as probiotics.
Maturational Signals from Commensal Microbes
Results from a number of experimental studies indicate that indigenous intestinal microbes provide a crucial inductive stimulus to morphologic and immunologic maturation of the gut (reviewed by Hooper [26]). Postnatal immune maturation guided by external stimuli from breast milk and intestinal bacteria is mandatory for healthy development of adequate protection against pathogens, for example, in the form of secretory IgA on the one hand and establishment of tolerance to dietary antigens and nonpathogenic commensal bacteria on the other. The glycosylation patterns of intestinal epithelial cells may be considered a marker for intestinal maturation: in the postnatal period the epithelial glycans predominantly terminate with sialic acid, but during weaning terminal fucose becomes dominant. However, such a shift in glycosylation patterns is not observed in animals raised under germ-free conditions [26]. It has been demonstrated that monocolonization of germ-free mice with Bacteroides thetaiotaomicron, a prominent component of gut microbiota after weaning in both mice and humans, leads to mature-like expression of fucosylated glycans in the ileal epithelium [27]. Furthermore, through the use of DNA microarray technique, it was discovered that such colonization is associated with significant changes in the expression of a number of host genes involved in a variety of intestinal functions, including nutrient absorption, metabolism, epithelial barrier function, and intestinal maturation [27]. Conversely, host epithelial cells and factors such as bile salts have been observed to modulate gene expression by the commensal microbe Lactobacillus plantarum [28]. Taken together, these data demonstrate the true symbiosis or mutual importance of the interaction between the microbiota and the host.
The crucial role of commensal microbes for normal immune maturation in postnatal life has been elucidated by a study in which mice kept in germ-free conditions exhibited impaired development of the intestinal immune system resulting in defective oral tolerance formation and allergic-type hypersensitivity [29]. Strikingly, however, the ability to establish oral tolerance was restored after colonization of the intestine with bifidobacteria early in the neonatal period. The precise molecular mechanisms of how intestinal microbes affect host immune physiology are not known, and it is likely that several distinct factors are involved. Bashir et al. [30] have demonstrated that allergic-type hypersensitivity to dietary antigens may be induced in mice with gut microbiota reduced by antibiotics. In the same series of studies, similar sensitization was also observed in TLR4-deficient animals with unaltered gut microbiota, and normal immune status could be rescued by administering CpG oligodeoxynucleotide, which stimulates TLR9. Taken together, these data suggest that recognition of commensal microbes and signaling through TLRs are necessary for the development of immune tolerance to dietary antigens.
The first demonstration of an exact molecular basis underlying the immunomodulatory properties of a colonizing microbe was provided by a report according to which delayed immune maturation in germ-free mice could be enhanced by colonization with the murine commensal microbe Bacteroides fragilis [31•]. Administration of a purified B. fragilis surface polysaccharide molecule (PSA) was sufficient to elicit significant immune maturation in germ-free animals, but the mechanism of immunomodulation by PSA is not yet known.
Although commensal bacteria do not under physiologic circumstances induce inflammatory immune reactions, active immune recognition processes are implicated in producing the developmental and homeostatic effects of host-commensal cross talk. Receptors homologous to human TLRs were first described in Drosophila as molecules involved in developmental processes. The question of whether there are endogenous TLR ligands in mammals remains open, but interesting data suggest links between vitamin D [32] or fatty acids [33] and TLR function. TLR activation by intestinal microbes is likely to play a role in intestinal maturation associated with microbial colonization. Initial interaction with Bifidobacterium lactis after monocolonization of germ-free rats with this commensal microbe has been shown to lead to TLR2-mediated transient activation of the MAPK and NF-κB pathways and proinflammatory cytokine gene expression in intestinal epithelial cells (IECs) [34]. However, this inflammatory immune activation is limited and does not result in tissue damage, and it may therefore be suggested that TLR activation is a physiologic phenomenon taking place during initial bacterial colonization. It may further be speculated that transient immune activation by colonizing commensal bacteria may be needed to induce tolerance toward the indigenous microbiota. In accord with this notion, Otte et al. [12] have reported that short-term stimulation of IECs with the TLR4 ligand LPS or the TLR2 ligand lipoteichoic acid activates IRAK and MAPK signaling and leads to IL-8 production. In contrast, continuous exposure to either of these bacterial products results in a state of hyporesponsiveness or tolerance to both TLR ligands. The expression of TLRs on the tolerized IECs is not altered, but the expression of the regulatory factor Tollip is significantly increased. Subsequently, LPS-induced hyporesponsiveness in IECs has been observed to extend to non-related immune activation by tumor necrosis factor (TNF)-α or PMA but be limited in scope to attenuating IL-8 production, whereas other proinflammatory responses remain intact [35]. Similar TLR ligand-induced cross tolerance to TLR activation has recently been described in IECs stimulated with bacterial DNA motifs binding to TLR9 [36••].
