Abstract
Rationale: Pulmonary hypertension (PH) is characterized by progressive increase in pulmonary artery pressure leading to right ventricular (RV) hypertrophy, RV failure, and death. Current treatments only temporarily reduce severity of the disease, and an ideal therapy is still lacking.
Objectives: Estrogen pretreatment has been shown to attenuate development of PH. Because PH is not often diagnosed early, we examined if estrogen can rescue preexisting advanced PH.
Methods: PH was induced in male rats with monocrotaline (60 mg/kg). At Day 21, rats were either treated with 17-β estradiol or estrogen (E2, 42.5 μg/kg/d), estrogen receptor-β agonist (diarylpropionitrile, 850 μg/kg/d), or estrogen receptor α-agonist (4,4',4"-[4-Propyl-(1H)-pyrazole-1,3,5-triyl] trisphenol, 850 μg/kg/d) for 10 days or left untreated to develop RV failure. Serial echocardiography, cardiac catheterization, immunohistochemistry, Western blot, and real-time polymerase chain reaction were performed.
Measurements and Main Results: Estrogen therapy prevented progression of PH to RV failure and restored lung and RV structure and function. This restoration was maintained even after removal of estrogen at Day 30, resulting in 100% survival at Day 42. Estradiol treatment restored the loss of blood vessels in the lungs and RV. In the presence of angiogenesis inhibitor TNP-470 (30 mg/kg) or estrogen receptor-β antagonist (PHTPP, 850 μg/kg/d), estrogen failed to rescue PH. Estrogen receptor-β selective agonist was as effective as estrogen in rescuing PH.
Conclusions: Estrogen rescues preexisting severe PH in rats by restoring lung and RV structure and function that are maintained even after removal of estrogen. Estrogen-induced rescue of PH is associated with stimulation of cardiopulmonary neoangiogenesis, suppression of inflammation, fibrosis, and RV hypertrophy. Furthermore, estrogen rescue is likely mediated through estrogen receptor-β.
Keywords: pulmonary hypertension, estrogen, neoangiogenesis, estrogen receptors, inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
Despite significant advances in cardiopulmonary research, pulmonary hypertension (PH) still remains a difficult disease to treat, as therapeutic strategies to simultaneously reduce pulmonary vascular damage and prevent right ventricular (RV) dysfunction are lacking. This debilitating disease occurs more frequently in women, yet both animal studies in classical models of PH and limited clinical data suggest protective effects of estrogens: the estrogen paradox in pulmonary hypertension.
What This Study Adds to the Field
Estrogen not only exerts beneficial effects on lung pathology but also protects the RV from PH-related complications in RV structure and function in rats treated with monocrotaline. This study demonstrates that estrogen rescues severe preexisting PH in rats, suggesting a possible role of estrogen for treatment of severe PH in patients. Because the rescue action of estrogen is likely mediated by estrogen receptor-β, selective estrogen receptor-β ligands may have value for rigorous preclinical evaluations and could provide a novel treatment modality for patients with pulmonary hypertension.
Pulmonary hypertension (PH) is a chronic lung disease characterized by pulmonary vascular remodeling and progressive increase in pulmonary artery pressure leading to right ventricular (RV) hypertrophy and RV failure (RVF). End-stage RVF has long been regarded as a terminal state of pathological cardiopulmonary remodeling, including fibrosis and chamber dilation, being unresponsive to available therapies. Advanced PH is most often treated with aggressive nonpharmacological therapies, such as lung transplantation, but this approach is only feasible for a fraction of patients. In the past decade, cell and gene therapies have shown great potential for treatment of PH in animal models (1, 2) and humans (3). However, effective pharmacological therapy for treatment of patients with advanced PH would be much more practical and much more cost effective. Several agents have been identified to attenuate the development of PH when the therapy is started before the initiating stimuli (4–6). Unfortunately, up to now, there has been no ideal pharmacological therapy to reverse advanced PH.
Although the incidence of PH remains higher in female patients (7), in various animal models females have been shown to be protected against PH (8–10), a phenomenon known as the “estrogen paradox” of PH. This sex difference in susceptibility to experimental PH has been suggested to be in part due to the action of estrogen (E2), as ovariectomy exacerbates PH and pretreatment with E2 and its metabolites attenuates the progression of different animal models of PH (4, 9, 11).
Because PH is not always diagnosed early, we tested the more clinically relevant hypothesis that E2 may also reverse preexisting severe PH and explored the molecular mechanisms involved in the rescue of preexisting PH by E2 therapy. We used the well-established model of monocrotaline (MCT)-induced PH in rats, which has been commonly used by many investigators (1, 12). We found that a 10-day E2 treatment significantly reversed MCT-induced preexisting PH by restoring lung and heart structure and function, resulting in 100% survival even after withdrawal of E2. Restoration of angiogenesis by E2 both in RV and lung is one of the key mechanisms in E2-induced rescue of PH. Furthermore, this rescue action of E2 is likely mediated through an estrogen receptor (ER)-β−dependent mechanism. Some of the results of these studies have been previously reported in the form of abstracts (13, 14).
Methods
Animals and Treatments
Male and female Sprague-Dawley rats (350–400 g) were used. Details of the treatments and criteria for survival measurements are given in the online data supplement. Protocols received institutional review and committee approval. The investigation conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (15).
Cardiac and Pulmonary Hemodynamics
B-mode, M-mode, and pulmonary pulse-wave Doppler echocardiography were performed using a VisualSonics Vevo 770 equipped with a 30-MHz linear transducer to accurately monitor the stage of the disease (see online data supplement for details).
Gross Histologic Evaluation
The RV wall, the left ventricular wall, and the interventricular septum (IVS) were dissected, and the ratio of the right ventricle to left ventricle (LV) plus septum weight (RV/[LV + IVS]) was calculated as an index of RV hypertrophy. Wet lung weight was determined by weighing the lung tissue.
Real-Time Polymerase Chain Reaction
Total RNA from lungs and RV were isolated using Trizol (Invitrogen) and reverse transcribed with gene-specific primers using the Omniscript RT kit (Qiagen). Controls were: (1) the reaction without reverse transcriptase, and (2) H2O instead of cDNA.
