Abstract
Elevated glucocorticoid (GC) activity may be involved in the development of the metabolic syndrome. Tissue GC exposure is determined by the tissue-specific GC-activating enzyme 11β-hydroxysteriod dehydrogenase type 1 (11β-HSD1) and the GC-inactivating enzyme 5α-reductase type 1 (5αR1), as well as 5β-reductase (5βR). Our aim was to study the effects of neonatal overfeeding induced by small litter rearing on the expression of GC-regulating enzymes in adipose tissue and/or liver and on obesity-related metabolic disturbances during development. Male Sprague-Dawley rat pup litters were adjusted to litter sizes of three (small litters, SL) or ten (normal litters, NL) on postnatal day 3 and then given standard chow from postnatal week 3 onward (W3). Small litter rearing induced obesity, hyperinsulinemia, and higher circulating corticosterone in adults. 11β-HSD1 expression and enzyme activity in retroperitoneal, but not in epididymal, adipose tissue increased with postnatal time and peaked at W5/W6 in both groups before declining. From W8, 11β-HSD1 expression and enzyme activity levels in retroperitoneal fat persisted at significantly higher levels in SL compared to NL rats. Hepatic 11β-HSD1 enzyme activity in SL rats was elevated from W3 to W16 compared to NL rats. Hepatic 5αR1 and 5βR expression was higher in SL compared to NL rats after weaning until W6, whereupon expression decreased in the SL rats and remained similar to that in NL rats. In conclusion, small litter rearing in rats induced peripheral tissue-specific alterations in 11β-HSD1 expression and activity and 5αR1 and 5βR expression during puberty, which could contribute to elevated tissue-specific GC exposure and aggravate the development of metabolic dysregulation in adults.
Introduction
The global epidemic of obesity and related metabolic disorders continues to progress at an alarming rate in both adults and children [1], [2]. Clinical and experimental studies have shown an association between the environments during the perinatal period and the development of features of metabolic syndrome, such as hypertension, type 2 diabetes, insulin resistance, and hyperlipidemia later in life [3]. In particular, nutritional status is an important factor. Under- or overnutrition during the prenatal period increases the risk of obesity and hyperinsulinemia [4]. In addition, rapid weight gain during infancy is consistently associated with increased subsequent risk for obesity [5]. Although the mechanisms behind the putative pathophysiological link between pre- and postnatal nutrition and obesity have not yet been established, glucocorticoids (GC) have been proposed as possible mediators of the permanent programming of obesity, insulin resistance, and other metabolic dysregulations [6].
GCs regulate, among other processes, adipose tissue differentiation, cellular lipid accumulation, and fat distribution. Alterations in central and peripheral GC activity have been described in both human obesity and animal models of obesity [7], [8]. At the tissue level, GC exposure is determined not only by circulating levels, but also by several enzymes that regulate local GC metabolism. In vivo, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) converts inactive GC to active GC (corticosterone or cortisol), which combine with glucocorticoid receptor (GR), and thus amplifies local GC action. 11β-HSD1 is mainly distributed in the liver, adipose tissue, and brain [9]. Adipose- or liver-specific 11β-HSD1 overexpression in transgenic mice exhibit elevated intra-adipose and portal corticosterone levels, abdominal obesity, dyslipidemia, insulin resistance, and hypertension [10], [11]. Previous studies showed higher 11β-HSD1 mRNA and/or activity in the adipose tissue of obese adults and children [12], [13], and 11β-HSD1 is positively associated with features of the metabolic syndrome in adults [14]. The principal enzymes responsible for GC catabolism in the liver are A-ring reductases (5α-reductase type 1 (5αR1) and 5β-reductase (5βR)). The activity and expression of these enzymes in the liver is higher in obese Zucker rats than in lean rats [8]; in humans, elevated hepatic 5αR1 activity is associated with insulin resistance [15] and both 5αR1 and 5βR activities are correlated with obesity [16]. 5αR1 is also expressed in adipose tissue, but is not dysregulated in rats or obese humans, at least not in subcutaneous fat [17]. The activities of both 11β-HSD1 and A-ring reductases can determine local tissue GC availability and also affect hypothalamic-pituitary-adrenal (HPA) axis activation; inhibition of 11β-HSD1 (e.g., decreased local GC generation) and activation of A-ring reductases (which enhances GC inactivation) may drive the HPA axis.
Studies have shown that local GC metabolism in adipose tissue and the liver is influenced by a number of factors, including age, nutrition, and hormones. Adipose tissue 11β-HSD1 expression [13] and enzyme activity (urinary terahydrocortisol [THF] and 5α-THF/tetrahydro-cortisone [THE] ratio) increases with age, particularly in boys [18]. GC and androgen hormones upregulate 11β-HSD1 [19] and growth hormone downregulates its expression [20]. Notably, GC and androgen appear to exert a depot-specific stimulation of the transformation of cortisone to cortisol within omental (i.e., visceral) adipose tissue [19]. Moreover, postnatal overfeeding of rats induces HPA axis hyperactivity and higher visceral 11β-HSD1 mRNA expression during adulthood [21]. Taken together, these observations strongly suggest the existence of a tight link between local GC production and hormone levels, age, and nutrition; this led us to hypothesize that the ontogeny of 11β-HSD1 in adipose tissue and liver and A-ring reductases in the liver is regulated by early postnatal overfeeding, and that puberty is a key phase of development in aberrant GC metabolism.
Methods
Animals and experimental design
All of the studies were approved by the University Committee on Use and Care of Animals, and were overseen by the Unit for Laboratory Animal Medicine of Nanjing Medical University (ID: 2008031801). Sprague-Dawley rats (Nanjing, Jiangsu, China) were maintained under controlled light (0600–1800 h) and temperature (22±2°C) conditions with free access to tap water and standard pellet diet.
