Abstract
The distribution of several pathogenic helminth infections coincides geographically with many devastating microbial diseases, including enteric bacterial infections. To dissect the mechanisms by which helminths modulate the host’s response to enteric bacteria and bacteria-mediated intestinal inflammation, we have recently established a coinfection model and shown that coinfection with the helminth Heligmosomoides polygyrus exacerbates colitis induced by infection with the Gram-negative bacterial pathogen Citrobacter rodentium. The disease severity of the coinfected mice was correlated with high Citrobacter loads in the gut, translocation of the bacteria into mucosal and systemic immune compartments, delayed bacterial clearance, and a significantly enhanced colonic TNF-α response. In the present study, using our in vivo coinfection model as well as in vitro approaches, we test the hypothesis that the phenotypic and functional alterations in macrophages induced by the helminth-driven T cell response may contribute to the observed alterations in the response to C. rodentium. We show that via a STAT6-dependent mechanism H. polygyrus coinfection results in a marked infiltration into the colonic lamina propria of F4/80+ cells that have the phenotype of alternatively activated macrophages. Functional analysis of these macrophages further shows that they are impaired in their killing of internalized bacteria. Yet, these cells produce an enhanced amount of TNF-α in response to C. rodentium infection. These results demonstrate that helminth infection can impair host protection against concurrent enteric bacterial infection and promote bacteria-induced intestinal injury through a mechanism that involves the induction of alternatively activated macrophages.
The major importance of helminth infections includes not only the direct pathogenic effect of the worms but also the modulatory role of the parasite on the host immune system, which may alter the response to other Ags or pathogens and cause additional immunopathology. Helminth infection has been shown to dampen Th1 reactions to other infections (1–4). This ability to attenuate damaging Th1-driven inflammatory responses in the host (5–8) has prompted the evaluation of helminth as a therapeutic agent for the treatment of some immune-mediated disorders, including certain types of inflammatory bowel diseases (9). Considering the profound immune activation and dysregulation induced by helminth parasites, the overlapping geographic distributions of helminth and bacterial infections, and the potential to modulate bacteria-associated intestinal inflammation, we have recently established a coinfection model system to facilitate detailed analysis of the combined effects of helminth and bacterial pathogens on host responses. This model involves two murine enteric infectious agents that induce distinct Th responses: 1) the Th2-inducing helminth Heligomosomoides polygyrus; and 2) the Th1-inducing bacterial pathogen Citrobacter rodentium (10, 11). C. rodentium is a mouse pathogen that colonizes the distal colon and causes pathological changes that are similar to those seen in some types of human infectious enteritis. It has been used as a model for studying host responses to human pathogens that use attaching and effacing lesion formation for epithelial colonization such as enteropathogenic Escherichia coli (EPEC), the most important causative agent of severe infantile diarrhea (10, 12–15). We recently showed that coinfection with H. polygyrus resulted in exacerbation of C. rodentium-induced colitis, which was associated with high bacterial loads in the gut, translocation into mucosal and systemic immune compartments, delayed bacterial clearance, and a significantly enhanced colonic TNF-α response (16).
Macrophages, the major population of tissue-resident mononuclear phagocytes, contribute significantly to the effector phase of the immune response, i.e., elimination of bacteria, and are also thought to be critical mediators of many chronic inflammatory diseases. Activation of macrophages by bacterial products (through TLR engagement) or proinflammatory stimuli such as Th1 cytokines leads to the development of the classically activated macrophages, which play an important role in the antimicrobial innate immunity of the host (17). In contrast, helminth infection and helminth infection-induced-Th2 cytokine responses have been suggested to affect macrophages by inducing the alternatively activated phenotype (18). One of the distinctive characteristics of this type of macrophage is the ability to suppress the proliferation of other cells with which they are cocultured (19, 20). They can negatively regulate classically activated macrophages and Th1 cell generation (18, 21). Recent evidence indicates that the alternatively activated macrophages can function as important effector cells mediating host protection against the nematode parasite (22). Although the results from these studies have begun shedding some light on our understanding of the functional differences between various macrophage subpopulation(s), the diverse activities and functional significance of alternatively activated macrophages are still not fully understood. Because exposure to helminth and simultaneous infections with other pathogens or Ags are common in developing countries and because helminths may have potential to be used as therapeutic agents for some immune mediated disorders, a better understanding of the mechanism(s) by which helminths modulate a host’s immune responses to other concomitant infections or Ags, such as vaccines, may have significant implications.
In the current study, we use the model of H. polygyrus and C. rodentium coinfection to determine the influence of potent helminth-induced Th2 activation signals on the phenotype and function of macrophages that are concomitantly exposed to enteric bacterial pathogens. We show that a primary infection with H. polygyrus induces the development of alternatively activated macrophages both mucosally and systemically. We further show that the exacerbated C. rodentium-mediated colitis that develops in H. polygyrus-coinfected mice correlates with a marked increase of F4/80+ macrophages in the colonic lamina propria (LP)3 via a STAT6-dependent mechanism. These cells display the phenotype of alternative activation based on the expression of a number of characteristic molecules. Functional analysis of these cells further shows that macrophages stimulated by helminth infection are impaired in their ability to kill internalized bacteria, yet produce an enhanced amount of TNF-α. These results, therefore, demonstrate a significant role for helminth-induced phenotypic and functional alterations of macrophages in the immune modulation of intestinal mucosal responses to concurrent enteric bacterial infection.
Materials and Methods
Mice
Six- to 8-wk old female BALB/c ByJ and STAT6 knockout (on BALB/c background) mice were purchased from The Jackson Laboratory, fed autoclaved food and water, and maintained in a specific pathogen-free facility at Massachusetts General Hospital (Charlestown, MA).
H. polygyrus infection
H. polygyrus was propagated as previously described and stored at 4°C until use (23). Mice were inoculated orally with 200 third-stage larvae (L3). Seven days following parasitic infection, a subset of the H. polygyrus-infected mice were fed C. rodentium.
C. rodentium infection
Mice were orally inoculated with C. rodentium (strain DBS100 from American Type Culture Collection). Bacteria were grown overnight in Luria broth (LB) and resuspended in PBS before infecting the mice (0.5 ml/mouse; ~5 × 108 CFU of C. rodentium). GFP-expressing C. rodentium (GFP-C. rodentium) (provided by Dr. L. Bry at Brigham and Women’s Hospital, Boston, MA) were grown overnight in LB containing carbenicillin (100 μg/ml) and resuspended in PBS before in vitro infection assay.
Macrophage isolation
At necropsy, mesenteric lymph node (MLN), spleen, and colon tissues were collected, and macrophages were isolated from various tissues. The colon tissues were removed and washed thoroughly with cold PBS without calcium and magnesium. Colonic epithelial cells and LP lymphocytes were removed. Briefly, colonic tissues were placed in 100 ml of 1 mM EDTA and incubated twice at 37°C with stirring. After the EDTA treatment, tissues were washed in complete DMEM (Invitrogen Life Technologies; complete DMEM contains 10% FCS, 10 mM HEPES, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-ME, 0.1 mM non-essential amino acids, and 1 mM sodium pyruvate) for 10 min at room temperature and then placed into 50 ml of DMEM containing 10% FCS and incubated for 15 min at 37°C with stirring. The tissues and medium were transferred to a 50-ml tube and shaken vigorously for 15 s, and the medium containing epithelial cells was removed. The MLN and spleen were dissected and the tissues were cut into small pieces and incubated in RPMI 1640 medium containing collagenase type VIII at 200 U/ml (Sigma-Aldrich) for 50 min at 37°C while stirring. Supernatants containing cells were collected and the cells were washed and then resuspended in complete RPMI 1640 medium. The cells were centrifuged over a 70/30% Percoll step gradient and the mononuclear cells were isolated from the gradient interphase. F4/80+ macrophages were purified with biotin-conjugated F4/80 (rat IgG2a; Caltag Laboratories) and streptavidin microbeads (Miltenyi Biotec) by using magnetic sorting (Miltenyi Biotec). The peritoneal macrophages were collected from mice infected with helminth (3 wk after H. polygyrus infection) or uninfected control mice. After incubation in complete DMEM for 2 h, nonadherent cells were removed by washing and the cells were cultured overnight. The adherent peritoneal macrophages were then used for in vitro infection experiments.