Western Blot Analysis
Standard Western blot analysis was performed using lung and RV lysates. The details of the Western blot analyses and the antibodies used are presented in the online data supplement.
Immunohistochemistry and Imaging
Whole hearts and lungs were fixed in 4% paraformaldehyde in 0.1 M Na2HPO4 and 23 mM NaH2PO4 (pH 7.4) for 4 hours on ice. The tissue was then immersed in ice-cold 20% sucrose overnight to cryoprotect, was mounted using OCT, and transversal 6- to 7-μm sections were obtained with a cryostat. Tissue sections were stained with immunofluorescence, immunoperoxidase, standard hematoxylin-eosin, and Masson trichrome staining. The images were acquired using a light microscope (Axiovert 135; Zeiss, Germany) or with a laser-scanning confocal microscope (Olympus).
Statistical Analysis
One-way analysis of variance was used to compare between groups and within the same group at different time points using SPSS13.0 for Windows. When significant differences were detected, individual mean values were compared by post hoc tests (Bonferroni correction) that allowed for multiple comparisons. P values less than 0.05 were considered statistically significant. Values are expressed as mean ± SE.
Results
Estrogen Reverses PH by Improving Cardiac and Pulmonary Structure and Function and Leads to 100% Survival
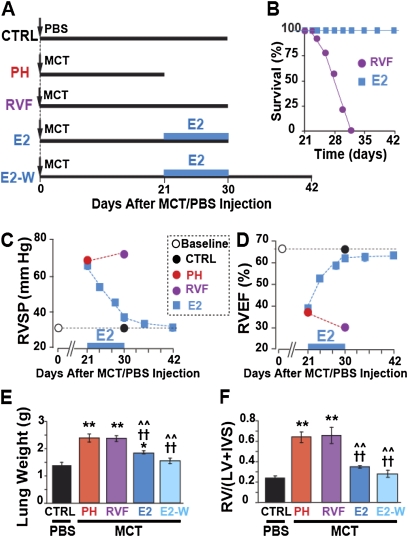
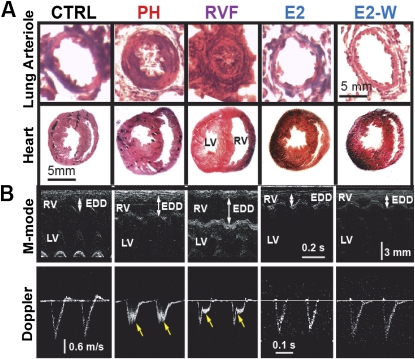
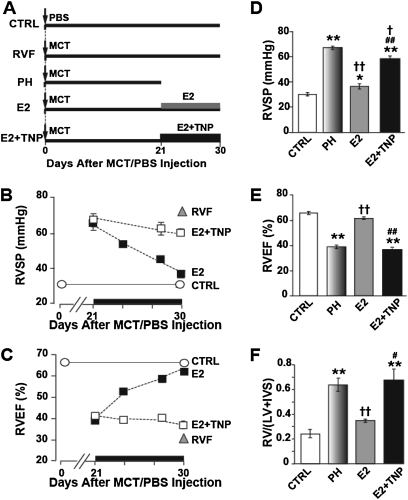
Male Sprague-Dawley rats were injected with a single dose of monocrotaline (MCT, 60 mg/kg, Figure 1A) to induce PH as has been extensively used by many investigators (1, 12). Two weeks after MCT injection, signs of PH started to appear together with RV hypertrophy, but the RV ejection fraction (RVEF) was still preserved (adaptive RV hypertrophy; see Table E1 in the online supplement). By day 21, severe PH had already been well established (PH group), as peak RV systolic pressure (RVSP) had increased from 31.1 ± 0.9 in control rats (CTRL) to 67.7 ± 1.1 mm Hg in the PH group (Figure 1C), and RVEF was reduced from 65.1 ± 1.7% in CTRL rats to 39.0 ± 0.6% in the PH group (Figure 1D). The severity of PH at Day 21 was further confirmed by (1) the increase in lung weight (from 1.37 ± 0.1 g in CTRL to 2.37 ± 0.09 g in PH, Figure 1E), (2) RV hypertrophy (RV/[LV+IVS] increased from 0.23 ± 0.02 in CTRL to 0.64 ± 0.05 in PH, Figure 1F), (3) pulmonary arteriolar medial hypertrophy (Figure 2A), and (4) appearance of a midsystolic notch on Doppler echocardiography of pulmonary artery flow (Figure 2B). These data clearly demonstrate the development of malignant PH by Day 21 (16) and agree with previous studies using this time point as an initiating point for therapy of advanced PH (12). Therefore, we started E2 therapy at Day 21 in one group of rats by subcutaneously implanting continuous-release E2 pellets (42.5 μg/kg/d) for 10 days from Days 21 to 30 (E2 group), and another group was left untreated to develop RV failure (RVF group) by Day 30. E2 not only prevented the progression to RVF but also it gradually improved RVSP and RVEF, as these parameters had returned to almost CTRL levels within 10 days of E2 treatment. In untreated animals, however, the RVSP significantly increased even further to 72.0 ± 1.4 mm Hg and RVEF significantly decreased to 30.4 ± 1.7% (Figures 1C and 1D). However, the lung weight (Figure 1E) and RV hypertrophy index (Figure 1F) did not increase after 21 days any further. E2 therapy was also associated with reversal of lung weight (1.83 ± 0.05 g vs. 2.37 ± 0.14 g at Day 21, Figure 1E) and regression of RV hypertrophy (RV/[LV+IVS] = 0.34 ± 0.01 g vs. 0.64 ± 0.05 at Day 21, Figure 1F). Moreover, pulmonary arteriolar medial hypertrophy was completely reversed (Figure 2A).
Figure 1.