Female rats were time-mated, and at postnatal day 3 (P3) male rat pup litters were randomly distributed among the mothers to adjust to litter sizes of three (small litter, SL) or ten (normal litter, NL) to induce early postnatal overfeeding or normal feeding [22], [23]. After weaning (P21), the pups of both litter groups were fed standard chow (15% calories as fat, 21% calories as protein and 64% calories as carbohydrate); all animals were housed three per cage post-weaning. Body weight, body length (nose to anus length) and food intake were monitored throughout life. As the puberty of rats is usually defined from postnatal days 21 to 60 [24], the animals were killed at postnatal weeks 3 (W3), 4, 5, 6, 8, and 16 between 0830 h and 1000 h after fasting overnight.
Serum and tissue collection
Rats were anesthetized with chloral hydrate (300 mg/kg body weight, i.p.) at 0830 h after overnight fasting (12 h) and blood samples were obtained from the right ventricle. The blood was centrifuged (2,000× g, 4°C, 15 min) and the separated serum was stored at −70°C for subsequent determination of serum insulin, corticosterone, and leptin. Epididymal and retroperitoneal white adipose tissue and liver samples were removed, snap-frozen in liquid nitrogen, and kept at −80°C until gene expression and activity analysis. Adipose tissue was weighed and frozen, except for a portion of adipose tissue, which was fixed for determination of adipocyte cross-sectional areas [25].
Histology
Adipose tissues were fixed in 10% formaldehyde in phosphate buffered saline (PBS), pH 7.4, for 24–48 h at room temperature. After fixation, tissues were dehydrated in graded ethanol, cleared in xylol, and embedded in paraffin at 60°C. The sections of 8 µm cut on the microtome (Leica, Wetzlar, Germany) were stained by hematoxylin and eosin (H/E), then analyzed with image software. At least 3 slices per adipose tissue sample, and 10 fields of vision each slice were accomplished to determine adipocyte cross-sectional areas.
Biochemical analysis
Serum glucose was determined with a hexokinase assay (Thermo Electron, Melbourne, Australia), triglyceride and cholesterol were measured using an Olympus AU400 analyzer with enzymatic reagents (Olympus America, New York, USA). Insulin concentration was determined by a radioimmunoassay (BNIBT, Beijing, China) according to company instructions, with a sensitivity limit of 10 pmol/L and an intra-assay coefficient of variation of 10% for the assay. Serum corticosterone (Cayman, Michigan, USA) and leptin (Millipore, Billerica, USA) was measured by enzyme-linked immunosorbent assays, sensitivity limits were 40 pg/ml for corticosterone and 0.04 ng/ml for leptin, and the intra-assay coefficients of variation for corticosterone and leptin were 7–9% and 2–3%, respectively.
Intraperitoneal glucose tolerance test measurement
The intraperitoneal glucose tolerance test (IPGTT) was performed as previously described [26]. Briefly, at W16 rats were fasted overnight, a fasting blood sample was taken from a tail vein, and then the rats were injected with 2.0 g D-glucose (50% stock solution in saline)/kg body weight intraperitoneally. Thereafter, blood samples were drawn at 30-, 60- and 120-min intervals after the glucose injection, and glucose levels were measured by a glucose meter (Accu-Chek; Roche, Mannheim, Germany).
Quantitative real-time PCR
Total RNA was isolated from adipose tissue and liver with Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions and quantified spectrophotometrically at OD260. The integrity of total RNA was assessed using agarose gel electrophoresis, and cDNA was synthesized using M-MLV reverse transcriptase (Promega, Southampton, UK) with 1.0 µg of the RNA sample as described by the manufacturer. PCR amplification using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers on a subset of the cDNA samples confirmed successful reverse transcription. Real-time PCR was performed using the SYBR GREEN ABI Prism 7500 sequence detector with cycling parameters of 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 sec, and 60°C for 1 min. The mRNA levels were normalized to the corresponding GAPDH mRNA levels. Data were analyzed with the 2−ΔΔct method [27]. The sequences of the primers are in Table 1.
Table 1. Primer sequences used for mRNA quantification by real-time PCR.
| Forward primer 5′–3′ | Reverse primer 5′–3′ | |
| 11β-HSD1 | GAA GAA GCA TGG AGG TCA AC | GCA ATC AGA GGT TGG GTC AT |
| 5αR1 | CTG TTT CCT GAC AGG CTT TGC | GCC TCC CCT GGG TAT CTT GT |
| 5βR | GCC TTT AAG CCT GGA GAG GAA | ACG TGG CAC ACA GAT TTG ATT |
| GR | GGG TAC TCA AGC CCT GGA ATG | CCC GTA ATG ACA TCC TGA AGC T |
| C/EBPα | GGC GGG AAC GCA ACA A | TCC ACG TTG CGC TGT TTG |
| C/EBPβ | TCT ACT ACG AGC CCG ACT GC | AGG TAG GGG CTG AAG TCG AT |
| GAPDH | CAA GTT CAA CGG CAC AGT CAA | TGG TGA AGA CGC CAG TAG ACT C |
11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; 5αR1, 5α-reductase type 1; 5βR, 5β-reductase; GR, glucocorticoid receptor; C/EBPα, CCAAT/enhancer-binding protein α; C/EBPβ, CCAAT/enhancer-binding protein β; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
11β-HSD1 enzyme activity
To estimate 11β-HSD1 protein, 11β-HSD1 activity was measured in adipose and hepatic tissue in the direction of the dehydrogenase reaction, because it is more stable in vitro than the reductase direction [28]. 11β-HSD1 enzyme activity measurements were performed essentially as previously described [29]. Briefly, tissue was homogenized in homogenization buffer (10% glycerol, 300 mM NaCl, 1 mM EDTA, and 50 mM Tris base pH 7.7) containing 1 mM dithiothreitol and then centrifuged at 4°C. Protein concentration was determined using a Pierce BCA protein assay kit with bovine serum albumin as the standard (Thermo Fisher Scientific, Rockford, IL, USA). Each sample was analyzed in duplicate, with the use of an internal control. Samples of adipose tissue (0.5 mg protein/ml) or liver (10 µg protein/ml) homogenate were incubated with 2 mM NADP and 100 nM 1,2,6,7-[3H]4-corticosterone (Amersham, Berkshire, UK). After incubation in a shaking water bath at 37°C for 2 h or 1 h for adipose tissue or liver, respectively, the reaction was interrupted; steroids were extracted with ethyl acetate and then dried, dissolved in ethanol, separated by thin-layer chromatography (mobile phase chloroform∶ethanol [92∶8]), and exposed to a phosphorimager tritium screen (GE Healthcare Europe, Freiburg, Germany). The TLC plates were then scanned and quantified using a Typhoon scanner (GE Healthcare Europe). 11β-HSD1 activity was expressed as percentage conversion of corticosterone into 11-dehydrocorticosterone.
