Abstract
Purpose
The disease outcome for patients with cancer is typically described in terms of estimated survival from diagnosis. Conditional probability offers more relevant information regarding survival for patients once they have survived for some time. We report conditional survival probabilities on the basis of 498 patients with glioblastoma multiforme receiving radiation and chemotherapy. For 1-year survivors, we evaluated variables that may inform subsequent survival. Motivated by the trend in data, we also evaluated the assumption of constant hazard.
Patients and Methods
Patients enrolled onto seven phase II protocols between 1975 and 2007 were included. Conditional survival probabilities and 95% CIs were calculated. The Cox proportional hazards model was used to evaluate prognostic values of age, Karnofsky performance score (KPS), and prior progression 1-year post diagnosis. To assess the constant hazard assumption, we used a likelihood-ratio test to compare the Weibull and exponential distributions.
Results
The probabilities of surviving an additional year given survival to 1, 2, 3, and 4 years were 35%, 49%, 69%, and 93%, respectively. For patients who survived for 1 year, lower KPS and progression were significantly predictive of shorter survival (both P < .001), but age was not (hazard ratio, 1.22 for a 10-year increase; P = .25). The Weibull distribution fits the data significantly better than exponential (P = .02), suggesting nonconstant hazard.
Conclusion
Conditional probabilities provide encouraging information regarding life expectancy to survivors of glioblastoma multiforme. Our data also showed that the constant hazard assumption may be violated in modern brain tumor trials. For single-arm trials, we advise using individual patient data from historical data sets for efficacy comparisons.
INTRODUCTION
At time of cancer diagnosis, patients may wish to learn an estimate of their life expectancy and how much survival may be prolonged if they undergo a specific cancer treatment. For many standard treatment regimens, physicians can refer to previously published clinical trials to obtain estimates of survival probabilities at the time of cancer diagnosis. The current standard of care for patients with newly diagnosed glioblastoma multiforme (GBM) includes the combination of radiation therapy and temozolomide (TMZ) followed by adjuvant TMZ.1 Stupp et al1 reported in their trial a median survival of 14.6 months and an estimated 2-year survival rate of 27% for this treatment combination. Although these estimates may be useful for general comparison and disease surveillance purposes, they are not as informative to patients who have survived for some time after diagnosis.
The estimates of subsequent survival probabilities after a patient has survived for a certain number of years are not directly available from the standard Kaplan-Meier curve. A useful quantity that addresses this question is the conditional probability. The conditional probability represents the probability of surviving to some specific years post diagnosis, given survival to a certain number of years. Several authors have studied the conditional probabilities of survival in patients with brain tumors. Hwang et al2 provided the conditional probabilities of survival within 6 years after craniotomy in 112 patients with primary supratentorial astrocytic tumors. Lin et al3 examined a series of 114 patients with anaplastic astrocytoma or GBM treated between 1981 and 2000. Davis et al4 reported the conditional probabilities of survival of patients with primary malignant brain tumors. These reports, however, included occurrences diagnosed more than a decade ago, when TMZ was unlikely to be part of the treatment regimen. As such, the estimates of survival probabilities may not be useful to recent brain tumor survivors.
This article has three objectives. First, we report conditional probability estimates on the basis of seven phase II trials in patients with newly diagnosed GBM or gliosarcoma. In the most recent three trials conducted at University of California, San Francisco (UCSF), patients were treated with interventions that were based on the Stupp regimen, each with an additional oral chemotherapy agent. Four remaining trials included patients receiving radiation therapy and concurrent or adjuvant chemotherapy. Second, we aim to provide insight into factors that may inform future prognosis among long-term GBM survivors. The third objective was motivated by the observed trend in the conditional probabilities in our data. In practice, investigators often rely on the constant hazard assumption (as a consequence of the exponential distribution) when designing trials and sometimes make this assumption in conducting the analysis. Although the violation of such assumption has been widely established in many cancer types, there is little documentation in the CNS tumors literature highlighting this phenomenon. We use more current GBM data as a concrete case study to demonstrate the violation of this assumption.