Estrogen (E2) rescues severe pulmonary hypertension (PH). (A) Experimental protocol: male rats were injected with monocrotaline (MCT) or phosphate-buffered saline (PBS) at Day 0. The thick horizontal lines represent the length of each experimental group. At Day 21, animals were either killed (PH group), or left untreated to develop right ventricular (RV) failure (RVF group). Both E2 and E2-W groups received E2 only from Day 21 to Day 30. All of the rats in the E2 group were killed at Day 30, whereas the rats in the E2-W group were kept for another 12 days until Day 42 after E2 withdrawal at Day 30. (B) Survival plot. (C) RV systolic pressure (RVSP) and (D) RV ejection fraction (RVEF) at baseline (open circle) and after a single MCT/PBS injection in control mice (CTRL; black circle), PH (red circle), RVF (purple circle), and E2-treated (blue squares). For simplicity, RVSP and RVEF are only shown at the end of the experiment, except for the E2-treated group, for which these parameters are shown as a function of time. The black dotted lines represent the baseline levels. (E) Lung weight and (F) RV hypertrophy index in CTRL (black bar), PH (red bar), RVF (purple bar), E2 (dark blue bar) and E2-W (light blue bar). *P < 0.05 versus CTRL, **P < 0.001 versus CTRL; ††P < 0.001 versus PH; ^^P < 0.001 versus RVF (n = 6–8 rats per group). IVS = interventricular septum; LV = left ventricular wall.
Figure 2.
Reversal of cardiopulmonary structure and function by estrogen (E2). (A) hematoxylin-eosin staining for lung arterioles and heart cross-sections in male rats. (B) Echocardiographic images of M-mode (upper panels) showing right ventricular (RV) end-diastolic diameter (EDD), left ventricular (LV) and interventricular septum (IVS), and pulse-wave Doppler (lower panels) in male rats. Yellow arrows show mid-systolic notch present in pulmonary hypertension (PH) and RV failure (RVF) only.
The improvements in lung and RV structure and function mediated by E2 led to 100% survival, as all E2-treated animals were alive at Day 30, whereas in untreated rats, the mortality started at Day 24 and sharply increased to 45% by Day 28 and to 75% by Day 30 (Figure 1B). Although RVF was associated with a significant reduction in weight gain during the last 10 days from Days 21 to 30 (9 ± 5 g vs. 39 ± 4 g in CTRL), E2 treatment significantly improved weight gain in this time interval (26 ± 5 g, Figure E1C).
Estrogen's Rescue of PH Persists Even after Estrogen Withdrawal
To investigate whether the improved structural and functional changes achieved by E2 therapy were maintained once E2 was no longer available, some E2-treated rats were kept for another 12 days until Day 42 after E2 withdrawal at Day 30 (E2-W group). Surprisingly, not only were the RVSP and RVEF maintained but also they improved even further and were fully restored to normal levels as in CTRL rats by Day 42 (Figures 1C and 1D; Figures E1A and E1B). The E2-induced reduction in lung weight and RV hypertrophy index decreased even further after E2 withdrawal: at Day 42, these parameters were not significantly different from the CTRL (lung weight = 1.54 ± 0.11 g in E2-W vs. 1.37 ± 0.1 g in CTRL; RV/[LV+IVS] = 0.28 ± 0.03 in E2-W vs. 0.23 ± 0.02 in CTRL; Figures 1E and 1F). All rats with PH treated with E2 for 10 days survived to at least 42 days after MCT injection, whereas none survived after Day 32 in the untreated PH group (Figure 1B). Hence, these data confirmed that E2 rescue of PH persisted even after discontinuation of E2 therapy, suggesting that short-term E2 therapy was sufficient to cure the disease.
Estrogen Reverses Pulmonary Inflammation and Inflammatory Signaling
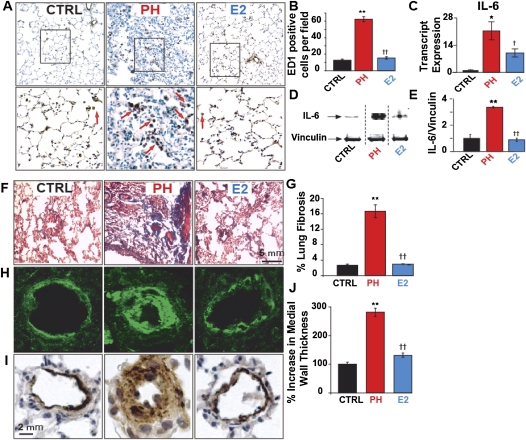
To gain insight into the cellular and molecular mechanisms of E2-induced rescue of PH, we first investigated whether suppression of inflammation by E2 could be involved in E2-induced rescue of PH. Lung sections were stained for ED1 (CD68), a marker for macrophages/monocytes. The number of ED1-positive inflammatory cells (both the alveolar macrophages and the perivascular macrophages) in rats with PH was increased fivefold (62.5 ± 3.16 in PH vs. 12.6 ± 1.42 cells/field in CTRL), and E2 fully reversed this increase (15.4 ± 1.4 cells/field, Figures 3A and 3B). Next, we examined the possible regulation of the proinflammatory cytokine IL-6 by E2 in the lungs (17). E2 partially reversed PH-induced approximately 22-fold up-regulation of IL-6 transcripts. IL-6 protein up-regulation in PH was also fully restored by E2 (Figures 3C–3E). In the PH group, lung fibrosis had increased considerably (16.6 ± 1.65% in PH vs. 2.6 ± 0.35% in CTRL), and E2 mediated a complete reversal of this fibrosis (2.93 ± 0.15%) (Figures 3F and 3G). Pulmonary arteriolar medial hypertrophy in the lungs of the PH group was significantly increased (281 ± 14% in PH vs. 100 ± 7% in CTRL) and was reversed by E2 (131 ± 7%) (Figures 3H–3J). RV fibrosis has been observed in biopsies from RV endomyocardial tissue of patients with PH (18). We also observed the presence of mild to moderate fibrosis in the RV sections of PH, but this was completely absent in E2-treated animals (data not shown). E2 was also able to reverse pulmonary fibrosis and inflammation in the lungs of females with PH (Figure E2).
Figure 3.