Western blotting
GR content was determined by Western blotting. Adipose tissues were homogenized in ice-cold Radio Immunoprecipitation Assay (RIPA) buffer to make whole cell lysates. Protein concentrations were determined using a Pierce BCA protein assay kit with bovine serum albumin as the standard (Thermo Fisher Scientific, Rockford, IL, USA). Protein of 40 µg was loaded in each well of 10% SDS–PAGE, separated, and transferred onto nitrocellulose (NC) filter membranes (0.45 µm; Millipore, Billerica, USA) at 4°C. The membranes were blocked in 5% non-fat milk for 1.5 h at room temperature and then blotted with a primary antibody to GR or actin (Santa Cruz, California, USA) at 1∶250 dilution overnight at 4°C. After being washed four times (10 min/wash) in PBST buffer at room temperature, the membrane was incubated with the second antibody for 1 h and immunoreactive bands were visualized using chemiluminescence. The intensity of target proteins (GR) and reference proteins (Actin) were quantified using Gel-Pro analyzer 4.0 software and the relative blackness of target proteins over reference protein was used to estimate the expression of GR.
Statistical methods
Results are expressed as mean ± SEM. Significant differences among different ages in each group were analyzed by using one-way analysis of variance (ANOVA) followed by post-hoc Fisher's least significance difference (LSD) test. Differences between SL and NL groups at corresponding time points were analyzed by unpaired Student's t-test. Serum glucose during IPGTT was analyzed using repeated-measures ANOVA followed by Fisher's test. P<0.05 was considered to be significant. For all statistical evaluations, SPSS for Windows was used (version 13.0 from SPSS, Chicago, IL, USA).
Results
Effects of early postnatal overfeeding on body weight
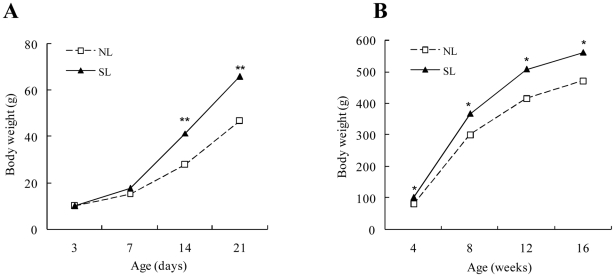
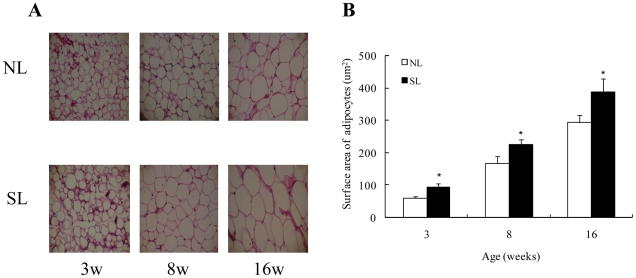
Body weight and fat pads increased with postnatal age in both groups. At postnatal day 14 (P14), body weight was higher in SL compared to NL rats (Fig. 1A); this persisted until W16 (Fig. 1B). The weight of retroperitoneal and epididymal fat pads was also higher in SL compared to NL rats from W3 to W16 (Table 2). When adjusted for body weight, retroperitoneal and epididymal fat weights in SL rats remained higher than in NL rats except for epididymal fat in W4 (Table 2). Throughout the study, the surface area of individual adipose cell increased with postnatal age in both groups (P<0.01), and SL rats had significantly higher average adipose cell cross-sectional areas than NL rats in both retroperitoneal and epididymal fat depots (Fig. 2A and 2B). There were no significant differences in body length between SL and NL rats from W3 to W16 (Table 3).
Figure 1. Body weight during the suckling (A) and post-suckling (B) periods in normal litters (NL) or small litters (SL) rats.
Data are expressed as mean ± SEM. Differences between groups at the corresponding time points were analyzed by unpaired Student's t-test. *P<0.05, **P<0.01 for SL vs. NL rats at corresponding time points (n = 6–9).
Table 2. Body fat weight (mg) at wk 3, 4, 5, 6, 8, and 16 in NL and SL rats.
| NL | SL | |
| Epididymal fat (%BW) | ||
| 3 wk | 136.73±18.27 (0.24±0.01) | 198.35±19.05* (0.30±0.02*) |
| 4 wk | 240.87±17.89 (0.31±0.02) | 300.00±12.82* (0.32±0.01) |
| 5 wk | 354.45±34.44 (0.26±0.03) | 550.32±15.28** (0.33±0.01*) |
| 6 wk | 588.67±46.79 (0.29±0.02) | 841.67±82.03* (0.36±0.01*) |
| 8 wk | 1638.96±168.76 (0.55±0.05) | 2639.09±135.48** (0.72±0.04*) |
| 16 wk | 5943.76±471.32 (1.16±0.10) | 7913.51±694.35* (1.41±0.07*) |
| Retroperitoneal fat (%BW) | ||
| 3 wk | 131.33±11.79 (0.23±0.01) | 228.33±37.37* (0.35±0.03**) |
| 4 wk | 248.32±22.84 (0.28±0.01) | 305.63±11.54* (0.32±0.01*) |
| 5 wk | 318.29±45.87 (0.23±0.03) | 620.37±50.70** (0.37±0.03**) |
| 6 wk | 554.33±78.06 (0.28±0.02) | 838.33±26.00** (0.36±0.01**) |
| 8 wk | 1797.63±212.56 (0.59±0.05) | 2751.31±134.63** (0.75±0.04*) |
| 16 wk | 6055.74±534.46 (1.18±0.11) | 9457.53±614.52** (1.68±0.11**) |
Data are expressed as mean ± SEM.