PATIENTS AND METHODS
Patient Population
This study was approved by the UCSF Committee on Human Research. Five hundred sixty-three adult patients with GBM (including one patient with gliosarcoma) enrolled on one of seven clinical protocols between 1975 and 2007 were identified. Trials and key characteristics are provided in Table 1, including the number of patients and number censored for survival. The four older trials (ie, 6G61, 6G91, 6G82-1, 8822) represent a combination of single-institution (UCSF) and multi-institutional trials. The multi-institutional trials were led by UCSF and were conducted through the former Northern California Oncology Group, a regional clinical trials consortium sponsored by the National Cancer Institute. All four protocols included provision for external-beam radiotherapy combined with concurrent or adjuvant chemotherapy. Because none of these protocols included TMZ, they are referred to as the pre-TMZ trials. The three recent trials (ie, TTRT, RTRT, OTRT) were phase II protocols conducted at UCSF that included TMZ.5–7 Treatment plans included conventional radiation and TMZ plus an additional oral chemotherapy agent. After a 2-week break on completion of radiotherapy, the added oral agent was to be coadministered with TMZ for at least 1 year unless disease progression or unacceptable toxicity occurred. Entry criteria for these three protocols were similar and included Karnofsky performance score (KPS) ≥ 60 and an estimated survival time of greater than 8 weeks. These three recent trials are referred to as the post-TMZ trials.
Table 1.
Treatment Plan for Studies Included in the Analysis
| Protocol by Study Type | No. of Patients | No. Censored | Treatment Plan |
||
|---|---|---|---|---|---|
| Radiotherapy Dose and Schedule | Concurrent/Adjuvant Chemotherapy | Enrollment Period | |||
| Pre-TMZ | |||||
| 6G61 | 59 | 3 | 60 Gy + hydroxyurea | BCNU v PCV | 1975-1981 |
| 6G91 | 70 | 5 | 60 Gy + hydroxyurea + misonidazole | Procarbazine, vincristine, BCNU, FU | 1979-1983 |
| 6G82-1 | 100 | 6 | 60 Gy at 1.8- to 2.0-Gy fractions + bromodeoxyyuridine | PCV | 1982-1988 |
| 8822 | 141 | 12 | 60 Gy at 1.8- to 2.0-Gy fractions + hydroxyurea | Thioguanine, BCNU | 1988-1991 |
| Post-TMZ | |||||
| TTRT | 67 | 4 | 60 Gy at 2.0-Gy/d × 5 d/wk × 6 wk | Temozolomide + thalidomide | 2000-2001 |
| RTRT | 61 | 1 | 60 Gy at 2.0-Gy/d × 5 d/wk × 6 wk | Temozolomide + cis-retinoic acid | 2001-2002 |
| OTRT | 65 | 9 | 59.4- to 61- Gy at 1.8- to 2.0 Gy/d × 5 d/wk × 6.5 wk | Temozolomide + erlotinib | 2004-2007 |
Abbreviations: BCNU, bischloronitrosourea; FU, fluorouracil; PCV, procarbazine, lomustine, and vincristine; TMZ, temozolomide.
Statistical Methods
The primary end point for all trials was overall survival, defined as the time from histologic diagnosis until death as a result of any cause. Patients not known to have died were censored for survival as of the last date known to be alive. Because only 7% (ie, 26 of 370) of patients were censored for survival in the pre-TMZ trials, survival data were not additionally updated for this analysis. For the post-TMZ trials, the cutoff date for survival data was May 2010. Survival rates were estimated by using the Kaplan-Meier method. The conditional probability of survival was defined as the probability of surviving to some Y years after diagnosis given survival to some X (X < Y) years and can be estimated from the data. For example, the conditional probability of surviving to 4 years given survival to 1 year was calculated by dividing the 4-year survival rate by the 1-year survival rate. Confidence intervals of the conditional probabilities were estimated by using the formulas given in Davis et al4 Specifically, the variance formula of the conditional probability is a variation of the usual Greenwood formula for the unconditional survival probability and can be derived by using the delta method.8 Confidence intervals then were obtained by the normal approximation. Of note, six trials (pre-TMZ studies + TTRT + RTRT) were combined for the purpose of conditional probability estimation because of their similar survival distributions. The most recent trial, OTRT, was reported separately because of its significant survival advantage compared with other trials (see Results).