FACS analysis
The expression of MHC II molecules was analyzed on Mac-1+ or F4/80+ macrophages in cell populations isolated from normal mice or mice infected with C. rodentium, H. polygyrus, or both at 10 days after bacterial infection. MLN were pooled from 3–5 mice per group and pressed through nylon mesh to prepare single cell suspension. In each of the tissue preparations the macrophages were identified with anti-CD11b (Mac-1) or F4/80 biotin and streptavidin-Cy3 labeled Abs (purchased from BD Pharmingen). MHC II molecules were identified with FITC-labeled anti-MHC II (IA). The stained samples were analyzed on a BD Biosciences FACScan with Cell Quest software. Dead cells and debris were excluded from analysis by gates set on forward and side angle light scatter.
Real-time quantitative RT-PCR
Total RNA was isolated from colon tissues and purified F4/80+ macrophages obtained from colonic LP, MLN, spleens or the peritoneal cavity using TRIzol reagent (Invitrogen Life Technologies) following the manufacturer’s instruction. cDNA was synthesized using 2 μg of target RNA (Ready-to-Go kit; GE Healthcare). The cDNA samples were then tested for the expression of iNOS, FIZZ1, YM1, and arginase 1 (Arg1) by real-time RT-PCR. Real-time RT-PCR was performed using SYBR Green PCR Master Mix for 38 cycles on an Opticon II DNA engine (MJ Research). β-Actin was used as an internal control. Negative controls included the amplification of samples without prior reverse-transcription reaction. LightCycler relative quantification software was used to normalize data to the same β-actin mRNA level. Reactions were set up using QuantiTect SYBR green RT-PCR kit reagents according to manufacturer’s instructions. Samples were run in triplicate. The sequences for the sense and antisense primers used to quantify mRNA are: β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′; Arg1, 5′-CAGAAGAATGGAAGAGTCAG-3′ and 5′-CAGATATGCAGGGAGTCACC-3′; Ym1, 5′-TCACAGGTCTGGCAATTCTTCTG-3′ and 5′-TTTGTCCTTAGGAGGGCTTCCTC-3′; Fizz1, 5′-TCCCAGATACTGATGAGA-3′ and 5′-CCACTCTGGATCTCCCAAGA-3′; and iNOS2, 5′-CTGGAGGAGCTCCTGCCTCATG-3′ and 5′-GCAGCATCCCCTCTGATGGTG-3′.
Gentamicin protection assay
Macrophages from MLN, spleen, or peritoneal cavity of helminth-infected or noninfected mice were isolated using density gradient centrifugation and plastic adherence. Briefly, the cells were incubated in a 24-well plate in complete DMEM for 2 h and nonadherent cells were removed by washing. The adherent cells were incubated in complete DMEM at 37°C overnight and then infected for 1 h with 107 C. rodentium (multiplicity of infection of 10:1) in antibiotic-free medium. After completion of the infection period the cells were washed with cold PBS (×3) and incubated with gentamicin-containing medium (100 μg/ml) for 2 h, which kills the extracellular bacteria. Because gentamicin is not cell permeable, intracellular bacteria are not killed by this antibiotic. The cells were then washed (×3) with sterile PBS and then lysed immediately in 0.2 ml of sterile 1% Triton X-100 in water or, after a further 4 h, in medium containing 10 μg/ml gentamicin. The lysates were mixed with 0.8 ml of PBS and serial dilutions were made before plating 100 μl of the appropriate dilutions on LB agar. Colonies were counted after overnight incubation at 37°C and the number of bacteria present inside the cells at each time point was calculated. At both 2 and 6 h after bacterial infection the culture supernatants were also collected for TNF-α determination.
Measurement of macrophage TNF-α production
TNF-α was measured using ELISA as previously described (16). ELISA capture (MP6-XT22) and biotinylated second Abs (C1150-14) were purchased from BD Pharmingen. Standard curves were obtained using recombinant murine TNF-α (BD Pharmingen).
Immunofluorescence microscopy
Colon tissues were frozen in Tissue-Tek OCT compound. Five-micrometer sections were cut on a cryostat and fixed in ice cold acetone. The sections were then washed and blocked with avidin/biotin blocking agent (Vector Laboratories). To examine intestinal macrophages, the sections were stained with FITC-labeled anti-mouse Mac-1 (BD Pharmingen) and/or biotin-labeled anti-mouse F4/80 (BD Pharmingen) followed by incubation with streptavidin-Cy3 (Cedarlane Laboratories). Alternatively activated macrophages were identified by staining with anti-F4/80 and anti-CD206-Alexa 647 (Serotec). TNF-α producing macrophages were identified by staining with anti-F4/80 or anti-CD206 together with anti-mouse TNF-α Alexa Fluor 488 (BD Biosciences). Sections were analyzed by immunofluorescence microscopy.
To further determine the functional impact of helminth infection on macrophages, peritoneal macrophages were collected from normal and helminth-infected mice, grown on coverslips, and infected with GFP-expressing C. rodentium. The cells were stained with anti-F4/80 or anti-CD206-Alexa-647 at both 2 and 6 h after infection. Internalization and elimination of bacteria by macrophages were visualized by detecting GFP-C. rodentium in infected macrophages.
Statistical analysis
All results were expressed as the mean ± SEM. N refers to the number of mice used. Statistical differences were determined using a two-tailed Student’s t test with GraphPad Prism (GraphPad Software). p < 0.05 was considered significant.
Results
Helminth-induced exacerbation of bacteria-mediated colitis is associated with an increased infiltration of macrophages into colonic tissue
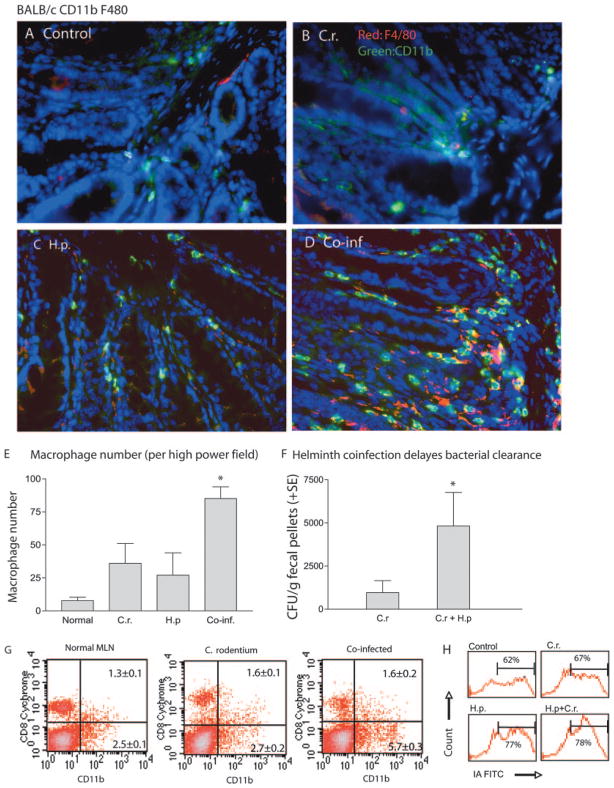
Infection with C. rodentium results in bacterial colonization of the intestine and colonic crypt hyperplasia. Recently, we showed that mice that were coinfected with an intestinal nematode parasite, H. polygyrus, developed an exacerbated C. rodentium-induced intestinal inflammation that was associated with higher bacterial loads, suppression of Th1 cytokine response (IFN-γ), and up-regulation of IL-4 and TNF-α response (16). Furthermore, in additional studies we observed that coinfection with both H. polygyrus and C. rodentium results in a significant augmentation in the number of dendritic cells (DCs) in the intestinal mucosa, providing evidence that H. polygyrus infection may alter the responses of innate immune cells to concurrent enteric bacterial pathogens (24). DCs play an important role in initiating and regulating host immune responses, whereas macrophages, the major population of tissue resident mononuclear phagocytes, contribute significantly to the effector phase, i.e., elimination of bacteria, and are also thought to be critical mediators of many chronic inflammatory diseases. In this study, we tested the hypothesis that intestinal helminth infection may induce phenotypic and functional alterations in macrophages, contributing to the observed impaired protection and the increase in disease severity in H. polygyrus-coinfected mice (16, 24). To determine whether helminth coinfection alters intestinal macrophage response to a subsequent enteric bacterial infection, we infected BALB/c mice with H. polygyrus and inoculated them with C. rodentium 7 days later. Using immunofluorescence microscopic techniques, we compared the abundance of macrophages in colonic tissue among various groups at 14 days after bacterial infection. Our results show that infection with C. rodentium alone results in an increase in intestinal F4/80+ macrophage compared with an uninfected control (Fig. 1). Helminth infection alone also induces increased colonic macrophage accumulation (Fig. 1C). More significantly, helminth and C. rodentium coinfection results in a pronounced increase in colonic LP macrophages as evidenced by an increase in CD11b+ and/or F4/80+ cells (a pan marker of macrophages) (Fig. 1D). The striking macrophage accumulation observed (quantitated in Fig. 1E) suggests that the small intestinal nematode infection (H. polygyrus) is capable of altering the macrophage response to concurrently exposed colonic bacterial pathogens. The increase in colonic macrophage infiltration detected in mice with coinfection of H. polygyrus and C. rodentium is correlated with impaired bacterial clearance (Fig. 1F) and a more severe C. rodentium-mediated intestinal injury, including epithelial cell hyperplasia, colonic crypt elongation, epithelial erosions, and edema of the gut wall (Ref. 16 and data not shown).
