Abstract
Rationale: Although recent work has shown that CD34 plays an important role in the trafficking of inflammatory cells during Th2-biased inflammatory responses, its role in Th1/Th17-biased disease as well as dendritic cell (DC) trafficking is unknown.
Objectives: We used CD34-deficient mice (Cd34−/−) to investigate the role of CD34 in the Th1/Th17-biased lung inflammatory disease, hypersensitivity pneumonitis (HP).
Methods: HP was induced in wild-type (wt) and Cd34−/− mice by repeated intranasal administration of Saccharopolyspora rectivirgula antigen. Lung inflammation was assessed by histology and analysis of bronchoalveolar lavage cells. Primary and secondary immune responses were evaluated by cytokine recall responses of pulmonary inflammatory cells as well as draining lymph node cells.
Measurements and Main Results: Cd34−/− mice were highly resistant to the development of HP and exhibited an inflammatory pattern more reflective of a primary response to S. rectivirgula rather than the chronic lymphocytosis that is typical of this disease. Cytokine recall responses from Cd34−/− lymph node cells were dampened and consistent with a failure of antigen-loaded Cd34−/− DCs to deliver antigen and prime T cells in the draining lymph nodes. In agreement with this interpretation, adoptive transfer of wt DCs into Cd34−/− mice was sufficient to restore normal sensitivity to HP. CD34 was found to be expressed by wt DCs, and Cd34−/− DCs exhibited an impaired ability to chemotax toward a subset of chemokines in vitro. Finally, expression of human CD34 in Cd34−/− mice restored normal susceptibility to HP.
Conclusions: We conclude that CD34 is expressed by mucosal DCs and plays an important role in their trafficking through the lung and to the lymph nodes. Our data also suggest that CD34 may play a selective role in the efficient migration of these cells to a subset of chemokines.
Keywords: cell migration, chemotaxis, dendritic cell, mast cell, inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
Dendritic cells (DCs) are known to be important contributors to lung inflammatory diseases, such as hypersensitivity pneumonitis (HP), through their key role in antigen presentation. In order to present antigen in the lymph nodes and subsequently induce T-cell responses, these cells are required to undergo several important migratory steps, which are crucial to normal DC function.
What This Study Adds to the Field
We show here for the first time that the stem cell antigen CD34 is expressed by lung mucosal DCs and that its expression is needed for migration of DCs from the lung to the lymph nodes in response to the HP antigen Saccharopolyspora rectivirgula. We also show that loss of CD34 leads to protection from development of HP and that this molecule plays an important role in the transition from a primary to a chronic T-cell response in HP.
Antigen-presenting cells (APCs) play a crucial role in the development of T cell–dependent adaptive immune responses (reviewed in Reference 1). In response to an antigen challenge in the lung, dendritic cells (DCs) are recruited to the alveoli, where they acquire antigen and migrate into the lymphatic circulation in response to several chemokines, including CCL19 (2). In the lymph nodes, DCs mature and up-regulate the expression of the costimulatory molecules CD80 and CD86, which facilitate their ability to induce proliferation of antigen-specific T cells (1). Stimulation of T cells via mature mucosal APCs plays a critical role in chronic T-cell inflammatory response in a number of pathologies, including hypersensitivity pneumonitis (HP) (3).
HP is a Th17-dependent inflammatory lung disease caused by an exaggerated reaction to inhaled antigens such as Saccharopolyspora rectivirgula (SR) (4, 5, and reviewed in Reference 6). This pathology is characterized by a chronic lymphocytosis, a Th1/Th17-biased cytokine response involving cytokines such as IFN-γ (7) and IL-12 (8) and the production of antigen-specific IgGs (6). The steps leading to development of this disease in humans are not well understood. Previous studies have suggested that the costimulatory pathway between APCs and T cells is altered in patients with HP (3, 9). Indeed, it has been reported that alveolar macrophages isolated from patients with HP have elevated levels of the costimulatory molecules CD80 and CD86 (9), and that blockade of the CD80/86–CD28 costimulation pathway leads to protection from experimental HP (3). Moreover, the adoptive transfer of CD4+ T cells primed with the SR antigen has been shown to transfer disease to naive animals, confirming the role of the antigen-specific T-cell responses in the pathogenesis of HP (10). Finally, there is experimental evidence to suggest that maturation of DCs via a viral infection can lead to an HP-prone, hyperresponsive lung environment (11, 12), as viral infections before exposure to the SR antigen in mice leads to more severe HP (11).
CD34 is the founding member of a family of heavily glycosylated cell-surface sialomucins. Although CD34 is commonly used as a marker of hematopoietic stem/progenitor cells, recent literature has shown that it is also expressed by subsets of mature cells, including mast cells (13), eosinophils (14, 15), microglia (16), skin Langerhans cells (17), and fibrocytes (18). Although the lack of CD34 does not result in an overt phenotype in adult mice at steady state (19), profound phenotypes are exhibited under inflammatory conditions in CD34-deficient (Cd34−/−) mice. For example, we have found that CD34 is essential for efficient trafficking of mast cells and eosinophils, particularly during Th2-type inflammatory responses, and that Cd34−/− mice are resistant to the development of allergic asthma (15, 19) and eosinophil-dependent colitis (20). Little, however, is known of the role of CD34 during the priming phase of a mucosal immune response.
Here we have used a classic mouse model of HP and Cd34−/− mice to investigate whether CD34 plays a role in mast cell– and eosinophil-independent lung inflammatory disease. We find that Cd34−/− mice are resistant to the development of HP and, in contrast to wild-type (wt) mice, develop inflammatory responses more typical of a primary immune response even after repeated exposure to SR antigen. Consistent with this observation, we find that CD34 is normally expressed by lung DCs and that in the absence of CD34, DCs fail to traffic efficiently through the lung in response to antigen exposure. In support of the concept that this reflects a cell-intrinsic defect in DCs, we find that purified splenic Cd34−/− DCs exhibit a profound defect in chemotactic migration in vitro. Finally, we show that expression of a human CD34 transgene (hCD34) in Cd34−/− mice reestablishes their susceptibility to HP. Our data are consistent with a specific role for CD34 in DC/APC trafficking into and out of the lung and in facilitating the development of chronic T-cell responses in HP. They also suggest that CD34 is, potentially, a therapeutic target for human pulmonary inflammatory disease. Some of these results were presented in the form of an abstract (21).
Methods
Mice
Mice were bred and maintained in a pathogen-free environment at The Biomedical Research Center (University of British Columbia). All protocols were approved by the local ethics committee. Cd34−/− mice were a gift from Dr. Tak Mak and were routinely backcrossed to control C57Bl/6 (wt) mice. Human transgenic CD34 mice (hCD34) were obtained from Dr. Daniel Tenen, Beth Israel Hospital, Harvard University (22) and crossed onto the Cd34−/− colony to generate hCD34+/mCd34−/− mice. hCD34 transgene expression in Cd34−/− mice was verified by reverse transcriptase–polymerase chain reaction (RT-PCR), using the following primers: 5′-GGC AAC AGC TCA ACC CA-3′ and 3′-C AGG ATT TTG AAC CCT CCG-5′. Mast cell–deficient, WBB6F1/J-KitW/KitW (hereafter referred to as W/Wv) were purchased from Jax Laboratories (Bar Harbor, ME). Wt littermates from this F1 hybrid strain were evaluated as control animals in all HP experiments
Induction of HP and Assessment of Lung Inflammation
HP was induced as previously described (3, 11, 12, 23) by injecting 50 μl of 4 mg/ml endotoxin-free SR antigen intranasally three times a week for 3 weeks. Four days after the last intranasal instillation, mice were anesthetized with ketamine/xylazine and blood was collected by cardiac puncture. After blood collection, a bronchoalveolar lavage (BAL) was performed by three subsequent introductions and aspirations of 1.0 ml sterile phosphate-buffered saline (PBS). The BAL fluid was set aside and used to determine the BAL fluid/serum total protein ratio as an indicator of lung vascular leakage. Total BAL cells were counted and differential counts were determined from cytospin preparations stained with modified May-Grunwald Giemsa stain (HemaStain Set; Fisher Scientific, Kalamazoo, MI). The lungs were excised and either kept for histological analysis or minced in 200 U/ml collagenase IV (Sigma-Aldrich, Oakville, ON, Canada) in Hanks’ balanced salt solution. The collagenase digestion was performed for 45 minutes at 37°C, and the cell suspension was separated by centrifugation on a 30% Percoll density gradient to isolate the inflammatory cell population.