To assess patient variables that would influence subsequent survival among long-term survivors, we undertook the landmark analysis approach.9–11 Because few patients survived more than 2 years, we focused on patients who survived beyond 1 year after diagnosis (approximately 60% of the patients were still alive at that time). We included only the post-TMZ trials because of the limited availability of KPS at 1 year in the pre-TMZ trials. All patients who were alive at 1 year post diagnosis were included in this analysis. Survival was counted from the 1-year landmark. Age was defined as patient age at baseline plus 1 year. KPS at the 1-year landmark was taken as the score recorded within the time interval 2 months before and after the 1-year time point. When multiple KPS records were available for a patient, the score closest to the 1-year landmark was used. Patients with a KPS value of 0 at the 1-year landmark were excluded from the analysis, as KPS = 0 was deterministic of the outcome of death. The Cox proportional hazards model was used to assess the prognostic value of age, KPS, and prior progression at the 1-year landmark, adjusting for study and surgical extent. In addition, to compare the hazard ratio (HR) between baseline and 1-year post diagnosis, a test of linear interaction between a binary time variable (dichotomized at 1-year landmark) and the covariate was performed, treating the corresponding covariate as time dependent. All P values are two sided.
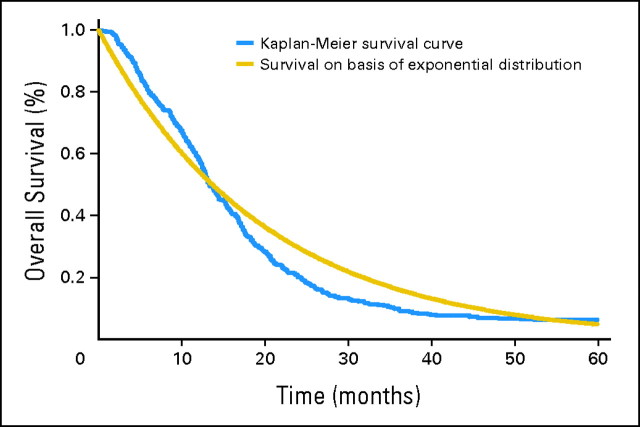
The evaluation of the constant hazard assumption used data from pre-TMZ trials, TTRT and RTRT. A likelihood ratio test was used to compare the Weibull and the exponential distributions. A plot was generated to compare the Kaplan-Meier curve and a survival curve that was based on the exponential distribution by estimating the hazard rate with its maximum likelihood estimate (Fig 1). Any discrepancy between the two curves would indicate a lack of fit of the exponential distribution.
Fig 1.
Kaplan-Meier curve versus a survival curve based on an exponential distribution assumption of time to death.
RESULTS
Patient Characteristics
Patient characteristics in each of the seven trials are listed in the Data Supplement. For the combined group, the median age was 54 years (range, 19 to 77 years), and the median baseline KPS was 90 (range, 60 to 100). Sixty-three percent of the patients were men, and 91% were white. Twelve percent of the patients had biopsy only before starting protocol treatment, 70% had subtotal resection, and the remaining 19% underwent gross total resection.