Discriminating Between Commensals and Pathogens
One of the most intriguing questions regarding indigenous intestinal microbiota is how the host immune system is capable of responding adequately to pathogens and containing potential threats by controlled inflammatory responsiveness without detrimental inflammatory reactivity against commensal microorganisms, which might lead to chronic intestinal inflammation. This phenomenon becomes even more puzzling considering that TLR ligands are present on both commensal and pathogenic microbes. IECs have been observed to be largely unresponsive to TLR ligands [37]. This observation has partly been explained by the fact that TLR expression on IECs is sparse. Furthermore, cytosolic PPRs are not in direct contact with commensal microbes, and some cell membrane-bound PPRs, such as TLR5, are predominantly expressed on the basolateral surface of polarized IECs and not on the apical surface exposed to luminal antigens when the epithelium is intact [38]. Disruption of intestinal epithelial barrier integrity, for example, by inflammation or pathogen invasion, however, allows binding to basolateral TLRs and leads to an inflammatory immune response. Lee et al. [36••] have recently demonstrated in a sophisticated series of studies that TLR responses are differentially regulated in epithelial cells depending on whether they originate from the apical or basolateral surface. Apical and basolateral TLR9 stimulation induces distinct responses despite similar expression of TLR9 in these compartments: in contrast to basolateral TLR9 ligation, apical TLR9 signaling fails to activate NF-κB or ERK and does not lead to secretion of the proinflammatory chemokine IL-8. Moreover, micro-array data from the same series of studies revealed that the profile of genes activated by apical versus basolateral TLR9 activation is strikingly different, with apical stimulation favoring induction of transcriptional regulators. These data may be interpreted to suggest that, whereas TLRs may not be able to distinguish between MAMPs present on pathogens and commensals as such, inflammatory responses are directed only against invading organisms penetrating the intestinal epithelial barrier. This notion is also consistent with inflammation directed toward commensal microbes observed in patients with IBD, in whom the gut barrier is compromised [39].
Despite recent advances, the role of TLR signaling and the proinflammatory transcription factor NF-κB in mucosal immune responses to commensals and pathogens remains elusive. Pathogen recognition by TLRs leads to an inflammatory immune response mediated by the NF-κB pathway, but certain enteropathogenic bacteria are able to suppress this pathway as a means to reduce host immune reaction to infection [40]. As discussed previously, nonpathogenic commensal microbes induce only transient activation of the NF-κB pathway in IECs. In addition to the data alluded to previously suggesting that this hyporesponsiveness toward commensal microbes may in part be achieved by downregulation of TLR signaling through Tollip [12], a number of other mechanisms by which commensal microbes may inhibit NF-κB activation have recently been discovered. Nonpathogenic Salmonella strains have been reported to prevent nuclear translocation of NF-κB in IECs via inhibition of IκB ubiquitination [41]. Secondly, apical but not basolateral TLR9 activation results in reduced NF-κB activation in IECs through inhibition of IκB degradation which is not due to a failure to ubiquitinate IκB [36••]. Thirdly, there are data indicating that the commensal B. thetaiotaomicron is able to inhibit NF-κB activation by exporting the transcription factor from the nucleus in an IκB-independent fashion via PPARγ [42]. According to a recent report, two distinct PRR signals may be required for the host to launch an inflammatory response: activation of caspase-1 by ligation of intracellular PRRs is necessary for posttranscriptional activation of IL-1β produced in response to TLR stimulation [43].