Reversal of lung inflammation and remodeling by estrogen (E2). (A) Lung sections of male rats stained for ED1 in control (CTRL), pulmonary hypertension (PH), and estrogen (E2) groups (upper panel) and at higher magnification of the respective fields (lower panel). Red arrows indicate the ED1-positive cells. (B) ED1-positive cells quantification per field in CTRL (black bar), PH (red bar), and E2 (blue bar). (C) Relative transcript expression of lung IL-6 in CTRL (black bar), PH (red bar), and E2 (blue bar), normalized to CTRL. (D) Representative immunoblots of lung lysates from CTRL, PH, and E2 labeled with anti–IL-6 and anti-vinculin antibodies. In this immunoblot and the subsequent immunoblots, all samples from CTRL, PH, and E2 groups were run on the same gel. Because we are only showing representative lanes from a total of three to five samples per group, some of the intervening lanes were not shown and are separated by a dotted line. (E) Western blot analysis of IL-6 protein in lung lysates normalized to vinculin in CTRL (black bar), PH (red bar), and E2 (blue bar). (F) Masson trichrome staining of lung sections in CTRL, PH, and E2; blue color indicates fibrosis. (G) Quantification of lung fibrosis showing percent lung fibrosis in CTRL, PH, and E2 groups. (H) Immunofluorescence labeling of pulmonary arterioles stained for smooth muscle actin (green) and (I) immunoperoxidase labeling of pulmonary arterioles stained with anti–smooth muscle actin antibody (brown) together with hematoxylin-stained nuclei (blue). (J) Bar graph for quantification of pulmonary arteriolar medial wall thickness in CTRL, PH, and E2 groups. *P < 0.05 versus CTRL, **P < 0.001 versus CTRL; †P < 0.05 versus PH, ††P < 0.001 versus PH (n = 4 animals per group).
Estrogen Stimulates Pulmonary and Cardiac Angiogenesis
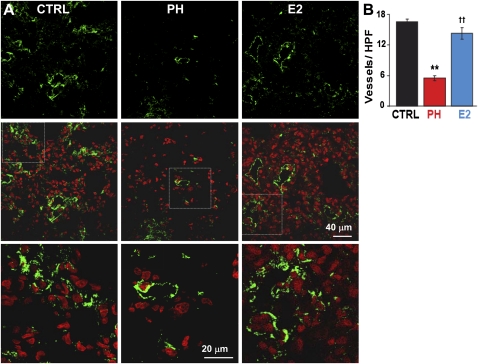
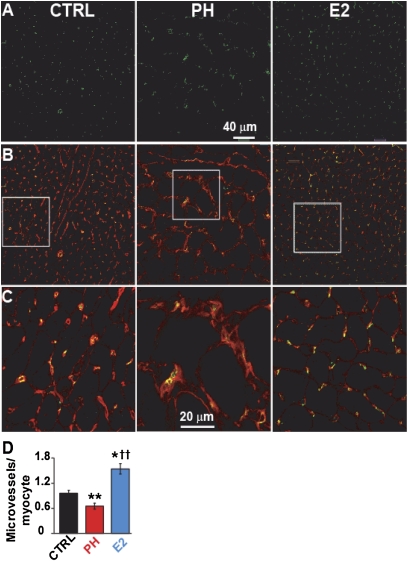
Stimulation of pulmonary neoangiogenesis has been suggested as a potential therapeutic strategy for treatment of PH (19). We examined whether stimulation of pulmonary neoangiogenesis by E2 participated in E2-induced rescue of PH. Pulmonary vessel density of small vessels up to 50 μm was significantly reduced by approximately threefold in PH (5.5 ± 0.5 vessels per high-power field in PH vs. 16.5 ± 0.5 in CTRL, Figures 4A and 4B). E2 reversed the loss of blood vessels associated with PH in lung (14.3 ± 1.4) (Figure 4B). Similar to male rats, E2 was also able to reverse the loss of vessels in the lungs of female rats with PH (Figure E3). Next, we examined whether E2 could also restore the loss of RV microvessels as reported in patients with PH as well (20). RV capillary density was significantly reduced by approximately 40% in PH (0.65 ± 0.06 microvessels per cardiomyocyte in PH vs. 0.95 ± 0.07 in CTRL, Figures 5A–5D). E2 not only completely reversed the loss of capillaries in RV but also significantly enhanced capillary density by approximately 50% compared with healthy CTRL (1.54 ± 0.12 in E2 vs. 0.95 ± 0.07 in CTRL) (Figure 5D). To further confirm the direct role of angiogenesis in the rescue action of E2, the animals were treated with E2 alone or E2 together with the angiogenesis inhibitor TNP-470 (30 mg/kg, Figure 6A). In the presence of TNP, E2 failed to rescue PH as RVSP (59.4 ± 1.8 mm Hg), RVEF (37.1 ± 1.8%), and RV hypertrophy index (0.68 ± 0.09) had not significantly improved after 10 days of E2 therapy (Figures 6B–6F). In fact, RVEF and RV hypertrophy index were not significantly different from PH. (Figures 6E and 6F). These data strongly support the vital role of angiogenesis in the rescue action of E2 in PH.
Figure 4.
Stimulation of pulmonary neoangiogenesis by estrogen (E2). (A) Single confocal images of lung sections of male rats immunostained for von Willebrand Factor (green, upper panels), overlay of von Willebrand factor and nuclei (stained red with TO PRO, middle panels), and at higher magnification of the respective fields (lower panels). (B) Quantification of vessels per high power field (HPF) in control (CTRL; black bar), pulmonary hypertension (PH; red bar), and E2 (blue bar). **P < 0.001 versus CTRL; ††P < 0.001 versus PH (n = 4 animals per group).
Figure 5.
Stimulation of cardiac neoangiogenesis by estrogen (E2). (A–C) Single confocal images of right ventricular sections of male rats immunostained for CD31 (green, A), overlay of CD31 and WGA (red, B) and at higher display magnification (C). (D) Quantification of microvessels/cardiomyocyte in control (CTRL; black bar), pulmonary hypertension (PH; red), and E2 (blue). *P < 0.05 versus CTRL, **P < 0.001 versus CTRL; ††P < 0.001 versus PH (n = 4 animals per group).
Figure 6.