FIGURE 1.
Helminth coinfection results in an increased number of macrophages in colonic LP. A–D, BALB/c mice were infected with H. polygyrus (200 L3) and inoculated with C. rodentium (5 × 108 CFU) 7 days later. Uninfected control mice (A) and mice infected with C. rodentium (C.r.) (B), H. polygyrus (H.p.) (C), or both (Co-inf, coinfected) (D) were sacrificed 2 wk afer bacterial infection. Five-micrometer sections of frozen colonic tissue (in OCT) were cut and fixed in ice-cold acetone. After washing with PBS, the sections were blocked with PBS and 1% BSA. The tissue sections were incubated with anti-CD11b-FITC (green) and biotin-labeled anti-F4/80 Ab followed by streptavidin-Cy3. The sections were analyzed by immunofluorescence microscopy. Magnification, ×400. All images were digitized and cropped in Adobe Photoshop LE 5.0 (Adobe Systems). E, The mean number of positive cells detected in each high power field (magnification, ×200) was determined by counting 10 fields from each sample (samples from three mice per group were counted). F, Mice that were coinfected with H. polygyrus and C. rodentium have higher bacterial output in the fecal pellets. The data shown are the number of bacteria recovered from fecal samples of C. rodentium-infected mice and coinfected mice at 3 wk postinfection. The data are represented as the mean ± SEM (n = 5–10 mice at each time point).*, p < 0.05 for a comparison of coinfected group vs every other group. G and H, FACS data show that helminth coinfection enhances the expansion (mean ± SE, n = 3 per group) and activation of macrophages in MLN. MLN cells were collected from noninfected and infected mice and stained for macrophages with FITC-labeled Ab or costained with PE-labeled anti-Mac-1 and FITC-labeled anti MHC II for the determination of activation of the cells. H, FACS data shows MHC II (IA) expression levels on a gated Mac-1 cell population.
To further examine the influence of helminth coinfection on mucosal macrophage responses to concurrent enteric bacterial infection, we compared the frequency and activation status of macrophages in the MLN among various groups by using FACS. The results in Fig. 1G show that helminth coinfection results in an increase in the frequency of CD11b+ macrophages in the MLN. H. polygyrus coinfection also enhances the activation status of macrophages as evidenced by up-regulation of the expression of MHC II (Fig. 1H).
Primary infection with H. polygyrus stimulates the development of alternatively activated macrophages in mice
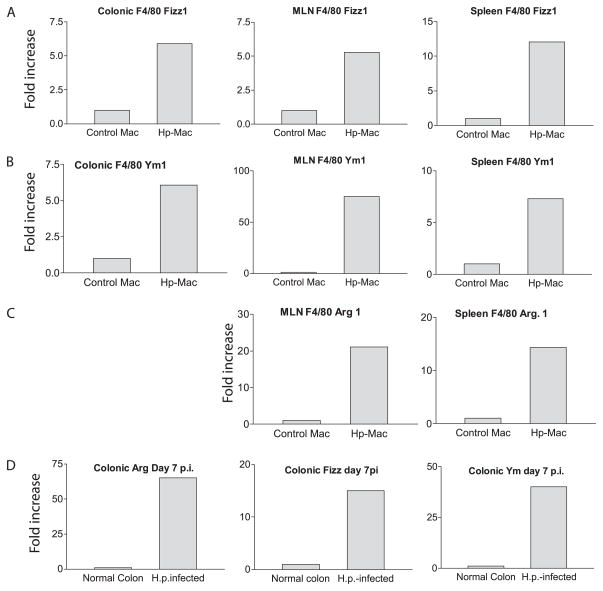
A series of recent studies have suggested that the Th2 cytokine response induced by nematode parasites stimulates the development of alternatively activated macrophages (25), a phenotype that is associated with the expression of a specific set of genes, including Ym1, Fizz1/REM-a, and Arg1 (26, 27). To determine whether primary infection with H. polygyrus, which is usually chronic in nature, induces the development of alternatively activated macrophages, we infected BALB/c mice with H. polygyrus and isolated F4/80+ macrophages 3–4 wk after infection. Gene expression for Fizz1, Ym1, and Arg1 from purified F4/80+ macrophages isolated from MLN, spleen, colonic LP, and peritoneal cavity of helminth-infected and uninfected mice was determined by real-time RT-PCR. The results show that F4/80+ macrophages isolated from various tissues of mice with primary H. polygyrus infection display a marked up-regulation of Fizz1 (Fig. 2A), Ym1 (Fig. 2B), and Arg1 (Fig. 2C) expression. This pattern of gene expression was observed in the colon as early as 7 days after helminth infection (Fig. 2D), the time at which C. rodentium was introduced. The data, therefore, demonstrate that a primary infection with the intestinal nematode parasite H. polygyrus induces the development of alternatively activated macrophages in mice both mucosally and systemically.
FIGURE 2.
A primary infection of H. polygyrus (Hp) up-regulates Ym1, Arg1, and Fizz1 expression in macrophages (Mac) of multiple tissues. A–C, Macrophages (were purified from spleen, MLN, and colonic LP of helminth-infected mice at 3–4 wk after infection or from normal control mice. FACS analysis of the purified macrophages revealed that they were >95% F4/80+CD11b+ cells (data not show). D, Colonic tissues were collected from control and helminth-infected mice (7 days after H. polygyrus (H.p.) infection). Total RNA was isolated from the purified macrophages and colonic tissues. Ym1, Arg1, and Fizz1 expression was determined using realtime PCR. Values are the fold increase compared with baseline obtained from uninfected control mice. The data shown are from one of two experiments performed showing similar results.