Histology
The lower left lung was collected and fixed in 10% formalin, embedded in paraffin, and cut longitudinally for histology sections. Slides were stained with hematoxylin and eosin and the severity of lung inflammation was assessed for the following criteria: (1) peribronchial infiltration, (2) parenchymal infiltration, (3) perivascular infiltration, (4) granulomas, and (5) general tissue damage. A score of 0 to 4 was blindly attributed to each criterion, for a possible total of 20 (0 = no sign of disease, 4 = maximum pathology).
Cytokine Measurements
Cells isolated either from the lungs or from the draining mediastinal lymph nodes of wt and Cd34−/− mice were plated at 500,000 cells/well in a 24-well plate in 1.0 ml RPMI supplemented with 10% fetal bovine serum (FBS)/1% penicillin/streptomycin. The specific recall immune responses were induced with increasing doses of SR antigen (0, 10, 100, and 200 μg/ml). Cytokine content in the supernatants was analyzed using a Cytokine Bead Array Inflammatory or Th1-Th2 cytokine kit (BD BioSciences, San Diego, CA) and samples were run on a FACS Calibur Machine.
Detection of SR-Specific IgGs
Plates were coated with the SR antigen (5 μg/ml) in carbonate coating buffer overnight at 4°C and washed with PBS-Tween 20 (0.05%). Serum and BAL fluid obtained from wt and Cd34−/− mice was added to the plates for 2 hours at 37°C, rinsed, and the SR-specific immunoglobulins were detected using biotin-conjugated rat anti-mouse mouse IgG2a (BD Pharmingen, SanDiego, CA), followed by streptavidin coupled to horseradish peroxidase, ABTS substrate (Sigma), and 2N H2SO4 as stopping solution. Absorbance was read at 450 nm using a standard spectrophotometer and compared between wt and Cd34−/− mice.
Identification and Culture of DCs and CD34 Expression
Cells were prepared from BAL, lung, and lymph node as described above and mouse bone marrow–derived DCs (BMDDC) were obtained via stimulation of bone marrow cells with 20 ng/ml mGM-CSF for 7 days. For the culture of human monocyte–derived DCs (MDDC), blood was obtained from healthy volunteers and the monocyte layer was isolated through Percoll gradient separation. Monocytes were cultured with 10 ng/ml hGM-CSF for 7 days, and MDDCs were later checked for CD11c expression by flow cytometry.
Single-cell suspensions were washed with PBS + 2% FBS, 2 mM ethylenediaminetetraacetic acid, and 0.5 mM sodium azide, and all subsequent steps were performed in this solution. Cells were blocked with anti-CD16/CD32 for 15 minutes at 4°C. Samples were subsequently stained with fluorophore-conjugated antibodies to: CD45, CD11c, CD11b, B220 (The Biomedical Research Center in-house antibody facility, Vancouver, BC), CD8a, CD80, CD86, MHC II (IAb), and mouse and human CD34 (BD Pharmingen, San Diego, CA) and examined using a FACS Calibur or LSRII instrument. DCs were identified as autofluorescence-negative, CD11c-positive, and MHCIIhi cells.
For detection of CD34 mRNA, total RNA was isolated from 2.5 × 105 BMDDC or FACS-sorted pulmonary DCs using Trizol reagent (Invitrogen Canada, Burlington, ON, Canada). RT was performed using the iScript cDNA synthesis kit according to the manufacturer's instructions (BIORAD, Mississauga, ON, Canada) and quantitative PCR performed using QuantiTect specific primers for detection of mouse CD34 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA (Qiagen, Montreal, PQ, Canada). ΔC(t) were obtained for CD34 and GAPDH mRNA expression and expression levels calculated and compared between wt and Cd34−/− mice using the Δ C(t) method (expression = 2(Ct(GAPDH)-(Ct(CD34)).
DC Adoptive Transfer
Flt3 ligand–producing melanoma cells were grown in vitro in complete RPMI media supplemented with 10% FBS and 1% penicillin/streptomycin. Next, 5 to 10 × 104 cells were subcutaneously injected in the lower back of wt C57Bl/6 mice or Cd34−/− mice. After tumor growth to approximately 1 cm diameter, mice were killed, spleens were excised, and DCs were isolated using a CD11c positive selection kit (Stem Cell Technologies, Vancouver, BC, Canada). The cell purity was verified and found to be between 96 and 98% DCs. Then 5 × 105 isolated DCs were intravenously injected into recipients 24 hours before the first intranasal instillation with the SR antigen. HP was then induced and analyzed as described above.
DC In Vivo and In Vitro Migration Essay
DC migration to the lymph nodes upon antigen acquisition was tested in vivo via intranasal administration of 50 μl fluorescein isothiocyanate (FITC)-coupled ovalbumin (OVA) (BD Pharmingen, San Diego, CA). The percentage of FITC+ myeloid (CD11b+) or nonmyeloid (CD11b−) DCs was evaluated in the cervical lymph nodes 18 hours after the administration of FITC-OVA via flow cytometry. Cervical lymph nodes were chosen for these experiments, (rather than mediastinal nodes) due to their accessibility in naive animals. For in vitro migration of DCs, CD11c+ splenic DCs were enriched from the spleens of wt and Cd34−/− mice injected with Flt3-ligand–producing melanomas and placed in the upper chamber of 24-well Biocoat Matrigel Invasion Chambers (BD Biosciences). Serial dilutions of CCL19 were added in the lower chamber as a chemoattractant. Chambers were incubated at 37°C + 5% CO2 for 18 hours. At the end of the incubation period, cells in lower chambers were recovered and evaluated. Latex beads were added to each sample for counting by flow cytometry.
Statistics
Statistical analysis was performed using an unpaired Student t test, comparing wt to Cd34−/− mice. Statistical significance was determined at P ≤ 0.05.