Estrogen (E2) fails to rescue pulmonary hypertension (PH) in the presence of angiogenesis inhibitor. (A) Experimental protocol. (B) Right ventricular systolic pressure (RVSP) and (C) RV ejection fraction (RVEF) at the end point of each experiment, except for E2-treated groups, for which the data are presented as a function of time. The solid lines represent the baseline levels. (D–F) RVSP, RVEF, and RV hypertrophy index RV/(left ventricular[LV]+ interventricular septum [IVS]) for control (CTRL; open bar), PH (shaded gray bar), E2 (gray bar), and E2+TNP (solid bar) groups at Day 30 except for PH group, which was at Day 21. *P < 0.05 versus CTRL, **P < 0.001 versus CTRL; †P < 0.05 versus PH, ††P < 0.001 versus PH; #P < 0.05 versus E2, ##P < 0.001 versus E2 (n = 3–8 animals per group).
Involvement of Estrogen Receptor β in the Rescue of PH by E2
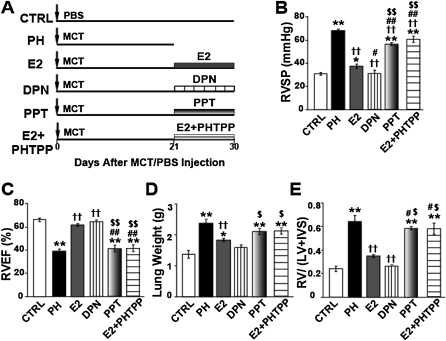
To investigate whether ERs are involved in the rescue action of E2, rats were treated with a selective agonist of ERα, 4,4',4"-(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT) (0.85 mg/kg/d), or a selective agonist of ERβ, diarylpropionitrile (0.85 mg/kg/d) at Day 21 after MCT injection for 10 days (Figure 7A). Diarylpropionitrile was as effective as E2, if not even better, in rescuing PH (RVSP = 34 ± 1 mm Hg, RV/[LV+IVS] = 0.30 ± 0.01, RVEF = 64 ± 4%, and lung weight = 1.5 ± 0.4 g), whereas PPT was not as effective as E2 (RVSP = 56 ± 1mm Hg, RV/[LV+IVS] = 0.60 ± 0.01, RVEF = 40 ± 4%, and lung weight = 2.0 ± 0.1 g). PPT was only able to reduce RVSP significantly compared with PH, but RVEF, lung weight, and RV hypertrophy index were not significantly different from PH (Figures 7B–7E). To further confirm the role of ERβ in the rescue action of E2, we treated rats with PH with E2 in the presence of the ERβ-specific antagonist PHTPP for 10 days. Interestingly, E2 could not rescue PH in the presence of PHTPP, as the beneficial effects of E2 on RV pressure, RV ejection fraction, lung weight, and RV hypertrophy were all abolished (RVSP = 60 ± 4 mm Hg, RV/[LV+IVS] = 0.58 ± 0.05, RVEF = 42 ± 1.7%, and lung weight = 1.93 ± 0.01 g) (Figures 7B–7E). These results suggest that the rescue of PH by E2 is most likely mediated via the ERβ.
Figure 7.
Selective estrogen receptor (ER)β agonist diarylpropionitrile (DPN) is as effective as estrogen (E2) in rescuing severe pulmonary hypertension (PH), and E2 fails to rescue PH in the presence of ERβ antagonist, PHTPP. (A) Experimental design. (B–E) Right ventricular systolic pressure (RVSP) (B), RV ejection fraction (RVEF) (C), lung weight (D), and RV hypertrophy index (E), at Day 30 except for PH, which was at Day 21 after monocrotaline (MCT) injection, for control (CTRL; open bar), PH (solid bar), E2 (gray bar), DPN (ERβ-agonist, vertical striped bar), PPT (ERα agonist, shaded gray bar), and E2+PHTPP (ERβ antagonist, horizontal striped bar) groups in male rats. *P < 0.05 versus CTRL, **P < 0.001 versus CTRL; ††P < 0.001 versus PH; #P < 0.05 versus E2, ##P < 0.001 versus E2; $P < 0.05 versus DPN, $$P < 0.001 versus DPN (n = 3–8 animals per group).
Discussion
The present study shows that E2 is a rescue agent for advanced preexisting PH. E2 reversed PH by improving cardiac and pulmonary structure and function leading to 100% survival even after withdrawal of E2. Our approach of starting E2 treatment after the establishment of fatal PH has major advantages over previous studies (4, 11) that used E2 therapy before the initiation of the disease, as our approach (1) is more practical for patients who already have severe PH, as this disease is not often diagnosed early; and (2) requires short duration and low dose of E2, thereby minimizing the chance of side effects, especially in women. Regarding the possible mechanisms involved in E2-induced rescue of PH, our data strongly support that the rescue action of E2 is mainly mediated through ERβ because (1) selective estrogen receptor-β agonist is able to rescue PH as efficiently as E2, and (2) E2 fails to rescue PH in the presence of ERβ selective antagonist (Figure 7). Stimulation of lung and RV angiogenesis by E2 is the other key mechanism involved in E2-induced rescue of PH (Figures 4–6). The beneficial effects of E2 in PH also seem to result from interplay of several factors, including suppression of lung inflammation and fibrosis as well as reversal of RV hypertrophy (Figure E4). Direct actions of E2 on the vessel wall of pulmonary arteries and arterioles have been described (21), but whether the improved RV function is simply a result of improved lung function or a direct action of E2 on the heart is not yet clear. As E2 was shown to attenuate LV hypertrophy of ventricular myocytes (22), we speculate that in addition to the action of E2 on the lung, E2 also directly acts on the RV to reverse RV hypertrophy (Figure 1F) and restores RV contractility and function (23) (Figure 1D).
Reverse Remodeling of Pulmonary Inflammation and Fibrosis by E2 Therapy
Inflammation plays an important role in the progression of PH in animal models as well as in patients with PH of various origins (24), and E2 has been shown to have antiinflammatory activity in many tissues such as the lung (25). The number of ED1-positive inflammatory cells was increased considerably in PH, and E2 treatment led to suppression of these inflammatory cells in the lungs both in males and females. E2 treatment also suppressed the up-regulation of lung IL-6 transcript and protein levels induced by PH (Figure 3). Therefore, suppression of inflammation and inflammatory mediators by E2 could be involved in E2-induced rescue of PH.