Both alternatively and classically activated macrophages are present in the colonic LP of mice coinfected with helminth and C. rodentium
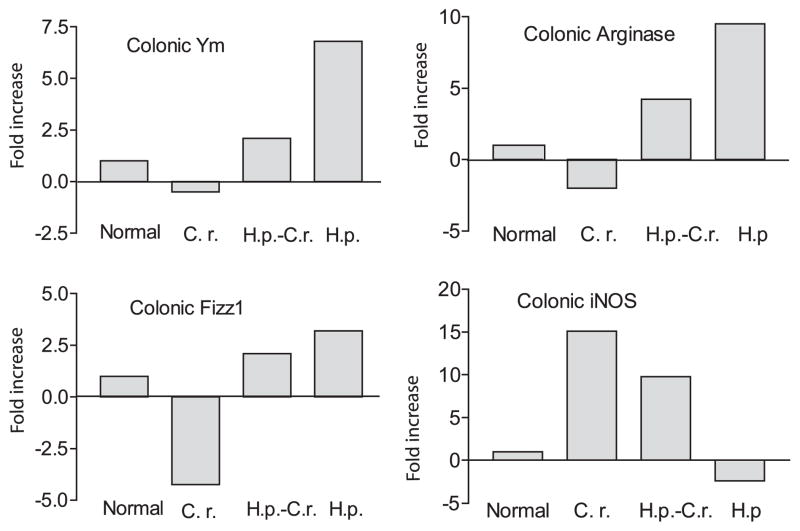
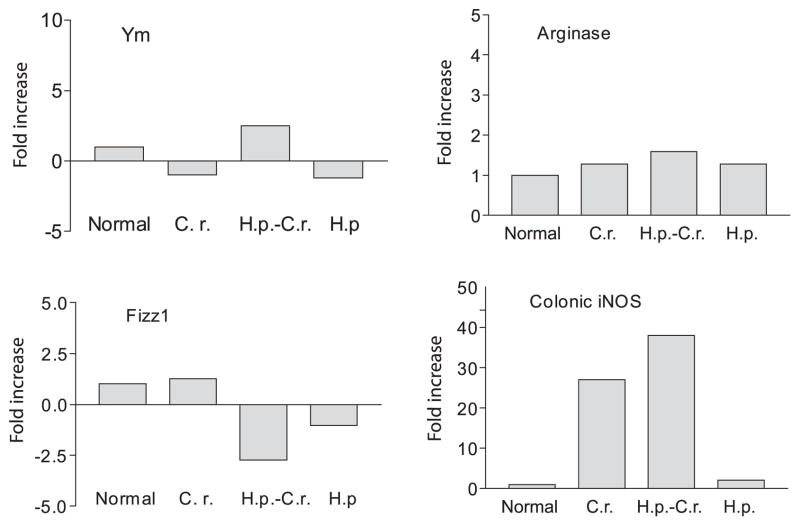
Next, we examined whether helminth infection induces phenotypical alterations in infiltrating macrophages in the intestinal mucosa in mice that were preinfected with H. polygyrus and subsequently exposed to C. rodentium. Using quantitative real-time RT-PCR, we analyzed the expression of macrophage genes associated with the alternatively activated phenotype (Arg1, Ym1, and Fizz1) as well as the expression of iNOS, a gene that is associated with classically activated macrophages, in the colonic tissues from normal, C. rodentium-infected, H. polygyrus-infected, and coinfected mice. As shown in Fig. 3, helminth infection alone induces a dramatic up-regulation of Arg1, Fizz1, and Ym1 expression, indicating the induction of alternatively activated macrophages in the colonic tissue of helminth-infected mice, whereas a marked decrease in these gene expression levels is detected in the colon of mice that are infected with C. rodentium. In contrast the expression of iNOS shows the inverse pattern, with low levels in helminth-infected mice and a marked up-regulation in C. rodentium-infected animals. These observations indicate that helminth and bacterial infection induced the development and/or recruitment of a macrophage population that is dominated by either alternatively activated or classically activated cells, respectively. In animals coinfected with C. rodentium and H. polygyrus, both types of macrophages are present in the colon, as indicated by increased expression of genes corresponding to the alternatively (Arg1, Ym1, and Fizz1) and classically (iNOS) activated phenotypes.
FIGURE 3.
Detection of alternatively and classically activated macrophages in colonic tissues. Colonic tissues were collected (2 wk after bacterial infection) from various groups. Total RNA was isolated. Gene expression for alternatively activated (Arg1, Fizz1 and Ym1) and classically activated macrophages (iNOS) of colonic tissues was determined using real-time RT-PCR. Values are the fold increase compared with baseline obtained from uninfected control mice. The data shown are from one of two experiments performed showing similar results. H.p., H. polygyrus; C.r., C. rodentium.
Helminth infection-associated macrophage recruitment in colon tissues of the coinfected host is STAT6 dependent
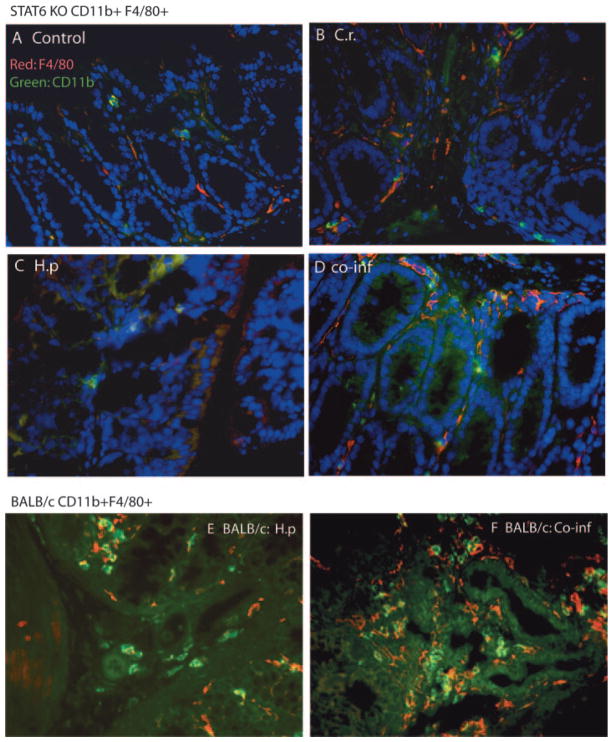
Prior reports have demonstrated that intestinal colonization with C. rodentium induces a significant CD3+ and CD4+ T cell infiltration into the colonic LP that stimulates a predominant Th1-type immune response in the intestine characterized by up-regulation of IFN-γ, TNF-α, and IL-12 (10, 11, 28, 29). Our recent study demonstrated that a helminth-induced Th2 response contributes significantly to the development of exacerbated bacteria-mediated intestinal injury (16). To determine whether the helminth-induced Th2 response contributes to the increased number of macrophages in mice coinfected with H. polygyrus and C. rodentium, we repeated the coinfection experiments described above by using STAT6 knockout (KO) mice, which are deficient in the functional differentiation of Th2 cells (30). As shown earlier, we observed that C. rodentium infection induced the development of colitis in STAT6 KO mice, whereas coinfection with the Th2-inducing helminth failed to induce the exacerbated bacteria-mediated intestinal injury (16). In addition, the results presented in Fig. 4 show that the increase in LP macrophage numbers seen in the H. polygyrus- and C. rodentium-coinfected WT (BALB/c) mice (Fig. 4, E and F) did not occur in the STAT6 KO animals (Fig. 4, A–D). More significantly, we observed that H. polygyrus infection fails to induce the development and accumulation of alternatively activated macrophages in the colon of STAT6 KO mice, as evidenced by the markedly attenuated change in expression of Ym1, Arg1, and Fizz1 (compare the data in Fig. 5 with those in Fig. 3). In contrast, the up-regulation of iNOS by either C. rodentium infection alone or C. rodentium plus H. polygyrus is intact and even accentuated in STAT6 KO mice, indicating the normal development of classically activated macrophages in these mice. These observations indicate that the observed helminth-induced increase in the appearance of alternatively activated macrophages in the colon involves a STAT6-dependent mechanism.
FIGURE 4.
Helminth-induced increase in macrophage numbers in colonic LP of mice is mediated through a STAT6-dependent mechanism. Uninfected STAT6 KO (A) mice and STAT6 KO mice infected with C. rodentium (C.r.) (B), H. polygyrus (H.p) (C), or both (co-inf, coinfected) (D) were sacrificed 2 wk after bacterial infection. BALB/c mice infected with H. polygyrus (E) and coinfected (F) were included as controls. Magnification, ×400. Five-micrometer sections of frozen colonic tissue (in OCT) were cut, fixed, and stained for CD11b (green) and F4/80 (red) as described in the Fig. 1 legend.