Overall Survival
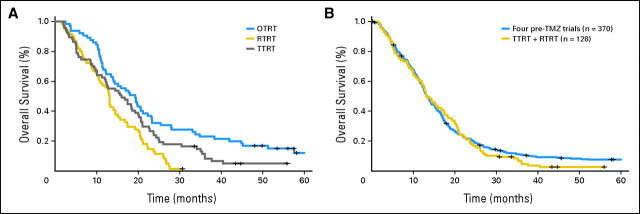
The estimated median survival and the survival rates at 26, 52, and 78 weeks for the pre-TMZ studies and for each recent UCSF study separately are listed in the Data Supplement. At the time of this analysis, 26 of 370 patients in the pre-TMZ trials were censored for survival with a median follow-up of 225 weeks (range, 1 to 881 weeks). One patient in RTRT was censored for survival at week 134, four patients in TTRT were censored with a median follow-up of 192 weeks (range, 146 to 243 weeks), and nine patients in OTRT were censored with a median follow-up of 250 weeks (range, 209 to 285 weeks). The latest study, OTRT, which combined the use of TMZ with erlotinib (an orally active selective inhibitor of the tyrosine kinase EGFR) during and after radiotherapy, was the only trial that successfully demonstrated prolonged survival when compared with its historical control, which consisted of TTRT and RTRT. In particular, this treatment combination was associated with the highest estimated survival at each time point that we investigated, with a median survival of 84 weeks. The estimated median survival for pre-TMZ trials, TTRT, and RTRT were 58, 70, and 57 weeks, respectively. Figure 2A presents the Kaplan-Meier curves for survival separately for each post-TMZ protocol. The OTRT study showed significant survival advantage compared with the other two trials (OTRT v TTRT P = .025; OTRT v RTRT P < .001). No statistically significant survival difference was found between TTRT and RTRT protocols (P = .07). Figure 2B shows the Kaplan-Meier curves of the pre-TMZ trials compared with the data combining TTRT and RTRT, suggesting that the survival distributions of these two cohorts of patients were comparable (P = .34). On the basis of these reasons, the pre-TMZ trials were combined with TTRT and RTRT for the conditional probability estimation. OTRT was reported separately because of its superior survival outcome. In general, survival probability seemed to decrease most rapidly in the first 2 years after initial diagnosis (Fig 2B). The second column of Table 2 gives the estimated survival probabilities separately for the combined data (pre-TMZ data + TTRT + RTRT) and OTRT; the observed 6-month and 1-, 2-, 3-, and 4-year survival rates were 80%, 58%, 20%, 10%, and 7%, respectively, for the combined data and 92%, 68%, 32%, 23%, and 16%, respectively, for OTRT.
Fig 2.
Kaplan-Meier curves for survival in (A) three post-temozolomide (TMZ) studies on the basis of the Stupp regimen (OTRT v TTRT, P = .025; OTRT v RTRT, P < .001; TTRT v RTRT, P = .07; all adjusting for age, Karnofsky performance score [KPS], and extent of resection) and (B) pre-TMZ trials v TTRT + RTRT (P = .34, adjusting for age, KPS, and extent of resection).
Table 2.
Conditional Probabilities of Survival at Various Time Points
| Time Point by Study Type (months) | Observed Survival |
Conditional Probability of Survival by Time Point (months) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 |
24 |
36 |
48 |
60 |
|||||||||
| % | 95% CI | No. at Risk* | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Pre-TMZ + TTRT + RTRT (n = 498) | |||||||||||||
| 6 | 80 | 77 to 84 | 398 | 72 | 68 to 77 | 25 | 21 to 29 | 12 | 9 to 16 | 8 | 6 to 11 | 8 | 5 to 11 |
| 12 | 58 | 54 to 63 | 286 | — | 35 | 29 to 40 | 17 | 12 to 21 | 12 | 8 to 16 | 11 | 7 to 15 | |
| 24 | 20 | 17 to 24 | 98 | — | — | 49 | 39 to 59 | 34 | 24 to 44 | 31 | 22 to 41 | ||
| 36 | 10 | 7 to 13 | 44 | — | — | — | 69 | 56 to 83 | 64 | 50 to 79 | |||
| 48 | 7 | 5 to 10 | 27 | — | — | — | — | 93 | 83 to 100 | ||||
| OTRT (n = 65) | |||||||||||||
| 6 | 92 | 86 to 99 | 60 | 73 | 62 to 85 | 35 | 23 to 47 | 25 | 14 to 36 | 18 | 8 to 28 | 12 | 3 to 22 |
| 12 | 68 | 57 to 80 | 44 | — | 48 | 33 to 62 | 34 | 20 to 48 | 24 | 11 to 37 | 17 | 4 to 29 | |
| 24 | 32 | 23 to 46 | 21 | — | — | 71 | 52 to 91 | 51 | 29 to 73 | 35 | 11 to 59 | ||
| 36 | 23 | 15 to 36 | 15 | — | — | — | 71 | 47 to 95 | 49 | 18 to 79 | |||
| 48 | 16 | 9 to 29 | 9 | — | — | — | — | 69 | 32 to 100 | ||||
Abbreviation: TMZ, temozolomide.