Sex differences have recently been proposed to exist in the development of lung fibrosis, as female mice are better protected against pulmonary fibrosis than male mice (26). In the present study, the increase in pulmonary fibrosis in our male PH group was almost completely reversed by E2. Reversal of cardiac fibrosis by E2 has been shown recently to be mediated by ERβ through suppression of transforming growth factor-β signaling. We believe that E2 may act through a similar ERβ-mediated pathway to reverse lung fibrosis as suggested by the recent reports (27, 28).
Stimulation of Pulmonary and Cardiac Angiogenesis by Estrogen
Loss of small blood vessels and impaired pulmonary angiogenesis contribute to the elevation of pulmonary pressures and progression of the PH. In the RV, loss of myocardial microvessels together with increased oxygen demand in patients with PH with normal coronary arteries has been shown to result in RV ischemia (20). The decrease in capillary density in the RV has been proposed to be due to insufficient up-regulation of angiogenic factors (29). Here we found that PH was associated with loss of small blood vessels both in lungs and in RV. E2 therapy reversed the loss of vessels associated with PH in the lungs in both male and female rats. E2 not only reversed the loss of blood vessels observed in the RV of the PH group, it also stimulated the growth of new capillaries beyond their levels in healthy CTRL rats. Stimulation of coronary angiogenesis in the LV has been shown to be a key event in maintaining heart function during the development of LV hypertrophy (30). These findings are not surprising as E2 is known to promote angiogenesis in several tissues (31–33). Therefore, increased myocardial blood vessels together with increased angiogenesis in the pulmonary circulation by E2 therapy may underlie the decrease in PH severity leading to improvements in cardiopulmonary structure and function. The fact that in the presence of TNP, a potent angiogenesis inhibitor, E2 failed to rescue PH strongly supports that stimulation of angiogenesis in both lung and RV by E2 is one of the key mechanisms in E2-induced rescue of PH.
The Rescue Action of Estrogen Is Likely Mediated through ERβ
E2 exerts its biological effects mainly via ERα and ERβ. Both receptor subtypes are present in lungs and heart (17, 34). ERβ has been shown to protect the lungs in a trauma-hemorrhage model (17) and the heart against ischemia/reperfusion injury and against pressure overload-induced LV failure (26, 35, 36). ERβ-knockout mice demonstrated abnormal vascular function and hypertension, increased mortality, and aggravation of heart failure (37, 38). Our data strongly support that the rescue action of E2 is mainly mediated through ERβ because (1) selective estrogen receptor β-agonist is able to rescue PH as efficiently as E2, and (2) E2 fails to rescue PH in the presence of an ERβ-selective antagonist. The ER–mediated action of estrogen has also been recently reported in another experimental model of PH induced by hypoxia (39). These researchers show that E2 attenuates hypoxia-induced pulmonary hypertension through an ER-dependent mechanism, as the preventive effect of E2 is abolished in the presence of E2 receptor antagonist ICI. The importance of receptor-specific actions of E2 in the protection against PH and RV dysfunction is in agreement with many studies demonstrating that the estrogen-mediated protection against vascular injury, hypertension, cardiac remodeling, and apoptosis are mediated through ERβ (26, 37, 40). However, Tofovic and colleagues speculated that the preventive action of E2 against PH is independent of its receptors because E2 metabolites have weak affinity for ERs (9). This speculation has been challenged recently as in the presence of COMT inhibitor OR-486, which inhibits the conversion of E2 to catechol and methoxyestrogen metabolites that exert their effects in an ER-independent manner, E2 was still able to attenuate the progression of PH and its beneficial effects were even potentiated (39).
The Estrogen Paradox in PH
The fact that E2 is rescuing PH may seem surprising due to the higher prevalence of idiopathic PH in young women (41). Contrary to humans, female rats with PH induced by either MCT or chronic hypoxia exhibit less severe PH than their male counterparts (1, 8, 12). This sex difference in susceptibility to PH has been suggested to be partly due to the protective action of E2, as E2 replacement attenuates the progression of PH in ovariectomized animals (42). Likewise, E2 treatment in male rats also slows down the development of PH (4). In addition to E2, estradiol metabolites, such as 2-methoxyestradiol (9), natural soybean-derived phytoestrogen, genistein (43), and a selective estrogen receptor modulator, raloxifene (44), all slow down the progression of MCT-induced or chronic hypoxia–induced PH in rodents.
How to explain the higher prevalence of idiopathic PH in young women? We favor the speculation by Lahm and colleagues (21), which states that a defect in the ERs, ER signaling, estrogen metabolism, or genetic susceptibility in certain women could make them more prone to this disease (45). Future studies are needed to investigate the possible correlation between plasma estrogen levels, pulmonary levels of ERβ and ERα, and the severity of PH in these women. The fact that (1) postmenopausal women have an increased risk for the development of pulmonary hypertension (46), and that (2) hormone replacement therapy may prevent the development of PH in these patients further supports the beneficial role of E2 in patients with PH (47). Other investigators have also suggested that severe PH has now become overwhelmingly a disease of postmenopausal women (48). In fact, despite higher incidence of the disease in female patients, as shown in the REVEAL registry of patients with PH (7), there is a clear shift in the mean age of diagnosis toward older age, particularly in the female patients from the United States (7).
Other Sex Steroids and PH
Similar to estrogen, progesterone also prevents the progression of experimental PH (49). Although testosterone has been shown to be a pulmonary vasodilator in isolated pulmonary arteries (49–51), male rats, which have higher levels of testosterone, do much worse than female rats in different experimental models of PH (8–10). Hansmann and colleagues showed male apoE–/– mice on high-fat diets developed more severe PH associated with insulin resistance and lower plasma adiponectin levels compared with female apoE–/– mice (52). Because testosterone inhibits the secretion of adiponectin in adipocytes, they hypothesized that elevation of this vasoprotective adipocytokine accounted for the less severe vascular phenotype in female apoE–/– mice. They treated male apoE–/– mice with rosiglitazone and documented eightfold higher plasma adiponectin levels, improved insulin sensitivity, and complete regression of PH (52). Taken together, testosterone does not seem to be beneficial in experimental PH. Low levels of testosterone in women are also unlikely to be the cause of female predominance of PH in patients (53).