FIGURE 5.
H. polygyrus infection fails to induce the development and accumulation of alternatively activated macrophages in the colon of STAT6 KO mice. Colonic tissues were collected from uninfected STAT6 KO mice (Normal), STAT6 KO mice infected with C. rodentium (C.r.), H. polygyrus (H.p.), or both (H.p.-C.r.) (2 wk after bacterial infection). Total RNA was isolated from the colonic tissues. Gene expression for alternatively activated macrophages (Arg1, Fizz1, and Ym1) and classically activated macrophages (iNOS) of colonic tissues was determined using real-time RT-PCR. Values are the fold increase compared with baseline obtained from uninfected control mice. The data shown are from one of two experiments performed showing similar results.
Helminth-stimulated macrophages display reduced microbicidal activity
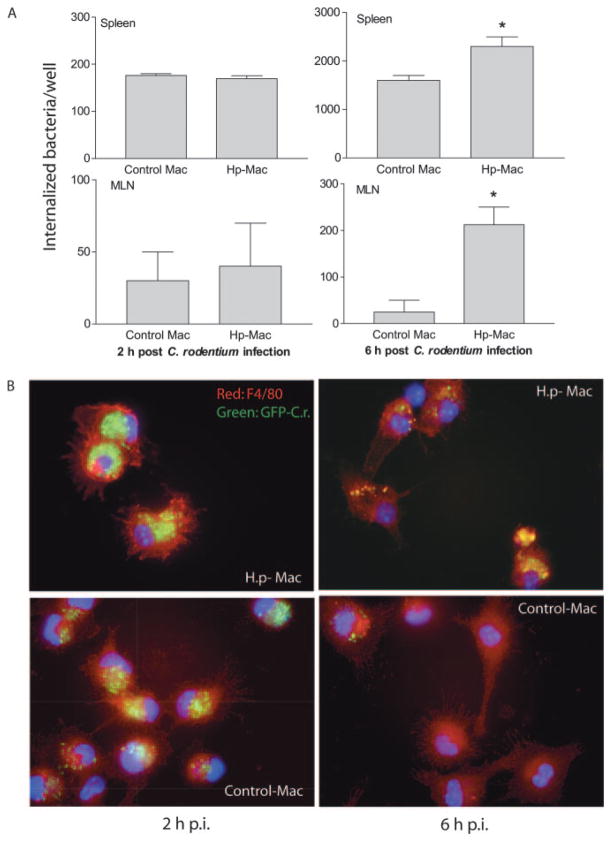
The immunohistology observations described above (Fig. 1) indicate a marked increase in colonic macrophages in helminth-coinfected mice. Despite this increase in macrophage number, our earlier studies showed that coinfected animals have elevated numbers of bacteria in the tissues (16), suggesting that the macrophages are unable to control bacterial multiplication effectively. To address this possibility, we isolated macrophages from the spleen and MLN of control and helminth-infected mice, infected the cells with C. rodentium in vitro, and used the gentamicin protection assay as a measure of macrophage microbicidal function (31). Our results show that macrophages isolated from helminth-infected mice (both spleen and MLN) internalized similar numbers of bacteria at 2 h after infection compared with cells isolated from normal control animals (Fig. 6A). However, at 6 h after the initial bacterial infection the number of viable bacteria recovered from macrophages that were isolated from the helminth-infected host was significantly higher compared with the macrophages isolated from normal mice (Fig. 6A), indicating an impaired bacterial killing capacity of helminth-stimulated macrophages.
FIGURE 6.
H. polygyrus (Hp) infection impairs the bacterial killing capacity of macrophages (Mac). Macrophages were isolated from spleen, MLN (A) and peritoneal cavity (B), incubated overnight in complete DMEM, and then exposed to C. rodentium (or GFP-expressing C. rodentium, ×10 bacteria/cell) for 1 h. After the completion of the infection period, the cells were incubated with gentamicin-containing medium, which kills extracellular bacteria. A, Two and 6 h after antibiotic treatment, the number of viable internalized bacteria recovered in macrophages from spleen and MLN was determined by plating the cell lysates onto LB plates. The data shown is the mean ± SE of triplicate cultures. *, p < 0.05 for a comparison of cells isolated from uninfected mice vs cells obtained from helminth-infected mice. B, Immunofluorescence microscopy data show the number of internalized bacteria in peritoneal macrophages (stained with anti-F4/80 and Cy3) from H. polygyrus (H.p.)-infected and uninfected mice at 2 and 6 h after C. rodentium-GFP infection (p.i., postinfection). Peritoneal macrophages from H. polygyrus-infected mice display an impaired ability to control growth of internalized bacteria compared with cells from uninfected control mice. The data shown are from one of three experiments performed showing similar results.
To further explore the functional alterations of macrophages induced by helminth infection, peritoneal macrophages from normal and H. polygyrus-infected mice were collected. Real-time RT-PCR analysis confirmed that peritoneal macrophages isolated from helminth-infected mice, similar to macrophages isolated from other sites (MLN, spleen, and colon) (Fig. 2), also displayed an alternatively activated phenotype as indicated by the up-regulation of Ym1, Fizz1, and Arg1 (data not shown). Using these readily available peritoneal macrophages, we were able to visualize the influence of helminth infection on the uptake and subsequent elimination of C. rodentium by macrophages in vitro by using GFP-expressing C. rodentium. To this end, peritoneal macrophages from both control and helminth-infected mice were collected and grown on cover-slips. After overnight incubation at 37°C, the cells were infected with GFP-expressing C. rodentium for 1 h and placed in gentamicin-containing medium. Two and 6 h after antibiotic treatment, the cells were stained by using anti-F4/80 mAb and streptavidin-Alexa 647. Immunofluorescence microscopic analysis showed that at the 2-h time point F4/80+ macrophages isolated from both helminth-infected and control mice internalized bacteria equally well, as evidenced by the detection of similar frequency and intensity of GFP-C. rodentium in macrophages (96 ± 3% of cells from control mice and 98 ± 2% of cells from helminth-infected mice were GFP+) (Fig. 6B). At the 6-h time point the number of cells that contained bacteria was significantly less in the case of the macrophages isolated from control animals (46 ± 9.0% of cells from control mice were GFP+ vs 95 ± 4.0% of cells from helminth-infected mice). This finding is in keeping with the results of the gentamicin protection assay described above (Fig. 6A) and further supports the idea that macrophages from helminth-infected mice are impaired in killing internalized bacteria. This impairment may contribute to the exacerbation of C. rodentium infection in helminth-coinfected mice.