*P<0.05,
**P<0.01 for SL vs. NL. n = 6–9 in each NL and SL group.
%BW, % of body weight.
Figure 2. Hematoxylin-eosin–stained sections (400×) (A) and average adipocyte cross-sectional area (B) in retroperitoneal adipose tissue of NL and SL rats from postnatal week 3 (W3) to W16.
Data are expressed as mean ± SEM. Differences between groups at the corresponding time points were analyzed using Student's unpaired t-test. *P<0.05 for SL vs. NL rats at the corresponding time point. (n = 6–9).
Table 3. Body length at wk 3, 4, 5, 6, 8, and 16 in NL and SL rats.
| NL | SL | |
| Body length (cm) | ||
| 3 wk | 13.88±0.24 | 14.2±0.31 |
| 4 wk | 15.98±0.24 | 16.12±0.41 |
| 5 wk | 18.01±0.35 | 18.33±0.29 |
| 6 wk | 20.60±0.31 | 20.75±0.19 |
| 8 wk | 24.17±0.27 | 23.58±0.32 |
| 16 wk | 26.50±0.29 | 26.44±0.34 |
Data are expressed as mean ± SEM. n = 6–9 in each NL and SL group.
SL rats repeatedly ate more chow compared to NL rats, and there were significant differences in food intake between the two groups in W3 and W4 (Table 4).
Table 4. Food intake at wk 3, 4, 5, 6, 8, and 16 in NL and SL rats.
| NL | SL | |
| Food intake (g/day) | ||
| 3 wk | 7.70±1.13 | 10.60±1.24** |
| 4 wk | 11.65±3.33 | 14.32±2.18* |
| 5 wk | 17.45±2.32 | 17.56±1.23 |
| 6 wk | 21.85±4.12 | 21.60±0.27 |
| 8 wk | 25.17±1.01 | 25.60±1.87 |
| 16 wk | 28.00±0.64 | 27.00±0.58 |
Data are expressed as mean ± SEM.
*P<0.05,
**P<0.01 for SL vs. NL. n = 6–9 in each NL and SL group.
Effect of postnatal overfeeding on biochemical measurements
There were no differences in serum glucose, triglyceride and cholesterol levels from W3 to W16 (data not shown). Insulin levels at W16 were higher in SL rats compared to NL rats (108.65±4.88 vs. 87.06±6.48 pmol/L, P<0.05), and the insulin-to-glucose ratio was higher in SL rats than in NL rats (15.62±1.43 vs. 12.5±1.08, P<0.05). When given an intraperitoneal glucose load at W16, SL rats had higher circulating levels of glucose compared to NL rats at 30 and 60 min and a higher area under the curve, and this may indicate a lower insulin sensitivity or an impaired insulin secretion (Fig. 3).
Figure 3. Intraperitoneal glucose tolerance test at W16 (2.0 g glucose/kg) in SL and NL rats.
Data are expressed as mean ± SEM and were analyzed by repeated measures ANOVA followed by a post-hoc LSD test. *P<0.05, **P<0.01 for SL vs. NL rats at the corresponding time points (n = 6–9).
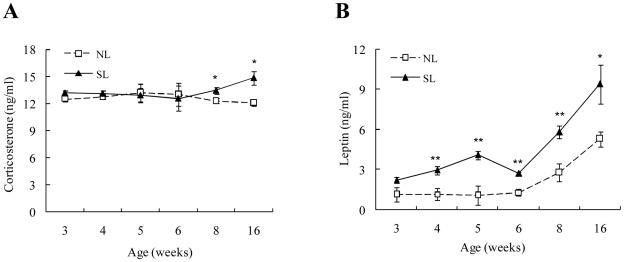
Circulating levels of corticosterone and leptin
The circulating corticosterone levels did not significantly change with postnatal age in the NL group, but increased in the SL group at W8 (although not at statistically significant levels) and W16 (P<0.05 vs. W3). W8 and W16 circulating corticosterone levels were higher in SL rats than in the NL rats at the corresponding time point (Fig. 4A). Serum leptin was increased during puberty in both groups (P<0.01), and SL rats exhibited significantly higher leptin levels from W4 to W16 (Fig. 4B) compared to NL rats.
Figure 4. Serum corticosterone (A) and leptin levels (B) from W3 to W16 in SL and NL rats.
Data are expressed as mean ± SEM. Differences between groups at corresponding time points were analyzed by unpaired Student's t-test. *P<0.05, **P<0.01 for SL vs. NL rats at the corresponding time points (n = 6).
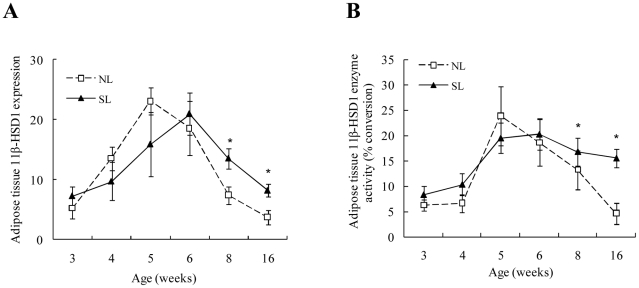
11β-HSD1 mRNA expression and activity in adipose tissue of NL and SL rats
In retroperitoneal adipose tissue, 11β-HSD1 mRNA expression increased with postnatal age in both NL and SL rats; the NL group peaked at W5 (P<0.05 vs. W3), while the SL group peaked at W6 (P<0.05 vs. W3). From W8 onward, 11β-HSD1 mRNA expression (Fig. 5A) and enzyme activity (Fig. 5B) remained higher in SL rats than NL rats. There were no significant differences in 11β-HSD1 mRNA expression and activity in epididymal fat.