Dehydroepiandrosterone (DHEA) can be metabolized to estrogens and androgens. In fact, Homma and colleagues detected higher plasma levels of estradiol and testosterone in DHEA-treated than in DHEA-untreated animals (54). Because testosterone does not seem to be beneficial in experimental PH, we speculate that the beneficial action of DHEA in experimental PH must have been due to conversion of DHEA to estrogen.
ERβ-Selective Ligands as Novel Therapeutic Agents for Patients with PH
Our findings raise the intriguing concept that E2 therapy may be of significant benefit for patients with severe PH. The rescue action of E2 is most likely mediated by ERβ, which has become a favorable therapeutic target due to its antiproliferative activity as opposed to ERα that promotes epithelial proliferation and has proestrogenic effects in the breasts and uterus (55, 56). Therefore, selective ERβ ligands could provide a novel treatment modality for patients with PH by minimizing estrogenic side effects, a concept that most definitely warrants further investigation.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants HL089876 and HL089876S1 (M.E).
Author contributions: Contributions to conception and design: M.E., S.U., A.v.d.L. Acquisition of data: S.U., A.I., H.M., R.D.N., F.M., J.L. Analysis and interpretation of data: S.U., R.D.N., M.E., A.I., F.M. Drafting the article or revising it critically for important intellectual content: M.E., S.U., A.v.d.L. All authors approved this version of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201101-0078OC on June 23, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani EH, Wagenaar GTM, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol 2009;297:H1606–H1616 [DOI] [PubMed] [Google Scholar]
- 2.Takemiya K, Kai H, Yasukawa H, Tahara N, Kato S, Imaizumi T. Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic Res Cardiol 2010;105:409–417 [DOI] [PubMed] [Google Scholar]
- 3.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol 2007;49:1566–1571 [DOI] [PubMed] [Google Scholar]
- 4.Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermal S, Ramwell PW. Protection by oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br J Pharmacol 1993;110:719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen CH, Leu S, Lin YC, Kao YH, Chang LT, Chua S, Fu M, Wu CJ, Sun CK, Yip HK. Sildenafil limits monocrotaline-induced pulmonary hypertension in rats through suppression of pulmonary vascular remodeling. J Cardiovasc Pharmacol 2010;55:574–584 [DOI] [PubMed] [Google Scholar]
- 6.Clozel M, Hess P, Rey M, Iglarz M, Binkert C, Qiu C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp Biol Med (Maywood) 2006;231:967–973 [PubMed] [Google Scholar]
- 7.Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, et al. The changing picture of pulmonary arterial hypertension (PAH) patients in the United States: how the REVEAL registry differs from historic and non-US contemporary registries. Chest 2011;139:128–137 [DOI] [PubMed] [Google Scholar]
- 8.Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol Heart Circ Physiol 1981;240:H62–H72 [DOI] [PubMed] [Google Scholar]
- 9.Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vascul Pharmacol 2006;45:358–367 [DOI] [PubMed] [Google Scholar]
- 10.Sweeney LB, Bogaard HJ, Natarajan R, Kraskauskas D, Voelkel NF. Female gender protects against angioproliferative pulmonary hypertension induced by VEGF receptor inhibition and hypoxic exposure. Am J Respir Crit Care Med 2009;179:A1826 [Google Scholar]
- 11.Xu DQ, Luo Y, Liu Y, Wang J, Zhang B, Xu M, Wang YX, Dong HY, Dong MQ, Zhao PT, et al. Beta-estradiol attenuates hypoxic pulmonary hypertension by stabilizing the expression of p27kip1 in rats. Respir Res 2010;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 2000;6:698–702 [DOI] [PubMed] [Google Scholar]
- 13.Umar S, Matori H, Iorga A, Partow-Navid R, Maltese F, Eghbali M. Estrogen rescues severe pulmonary hypertension and increases survival through stimulation of neoangiogenesis and suppression of inflammation [abstract]. Circulation 2010;122:A18969 [Google Scholar]
- 14.Umar S, Nadadur RD, Matori H, Partow-Navid R, Iorga A, Maltese F, Eghbali M. Complete reversal of cardiac and pulmonary remodelling associated with severe pulmonary arterial hypertension by estrogen therapy [abstract]. Am J Respir Crit Care Med 2011;183:A2522 [Google Scholar]
- 15.National Institute of Health guide for the care and use of laboratory animals 1996. NIH publication; 85–23 [Google Scholar]
- 16.Koskenvuo J, Mirsky R, Zhang Y, Angeli F, Jahn S, Alastalo TP, Schiller N, Boyle A, Chatterjee K, De Marco T, et al. A comparison of echocardiography to invasive measurement in the evaluation of pulmonary arterial hypertension in a rat model. Int J Cardiovasc Imaging 2010;26:509–518 (formerly Cardiac Imaging) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu HP, Hsieh YC, Suzuki T, Shimizu T, Choudhry MA, Schwacha MG, Chaudry IH. Salutary effects of estrogen receptor-beta agonist on lung injury after trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol 2006;290:L1004–L1009 [DOI] [PubMed] [Google Scholar]
- 18.Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, Robertson AD, Voelkel NF, et al. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest 1997;100:2315–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell AIM, Zhao Y, Sandhu R, Stewart DJ. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension. Circulation 2001;104:2242–2248 [DOI] [PubMed] [Google Scholar]
- 20.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol 2001;38:1137–1142 [DOI] [PubMed] [Google Scholar]
- 21.Lahm T, Crisostomo PR, Markel TA, Wang M, Weil BR, Novotny NM, Meldrum DR. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit Care Med 2008;36:2174–2183 [DOI] [PubMed] [Google Scholar]
- 22.Babiker FA, De Windt LJ, van Eickels M, Thijssen V, Bronsaer RJ, Grohe C, van Bilsen M, Doevendans PA. 17beta-estradiol antagonizes cardiomyocyte hypertrophy by autocrine/paracrine stimulation of a guanylyl cyclase A receptor-cyclic guanosine monophosphate-dependent protein kinase pathway. Circulation 2004;109:269–276 [DOI] [PubMed] [Google Scholar]