Helminth stimulated macrophages are an important source of TNF-α in coinfected mice
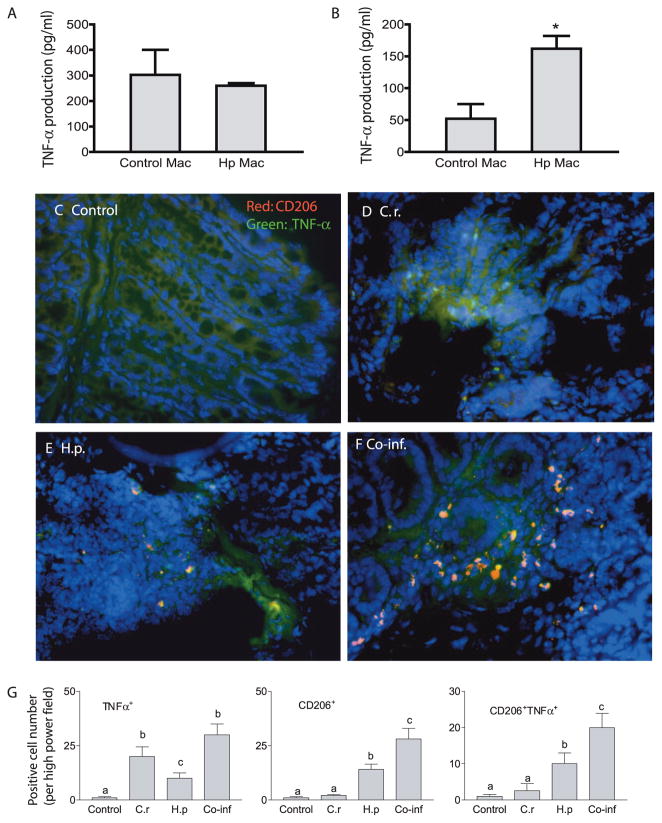
We have recently observed that helminth coinfection significantly exacerbated bacteria-mediated colitis and up-regulated colonic TNF-α expression (16). Because increased expression of TNF-α has been implicated in the pathogenesis of intestinal inflammation in both experimental models and human clinical studies (32, 33), we were interested in determining the effect of helminth infection on macrophage production of this cytokine. In initial in vitro experiments we collected F4/80+ macrophages from the colon, MLN, and spleen of helminth-infected as well as control mice and infected the cells with C. rodentium. As shown in Fig. 7A, we found that macrophages isolated from helminth-infected mice produced a similar level of TNF-α in response to C. rodentium infection compared with cells isolated from normal mice at 2 h after bacterial infection. However, at 6 h after infection a significantly higher amount of TNF-α production was detected in helminth-stimulated macrophages than in control cells (Fig. 7B), correlating with the increased bacterial load in these cells (Fig. 6A).
FIGURE 7.
Helminth-induced, alternatively activated macrophages (Mac) (CD206+) produce TNF-α in response to C. rodentium (C.r.) exposure. A and B, Macrophages were isolated from MLN of uninfected (Control) and H. polygyrus (Hp)-infected mice, incubated overnight in complete DMEM, and then exposed to C. rodentium for 1 h. Two (A) and 6 h (B) after infection, culture supernatants were collected. TNF-α production of MLN macrophages was measured by ELISA. The data shown are the mean ± SE of triplicate cultures. *, p < 0.05 for a comparison of cells isolated from uninfected mice vs cells obtained from helminth-infected mice. C–F, Colon tissues were prepared as in Fig. 1. The tissues were stained with anti-CD206 Ab (red) and anti-TNF-α (green). The immunofluorescence microscopic analysis showed that C. rodentium infection induced TNF-α production by cells other than CD206+ cells (D). H. polygyrus infection results in the induction of TNF-α producing cells that express the alternatively activated phenotype (CD206+) (E). Helminth and C. rodentium coinfection (F) resulted in a marked increase in alternatively activated macrophages that produce TNF-α in colon tissues. G, The mean number of TNF-α+, CD206+, and CD206+TNF-α+ cells detected in each high power field (×200) by counting five fields from each sample (three mice from each group were counted). Different letters represent significant differences ( p < 0.05). Co-inf, coinfected.
To further examine the impact of helminth infection on the macrophage TNF-α response to enteric bacterial pathogens, we took an in vivo approach. We repeated the coinfection experiments and determined the contribution of helminth-induced, alternatively activated macrophages to the observed TNF-α response in the coinfected host. Colon sections of mice from different groups were costained with CD206 (used as a marker of alternatively activated macrophages) and anti-TNF-α Abs. Our results from immunofluorescence microscopic analysis show that C. rodentium infection results in the appearance of TNF-α producing cells, most of which were CD206+ (Fig. 7, C–G). We also observed a low number of CD206+ TNF-α expressing cells in helminth-infected mice. More dramatically, however, we found that in helminth- and C. rodentium-coinfected mice there was a significant increase in CD206+ cells, i.e., alternatively activated macrophages, that produce TNF-α. These observations indicate that alternatively activated macrophages are an important source of TNF-α in the colons of coinfected mice.
Exacerbated colonic pathology in mice with helminth coinfection is caused by uncontrolled C. rodentium infection
The observations of increased disease severity in coinfected mice and high bacterial output (Fig. 1F) and translocation into mucosal and systemic immune compartments suggest that coinfected mice were less able to control the bacterial infection (16). It is known that macrophages can be activated by microbial pattern-recognition receptors such as TLRs, produce proinflammatory cytokines, and play a role in bacterial recognition and elimination. We now provide evidence to suggest that helminth coinfection induces the development of alternatively activated macrophages with impaired antimicrobial function. This leads to an uncontrolled infection of bacteria that have translocated through the disrupted intestinal epithelium, contributing to exacerbated bacteria-mediated colitis. This hypothesis predicts that reducing bacterial number would ameliorate the colitis seen in the coinfected animals. To directly test whether a failure to effectively control enteric bacteria contributes to increased disease severity in coinfected mice, we performed another set of coinfection experiments with the modification that starting at 1 wk after bacterial infection some coinfected mice were given drinking water containing oxytetracycline (0.8 mg/ml; Sigma) for 1 wk, which was shown previously to be effective in controlling C. rodentium infections (34). As shown in Fig. 8, we observed as anticipated that helminth-coinfected mice developed more severe intestinal pathology than mice with C. rodentium infection alone (Fig. 8). Interestingly, our results further show that antibiotic-mediated bacterial clearance in coinfected mice was associated with a significant reduction in the colitis (Fig. 8). These results support the idea that increased disease severity in coinfected mice is due to an inability to effectively control enteric bacterial infection.
FIGURE 8.
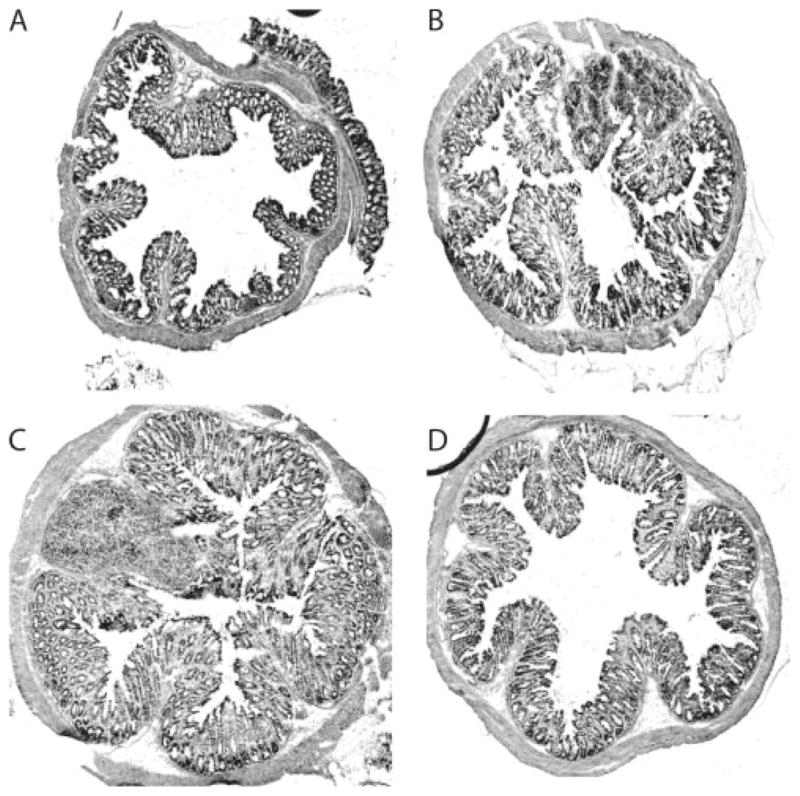
Helminth coinfection results in increased colonic inflammation due to impaired ability to control bacterial infection. Colon tissue was prepared from uninfected (A), C. rodentium-infected (B), helminth-and C. rodentium-coinfected (C), and coinfected and antibiotic-treated mice (D). Five-micrometer sections of frozen colonic tissue (in OCT) were cut, fixed, and stained with H&E. The figures shown are representative histology of the distal colon 2 wk after C. rodentium infection.