Indicates number still alive and not censored.
Conditional Probabilities of Survival
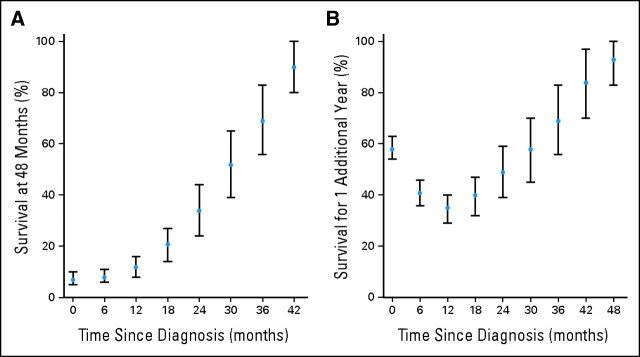
Table 2 gives the conditional probabilities of survival at various time points for the cohort combining pre-TMZ data, TTRT, and RTRT (top) and OTRT (bottom) separately. For example, the conditional probability of surviving to 4 years after survival to 2 years (ie, surviving an additional 2 years) in the combined cohort was 34% (95% CI, 24% to 44%). This was markedly higher than the observed 4-year survival rate of 7% (95% CI, 5% to 10%). Conditional probabilities of other time points can be obtained similarly. Figure 3A presents the probabilities of survival to 4 years post diagnosis for this cohort as the number of months after diagnosis increases.
Fig 3.
(A) Conditional probability of survival at 4 years given survival to various time points, and (B) conditional probability of surviving an additional year at various time points. Note that these data combine pre-temozolomide, TTRT, and RTRT trials. The dots represent the probability point estimates, and the vertical bars represent the 95% CIs of the corresponding point estimates.
The conditional probability of survival also gives a prediction of surviving the next year for GBM survivors. For example, in the combined cohort of patients on pre-TMZ, TTRT, and RTRT, the conditional probabilities of surviving an additional year given survival to 1, 2, 3, and 4 years after diagnosis were 35%, 49%, 69%, and 93%, respectively. Figure 3B depicts the conditional probabilities of living an additional year given survival at various time points after diagnosis. Interestingly, compared with the unconditional probability of surviving 1 year after diagnosis (58%), there appears to be first a decrease in the conditional probability of surviving an additional year at 6 months and 1 year, followed by an increasing trend after 1.5 years. At 3 years, the conditional probability of surviving an additional year (69%) has exceeded the unconditional 1-year survival rate. This indicates that the estimated 1-year survival rate for a patient who had already lived for 3 years may be higher than a patient who was recently diagnosed.
Assessment of Prognostic Values of Patient Factors According to the 1-Year Landmark
By using three recent UCSF trials, we evaluated whether putative prognostic variables, such as age, KPS, and progression status, were predictive of subsequent survival at the 1-year landmark. At the 1-year time point, KPS was available for 85 patients. Seventy (82%) of these patients had a KPS of 70 or higher. Ten of these patients died around the 1-year landmark (KPS = 0). Patients who died or were lost to follow-up within 1 year post diagnosis were excluded from the analysis, and survival was measured from the 1-year time point. Table 3 presents the results of the Cox proportional hazards models side-by-side with clinical factors at baseline (left) and at the 1-year time point (right). As expected, baseline KPS and age were both significantly predictive of survival (KPS HR, 0.98; KPS P = .04; age [10 years] HR, 1.34; age [10 years] P < .001). At the 1-year landmark, lower KPS and prior progression were significantly associated with higher risk of death (P < .001 for both variables). However, age did not reach statistical significance (HR [10 years], 1.22; P = .25). The test of interaction indicated that the HR of age at baseline was significantly different from that at the 1-year landmark (P = .001). Although the extent of resection was not predictive of survival at both time points, we note the drastic difference in the HR estimates comparing biopsy to subtotal resection at the two time points (baseline HR, 1.52; 1-year HR, 0.4). Although the reason for this reversed effect is unclear, this difference is less persuasive on the basis of the marginally significant P value (test of interaction P = .04) and the limited sample size available in the biopsy category at the 1-year landmark (n = 7).