Figure 5. Retroperitoneal adipose tissue 11β-HSD1 mRNA expression (A) and enzyme activity (B) from W3 to W16 in SL and NL rats.
Data are expressed as mean ± SEM. Differences between groups at corresponding time points were analyzed by unpaired Student's t-test. *P<0.05 for SL vs. NL rats at the corresponding time points (n = 6–9).
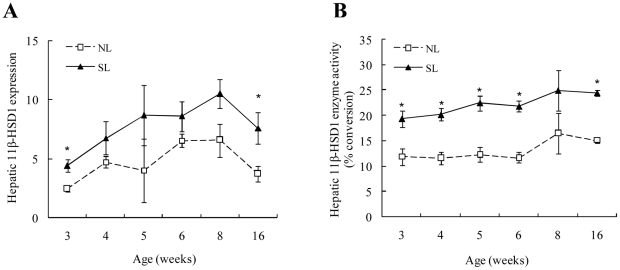
Hepatic 11β-HSD1 and A-ring reductases in NL and SL rats
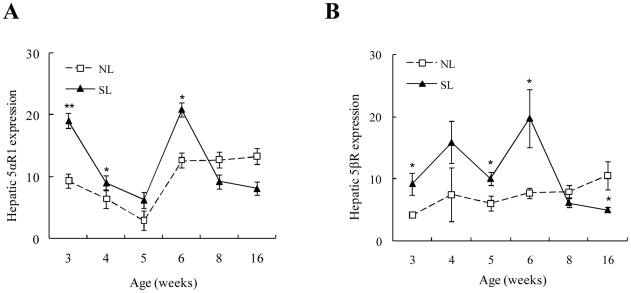
11β-HSD1 mRNA expression and activity in the liver did not change with increasing postnatal age in either NL or SL rats. However, hepatic 11β-HSD1 mRNA expression was higher in SL rats compared to NL rats at W3 and W16 (Fig. 6A), and hepatic 11β-HSD1 activity was significantly elevated in SL rats from W3 to W16 compared to NL rats (except for W8) (Fig. 6B). Hepatic 5αR1 decreased with postnatal age and reached minimum levels at W5 in both the NL and SL groups (P<0.05 vs. W3) (Fig. 7A). 5βR mRNA expression did not significantly change with time in NL rats. Compared to the NL rats, hepatic 5αR1 (Fig. 7A) and 5βR mRNA expression (Fig. 7B) was higher in SL rats at W3, 4, and 6 and W3, 5, and 6, respectively, but decreased at W8, whereupon there were no apparent differences between the NL and SL groups. 5βR mRNA expression was actually lower in SL rats compared to NL rats in W16 (P<0.05).
Figure 6. Hepatic 11β-HSD1 mRNA expression (A) and enzyme activity (B) from W3 to W16 in SL and NL rats.
Data are expressed as mean ± SEM. Differences between groups at corresponding time points were analyzed by unpaired Student's t-test. *P<0.05 SL vs. NL groups at the corresponding time points (n = 6–9).
Figure 7. Hepatic 5αR1 (A) and 5βR (B) mRNA expression from W3 to W16 in SL and NL rats.
Data are expressed as mean ± SEM. Differences between groups at corresponding time points were analyzed by unpaired Student's t-test. *P<0.05, **P<0.01 for SL vs. NL groups at the corresponding time points (n = 6–9).
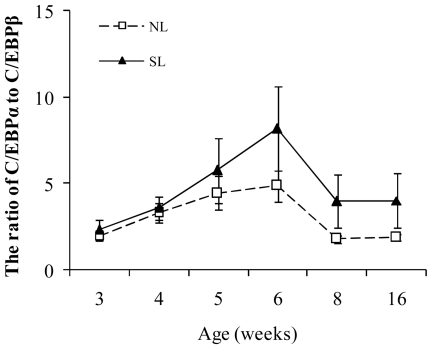
C/EBPα and C/EBPβ in adipose tissue of NL and SL rats
CCAAT/enhancer-binding protein α (C/EBPα) has been shown to be a potent activator of the basal transcription of 11β-HSD1 whereas CCAAT/enhancer-binding protein β (C/EBPβ) is only a weak activator and competing with the strong activation of 11β-HSD1 promoter activity mediated by C/EBPα [30], [31], therefore, the transcriptional activity of 11β-HSD1 was assessed by the ratio of C/EBPα to C/EBPβ rather than measuring a single transcription factor alone. In retroperitoneal adipose tissue, the ratio of C/EBPα to C/EBPβ increased with postnatal age and peaked at W6 in SL (P<0.05) and NL (P<0.05) rats respectively. Although the C/EBPα to C/EBPβ ratio in SL rats was higher than in NL rats at every time-point, the difference did not reach significance (Fig. 8). There were no significant differences in the ratio of C/EBPα to C/EBPβ in epididymal adipose tissue with regard to either age or litter size (data not shown).
Figure 8. The ratio of C/EBPα to C/EBPβ mRNA expression in retroperitoneal adipose tissue from W3 to W16 in SL and NL rats.
Data are expressed as mean ± SEM. Differences between groups at corresponding time points were analyzed by unpaired Student's t-test (n = 6–9).
GR mRNA and protein expression in adipose tissue and liver of NL and SL rats
There were no significant differences in GR mRNA and protein expression in liver, retroperitoneal and epididymal adipose tissue, with regard to either age or litter size (data not shown).