Results
CD34 Is Required for Lung Inflammation in Response to the SR Antigen
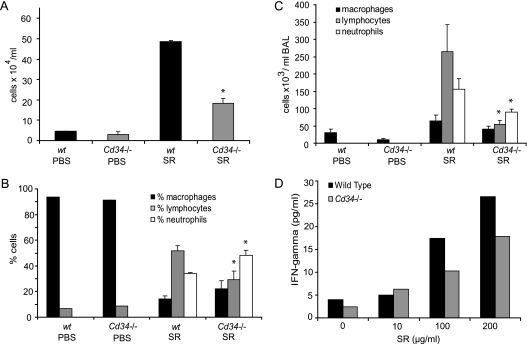
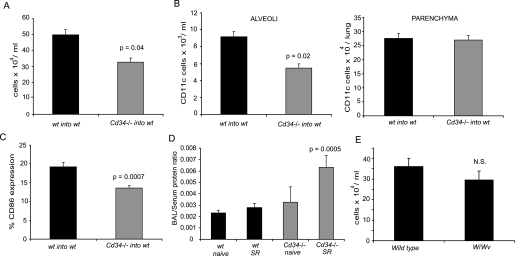
To test the role of CD34 in the development of Th1/Th17 lung inflammatory disease, we used a well-described Farmer's lung model of HP (3, 11, 12, 23). The development of pulmonary inflammation was first assessed through analysis of the BAL cellular content. As shown in Figures 1A–1C, the cells in the alveoli of naive animals (both wt and Cd34−/−) are almost exclusively resident alveolar macrophages. In response to the repeated inhalation of SR, wt mice exhibit a strong recruitment of inflammatory cells into the alveoli (Figure 1A) characterized by a lymphocyte infiltration (Figures 1B and 1C). In contrast, Cd34−/− mice challenged with the SR antigen consistently show reduced accumulation of total inflammatory cells compared with wt mice (P = 0.02), primarily reflected in reduced numbers of lymphocytes (P = 0.002) and neutrophils (P = 0.05). Although the numbers of all inflammatory cell subsets are reduced, when the frequencies of cell subsets are compared (Figure 1C), Cd34−/− mice consistently exhibit a lower frequency of lymphocytes (P = 0.02) and a compensatory increase in the frequency of neutrophils (P = 0.02) compared with wt mice. This skewed ratio of lymphocytes to neutrophils observed in the Cd34−/− mice is reminiscent of a wt mouse response during early priming in the HP model (24) and suggests that Cd34−/− mice fail to progress to the chronic lymphocytosis stage after repeated exposure to SR.
Figure 1.
Loss of CD34 expression leads to attenuated development of hypersensitivity pneumonitis (HP). (A) Total cells counts from bronchoalveolar lavage (BAL) of wild-type (wt) and Cd34−/− mice challenged with either phosphate-buffered saline (PBS) or with the Saccharopolyspora rectivirgula (SR) antigen. (B) Frequency of macrophages, lymphocytes, and neutrophils in BAL of wt and Cd34−/− mice. (C) Total number of macrophages, lymphocytes, and neutrophils in BAL of wt and Cd34−/− mice challenged with PBS or the SR antigen. (A–C) Data are presented as mean ± SEM and are representative of four independent experiments. (D) IFN-γ production by lung inflammatory cells in response to the SR antigen. Data are representative of three separate recall experiments. *P < 0.05, n = 6–7 mice per group.
The lung inflammatory cell cytokine recall response is an indicator of the activation status of the local inflammatory cells. To further characterize the pathology in Cd34−/− mice, inflammatory cells from the lungs were isolated and stimulated with increasing doses of SR in vitro to evaluate the antigen-specific IFN-γ production, a cytokine involved in development of HP (4). As shown in Figure 1D, the Cd34−/− inflammatory cells produce less IFN-γ in response to SR, supporting the conclusion that lung inflammation is lower in Cd34−/− mice compared with their wt counterparts (cells pooled from 5–6 mice, representative result of three separate HP experiments).
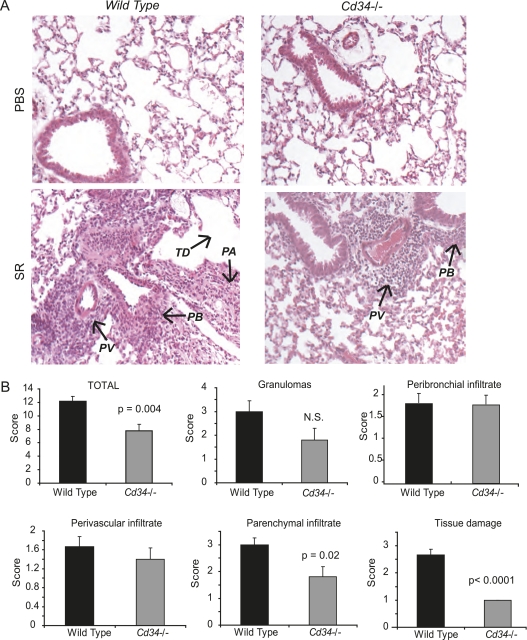
The reduced pulmonary inflammation in Cd34−/− mice was further confirmed via analysis of hematoxylin and eosin–stained lung sections (Figure 2), using a well-described method of lung histology analysis (3, 15, 25, 26). Briefly, slides were evaluated for the level of peribronchial, perivascular, and parenchymal (tissue and alveoli) infiltration (see Methods). The presence of granulomas as well as general tissue damage (damaged alveoli, damaged epithelium, overall quality of anatomic structures) were also evaluated. Administration of SR in wt mice induces a robust inflammatory cell infiltration, tissue damage, and granuloma formation. Although Cd34−/− mice exhibit similar levels of peribronchial and perivascular infiltration (Figure 2B), they show significantly less parenchymal infiltration (P = 0.02) and reduced tissue damage (P < 0.0001) for an attenuated total histological score (P = 0.004).
Figure 2.
Histological analysis of lungs from wild-type (wt) and Cd34−/− mice challenged with phosphate-buffered saline (PBS) or the Saccharopolyspora rectivirgula (SR) antigen. (A) Hematoxylin and eosin–stained lung sections obtained from wt and Cd34−/− mice challenged intranasally with PBS or the SR antigen. PA = parenchymal infiltrate; PB = peribronchial infiltrate; PV = perivascular infiltrate; TD = tissue damage. (B) Histological score obtained in four animals, in which the following parameters were evaluated on a scale of 1 to 4 (naive to most severe): parenchymal infiltrate, perivascular infiltrate, peribronchial infiltrate, tissue damage, presence of granulomas, and all of the above combined for the total histological score. Data are presented as mean ± SEM, and are representative of two separate experiments. n = 4 mice per group.
Mucosal DCs Express CD34
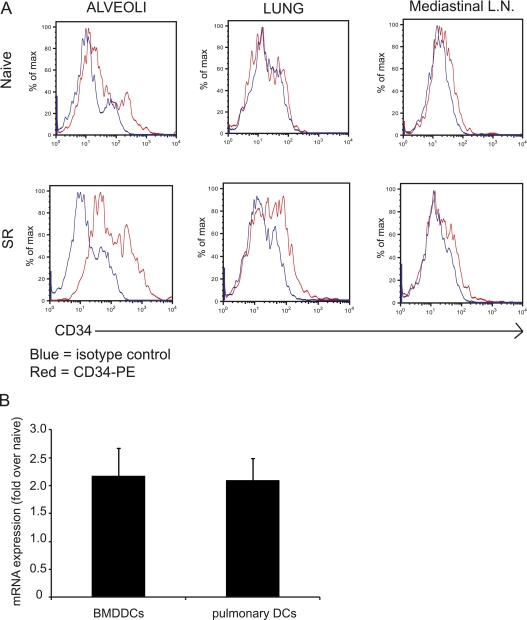
Although we and others have found CD34 to be expressed on mast cells and eosinophils (13–15), we have, to date, failed to detect CD34 on cell types known to be involved in Th1/Th17 inflammatory responses (such as macrophages, neutrophils, and lymphocytes). Because Cd34−/− mice are resistant to the development of HP, and because mucosal DC/APCs play an important role in HP pathogenesis, we evaluated CD34 expression on mucosal DCs isolated from the alveoli, lung tissue (parenchyma), and the draining mediastinal lymph nodes by flow cytometry. Mucosal DCs were identified as autofluorescence-negative, CD11c+/MHCIIhi cells, (as described in References 27–29). As shown in Figure 3A, in naive animals, alveolar DCs express low levels of CD34, whereas nonalveolar parenchymal DCs and DCs isolated from the lymph nodes are CD34-negative. Intriguingly, CD34 expression is up-regulated in response to the SR antigen on the alveolar DCs, mildly up-regulated on parenchymal DCs, and not up-regulated on lymph node DCs.