Given the accumulating data indicating that mucosal tolerance to commensal microbes is the result of attenuated TLR signaling and inhibition of NF-κB activation, it is puzzling that mice with IEC-specific inhibition of NF-κB resulting from conditional ablation of the IKK regulatory subunit NEMO develop severe intestinal inflammation resembling IBD in early life [44]. The colitis in NEMO-deficient animals is associated with increased expression of inflammatory cytokines and chemokines, including IL-6, IL-8, MCP-1, and TNF-α. Moreover, depletion of MyD88 function protects NEMO-deficient mice from colitis, which may be interpreted to imply that TLR signaling by commensal bacteria is necessary for the development of intestinal inflammation that spontaneously develops in the absence of NF-κB activation. Unfortunately, the phenotype of NEMO-deficient animals raised in germ-free conditions has not been reported. Perplexingly, recognition of commensal bacteria through TLRs appears to be mandatory for protection against epithelial injury induced by dextran sodium sulfite (DSS); mice deficient in TLR2, TLR4, or MyD88 exhibit increased morbidity and mortality from DSS colitis, according to a report based on a series of studies conducted by Rakoff-Nahoum et al. [45••].
The discrepancies between studies conducted using IECs and animal models may at least partially be explained by the fact that the immunomodulatory effects of commensal microbes extend beyond epithelial cells. In the intestinal epithelium, DC singe stand sample antigens passing through M cells or directly from within the intestine by opening tight junctions between IECs and extending dendrites into the gut lumen [46]. Microbial stimulation through TLRs controls DC activation, maturation, and migration at least in part through NF-κB and MAPK-dependent mechanisms [47–49]. Intestinal DCs may retain ingested live commensal bacteria within them, migrate to the mesenteric lymph nodes, and induce secretory IgA production without systemic immune activation [50]. These data are consistent with the general notion that, under normal circumstances, immune responsiveness toward commensal microbes is restricted to the intestinal mucosa without systemic inflammatory reactions.
DCs play an important role in determining the type of adaptive immune response elicited upon microbial contact [51]. Lamina propria CD11c+ cells do not express TLR4, nor do they generate an inflammatory response against commensal microbes, but instead they recognize pathogenic bacteria through TLR5 and secrete proinflammatory cytokines [52]. Recently, PSA, a bacterial polysaccharide produced by the commensal microbe B. fragilis, has been observed to modulate the interaction between DCs and T cells in a TLR2-dependent manner [53]. Interaction between DCs and commensal microbes appears also to play a role in tolerance toward intestinal microbiota or dietary antigens. Stimulation of DCs through TLR9 reportedly induces the development of regulatory CD4+CD25+ T cells, which suppress inflammatory immune reactivity against indigenous intestinal microbes or dietary antigens via the production of IL-10 and TGF-β [54]. However, TLR stimulation may also induce DCs to produce IL-6, which is required to inhibit CD4+CD25+ T-cell–mediated immunosuppression and allow pathogen-specific adaptive immune responsiveness [55]. Thus, DCs act as critical sentinels and determine whether mucosal contact with microbes results in a tolerogenic or local protective immune response or if a systemic inflammatory reaction is to ensue.
Conclusions
Commensal microbes have a profound impact on intestinal physiology and mucosal immune functions in particular. Colonizing bacteria provide the immature neonatal gut immune system with signals that induce transient immune activation, leading to changes in epithelial glycosylation patterns, functional maturation, and tolerance toward the indigenous microbiota. TLR activation by colonizing bacteria leads to subsequent cross hyporeactivity to TLR ligands, but the mechanisms of this tolerance are not fully understood. Intriguingly, recognition of pathogens bearing TLR ligands thought to be indistinguishable from those on commensal microbes leads to protective inflammatory immune reactions. One solution to this paradox has been provided by data according to which apical TLR stimulation—microbes residing in the gut lumen—do not induce the inflammatory NF-κB pathway, whereas similar activation on the basolateral surface by invading or translocated microorganisms leads to NF-κB activation (Fig. 1) [36••]. In addition to this observation, different species of commensal microbes as well as some pathogens have developed a number of strategies through which they can inhibit the NF-κB pathway. All of this notwithstanding, some level of TLR activation by commensal microbes is required for the maintenance of the noninflammatory tone of the intestinal immune system. The complexity of the indigenous intestinal microbiota should be born in mind, and it may be hypothesized that under physiologic conditions both activation and inhibition of proinflammatory signaling pathways by distinct commensal species produce the net effect of dynamic intestinal homeostasis.