Discussion
Manipulation of rat litter rearing during the postnatal period has been widely used to examine the effects of early overfeeding and has been shown to have long-term effects on body weight, appetite, and glucose tolerance [21], [22], [32]. Consistent with these reports, we confirmed that early postnatal overfeeding in rats can induce obesity in adults, as well as high circulating leptin and insulin resistance. Our most important finding was that peripheral 11β-HSD1 and 5αR1 and 5βR expression had different developmental patterns in SL rats compared to NL rats. 11β-HSD1 expression and activity in visceral adipose tissue was persistently higher from W8 afterward in SL rats compared to NL rats. Moreover, hepatic 11β-HSD1 expression and activity increased from W3 in SL rats, while A-ring reductase levels were higher in SL rats until W6, but the difference disappeared later in puberty (W8). At the same time (W8), the concentration of circulating corticosterone was increased in SL rats. These data suggest that early postnatal overfeeding induced by small litter rearing induces dysregulation of peripheral GC metabolism at puberty, which may contribute to obesity and metabolic alterations in adult rats.
In the present study, adipose tissue 11β-HSD1 expression appeared to be depot-specific in both SL and NL rats, since 11β-HSD1 expression was increased with age before puberty in retroperitoneal fat (visceral adipose tissue), but not in epididymal fat (which is not visceral), consistent with studies of this gene in sheep and children [13], [33]. Adipose tissue depot-specific dysregulation of 11β-HSD1 has been reported by others [19], [34] and may involve depot-specific promoter activation of C/EBPs [35]. In the present study, the ratio of C/EBPα to C/EBPβ increased with postnatal age in retroperitoneal adipose tissue, while no change was seen in epididymal adipose tissue. However, there were no difference in the ratio of C/EBPα to C/EBPβ between SL and NL rats, and other transcription factors may thus be involved in this process and this needs further research. The increase in visceral adipose 11β-HSD1 activity with age may contribute to local activation of GC, which in turn may promote preadipocyte differentiation and lipid accumulation [9], suggesting an important role for 11β-HSD1 in fat accumulation and distribution during development.
Interestingly, there were different developmental patterns in visceral adipose 11β-HSD1 expression and activity after puberty onset in NL compared to SL rats. 11β-HSD1 in NL rats began to decline earlier (W6) than in SL rats; thus, SL rats had higher visceral adipose 11β-HSD1 activity from W8 to adulthood. A large number of animal models have confirmed that high expression of 11β-HSD1 in visceral fat is closely related to obesity, insulin resistance, and lipid metabolism dysregulation. Thus, it is possible that overfeeding during lactation may underlie the increase in visceral fat GC activity at puberty and make SL rats more prone to fat accumulation and obesity, similar to what is observed in adipose tissue-selective overexpression of 11β-HSD1 in a transgenic mouse model [10]. However, the regulatory mechanisms of adipose 11β-HSD1 activity in SL rats during puberty is unclear, it is plausible that the factors derive from the adipose tissue itself, e.g. through different adipocytokines [36], [37], but this matter can only be concluded though future, more mechanistic, studies.
We found no differences in circulating corticosterone levels between the two groups in the earlier stages of puberty, and this correlates with a lack of change in GR expression in the liver. Importantly, we found that hepatic 11β-HSD1 mRNA was increased at W3 and W16 whereas activity was increased in SL rats from weaning to adulthood, as reported previously in db/db mice [38] and marmosets prenatally programmed with GC [39]. In the majority of obesity animal models, however, hepatic 11β-HSD1 expression and/or activity are unchanged [40] or downregulated [41]. This inconsistency may due to different time windows of exposure of overnutrition. Our animal model underwent general nutritional excess during lactation, while other obese animal models are usually manipulated in later puberty or later on [40], [42]. Perinatal nutrition may thus be pivotal in regulating hepatic 11β-HSD1 development. Mouse transgenic overexpression of 11β-HSD1 in the liver results in insulin resistance, dyslipidemia, hypertension, and fatty liver [11]. The increase in liver 11β-HSD1 may therefore be important for the development of insulin resistance in the present study. However, biochemical measures of serum triglycerides and cholesterol did not reveal any differences between the groups. The reason behind this inconsistency is currently unknown.
Circulating GC is inactivated by A-ring reductases in the liver. Studies of obese animals [8], [43] and humans revealed higher expression of hepatic 5α-reductase and normal circulating GC [15], while in hypertension patients, 5βR activity was decreased [44]. In the present study, SL rats showed higher hepatic A-ring reductase expression compared to NL rats in prepuberty. Higher A-ring reductase actvity may consequently increase GC clearance and lead to a compensatory activation of the HPA axis and reactivation of GC by 11β-HSD1 in the liver. However, the differences in A-ring reductase expression disappeared at the end of puberty and serum corticosterone was elevated. Since elevated GC actions influence pathways of hepatic glucose metabolism [45], this may aggravate metabolic disorders in SL rats. Taken together, the developmental ontogeny of GC metabolism in the liver could reflect the pathological process of metabolic disease in SL. Little is known about the regulatory mechanisms behind 5αR1 and 5βR expression during postnatal development. Androgens are potent regulators of 5αR1 [46], [47], and temporal 5αR1 expression during development may reflect the action of sex hormones, as W5 is near the onset of puberty and is accompanied by increasing androgen hormone levels [48]. Thus, investigating the developmental changes in liver 5αR1 in castrated rats is warranted in future research.
It is well known that variations in maternal care affect the development of individuals in neuroendocrine stress systems, e.g. the HPA-axis, in rats [49]. In our study, there may be subtle differences in mother-pup interactions such as maternal licking and grooming between SL and NL rats. Therefore, other factors beside nutritional factors may be involved in the changes in GC metabolism, and this needs further studies.
In conclusion, small litter rearing in rats can induce obesity and alter GC metabolism in adults. During puberty, increased 11β-HSD1 activity and decreased 5αR1 and 5βR expression in peripheral tissue can to contribute to dysregulated GC metabolism and hyperactivity of the HPA-axis, and may aggravate the pathological processes associated with nutritional programming in obese organisms. Furthermore, the developmental patterns of GC metabolism suggest that early interventions should be considered and brought into action before the onset of puberty.