Figure 3.
CD34 expression on mucosal dendritic cells (DCs). (A) Flow cytometry histograms showing expression of CD34 on DCs (CD45+/CD11c+/MHCIIhi) cells isolated from either the bronchoalveolar lavage (BAL) (alveoli), lung, or mediastinal lymph nodes of mice that were exposed to Saccharopolyspora rectivirgula (SR) or vehicle (phosphate-buffered saline). These data are representative of four to five individual mice and of two independent experiments. (B) Relative CD34 mRNA expression found in SR-stimulated bone marrow–derived DCs (BMDDCs) and pulmonary DCs isolated from wild-type (wt) hypersensitivity pneumonitis (HP) mice. Results are expressed as fold of expression over naive cells.
The expression of CD34 on DCs was confirmed through detection of mRNA using quantitative RT-PCR (Figure 3B). PCR products for CD34 mRNA in unstimulated BMDDCs or naive lung-isolated DCs were detectable at 22 cycles and 30 cycles, respectively, with ΔC(t)s of 5.18 and 9.35 over GAPDH expression. Stimulation of BMDDCs with SR and exposure of mice to the SR antigen induced an approximately two-fold increase in mRNA expression compared with naive cells and naive mice (Figure 3B) (n = 3). As expected, no CD34 transcript was detected in Cd34−/− DCs (data not shown).
CD34 Deficiency Alters DC Emigration and Maturation in the Lung in Response to the SR Antigen
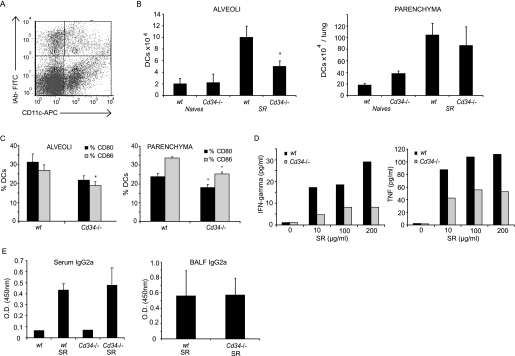
DC trafficking in the lung during inflammation is an intricate phenomenon involving several migratory steps, including migration from the blood to the parenchyma, the parenchyma to the alveoli, and finally the alveoli to the lymphatic endothelia to enter the lymphatic circulation and to reach the lymph nodes. Based on our observation that CD34 is expressed by lung DCs after inflammatory stimuli and previous work showing its importance in trafficking of mast cells and eosinophils to the lung (15), we tested whether CD34 expression affects the trafficking of DCs through the lung in HP. DCs were identified as autofluorescence-negative, CD11c+/MHCII (IAb)hi (Figure 4A), as previously described (27–29). We evaluated their recruitment to the alveoli and parenchyma based on the frequency of DCs and the total number of inflammatory cells in the BAL (alveoli) and lung parenchyma preparations (Figure 4B). We observed that lack of CD34 leads to a significant decrease in DC numbers in the alveoli (P = 0.009) but not in the lung parenchyma, where either equal numbers or, in some experiments, increased numbers of DCs are observed (for an example of increased numbers, see Figure 7). It is noteworthy that the number of DC progenitors in the bone marrow and blood is not altered in Cd34−/− mice compared with wt mice in response to the SR antigen (data not shown). Intriguingly, the maturation state of the CD11c+ cells (Figure 4C) present in the alveoli and lung tissue is altered in Cd34−/− mice compared with wt, as shown by lower expression of CD80, CD86, and MHCII (P < 0.05).
Figure 4.
CD34 and dendritic cell (DC) accumulation, maturation, and trafficking. (A) DCs were identified in the alveoli and parenchyma as autofluorescence-negative/CD11c+/MHCIIhi population (top right section). (B) Total numbers of DCs in the alveoli and parenchyma of wild-type (wt) and Cd34−/− mice. (C) CD80 and CD86 expression in alveolar and parenchymal DCs. (D) Tumor necrosis factor (TNF) and IFN-γ production measured in recall assays performed on lymph node cells. (E) Amount of Saccharopolyspora rectivirgula (SR)-specific IgG2a detected in the serum and bronchoalveolar lavage fluid (BALF) of wt and Cd34−/− mice. (A–C, E) Data are presented as mean ± SEM and are representative of four independent experiments. n = 4–6 mice per group. (D) Data are representative of three separate experiments performed on cells pooled from four to six mice in each experiment.
Figure 7.
Expression of human CD34 in Cd34−/− mice restores disease sensitivity. (A) Isotype control and (B) CD34 expression in unstimulated and (C) 50 μg/ml Saccharopolyspora rectivirgula (SR)-stimulated human monocyte–derived dendritic cells (DCs). (D) Total number of bronchoalveolar lavage (BAL) cells, (E) percentage of leukocyte subsets, and (F) total number of lymphocytes, macrophages, and neutrophils/ml found in the BAL of wild-type (wt), Cd34−/−, and Cd34−/− mice expressing the human CD34 gene (Cd34−/−/hCd34+). (G) Total number of DCs found in the alveoli and (I) parenchyma of wt, Cd34−/−, and Cd34−/−/hCd34+ mice. (H) Percentage of DCs in the parenchyma of wt, Cd34−/−, and Cd34−/−/hCd34+ mice. Data are presented as mean ± SEM, and are representative of three separate experiments. n = 6 mice per group. *P < 0.05.
Although some local antigen presentation can occur in the lung lymphoid follicles, the majority of antigen presentation and T-cell activation occurs in the draining mediastinal lymph nodes (reviewed in Reference 1). To test whether the trafficking of antigen-presenting cells to the draining lymph nodes is affected in Cd34−/− mice, we tested the antigen-specific recall response of wt and Cd34−/− mediastinal lymph node cells isolated from mice challenged with SR. We noted a dose-dependent, antigen-specific increase in the production of tumor necrosis factor and IFN-γ from wt mice (Figure 4D; representative experiment of three separate recall experiments), suggesting normal antigen presentation and T-cell priming in wt lymph nodes. In contrast, the cytokine response in Cd34−/− total lymph node cells is consistently lower than in wt cells. This is likely not due to a defect in Th1 or Th2 polarization in Cd34−/− mice, as we have observed that, on a per-cell basis, splenocytes from mice lacking CD34 expression can produce equal levels of IFN-γ, TNF, IL-10, and IL-5 in response to an anti-CD3 stimulation (unpublished observation). This is further supported by the fact that we detect equivalent levels of SR antigen-specific IgG2a in serum and BAL fluid of wt and Cd34−/− mice (Figure 4E) along with similar levels of B cells in the parenchyma (data not shown) suggesting that local antigen priming in lung lymphoid aggregates may occur normally and that the altered recall response in Cd34−/− draining lymph node cells is likely due to a lower trafficking of antigen-loaded DCs to the lymph nodes.
Taken together, our data suggest that CD34 is required for efficient trafficking of DCs through the lung and that DCs are retained in the lung parenchyma of Cd34−/− mice in response to SR rather than migrating to the lymph nodes to stimulate the antigen-specific T cell response.