Discussion
Intestinal helminth infections have been shown to have a significant impact on the occurrence and course of a number of other illnesses. Cross-regulatory suppression of Th1 immunity by the helminth-driven Th2 response has been suggested to alter the host response to concurrent bacterial infections. This may be particularly important in the developing world where chronic helminth infection coexists commonly with enteric bacterial pathogens. In contrast, recent evidence indicates that in the developed world a complete absence of helminth infection may predispose individuals to certain immune-mediated disorders (35). The ability of helminths to attenuate damaging Th1-driven inflammatory responses in the host (5–8) has prompted the evaluation of helminths as therapeutic agents for the treatment of some immune-mediated disorders, including inflammatory bowel disease (9). Thus, a better understanding of immune modulation by helminths will have important public health implications. The coinfection model that we recently developed allows us to explore the mechanisms by which helminths affect the capacity of the host to develop an appropriate immune response to other concomitant pathogens or Ags. Using this model we have shown that coinfection with H. polygyrus results in alterations in mucosal responses to concomitant bacterial pathogens and the development of an exacerbated bacteria-mediated intestinal injury (16). Furthermore, our recent study provided evidence to indicate that helminth-primed DCs are involved in the dampening of protective Th1 responses. This in turn may allow for increased colonization and proliferation of the microorganisms and a delayed clearance of the infecting bacteria, leading to more severe tissue damage and disease (24). In the current study, we have expanded our previous observations and investigated the role of helminth-stimulated macrophages in this process and examined the influence of potent helminth-induced Th2 activation signals on the phenotype and function of macrophages. Because macrophages in the intestinal mucosal are strategically positioned in the LP in close proximity to enteric bacteria that have breached the epithelium, these cells can contribute significantly to the effector phase of the immune response, i.e., elimination of bacteria, and they also act as critical mediators of many chronic inflammatory diseases.
We show that a primary H. polygyrus infection, which is chronic in nature, induces the development of alternatively activated macrophages in both mucosal sites (MLN and LP) and systemic compartments such as the spleen and peritoneal cavity. We further show that the exacerbated C. rodentium-mediated colitis that develops in H. polygyrus-coinfected mice correlates with the marked accumulation of alternatively activated macrophages in colonic LP via a STAT6-dependent mechanism. Functional analysis indicates that these helminth-stimulated macrophages have an impaired ability to effectively control the multiplication of phagocytosed C. rodentium. Presumably as a result of the increased bacterial load, these cells produce increased amounts of TNF-α, a cytokine that has a well-established role in intestinal and other types of inflammation (32, 33). Together with our earlier work showing a STAT6-dependent exacerbation of Citrobacter-induced colitis by H. polygyrus, the findings presented here suggest that helminth-induced, alternative macrophage activation contributes importantly to this process. Thus, our results provide a mechanistic explanation for the enhanced bacterial infection and exacerbated bacteria-induced intestinal injury in mice that are coinfected with H. polygyrus. Other effects of helminth infection on the response to Citrobacter may also play a role and are the subject of ongoing investigations in our laboratory.
The nature of microbial Ags and T cell-derived cytokines can have a profound influence on the heterogeneity and activation status of macrophages. Our observation of the STAT6-dependent accumulation of colonic alternatively activated macrophages in the coinfected host demonstrates a role for the helminth-induced Th2 response in the modulation of macrophage phenotype and the outcome of a concurrent enteric bacterial infection. In line with our observations, a recent study that used an anti-helminthic drug-abbreviated infection protocol showed that, during H. polygyrus challenge infection, IL-4 production by memory CD4+ T cells in the small intestine led to a localized accumulation of alternatively activated macrophages in a STAT6-dependent fashion (22). In addition, recent evidence suggests that STAT6 may directly control the transcriptional activation of genes implicated in the alternatively activated phenotype such as Arg1 (36) and Tfec (37). Helminths may also influence macrophages directly, independently of effects on T cells. A recent study demonstrated that the helminth structural molecule chitin, a biopolymer of N-acetyl-β-D-glucosamine, has the ability to act on macrophages to induce the alternatively activated phenotype (38). These observations raise the possibility that helminth parasites may induce the development of alternatively activated macrophages locally in the small intestine and that these cells may circulate to the colonic tissue and systemic sites. However, our observations from STAT6-deficient mice showing a defect in the induction of alternatively activated macrophages during helminth infection support the idea that the helminth-induced Th2 response is responsible for the development of these cells. Therefore, in our model system it is likely that helminth-induced Th2 cells that differentiate in the small intestine or GALT migrate to the colon and elsewhere and influence macrophage phenotype and function at these distant sites.
In our current study, we also show that despite the inability to develop alternatively activated macrophages during helminth infection, STAT6 KO mice that were infected with C. rodentium alone or coinfected with helminth produced a robust, classically activated macrophage response (Fig. 5). The failure of helminth-induced, alternative macrophage activation, together with the uncompromised classical pathway of macrophage activation, provides an explanation for the observation that H. polygyrus infection in these animals is not associated with poor control of Citrobacter multiplication and the corresponding exacerbation of the bacterial colitis.
Although helminth-induced, alternatively activated macrophages have been suggested to act as an effector cell population contributing to helminth parasite elimination (22), our results presented here demonstrate that the macrophages stimulated by helminth infection are unable to effectively control the concomitant enteric bacterial pathogens. This conclusion is supported by the results from two different in vitro assays showing the presence of a significantly higher number of intracellular viable bacteria in macrophages from helminth-infected mice (Fig. 6, A and B). These results are consistent with a recent report that showed that alternative activation of macrophages by the Th2 cytokine IL-4 supported the growth of the intracellular pathogen Mycobacterium tuberculosis in association with impaired NO production (39). Alternatively activated macrophages are known to produce a reduced amount of NO (40), but it remains to be determined whether this characteristic accounts for the impaired clearance of C. rodentium by these cells. Our ongoing studies are exploring the basis for the compromised antimicrobial activity of helminth-stimulated macrophages in controlling the growth of phagocytosed C. rodentium. Regardless of the precise mechanisms for the reduced killing of C. rodentium, it is clear that the resultant increase in tissue bacterial load directly contributes to the worsening of colitis. When we reduced the number of bacteria by antibiotic treatment in coinfected mice, the intestinal inflammation was significantly attenuated (Fig. 8).
Helminth-induced, alternatively activated macrophages contribute not only to the impaired host protection against concomitant enteric bacterial infection but also to the enhanced production of TNF-α in response to the bacteria. Although current views suggest that the TNF-α response is associated with activation of classically activated macrophages during microbial infections, the results from the present study provide in vitro and in vivo evidence to indicate that helminth-stimulated, alternatively activated macrophages are also important sources of TNF-α in coinfected mice (Fig. 7). These observations are supported by a recent report showing that macrophages pretreated with various cytokines (including the Th2 cytokine IL-4) and then restimulated with LPS produced an enhanced amount of the proinflammatory cytokines TNF-α and IL-12 while retaining the alternatively activated phenotype (41). Our results suggest, therefore, that an increase in the number of helminth-induced, alternatively activated macrophages may contribute significantly to the observed exacerbated intestinal injury in coinfected mice by the production of TNF-α. Factors other than increased TNF-α production may also play a role in the helminth-induced worsening of Citrobacter colitis.
Quantitative real-time RT-PCR analysis of colon tissues shows an up-regulation of expression of various genes that are associated with alternatively activated macrophages in the colons of H. polygyrus-infected and -coinfected, but not in C. rodentium-infected, mice (Fig. 3). In C. rodentium-infected mice the expression of iNOS, a classically activated macrophage-associated gene, was significantly up-regulated. In helminth-coinfected mice the gene expression pattern associated with both types of macrophages was detected, suggesting that in these mice the driving forces for both classical and alternative activation are present. It is possible that in addition to impaired bacterial killing capacity, alternatively activated macrophages present at the site of bacterial infection may influence the functional properties of the other cell types in the vicinity, including macrophages. By releasing IL-10 and CCL17 (42), alternatively activated cells can exert suppressive effects on classically activated macrophages. In addition, alternatively activated macrophages may suppress CD4 T cell responses by a TGF-β-dependent mechanism (43).