Table 3.
Multivariable Cox Proportional Hazards Analysis of Survival at Baseline and at 1-Year Landmark on the Basis of Three Recent UCSF Post-TMZ Trials
| Variable | Baseline Analysis (n = 192)* |
1-Year Landmark (n = 74)† |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Karnofsky performance score‡ | 0.98 | 0.96 to 0.99 | .04 | 0.95 | 0.92 to 0.97 | < .001 |
| Age, 10-year units | 1.34 | 1.22 to 1.63 | < .001 | 1.22 | 0.9 to 1.79 | .25 |
| Progression status at 1 year§ | — | — | 4.78 | 2.31 to 9.88 | < .001 | |
| Extent of resection∥ | ||||||
| Biopsy | 1.52 | 0.99 to 2.31 | .052 | 0.4 | 0.15 to 1.09 | .07 |
| Gross total | 0.89 | 0.64 to 1.26 | .53 | 1.22 | 0.7 to 2.14 | .48 |
| Study¶ | ||||||
| RTRT | 2.14 | 1.46 to 3.14 | < .001 | 1.42 | 0.69 to 2.91 | .34 |
| TTRT | 1.56 | 1.08 to 2.25 | .02 | 0.99 | 0.52 to 1.86 | .97 |
Abbreviations: UCSF, University of California, San Francisco; TMZ, temozolomide; HR, hazard ratio.
Survival was measured from time of initial diagnosis.
Survival was measured from the 1-year time point. All patients who died or were censored for survival before 1 year were excluded.
For the 1-year landmark analysis, Karnofsky performance score recorded within the interval 2 months before and after the 1-year time point was used. When multiple records are available for a patient, the score closest to the 1-year landmark was used.
Reference value: none.
Reference value: subtotal.
Reference value: OTRT.
Test for Constant Hazard of Death Assumption (Exponential Distribution)
The likelihood ratio test comparing the Weibull and the exponential distributions indicated that the Weibull distribution fits the data significantly better (P = .02), demonstrating the violation of the constant hazard assumption. Figure 1 presents the comparison of the Kaplan-Meier curve and a survival curve on the basis of the exponential distribution assumption. The departure of the Kaplan-Meier curve from the curve that is based on the exponential distribution illustrates the nature of this difference in this population.
DISCUSSION
Disease outcome for patients with cancer is typically described in terms of estimated survival rates that are based on the Kaplan-Meier method. These estimates are calculated with respect to the time of initial cancer diagnosis and may not be pertinent to patients who have survived for a period of time and wish to know their remaining life expectancy. Conditional probability offers more relevant information regarding subsequent survival for cancer survivors. In this study, we reported the conditional survival probabilities on the basis of seven phase II trials in patients with newly diagnosed GBM receiving radiation therapy and chemotherapy.
Our data suggested that the survival probabilities decreased most rapidly in the first 2 years after diagnosis and leveled off in subsequent years. This suggests a nonconstant hazard of death over time and indicates that the prognosis of patients with GBM surviving the first 2 years after diagnosis may be more optimistic. This observation is in accordance with the report by Davis et al,4 although their study included patients with malignant brain and other CNS tumors. As expected, the survival for patients with GBM patients is discouraging: 1-, 2-, 3-, and 4-year estimates were 58%, 20%, 10%, and 7%, respectively. However, the conditional probability of surviving an additional year after survival to 3 years post diagnosis exceeds the 1-year survival rate, providing evidence that the future prognosis of a patient who had survived for 3 years may be as good as those recently diagnosed.