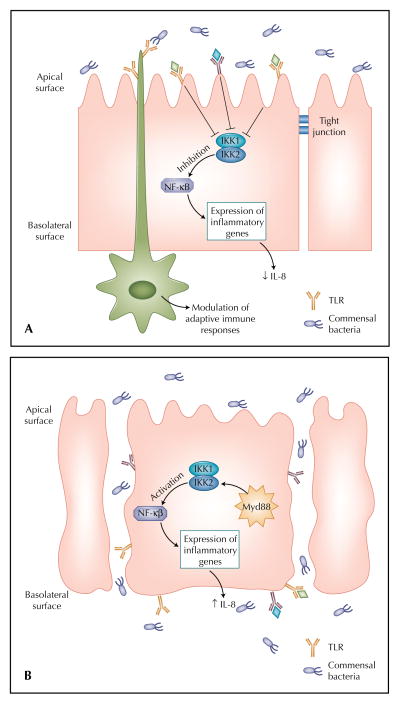
Figure 1.
Recognition of microbes in the intestinal mucosa. When the intestinal mucosal barrier is intact (A), bacteria come into contact with the epithelial cell solely on the apical membrane. The expression of Toll-like receptors (TLRs) on the apical surface is sparse, and TLR ligation results in inhibition of the proinflammatory nuclear factor kappa B (NF-κB) pathway. Dendritic cells sample intestinal contents by opening tight junctions and extending dendrites expressing TLRs to the gut lumen. When the integrity of the epithelium is compromised by immaturity or inflammation (B), bacteria translocate within and between enterocytes and bind to TLRs on the basolateral surface of the epithelial cells. An inflammatory immune response is launched via the NF-κB pathway. IL—interleukin.
The use of specific commensal microbial strains as probiotics has shown promising potential in prevention and treatment of such diverse conditions as NEC, IBD, infectious diarrhea, and allergic disease [25]. Recent advances in research reviewed in the present article have for the first time identified microbial molecular structures and host counterparts involved in host-commensal bacterial cross talk, and undoubtedly more such molecular mechanisms will be discovered in the future. Understanding these mechanisms through which indigenous microbes affect host physiology provides the possibility to design exact interventions to combat immunoinflammatory intestinal conditions or to support healthy immune maturation in infancy.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Rautava S, Kalliomaki M, Isolauri E. New therapeutic strategy for combating the increasing burden of allergic disease: Probiotics-A Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota (NAMI) Research Group report. J Allergy Clin Immunol. 2005;116:31–37. doi: 10.1016/j.jaci.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnare M, Barton GM, Holt AC, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 4.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182–1193. doi: 10.1136/gut.2004.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 8.Hart AL, Al-Hassi HO, Rigby RJ, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 11.O’Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–290. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 12.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Haller D, Jobin C. Interaction between resident luminal bacteria and the host: can a healthy relationship turn sour? J Pediatr Gastroenterol Nutr. 2004;38:123–136. doi: 10.1097/00005176-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 15.Girardin SE, Boneca IG, Carneiro LA, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 16.Chin AI, Dempsey PW, Bruhn K, et al. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 17.Eder W, Klimecki W, Yu L, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113:482–488. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- 18.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Tannock GW. What immunologists should know about bacterial communities of the human bowel. Semin Immunol. 2006;19:94–105. doi: 10.1016/j.smim.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Matsumiya Y, Kato N, Watanabe K, Kato H. Molecular epidemiological study of vertical transmission of vaginal Lactobacillus species from mothers to newborn infants in Japanese, by arbitrarily primed polymerase chain reaction. J Infect Chemother. 2002;8:43–49. doi: 10.1007/s101560200005. [DOI] [PubMed] [Google Scholar]
- 21.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Bennet R, Eriksson M, Nord CE, Zetterstrom R. Fecal bacterial microflora of newborn infants during intensive care management and treatment with five antibiotic regimens. Pediatr Infect Dis. 1986;5:533–539. doi: 10.1097/00006454-198609000-00009. [DOI] [PubMed] [Google Scholar]
- 24.de la Cochetiere MF, Piloquet H, des Robert C, et al. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: the putative role of Clostridium. Pediatr Res. 2004;56:366–370. doi: 10.1203/01.PDR.0000134251.45878.D5. [DOI] [PubMed] [Google Scholar]