CD34 Expression by Hematopoietic Cells Is Key to the Development of HP
CD34 is expressed on several hematopoietic lineages as well as on some of the non-hematopoietic microenvironmental cells (vascular and lymphatic endothelia, for example). We have previously shown that Cd34−/− animals have an intrinsic defect in vascular integrity (30). Therefore, the defective response to SR observed in Cd34−/− mice could, potentially, reflect an upstream defect in the vascular endothelial barrier function. To address this possibility, we generated bone marrow chimeric mice to differentiate between the contributions of the hematopoietic versus nonhematopoietic expression of CD34 in the protection observed in Cd34−/− mice. Briefly, wt Ly5.1 mice were lethally irradiated (1,200 rads) and reconstituted intravenously with 5 × 106 cells of Cd34−/− Ly5.2 bone marrow or wt Ly5.2 bone marrow. Reconstitution was verified 12 week post-transplant, and only mice with more than 90% chimerism were studied further. HP was induced in chimeric mice and the pulmonary inflammation was assessed as described above. We find that wt mice reconstituted with wt bone marrow develop a robust inflammatory response (Figure 5), whereas the response was attenuated in wt mice reconstituted with Cd34−/− bone marrow (P = 0.04). When the frequency and maturation state of DCs in the BAL and lung parenchyma of SR-challenged chimeric mice was assessed we found that, similar to mice with ubiquitous deletion of CD34, wt mice reconstituted with Cd34−/− bone marrow have lower numbers of DCs in the alveoli compared with wt mice reconstituted with wt bone marrow (Figure 5B) (P = 0.02), but similar or higher numbers of DCs in the parenchyma (Figure 5C). Finally, similar to ubiquitous Cd34−/− mice, DCs from wt mice reconstituted with Cd34−/− bone marrow express lower levels of CD86 compared with mice reconstituted with wt bone marrow. All in all, these results strengthen the conclusion that loss of CD34 on the hematopoietic compartment (and specifically on DCs) is responsible for the protective phenotype observed in Cd34−/− mice.
Figure 5.
Development of hypersensitivity pneumonitis (HP) in bone marrow chimeric and W/Wv mice. (A) Total cells found in the bronchoalveolar lavage (BAL) of mice transplanted with wild-type (wt) or Cd34−/− bone marrow and challenged with the Saccharopolyspora rectivirgula (SR) antigen. (B) Total number of CD11c+ cells found in the alveoli or parenchyma of bone marrow chimeric mice. (C) Percent of dendritic cells (DCs) expressing the maturation marker CD86 isolated from the parenchyma of bone marrow chimeric mice. (D) Lung vascular permeability in response to the SR antigen, as determined by total protein leakage in the alveoli and expressed as the BAL fluid/serum total protein ratio. (E) Total cells found in the BAL of mast cell–deficient W/Wv mice and their wt littermates challenged intranasally with the SR antigen. Data are presented as mean ± SEM and are representative of two separate experiments. n = 4–6 mice per group.
Nevertheless, because CD34 is expressed on the vascular endothelia and we have recently demonstrated increased vascular permeability in Cd34−/− mice in response to local inflammation (30), we tested whether these mice also exhibit any defects in vascular permeability in response to the SR antigen by calculating the BAL fluid/total serum protein ratio as an indicator of lung vascular leakage (31). In wt mice, SR induced an increase in vascular permeability compared with naive mice (Figure 5D). Consistent with our previous observations, vascular permeability in response to SR was higher in Cd34−/− mice (P = 0.0005). However, although we do detect greater vascular permeability when CD34 is deleted ubiquitously, our chimeric data suggest the protection of HP in Cd34−/− mice reflects a hematopoietic-specific lesion.
Mast Cells Are Not Required for Development of HP
Previously, we showed that CD34 is expressed on mast cells and that loss of this sialomucin leads to impaired trafficking of these cells, in vivo. Although mast cells have not previously been reported to play a significant role in the development of HP, we formally addressed this possibility by inducing HP in mast cell–deficient W/Wv mice (32), which have been used extensively to evaluate the importance of mast cells in a variety of diseases (33–35). We observe no significant changes in the total number of inflammatory cells (Figure 5E) or in the frequency of macrophages, lymphocytes, or neutrophils (data not shown) in W/Wv mice compared with their littermate control mice. This suggests that mast cells, as a lineage, are dispensable for development of HP and that loss of CD34 expression on mast cells could not account for the phenotype observed in Cd34−/− mice.
Adoptive Transfer of WT DCs into Cd34−/− Mice Restores Susceptibility to Disease
Because it is known that intravenously transferred DCs can localize to the lung shortly after transfer (36, 37), we reasoned that if trafficking of DCs in response to antigen is deficient in Cd34−/− mice, then addition of intravenous wt DCs before antigen exposure would restore susceptibility to disease. To generate large quantities of DCs for transplantation, Flt-3 ligand-producing B16 melanoma cells were injected in wt mice and tumors were allowed to grow for 2 weeks. As reported previously, this resulted in a potent, Flt-3 ligand-dependent expansion of tissue DCs (38). Splenic preparations were then enriched for CD11c+ cells, which were subsequently injected intravenously into wt or Cd34−/− mice 24 hours before the first challenge with the SR antigen.
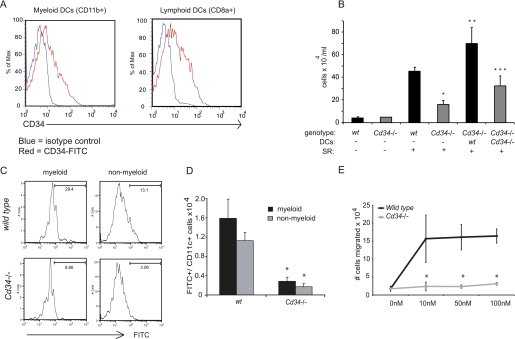
Before transfer, DC preparations were evaluated by flow cytometry and found to contain myeloid CD11b+ DCs as well as lymphoid CD8+ DCs, both expressing intermediate levels of CD34 (Figure 6A). Because the same subtypes are naturally found in the BAL and parenchyma of SR-treated mice, we believed that these would likely complement the defective DC subsets in Cd34−/− mice. As per previous experiments, we note that Cd34−/− mice develop a lower inflammatory cell infiltration in response to SR than wt control mice (Figure 6B). Interestingly, transfer of wt DCs into Cd34−/− mice induces a robust response to the SR antigen, whereas the response induced by the transfer of Cd34−/− DCs into Cd34−/− recipients was lower. This result indicates that the phenotype observed in Cd34−/− mice can be reversed solely by the addition of wt DCs before antigen exposure. This also suggests that expression of CD34 on DCs alone can account for the protective phenotype observed in Cd34−/− mice and that the defect observed in Cd34−/− mice likely occurs downstream of DC progenitor production in the bone marrow and recruitment to the peripheral blood.
Figure 6.
Adoptive transfer of wild-type (wt) dendritic cells (DCs) into Cd34−/− mice restores disease. (A) Representative example of CD34 expression in the two DC subtypes found in the splenic preparation (CD11b+ = myeloid; CD8+ = lymphoid). (B) Total inflammatory cells found in the bronchoalveolar lavage (BAL) of mice adoptively transferred with wt and Cd34−/− DCs. Data are presented as mean ± SEM and are representative of four separate experiments. n = 4–6 mice per group. *P < 0.05, Cd34−/− compared with wt. **P < 0.05, Cd34−/− + wt DCs compared with Cd34−/− alone. ***P < 0.05, Cd34−/− + wt DCs compared with Cd34−/− + Cd34−/− DCs. (C) Flow cytometry plots representing fluorescein isothiocyanate (FITC)+ myeloid (CD11b+) and nonmyeloid (CD11bneg)/CD11c+ lymph node cells. (D) Numbers of Cd11c+/FITC+ cells in the cervical lymph nodes isolated from wt and Cd34−/− mice. (E) Splenic DCs were derived from wt and Cd34−/− mice and examined for their ability to chemotax across matrigel-coated transwells in response to increasing concentrations of CCL19.