Acknowledgments
We thank Junle Wang (Department of Clinical Laboratories, Nanjing Children's Hospital) and Yongquan Zhang (Department of Clinical Laboratories, the Second Affiliated Hospital of Nanjing Medical University) for their technical assistance, and Associate Professor Baoqing Mo (Department of Public Health, Nanjing Medical University) for help with statistics.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Natural Science Foundation of the Jiangsu Health Department of China (2009) and the Scientific Research Foundation for Returned Overseas Scholars of the Ministry of Education of China (2009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 2.Cali AM, Caprio S. Obesity in children and adolescents. J Clin Endocrinol Metab. 2008;93:S31–S36. doi: 10.1210/jc.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009;5:604–610. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 4.Dunger DB, Salgin B, Ong KK. Session 7: Early nutrition and later health early developmental pathways of obesity and diabetes risk. Proc Nutr Soc. 2007;66:451–457. doi: 10.1017/S0029665107005721. [DOI] [PubMed] [Google Scholar]
- 5.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 6.Metges CC. Early nutrition and later obesity: animal models provide insights into mechanisms. Adv Exp Med Biol. 2009;646:105–112. doi: 10.1007/978-1-4020-9173-5_11. [DOI] [PubMed] [Google Scholar]
- 7.Stimson RH, Walker BR. Glucocorticoids and 11beta-hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Minerva Endocrinol. 2007;32:141–159. [PubMed] [Google Scholar]
- 8.Livingstone DE, Jones GC, Smith K, Jamieson PM, Andrew R, et al. Understanding the role of glucocorticoids in obesity: tissue-specific alterations of corticosterone metabolism in obese Zucker rats. Endocrinology. 2000;141:560–563. doi: 10.1210/endo.141.2.7297. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, et al. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 10.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 11.Paterson JM, Morton NM, Fievet C, Kenyon CJ, Holmes MC, et al. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci U S A. 2004;101:7088–7093. doi: 10.1073/pnas.0305524101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Lindquist S, Chen R, Myrnas T, Angsten G, et al. Depot-specific messenger RNA expression of 11 beta-hydroxysteroid dehydrogenase type 1 and leptin in adipose tissue of children and adults. Int J Obes (Lond) 2007;31:820–828. doi: 10.1038/sj.ijo.0803470. [DOI] [PubMed] [Google Scholar]
- 14.Morton NM, Seckl JR. 11beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res. 2008;36:146–164. doi: 10.1159/000115363. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson JW, Finney J, Gay C, Hughes BA, Hughes SV, et al. Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes. 2008;57:2652–2660. doi: 10.2337/db08-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westerbacka J, Yki-Jarvinen H, Vehkavaara S, Hakkinen AM, Andrew R, et al. Body fat distribution and cortisol metabolism in healthy men: enhanced 5beta-reductase and lower cortisol/cortisone metabolite ratios in men with fatty liver. J Clin Endocrinol Metab. 2003;88:4924–4931. doi: 10.1210/jc.2003-030596. [DOI] [PubMed] [Google Scholar]
- 17.Barat P, Livingstone DE, Elferink CM, McDonnell CR, Walker BR, et al. Effects of gonadectomy on glucocorticoid metabolism in obese Zucker rats. Endocrinology. 2007;148:4836–4843. doi: 10.1210/en.2007-0597. [DOI] [PubMed] [Google Scholar]
- 18.Wiegand S, Richardt A, Remer T, Wudy SA, Tomlinson JW, et al. Reduced 11beta-hydroxysteroid dehydrogenase type 1 activity in obese boys. Eur J Endocrinol. 2007;157:319–324. doi: 10.1530/EJE-07-0193. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Hou M, Sun B, Buren J, Zhang L, et al. Testosterone stimulates adipose tissue 11beta-hydroxysteroid dehydrogenase type 1 expression in a depot-specific manner in children. J Clin Endocrinol Metab. 2010;95:3300–3308. doi: 10.1210/jc.2009-2708. [DOI] [PubMed] [Google Scholar]
- 20.Agha A, Monson JP. Modulation of glucocorticoid metabolism by the growth hormone - IGF-1 axis. Clin Endocrinol (Oxf) 2007;66:459–465. doi: 10.1111/j.1365-2265.2007.02763.x. [DOI] [PubMed] [Google Scholar]
- 21.Boullu-Ciocca S, Dutour A, Guillaume V, Achard V, Oliver C, et al. Postnatal diet-induced obesity in rats upregulates systemic and adipose tissue glucocorticoid metabolism during development and in adulthood: its relationship with the metabolic syndrome. Diabetes. 2005;54:197–203. doi: 10.2337/diabetes.54.1.197. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues AL, de Moura EG, Passos MC, Dutra SC, Lisboa PC. Postnatal early overnutrition changes the leptin signalling pathway in the hypothalamic-pituitary-thyroid axis of young and adult rats. J Physiol. 2009;587:2647–2661. doi: 10.1113/jphysiol.2009.169045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velkoska E, Cole TJ, Dean RG, Burrell LM, Morris MJ. Early undernutrition leads to long-lasting reductions in body weight and adiposity whereas increased intake increases cardiac fibrosis in male rats. J Nutr. 2008;138:1622–1627. doi: 10.1093/jn/138.9.1622. [DOI] [PubMed] [Google Scholar]
- 24.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 25.Chen HC, Farese RJ. Determination of adipocyte size by computer image analysis. J Lipid Res. 2002;43:986–989. [PubMed] [Google Scholar]
- 26.Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149:5348–5356. doi: 10.1210/en.2008-0582. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakshmi V, Monder C. Purification and characterization of the corticosteroid 11 beta-dehydrogenase component of the rat liver 11 beta-hydroxysteroid dehydrogenase complex. Endocrinology. 1988;123:2390–2398. doi: 10.1210/endo-123-5-2390. [DOI] [PubMed] [Google Scholar]