- 25.Rautava S. Potential uses of probiotics in the neonate. Semin Fetal Neonatal Med. 2007;12:45–53. doi: 10.1016/j.siny.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12:129–134. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 28.Bron PA, Marco M, Hoffer SM, et al. Genetic characterization of the bile salt response in Lactobacillus plantarum and analysis of responsive promoters in vitro and in situ in the gastrointestinal tract. J Bacteriol. 2004;186:7829–7835. doi: 10.1128/JB.186.23.7829-7835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sudo N, Sawamura S, Tanaka K, et al. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–1745. [PubMed] [Google Scholar]
- 30.Bashir ME, Louie S, Shi HN, et al. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 31•.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. This report provides a molecular basis for one of the mechanisms through which colonizing commensal bacteria modulate host immunophisology. [DOI] [PubMed] [Google Scholar]
- 32.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Zhao L, Youn HS, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz PA, Hoffmann M, Szcesny S, et al. Innate mechanisms for Bifidobacterium lactis to activate transient pro-inflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology. 2005;115:441–450. doi: 10.1111/j.1365-2567.2005.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savidge TC, Newman PG, Pan WH, et al. Lipopolysaccharide-induced human enterocyte tolerance to cytokine-mediated interleukin-8 production may occur independently of TLR-4/MD-2 signaling. Pediatr Res. 2006;59:89–95. doi: 10.1203/01.pdr.0000195101.74184.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Lee J, Mo JH, Katakura K, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. Distinct responses to TLR activation on apical versus basolateral surfaces of polarized epithelial cells are described in this paper. This phenomenon is crucial for understanding how TLR ligands on commensal and pathogenic bacteria induce tolerance and inflammation, respectively. [DOI] [PubMed] [Google Scholar]
- 37.Melmed G, Thomas LS, Lee N, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 38.Gewirtz AT, Navas TA, Lyons S, et al. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 39.Malin M, Isolauri E, Pikkarainen P, et al. Enhanced absorption of macromolecules. A secondary factor in Crohn’s disease. Dig Dis Sci. 1996;41:1423–1428. doi: 10.1007/BF02088568. [DOI] [PubMed] [Google Scholar]
- 40.Collier-Hyams LS, Zeng H, Sun J, et al. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 41.Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 42.Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol Jan. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 43.Kanneganti TD, Lamkanfi M, Kim YG, et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 45••.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. The importance of TLR signaling induced by commensal microbes to intestinal immune homeostasis is demonstrated in this report. [DOI] [PubMed] [Google Scholar]
- 46.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 47.Michelsen KS, Aicher A, Mohaupt M, et al. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J Biol Chem. 2001;276:25680–25686. doi: 10.1074/jbc.M011615200. [DOI] [PubMed] [Google Scholar]
- 48.Turnbull EL, Yrlid U, Jenkins CD, Macpherson GG. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J Immunol. 2005;174:1374–1384. doi: 10.4049/jimmunol.174.3.1374. [DOI] [PubMed] [Google Scholar]
- 49.An H, Yu Y, Zhang M, et al. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002;106:38–45. doi: 10.1046/j.1365-2567.2002.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 51.Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Curr Opin Gastroenterol. 2006;22:354–360. doi: 10.1097/01.mog.0000231807.03149.54. [DOI] [PubMed] [Google Scholar]
- 52.Uematsu S, Jang MH, Chevrier N, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q, McLoughlin RM, Cobb BA, et al. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligode-oxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 55.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]