In Vivo Trafficking of DCs to the Lymph Nodes Is Impaired in Cd34−/− Mice
To directly address the hypothesis that DC trafficking to the lymph nodes on antigen uptake is impaired in the absence of CD34 expression, the in vivo trafficking of intranasally instilled OVA-FITC to the lymph nodes was evaluated in wt and Cd34−/− mice. Briefly, wt and Cd34−/− mice were challenged intranasally with OVA coupled to the FITC fluorochrome. Eighteen hours post OVA challenge, the number of FITC+ myeloid (CD11b+) and nonmyeloid (CD11blow/−) DCs were analyzed in the cervical lymph nodes (Figures 6C and 6D). Interestingly, we observed lower numbers of FITC+ myeloid (CD11b+) and nonmyeloid (CD11b−) DCs in the lymph nodes isolated from Cd34−/− animals compared with wt (Figure 6B), suggesting a defect in Cd34−/− DCs trafficking from the alveoli to the lymph nodes on antigen uptake. This was not due to an inability to uptake the antigen in the absence of CD34, because FITC+ DCs were found in the lung of Cd34−/− mice (data not shown). Taken together, these data suggest that CD34 is required for efficient trafficking from the alveoli to the lymph nodes.
In Vitro Chemotaxis Is Defective in Cd34−/− DCs
To test whether the impaired ability of Cd34−/− DCs to migrate to the lymph nodes in vivo reflected a cell-autonomous defect, we examined the chemotactic capabilities of purified wt and Cd34−/− DCs in vitro. Briefly, wt or Cd34−/− DCs were prepared from the spleens of mice as described in the Methods. These DCs were then placed in the upper chamber of Matrigel-coated transwells and monitored for their ability to migrate through these chambers in response to increasing doses of the DC chemotactic agent CCL19. We chose CCL19 for these assays because we found that lung CCL19 mRNA expression was up-regulated in the HP model (data not shown); moreover, this chemokine is specifically expressed in the lymphoid tissue (39), involved in DC trafficking from the lung to the lymph nodes, and increased in another interstitial lung disease (2, 40), which make it a candidate of choice for in vitro testing of DC chemotaxis from the lungs to the lymph nodes. As shown in Figure 6E, although wt DCs showed a clear dose-dependent ability to chemotax to CCL19, Cd34−/− DCs were found to be defective in their ability to migrate to this chemokine over a range of concentrations. Surprisingly, wt and Cd34−/− DCs showed an equal ability to chemotax to SDF-1/CXCL12 (data not shown). We conclude that the impaired ability of Cd34−/− DCs to traffic in vivo reflects a selective and cell-intrinsic defect in the ability to sense and appropriately respond to specific chemotactic signals.
Expression of Human CD34 Compensates for Lack of Mouse CD34
Because lack of CD34 in mice leads to attenuated inflammation in this model we hypothesized that human CD34 may be a general therapeutic target for dampening pulmonary inflammatory disease. To first determine whether CD34 is expressed by human DCs, blood was obtained from five healthy individuals and MDDCs were generated and tested for CD11c expression (data not shown) and found to be more than 85% positive for the DC marker. Results for CD34 expression on CD11c+ cells are presented in Figures 7A–7C. We observed that CD34 is expressed on MDDCs (as shown in Figure 7B), and, as previously observed in mouse DCs, is up-regulated upon in vitro stimulation with the HP antigen SR. Indeed, we observed an increase in the number of CD34-expressing cells as well as an increase in the mean fluorescence of CD34+ cells on exposure to SR (Figure 7C).
To test whether human CD34 plays a similar role to the mouse form of CD34 in the HP model, we crossed Cd34−/− animals to mice bearing a human genomic BAC clone (hCD34+ mice) carrying the entire human CD34 gene as well as the appropriate regulatory sequences required for the normal human expression pattern (22). These mice (hCD34+/mCd34−/−) were then challenged with the SR antigen to test whether expression of the human form of CD34 could reestablish sensitivity to HP. As presented in Figure 7D, we again observe that Cd34−/− mice develop milder inflammation compared with wt mice, characterized by a lower number of total cells in the BAL (Figure 7D) (P = 0.05), a lower percentage (Figure 7E) and total number of lymphocytes/ml (Figure 7F) (P = 0.007 for %; P = 0.009 for number of lymphocytes/ml; n = 6) and a higher percentage of neutrophils (P = 0.003). In contrast, hCD34+/mCd34−/− mice mount a response that is indistinguishable from wt mice, characterized by an equivalent total number of inflammatory cells as well as the expected lymphocytosis. This result suggests that the expression of hCD34 in mice can compensate for loss of mouse CD34.
Consistent with a role for human CD34 in DC trafficking, we noted that expression of hCD34 also reverses the blockade in DC recruitment to the alveoli observed in Cd34−/− mice (Figure 7H). As noted above, here and in previous experiments we observe an atypical accumulation of DCs (percentage and total number of cells Figures 7H and 7I) in the parenchyma of Cd34−/− mice, suggesting that Cd34−/− DCs tend to accumulate in the parenchyma rather than the lymph nodes. Intriguingly, this too was completely reversed by expression of the hCD34 transgene. Taken together, these data suggest that human CD34 is expressed by DCs, up-regulated on stimulation, facilitates DC trafficking, and acts in an equivalent manner to mouse CD34 in this model.
Discussion
We recently showed that CD34 plays a critical role in facilitating the trafficking of mast cells and eosinophils to the lung and thereby regulates the pathology associated with allergic asthma (15). Because eosinophils and mast cells play a more prominent role in Th2-skewed diseases and are not believed to have a major role in HP, we reasoned that Cd34−/− mice would respond in a similar manner to wt mice in this Th1/Th17-dependent disease. Surprisingly, we found that Cd34−/− mice were also protected from HP, and this led us to investigate whether CD34 is expressed by other cell types involved in development of HP, including lung DCs. This is, to our knowledge, the first report to document CD34 expression on mucosal DCs, and we provide clear data to suggest that CD34 affects trafficking of these cells into the lung and the subsequent pathology associated with this model of pulmonary inflammation.
Several lines of evidence suggest that protection in this model is due selectively to loss of CD34 on DCs and not loss on other hematopoietic or nonhematopoietic cell types. First, although we have previously shown that loss of CD34 on vascular endothelial cells leads to enhanced permeability during inflammation (30), this does not appear to be responsible for the attenuated inflammation observed here because transplantation of Cd34−/− hematopoietic cells into wt recipients is sufficient to transfer resistance. We also show that the phenotype is unlikely to be due to loss of CD34 from other CD34-expressing hematopoietic candidates like mast cells because our experiments in mast cell–deficient W/Wv mice suggests they are dispensable for this disease. Likewise, eosinophils are not observed during HP development, arguing against a role for these cells in this disease. Finally, we have shown that transfer of wt DCs into CD34-deficient animals is sufficient to restore susceptibility to disease. In aggregate, these data strongly argue that the lack of CD34 on DCs, specifically, is sufficient to attenuate inflammation in this model and that normal DC function is essential for the development of inflammation in HP.