In conclusion, our investigation provides evidence suggesting that helminth coinfection can impair host protection against concurrent enteric bacterial infection and promote bacteria-induced intestinal injury through a mechanism that involves the induction of alternatively activated macrophages with a reduced bactericidal activity. The results from our recent studies together with the current investigation provide evidence to indicate that the immunomodulation of bacterial infection and bacteria-mediated intestinal inflammation by helminth parasites involves multiple mechanisms, including regulatory as well as effector cells in both the innate and the adaptive immune systems. The results described in this study, along with ongoing study of the coinfection model, will provide a framework for a better understanding of the immunomodulatory effects of helminths, yielding information not only for establishing novel and more effective treatments for immune-mediated diseases but also for the design of effective intestinal vaccines for the prevention and treatment of microbial diseases in the areas where multiple infections coexist.
Acknowledgments
We thank Dr. L. Bry (Brigham and Women’s Hospital) for providing the GFP-expressing C. rodentium.
Footnotes
This work was supported in part by the Clinical Nutrition Research Center at Harvard (P30 DK 40561) and a RO1 grant (to B.J.C.). H.N.S. is a recipient of National Institutes of Health KO1 Award (DK059996). M.W. is sponsored by Fogarty International Maternal and Child Health Research and Training Program (TW001265). O.F.-J. is sponsored by the Harvard Medical School Training Program in Nutrition and Metabolism (5 T32 HD052961).
Abbreviations used in this paper: LP, lamina propria; Arg1, arginase 1; DC, dendritic cell; iNOS, inducible NO synthase; KO, knockout; LB, Luria broth; MLN, mesenteric lymph node.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Actor JK, Shirai M, Kullberg MC, Buller ML, Sher A, Berzofsky JA. Helminth infection results in decreased virus specific CD8+ cytotoxic T cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo MI, Bliss SK, Suzuki Y, Alcaraz A, Denkers EY, Pearce E. Interleukin-12 promotes pathologic liver changes and death in mice coinfected with Schistosoma mansoni and Toxoplasma gondii. Infect Immun. 2001;69:1454–1462. doi: 10.1128/IAI.69.3.1454-1462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady MT, O’Neill SM, Dalton JP, Mills K. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect Immun. 1999;67:5372–5378. doi: 10.1128/iai.67.10.5372-5378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansfield LS, Gauthier DT, Abner SR, Jones KM, Wilder SR, Urban J., Jr Enhancement of disease and pathology by synergy of Trichuris suis and Campylobacter jejuni in the colon of immunologically naive swine. Am J Trop Med Hyg. 2003;68:70–80. [PubMed] [Google Scholar]
- 5.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 6.Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, Collins S. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002;70:5931–5937. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, Van Marck EA, Pelckmans PA. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53:99–107. doi: 10.1136/gut.53.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Weinstock J. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol. 2003;284:G385–G391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 9.Summers RW, Elliott DE, Urban JF, Thompson RA, Weinstock J. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Vallance BA, Deng W, Knodler LA, Finlay BB. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect Immun. 2002;70:2072–2081. doi: 10.1128/IAI.70.4.2070-2081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goncalves NS, Ghaem-Maghami M, Monteleone G, Frankel G, Dougan G, Lewis DJ, Simmons CP, MacDonald T. Critical role for tumor necrosis factor α in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect Immun. 2001;69:6651–6659. doi: 10.1128/IAI.69.11.6651-6659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003;71:5077–5086. doi: 10.1128/IAI.71.9.5077-5086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallance BA, Chan C, Robertson ML, Finlay BB. Enteropathogenic and enterohemorrhagic Escherichia coli infections: emerging themes in pathogenesis and prevention. Can J Gastroenterol. 2002;16:771–778. doi: 10.1155/2002/410980. [DOI] [PubMed] [Google Scholar]
- 14.Luperchio S, Newman JV, Dangler CA, Schrenzel MD, Brenner DJ, Steigerwalt AG, Schauer DB. Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, exhibits clonality: synonymy of Citrobacter rodentium and mouse-pathogenic Escherichia coli. J Clin Microbiol. 2000;38:4343–4350. doi: 10.1128/jcm.38.12.4343-4350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luperchio S, Schauer D. Molecular pathogenesis of Citrobacter rodentium and transmissible murine molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 2001;3:333–340. doi: 10.1016/s1286-4579(01)01387-9. [DOI] [PubMed] [Google Scholar]
- 16.Chen CC, Louie S, McCormick BA, Walker AW, Shi HN. Concurrent infection of an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect Immun. 2005;73:5468–5481. doi: 10.1128/IAI.73.9.5468-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 18.Goerdt S, Orfanos C. Other functions, other genes: alternative activation of Ag-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 19.Loke P, MacDonald AS, Robb AO, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell to cell contact. Eur J Immunol. 2000;30:2669–2678. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Allen J, Lawrence R, Maizels R. Antigen presenting cells from mice harboring the filarial nematode: Brugia malayi, prevent cellular proliferation but not cytokine production. Int Immunol. 1996;8:143–151. doi: 10.1093/intimm/8.1.143. [DOI] [PubMed] [Google Scholar]
- 21.Herbert DR, Hölscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 22.Anthony RM, Urban JF, Farhang A, Hamed HA, Rozo CT, Boucher J, Van Rooijen N, Gause WC. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi HN, Scott ME, Stevenson MM, Koski KG. Zinc deficiency impairs T cell function in mice with primary infection of Heligmosomoides polygyrus (Nematoda) Parasite Immunol. 1994;16:339–350. doi: 10.1111/j.1365-3024.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen CC, Louie S, McCormick BA, Walker WA, Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol. 2006;176:472–483. doi: 10.4049/jimmunol.176.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 26.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7–18. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noël W, Raes G, Ghassabeh GH, Baetselier PD, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004;20:126–133. doi: 10.1016/j.pt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Higgins LM, Frankel G, Connerton I, Goncalves NS, Dougan G, MacDonald TT. Role of bacterial intimin in colonic hyperplasia and inflammation. Science. 1999;285:588–591. doi: 10.1126/science.285.5427.588. [DOI] [PubMed] [Google Scholar]
- 29.Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat 6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity. 1996;4:313–318. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 31.Rhee S, Walker W, Cherayil BJ. Developmentally regulated intestinal expression of IFN-γ and its target genes and the age-specific response to enteric Salmonella infection. J Immunol. 2005;175:1127–1136. doi: 10.4049/jimmunol.175.2.1127. [DOI] [PubMed] [Google Scholar]
- 32.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 33.Möller B, Villiger PM. Inhibition of IL-1, IL-6, and TNF-α in immune-mediated inflammatory diseases. Springer Semin Immunopathol. 2006;27:391–408. doi: 10.1007/s00281-006-0012-9. [DOI] [PubMed] [Google Scholar]
- 34.de LaPuene-Redondo VA, Gutierrez-Martin CB, Perez-Martinez C, del Blanco NG, Garcia-Iglesias MJ, Perez-Garcia CC, Rodriguez-Ferri EF. Epidemic infection caused by Citrobacter rodentium in a gerbil colony. Vet Rec. 1999;145:400–403. doi: 10.1136/vr.145.14.400. [DOI] [PubMed] [Google Scholar]
- 35.Yazdanbakhsh M, Kremsner PG, van Ree RR. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 36.Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM., Jr Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPβ. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Rehli M, Sulzbacher S, Pape S, Ravasi T, Wells CA, Heinz S, Sollner L, El Chartouni C, Krause SW, Steingrimsson E, et al. Transcription factor Tfec contributes to the IL-4-inducible expression of a small group of genes in mouse macrophages including the granulocyte colony-stimulating factor receptor. J Immunol. 2005;174:7111–7122. doi: 10.4049/jimmunol.174.11.7111. [DOI] [PubMed] [Google Scholar]
- 38.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, Kaufmann SH. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 40.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 41.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 42.Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–1413. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- 43.Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol. 2006;176:6918–6927. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]