In this report, we also evaluated potential prognostic variables among patients who have survived for 1 year after diagnosis. Our analysis revealed that KPS and progression at the 1-year landmark were highly predictive of subsequent survival, but age was not. The comparison of the HR from the two time points suggests that older age may be less predictive of worse future survivorship after surviving 1 year, although the HR estimate was greater than 1 for both analyses. Itis also important to note that the lack of statistical significance for age at the 1-year landmark may be attributed in part to the decrease in effective sample size (N = 74). Moreover, the finding that age is not statistically associated with survival on the basis of the 1-year analysis does not imply that age is not a clinically important prognosticator for 1-year survivors.
Finally, we used these data to evaluate the standard assumption of constant hazard rate on the basis of an exponential distribution. Although the violation of constant hazard has been well documented in many cancer types, there is currently a paucity of GBM literature highlighting this phenomenon. This is especially pertinent after the trial by Stupp1 changed the standard of care for patients with newly diagnosed GBM. The purpose of this analysis is to use a patient series on a large GBM trial at our institution to provide updated information about the shape of the survival distribution in modern brain tumor trials. The constant hazard assumption was found to be invalid in our data. The violation of this assumption would affect the precision of the power estimate. Hence, although the simplifying exponential assumption may be useful for sample size planning, caution should be exercised to ensure its validity. In addition, in analyzing efficacy results, the use of individual patient data either from concurrent or historical controls for efficacy comparisons provides a relatively robust outcome, because the standard log-rank test and the Cox proportional hazards models used do not rely on the constant hazard assumption.
Our study only included patients who were enrolled on clinical trials. Because these patients typically carry better prognoses than the general disease population, the conditional survival estimates reported here may not be generalizable to the majority of patients with GBM. Future research should consider utilizing data from publicly available cancer registries, such as Surveillance, Epidemiology, and End Results, for which long-term follow-up is available on a large series of patients.
In conclusion, conditional probabilities can provide encouraging information regarding life expectancy to GBM survivors. To our knowledge, this report encompasses the largest, and most homogeneous, GBM patient series enrolled on clinical trials used to date for this purpose.
Acknowledgment
We thank Ilona Garner, Department of Neurological Surgery, University of California, San Francisco, for editorial support.
Footnotes
See accompanying article on page 4151
Supported by National Institutes of Health Brain Tumor Specialized Program of Research Excellence Grant No. P50 CA097257.
Presented at the 15th Scientific Meeting of the Society of Neuro-Oncology, November 18-21, 2010, Montreal, Quebec, Canada.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Susan M. Chang, Schering-Plough, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Mei-Yin C. Polley, Kathleen R. Lamborn, Michael Prados
Administrative support: Michael Prados
Provision of study materials or patients: Susan M. Chang, Nicholas Butowski, Jennifer L. Clarke, Michael Prados
Collection and assembly of data: Mei-Yin C. Polley, Susan M. Chang, Nicholas Butowski, Michael Prados
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Hwang SL, Yang YH, Lieu AS, et al. The conditional survival statistics for survivors with primary supratentorial astrocytic tumors. J Neurooncol. 2000;50:257–264. doi: 10.1023/a:1006484220764. [DOI] [PubMed] [Google Scholar]
- 3.Lin CL, Lieu AS, Lee KS, et al. The conditional probabilities of survival in patients with anaplastic astrocytoma or glioblastoma multiforme. Surg Neurol. 2003;60:402–406. doi: 10.1016/s0090-3019(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 4.Davis FG, McCarthy BJ, Freels S, et al. The conditional probability of survival of patients with primary malignant brain tumors. Cancer. 1999;85:485–491. [PubMed] [Google Scholar]
- 5.Butowski N, Prados MD, Lamborn KR, et al. A phase II study of concurrent temozolomide and cis-retinoic acid with radiation for adult patients with newly diagnosed supratentorial glioblastoma. Int J Radiat Oncol Biol Phys. 2005;61:1454–1459. doi: 10.1016/j.ijrobp.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Chang SM, Lamborn KR, Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;60:353–357. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collett D. Modelling Survival Data in Medical Research. Chapman and Hall/CRC CRC Press; 2003. [Google Scholar]
- 9.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 10.Mantel N, Byar DP. Evaluation of response-time data involving transient states: An illustration using heart-transplant data. J Am Stat Assoc. 1974;69:81–86. [Google Scholar]
- 11.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26:3913–3915. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]