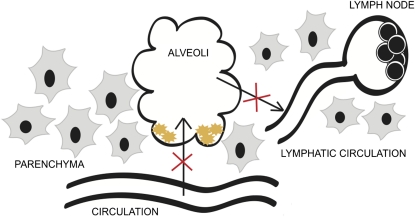
Our results are also consistent with a key role for CD34 in DC trafficking from the lungs to the lymph nodes. First, the fact that the expression level of CD34 (1) is higher on the alveolar and parenchymal DCs compared with those isolated from the lymph nodes, and (2) increases after challenges with the SR antigen would argue that this molecule is important in trafficking in response to antigen uptake. This is also supported by the fact that we consistently note equal or higher numbers of DCs in the lung parenchyma but normal numbers of bone marrow and blood DC progenitors. We therefore hypothesize that CD34 is up-regulated by DC activation, possibly on antigen capture, and thereby facilitates DC migration through the lung to the lymph nodes. Finally, we find CD34 is down-regulated on lymph node DCs, possibly reflecting a step in the maturation process to halt further migration (Figure 8).
Figure 8.
Schematic representation of the lesions in CD34−/− dendritic cell (DC) function in vivo. The red crosses indicate the hypothesized defect in Cd34−/− mice. Normally, DC precursors leave the circulation, enter the lung parenchyma, and subsequently cross the basement membrane and epithelia to enter the alveolar space. Upon antigen uptake these cells then traffic back into the lung parenchyma and enter the lymphatic circulation on their way to the draining lymph node for antigen presentation to T cells. Cd34−/− DCs fail to efficiently traffic from the alveolar space and migrate to the draining node and instead accumulate in the parenchyma. Likewise, recruited DCs from the circulation accumulate in the parenchyma and fail to enter the alveolar space in response to inflammation.
This model is supported by several additional observations, including the attenuated antigen-specific cytokine recall response in the draining mediastinal lymph nodes of Cd34−/− mice and the ability to override defective HP responses in Cd34−/− mice through transplantation of wt DCs. Finally, the observed inability of Cd34−/− DCs to efficiently carry FITC-labeled antigen from the alveoli to the lymph nodes strongly suggests that lack of CD34 expression alters the capacity of DCs to traffic efficiently from the lung to the lymph nodes. This would explain the accumulation of DCs observed in the parenchyma of Cd34−/− mice as well as the failure to induce chronic antigen-specific T-cell responses in Cd34−/− mice. Indeed, we consistently observe that the early neutrophilic response to SR in these mice fails to evolve into a T-cell alveolitis typical of the wt type disease.
Our in vitro data argue that CD34 plays a cell-intrinsic role in facilitating efficient DC chemotaxis, because the migration of Cd34−/− DCs to CCL19 is highly impaired. The fact that this occurs in vitro and in the absence of selectins (the only known potential ligand for CD34) would argue against a selectin-dependent adhesion effect and for a more subtle effect on chemokine gradient detection. Although CCL19 has also been reported to activate DC maturation (41), it is unlikely that Cd34−/− mice have an intrinsic defect in the ability to present antigen because we have previously shown that, when primed intraperitoneally, inflammatory cells isolated from Cd34−/− mice can produce cytokines in an antigen-specific fashion at normal levels in a mouse model of asthma (15). Similarly, in the present studies we detect wt levels of SR-specific IgG2a in the serum of Cd34−/− animals, suggesting that Cd34−/− antigen-presenting cells can efficiently present the antigen locally in lymphoid follicles. Taken together, our data suggest that CD34 expression on DCs is important for trafficking in response to an antigen challenge and is essential for the subsequent T-cell antigen-specific response.
It is noteworthy that several recent studies have also suggested a role for transmembrane sialomucins in increasing the fidelity of chemokine receptor signaling, and enhancing chemotaxis (42, 43). Indeed, the cell surface sialomucin CD164 enhances the ability of human hematopoietic precursor cells to migrate in response to CXCL12, through a coassociation between CD164 and the CXCL12 receptor (42). Similarly, we have shown that hematopoietic progenitors lacking podocalyxin, a close relative of CD34, also exhibit an impaired ability to migrate to CXCL12 (unpublished observation). Likewise, it was recently shown that P-selectin glycoprotein ligand 1 (PSGL-1) enhances the binding of CCL19 and CCL21 to T cells, thereby enhancing chemotaxis (43). In summary, the ability of hematopoietic sialomucins to enhance chemotaxis is an emerging theme in blood cell migration and the data presented here offer a further example of this concept. It will now be important to ascertain the precise lesion in signal transduction that leads to this defect.
Our results also suggest that targeting DCs, and specifically CD34 expression on DCs, could lead to efficient treatment of DC-dependent pulmonary inflammatory disease. Because the ectopic expression of human CD34 on the mouse Cd34−/− background leads to the complete reversal of the phenotype in response to the SR antigen, it is likely that the human form of CD34 acts in a similar fashion to modulate lung inflammation. This quality suggests that hCD34 could be an appealing target for treatment of human HP and possibly other lung inflammatory diseases, including allergic asthma (15). The fact that CD34 deletion does not lead to any obvious defect in mice during development, or at steady state in the adult, suggests that CD34-based therapies are likely to be well-tolerated and that the humanized CD34 mouse described here may provide an ideal model for evaluating the efficacy of such therapies.
Acknowledgments
The authors thank Anne Meriaux, Helen Merkens, Kay Jian, and Shannon Russell for their technical support; Andrew Johnson, David Marsolais, and Marc Veillette for their assistance in design and analysis of FACS data; and Les Rollin for expert animal care.
Footnotes
Supported by National Institutes of Health grant HL56745 (D.G.T.) and Canadian Institute for Health Research and AllerGen Network grants (K.M.M.). Salary support for M.-R.B. was provided by the Fonds de Recherche en Santé du Québec and Michael Smith Foundation for Health Research. Salary support for J.L.B. was provided by the Multiple Sclerosis Foundation of Canada. K.M.M. is a Michael Smith Foundation for Health Research Senior Scholar.
Author contributions: M.-R.B. and J.L.B.: design, experiments, analysis, and interpretation. M.J.G.: experiments and analysis. E.L. and D.G.T.: hCD34 transgenic mouse production and evaluation. M.G. and Y.C.: production of the SR antigen, analysis, and manuscript. K.M.M.: supervision, design, analysis, and interpretation.