- 29.Buren J, Bergstrom SA, Loh E, Soderstrom I, Olsson T, et al. Hippocampal 11beta-hydroxysteroid dehydrogenase type 1 messenger ribonucleic acid expression has a diurnal variability that is lost in the obese Zucker rat. Endocrinology. 2007;148:2716–2722. doi: 10.1210/en.2006-0897. [DOI] [PubMed] [Google Scholar]
- 30.Williams LJ, Lyons V, MacLeod I, Rajan V, Darlington GJ, et al. C/EBP regulates hepatic transcription of 11beta-hydroxysteroid dehydrogenase type 1. A novel mechanism for cross-talk between the C/EBP and glucocorticoid signaling pathways. J Biol Chem. 2000;275:30232–30239. doi: 10.1074/jbc.M001286200. [DOI] [PubMed] [Google Scholar]
- 31.Balazs Z, Schweizer RA, Frey FJ, Rohner-Jeanrenaud F, Odermatt A. DHEA induces 11 -HSD2 by acting on CCAAT/enhancer-binding proteins. J Am Soc Nephrol. 2008;19:92–101. doi: 10.1681/ASN.2007030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plagemann A, Harder T, Brunn M, Harder A, Roepke K, et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587:4963–4976. doi: 10.1113/jphysiol.2009.176156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gnanalingham MG, Mostyn A, Symonds ME, Stephenson T. Ontogeny and nutritional programming of adiposity in sheep: potential role of glucocorticoid action and uncoupling protein-2. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1407–R1415. doi: 10.1152/ajpregu.00375.2005. [DOI] [PubMed] [Google Scholar]
- 34.Baudrand R, Carvajal CA, Riquelme A, Morales M, Solis N, et al. Overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in hepatic and visceral adipose tissue is associated with metabolic disorders in morbidly obese patients. Obes Surg. 2010;20:77–83. doi: 10.1007/s11695-009-9937-0. [DOI] [PubMed] [Google Scholar]
- 35.Apostolova G, Schweizer RA, Balazs Z, Kostadinova RM, Odermatt A. Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E957–E964. doi: 10.1152/ajpendo.00442.2004. [DOI] [PubMed] [Google Scholar]
- 36.Dieudonne MN, Sammari A, Dos SE, Leneveu MC, Giudicelli Y, et al. Sex steroids and leptin regulate 11beta-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: Sex specificities. J Steroid Biochem Mol Biol. 2006;99:189–196. doi: 10.1016/j.jsbmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Ishii-Yonemoto T, Masuzaki H, Yasue S, Okada S, Kozuka C, et al. Glucocorticoid reamplification within cells intensifies NF-kappaB and MAPK signaling and reinforces inflammation in activated preadipocytes. Am J Physiol Endocrinol Metab. 2010;298:E930–E940. doi: 10.1152/ajpendo.00320.2009. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Nakagawa Y, Wang Y, Li R, Li X, et al. Leptin activation of corticosterone production in hepatocytes may contribute to the reversal of obesity and hyperglycemia in leptin-deficient ob/ob mice. Diabetes. 2003;52:1409–1416. doi: 10.2337/diabetes.52.6.1409. [DOI] [PubMed] [Google Scholar]
- 39.Nyirenda MJ, Carter R, Tang JI, de Vries A, Schlumbohm C, et al. Prenatal programming of metabolic syndrome in the common marmoset is associated with increased expression of 11beta-hydroxysteroid dehydrogenase type 1. Diabetes. 2009;58:2873–2879. doi: 10.2337/db09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton NM, Ramage L, Seckl JR. Down-regulation of adipose 11beta-hydroxysteroid dehydrogenase type 1 by high-fat feeding in mice: a potential adaptive mechanism counteracting metabolic disease. Endocrinology. 2004;145:2707–2712. doi: 10.1210/en.2003-1674. [DOI] [PubMed] [Google Scholar]
- 41.Drake AJ, Livingstone DE, Andrew R, Seckl JR, Morton NM, et al. Reduced adipose glucocorticoid reactivation and increased hepatic glucocorticoid clearance as an early adaptation to high-fat feeding in Wistar rats. Endocrinology. 2005;146:913–919. doi: 10.1210/en.2004-1063. [DOI] [PubMed] [Google Scholar]
- 42.Shin AC, MohanKumar SM, Sirivelu MP, Claycombe KJ, Haywood JR, et al. Chronic exposure to a high-fat diet affects stress axis function differentially in diet-induced obese and diet-resistant rats. Int J Obes (Lond) 2010;34:1218–1226. doi: 10.1038/ijo.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livingstone D, Grassick S, Currie G, Walker B, Andrew R. Dysregulation of glucocorticoid metabolism inmurine obesity:comparable effedts of leptin resistance and deficiency. J Endocrinol. 2009 doi: 10.1677/JOE-09-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campino C, Carvajal CA, Cornejo J, San MB, Olivieri O, et al. 11beta-hydroxysteroid dehydrogenase type-2 and type-1 (11beta-HSD2 and 11beta-HSD1) and 5beta-reductase activities in the pathogenia of essential hypertension. Endocrine. 2010;37:106–114. doi: 10.1007/s12020-009-9269-9. [DOI] [PubMed] [Google Scholar]
- 45.Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol. 2008;197:189–204. doi: 10.1677/JOE-08-0054. [DOI] [PubMed] [Google Scholar]
- 46.Gustafsson JA, Stenberg A. Irreversible androgenic programming at birth of microsomal and soluble rat liver enzymes active on androstene-3,17-dione and 5alpha-androstane-3alpha,17beta-diol. J Biol Chem. 1974;249:711–718. [PubMed] [Google Scholar]
- 47.El-Awady MK, El-Garf W, El-Houssieny L. Steroid 5alpha reductase mRNA type 1 is differentially regulated by androgens and glucocorticoids in the rat liver. Endocr J. 2004;51:37–46. doi: 10.1507/endocrj.51.37. [DOI] [PubMed] [Google Scholar]
- 48.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- 49.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]