- 23.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, et al. Sex hormones are associated with right ventricular structure and function: The MESA-right ventricle study. Am J Respir Crit Care Med 2011;183:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 2003;22:358–363 [DOI] [PubMed] [Google Scholar]
- 25.Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol 2005;288:C881–C890 [DOI] [PubMed] [Google Scholar]
- 26.Brass DM, McGee SP, Dunkel MK, Reilly SM, Tobolewski JM, Sabo-Attwood T, Fattman CL. Gender influences the response to experimental silica-induced lung fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2010;299:L664–L671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 2010;298:R1597–R1606 [DOI] [PubMed] [Google Scholar]
- 28.Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol 2010;24:2152–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009;120:1951–1960 [DOI] [PubMed] [Google Scholar]
- 30.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 2005;115:2108–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodger FE, Young FM, Fraser HM, Illingworth PJ. Endothelial cell proliferation follows the mid-cycle luteinizing hormone surge, but not human chorionic gonadotrophin rescue, in the human corpus luteum. Hum Reprod 1997;12:1723–1729 [DOI] [PubMed] [Google Scholar]
- 32.Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-{alpha} in a rodent experimental stroke model. Stroke 2005;36:337–341 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Jin X, Zeng Z, Liu W, Wang B, Wang H. Estrogen-replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing SDF-1 and estrogen receptor expression. Microvasc Res 2009;77:71–77 [DOI] [PubMed] [Google Scholar]
- 34.Grohe C, Kahlert S, Lobbert K, Vetter H. Expression of oestrogen receptor alpha and beta in rat heart: role of local oestrogen synthesis. J Endocrinol 1998;156:R1–R7 [DOI] [PubMed] [Google Scholar]
- 35.Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohe C, Doevendans PA. Estrogen receptor beta protects the murine heart against left ventricular hypertrophy. Arterioscler Thromb Vasc Biol 2006;26:1524–1530 [DOI] [PubMed] [Google Scholar]
- 36.Wang M, Crisostomo PR, Markel T, Wang Y, Lillemoe KD, Meldrum DR. Estrogen receptor beta mediates acute myocardial protection following ischemia. Surgery 2008;144:233–238 [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 2002;295:505–508 [DOI] [PubMed] [Google Scholar]
- 38.Pelzer T, Loza PAA, Hu K, Bayer B, Dienesch C, Calvillo L, Couse JF, Korach KS, Neyses L, Ertl G. Increased mortality and aggravation of heart failure in estrogen receptor-{beta} knockout mice after myocardial infarction. Circulation 2005;111:1492–1498 [DOI] [PubMed] [Google Scholar]
- 39.Lahm T, Albrecht M, Fisher A, Justice M, Van Demark M, Patel N, Presson RG, Jr, Petrache I. 17-beta estradiol (E2) attenuates hypoxia-induced pulmonary hypertension through an estrogen receptor-dependent, but E2 metabolite-independent mechanism. Am J Respir Crit Care Med 2011;183:A2521 [Google Scholar]
- 40.Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen signaling via estrogen receptor {beta}. J Biol Chem 2010;285:39575–39579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De MT. Pulmonary arterial hypertension and women. Cardiol Rev 2006;14:312–318 [DOI] [PubMed] [Google Scholar]
- 42.Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol 2001;280:L88–L97 [DOI] [PubMed] [Google Scholar]
- 43.Homma N, Morio Y, Takahashi H, Yamamoto A, Suzuki T, Sato K, Muramatsu M, Fukuchi Y. Genistein, a phytoestrogen, attenuates monocrotaline-induced pulmonary hypertension. Respiration 2006;73:105–112 [DOI] [PubMed] [Google Scholar]
- 44.Nishida M, Hasegawa Y, Tanida I, Nakagawa E, Inaji H, Ohkita M, Matsumura Y. Preventive effects of raloxifene, a selective estrogen receptor modulator, on monocrotaline-induced pulmonary hypertension in intact and ovariectomized female rats. Eur J Pharmacol 2009;614:70–76 [DOI] [PubMed] [Google Scholar]
- 45.Morse JH, Horn EM, Barst RJ. Hormone replacement therapy: a possible risk factor in carriers of familial primary pulmonary hypertension. Chest 1999;116:847. [DOI] [PubMed] [Google Scholar]
- 46.Scorza R, Caronni M, Bazzi S, Nador F, Beretta L, Antonioli R, Origgi L, Ponti A, Marchini M, Vanoli M. Post-menopause is the main risk factor for developing isolated pulmonary hypertension in systemic sclerosis. Ann NY Acad Sci 2002;966:238–246 [DOI] [PubMed] [Google Scholar]
- 47.Beretta L, Caronni M, Origgi L, Ponti A, Santaniello A, Scorza R. Hormone replacement therapy may prevent the development of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Scand J Rheumatol 2006;35:468–471 [DOI] [PubMed] [Google Scholar]
- 48.Taraseviciute A, Voelkel NF. Severe pulmonary hypertension in postmenopausal obese women. Eur J Med Res 2006;11:198–202 [PubMed] [Google Scholar]
- 49.Tofovic PS, Zhang X, Petrusevska G. Progesterone inhibits vascular remodeling and attenuates monocrotaline-induced pulmonary hypertension in estrogen-deficient rats. Prilozi 2009;30:25–44 [PubMed] [Google Scholar]
- 50.Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS. Pulmonary vasodilatory action of testosterone: evidence of a calcium antagonistic action. J Cardiovasc Pharmacol 2002;39:814–823 [DOI] [PubMed] [Google Scholar]
- 51.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res 2001;33:645–652 [DOI] [PubMed] [Google Scholar]
- 52.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 2007;115:1275–1284 [DOI] [PubMed] [Google Scholar]
- 53.Pugh ME, Hemnes AR. Development of pulmonary arterial hypertension in women: interplay of sex hormones and pulmonary vascular disease. Womens Health (Lond Engl) 2010;6:285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Homma N, Nagaoka T, Karoor V, Imamura M, Taraseviciene-Stewart L, Walker LA, Fagan KA, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am J Physiol Lung Cell Mol Physiol 2008;295:L71–L78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 2004;64:423–428 [DOI] [PubMed] [Google Scholar]
- 56.Warner M, Gustafsson JK. The role of estrogen receptor beta (ERbeta) in malignant diseases–a new potential target for antiproliferative drugs in prevention and treatment of cancer. Biochem Biophys Res Commun 2010;396:63–66 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.