Originally Published in Press as DOI: 10.1164/rccm.201011-1764OC on June 3, 2011
Author disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cook DN, Bottomly K. Innate immune control of pulmonary dendritic cell trafficking. Proc Am Thorac Soc 2007;4:234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibejova A, Mrazek F, Subrtova D, Sekerova V, Szotkowska J, Kolek V, du Bois RM, Petrek M. Expression of macrophage inflammatory protein-3 beta/CCL19 in pulmonary sarcoidosis. Am J Respir Crit Care Med 2003;167:1695–1703 [DOI] [PubMed] [Google Scholar]
- 3.Israel-Assayag E, Fournier M, Cormier Y. Blockade of T cell costimulation by CTLA4-Ig inhibits lung inflammation in murine hypersensitivity pneumonitis. J Immunol 1999;163:6794–6799 [PubMed] [Google Scholar]
- 4.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, Born WK, O'Brien RL, Fontenot AP. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol 2009;182:657–665 [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi AD, Fong DJ, Oak SR, Trujillo G, Flaherty KR, Martinez FJ, Hogaboam CM. Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am J Respir Crit Care Med 2009;179:705–716 [DOI] [PubMed] [Google Scholar]
- 6.Girard M, Israel-Assayag E, Cormier Y. Pathogenesis of hypersensitivity pneumonitis. Curr Opin Allergy Clin Immunol 2004;4:93–98 [DOI] [PubMed] [Google Scholar]
- 7.Gudmundsson G, Hunninghake GW. Interferon-gamma is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest 1997;99:2386–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudmundsson G, Monick MM, Hunninghake GW. IL-12 modulates expression of hypersensitivity pneumonitis. J Immunol 1998;161:991–999 [PubMed] [Google Scholar]
- 9.Israel-Assayag E, Dakhama A, Lavigne S, Laviolette M, Cormier Y. Expression of costimulatory molecules on alveolar macrophages in hypersensitivity pneumonitis. Am J Respir Crit Care Med 1999;159:1830–1834 [DOI] [PubMed] [Google Scholar]
- 10.Schuyler M, Gott K, Cherne A, Edwards B. Th1 CD4+ cells adoptively transfer experimental hypersensitivity pneumonitis. Cell Immunol 1997;177:169–175 [DOI] [PubMed] [Google Scholar]
- 11.Cormier Y, Israel-Assayag E, Fournier M, Tremblay GM. Modulation of experimental hypersensitivity pneumonitis by Sendai virus. J Lab Clin Med 1993;121:683–688 [PubMed] [Google Scholar]
- 12.Girard M, Israel-Assayag E, Cormier Y. Mature CD11c(+) cells are enhanced in hypersensitivity pneumonitis. Eur Respir J 2009;34:749–756 [DOI] [PubMed] [Google Scholar]
- 13.Drew E, Merkens H, Chelliah S, Doyonnas R, McNagny KM. CD34 is a specific marker of mature murine mast cells. Exp Hematol 2002;30:1211. [DOI] [PubMed] [Google Scholar]
- 14.Radinger M, Johansson AK, Sitkauskiene B, Sjostrand M, Lotvall J. Eotaxin-2 regulates newly produced and CD34 airway eosinophils after allergen exposure. J Allergy Clin Immunol 2004;113:1109–1116 [DOI] [PubMed] [Google Scholar]
- 15.Blanchet MR, Maltby S, Haddon DJ, Merkens H, Zbytnuik L, McNagny KM. CD34 facilitates the development of allergic asthma. Blood 2007;110:2005–2012 [DOI] [PubMed] [Google Scholar]
- 16.Ladeby R, Wirenfeldt M, Dalmau I, Gregersen R, Garcia-Ovejero D, Babcock A, Owens T, Finsen B. Proliferating resident microglia express the stem cell antigen CD34 in response to acute neural injury. Glia 2005;50:121–131 [DOI] [PubMed] [Google Scholar]
- 17.Erdag G, Qureshi HS, Patterson JW, Wick MR. CD34-positive dendritic cells disappear from scars but are increased in pericicatricial tissue. J Cutan Pathol 2008;35:752–756 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–389 [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Andrew DP, Gonzalo JA, Fukumoto M, Spellberg J, Hashiyama M, Takimoto H, Gerwin N, Webb I, Molineux G, et al. CD34-deficient mice have reduced eosinophil accumulation after allergen exposure and show a novel crossreactive 90-kD protein. Blood 1996;87:3550–3562 [PubMed] [Google Scholar]
- 20.Maltby S, Wohlfarth C, Gold M, Zbytnuik L, Hughes MR, McNagny KM. CD34 is required for infiltration of eosinophils into the colon and pathology associated with DSS-induced ulcerative colitis. Am J Pathol 2010;177:1244–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchet MR, Bennett J, Gold M, Girard M, Cormier Y, McNagny K. CD34 mediates dendritic cells trafficking in hypersensitivity pneumonitis [abstract]. Am J Respir Crit Care Med 2009;179:A4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuno Y, Iwasaki H, Huettner CS, Radomska HS, Gonzalez DA, Tenen DG, Akashi K. Differential regulation of the human and murine CD34 genes in hematopoietic stem cells. Proc Natl Acad Sci USA 2002;99:6246–6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanchet MR, Israel-Assayag E, Cormier Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am J Respir Crit Care Med 2004;169:903–909 [DOI] [PubMed] [Google Scholar]
- 24.Nance SC, Yi AK, Re FC, Fitzpatrick EA. MyD88 is necessary for neutrophil recruitment in hypersensitivity pneumonitis. J Leukoc Biol 2008;83:1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naus S, Blanchet MR, Gossens K, Zaph C, Bartsch JW, McNagny KM, Ziltener HJ. The metalloprotease-disintegrin ADAM8 is essential for the development of experimental asthma. Am J Respir Crit Care Med 2010;181:1318–1328 [DOI] [PubMed] [Google Scholar]
- 26.Blanchet MR, Israel-Assayag E, Cormier Y. Modulation of airway inflammation and resistance in mice by a nicotinic receptor agonist. Eur Respir J 2005;26:21–27 [DOI] [PubMed] [Google Scholar]
- 27.Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A 2004;61:170–177 [DOI] [PubMed] [Google Scholar]
- 28.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol 2010;185:3426–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest 2009;119:3723–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanchet MR, Gold M, Maltby S, Bennett J, Petri B, Kubes P, Lee DM, McNagny KM. Loss of CD34 leads to exacerbated autoimmune arthritis through increased vascular permeability. J Immunol 2010;184:1292–1299 [DOI] [PubMed] [Google Scholar]
- 31.Witzenrath M, Schmeck B, Doehn JM, Tschernig T, Zahlten J, Loeffler JM, Zemlin M, Muller H, Gutbier B, Schutte H, et al. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit Care Med 2009;37:642–649 [DOI] [PubMed] [Google Scholar]
- 32.Nakano T, Kanakura Y, Nakahata T, Matsuda H, Kitamura Y. Genetically mast cell-deficient W/Wv mice as a tool for studies of differentiation and function of mast cells. Fed Proc 1987;46:1920–1923 [PubMed] [Google Scholar]
- 33.Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, Ishizaka K. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J Immunol 2000;164:3855–3861 [DOI] [PubMed] [Google Scholar]
- 34.Bennett JL, Blanchet MR, Zhao L, Zbytnuik L, Antignano F, Gold M, Kubes P, McNagny KM. Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J Immunol 2009;182:5507–5514 [DOI] [PubMed] [Google Scholar]
- 35.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 2002;297:1689–1692 [DOI] [PubMed] [Google Scholar]
- 36.Koya T, Matsuda H, Takeda K, Matsubara S, Miyahara N, Balhorn A, Dakhama A, Gelfand EW. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J Allergy Clin Immunol 2007;119:1241–1250 [DOI] [PubMed] [Google Scholar]
- 37.Liu P, Li J, Yang X, Shen Y, Zhu Y, Wang S, Wu Z, Liu X, An G, Ji W, et al. Helminth infection inhibits airway allergic reaction and dendritic cells are involved in the modulation process. Parasite Immunol 2010;32:57–66 [DOI] [PubMed] [Google Scholar]
- 38.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res 2000;60:3239–3246 [PubMed] [Google Scholar]
- 39.Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol 1997;158:1033–1036 [PubMed] [Google Scholar]
- 40.Khader SA, Rangel-Moreno J, Fountain JJ, Martino CA, Reiley WW, Pearl JE, Winslow GM, Woodland DL, Randall TD, Cooper AM. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J Immunol 2009;183:8004–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR, Dietmeier K, Ivanova L, Pfister T, Vogt L, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity 2005;22:493–505 [DOI] [PubMed] [Google Scholar]
- 42.Forde S, Tye BJ, Newey SE, Roubelakis M, Smythe J, McGuckin CP, Pettengell R, Watt SM. Endolyn (CD164) modulates the CXCL12-mediated migration of umbilical cord blood CD133+ cells. Blood 2007;109:1825–1833 [DOI] [PubMed] [Google Scholar]
- 43.Veerman KM, Williams MJ, Uchimura K, Singer MS, Merzaban JS, Naus S, Carlow DA, Owen P, Rivera-Nieves J, Rosen SD, et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol 2007;8:532–539 [DOI] [PubMed] [Google Scholar]