Abstract
Most of the studies investigating the effectiveness of blocking the leukotriene B4 (LTB4) receptor 1 (BLT1) have been performed in models of primary or acute allergen challenge. The role of the LTB4-BLT1 pathway in secondary challenge models, where airway hyperresponsiveness (AHR) and airway inflammation have been established, has not been defined. We investigated the effects of blocking BLT1 on early- and late-phase development of AHR and airway inflammation in previously sensitized and challenged mice. Female BALB/c mice were sensitized (Days 1 and 14) and challenged (primary, Days 28–30) with ovalbumin. On Day 72, mice were challenged (secondary) with a single OVA aerosol, and the early and late phases of AHR and inflammation were determined. Specific blockade of BLT1 was attained by oral administration of a BLT1 antagonist on Days 70 through 72. Administration of the antagonist inhibited the secondary ovalbumin challenge–induced alterations in airway responses during the late phase but not during the early phase, as demonstrated by decreases in AHR and in bronchoalveolar lavage neutrophilia and eosinophilia 6 and 48 hours after secondary challenge. The latter was associated with decreased levels of KC protein, macrophage inflammatory protein 2, and IL-17 in the airways. These data identify the importance of the LTB4-BLT1 pathway in the development of late–phase, allergen-induced airway responsiveness after secondary airway challenge in mice with established airway disease.
Keywords: LAR, EAR, established asthma, BLT1 antagonist
Allergic asthma is a complex syndrome characterized by airway obstruction, airway inflammation, and airway hyperresponsiveness (AHR) (1, 2). Many investigations have reported that T cells and the CD4+ and CD8+ T cell subsets are central to the development of AHR and eosinophilic inflammation (1–9) through the production of cytokines, especially IL-13 (6–9).
In patients with allergic asthma, allergen challenge leads to an early asthmatic response (EAR), occurring within 15 to 30 minutes after allergen challenge. About 60% of patients develop a late asthmatic response (LAR), which occurs approximately 3 to 5 hours after allergen challenge and is characterized by airway obstruction and increased airway inflammation (10, 11). Similarly, in mice with established airway disease, allergen challenge can evoke an EAR and a LAR (12), followed by the development of sustained AHR (13). We have previously shown important differences when a primary challenge approach was compared with mice that had previously been sensitized and challenged and later provoked with a single airway challenge (secondary challenge) (13). It has been shown that neutrophils increase in bronchoalveolar lavage (BAL) fluid 6 hours after provocation, whereas eosinophils increase 48 hours after provocation (14).
Leukotriene B4 (LTB4) is a proinflammatory lipid mediator that is derived from membrane phospholipids (15–17). LTB4 activates leukocytes through a G protein–coupled cell surface receptor, BLT1, and as a major chemoattractant results in granulocyte and macrophage accumulation at sites of inflammation (18–21). We and others have recently shown that the LTB4-BLT1 pathway may be important in effector T-cell movement to sites of acute inflammation, critical to the development of AHR and inflammation (22–29). However, the role of the LTB4-BLT1 pathway on established asthma, including EAR and LAR, has not been elucidated.
The aim of the present study was to evaluate the role of LTB4 on airway function and lung inflammation in established disease, after provocative allergen challenge in previously sensitized and challenged mice, monitoring EAR and LAR. To assess LAR, we investigated allergen-induced airway obstruction, AHR, and airway inflammation 6 hours after secondary challenge when neutrophilic inflammation was predominant in the alveolar space. We then evaluated the development of AHR and inflammation 48 hours after secondary challenge when increased eosinophilic inflammation was observed. Although EAR was not affected, blocking the LTB4-BLT1 pathway significantly suppressed LAR, including AHR and inflammation, indicating that controlling the LTB4-BLT1 pathway could provide a novel therapeutic approach for the treatment of established asthma.
Materials and Methods
An expanded description of methods can be found in the online supplement.
Animals
Female BALB/c mice were maintained on ovalbumin (OVA)-free diets and under a protocol approved by the Institutional Animal Care and Use Committees of the Okayama University (Okayama, Japan) and National Jewish Health (Denver, CO).
Experimental Protocol: Sensitization and Airway Challenge
Mice (8–10 wk old) were sensitized and challenged to OVA as described previously (12). In the secondary challenge protocol, to assess EAR, mice were exposed to OVA (5% in saline) for 5 minutes 6 weeks after the primary challenge was completed, and assessments of airway function were begun immediately (12). To assess LAR, 6 weeks after the primary challenge, mice were exposed to a single OVA challenge (1% in saline), and lung resistance (RL), airway reactivity, and tissues were assessed 6 or 48 hours later (12).
Administration of the LTB4 Receptor Antagonist
The LTB4 receptor (BLT1) antagonist (CP105,696; provided by Pfizer Pharmaceuticals, New York, NY) (30) or vehicle was administered by gavage at 50 mg/kg (100 μl of carboxy-methyl cellulose) (Abbott Laboratories), 48, 24, and 2 hours before the secondary challenge.
Determination of Airway Function
A FlexiVent small-animal ventilator (SCIREQ, Montreal, PQ, Canada) was used to assess airway function (31,32). For EAR, animals were provoked with OVA (5% in saline) for 5 minutes, and RL was measured every minute for 60 minutes. For LAR, RL 6 hours after secondary OVA challenge was measured. Airway responsiveness was assessed as a change in airway function after challenge with aerosolized methacholine (MCh). Maximum values of RL were recorded and expressed as percent change from baseline after saline aerosol. There were no significant differences in baseline values among the different groups.
BAL
After assessment of AHR, lungs were lavaged via the tracheal tube with Hanks' balanced salt solution (2 × 1 ml; 37°C). The number of cells in the BAL fluid was determined. Cytospin slides were stained and differentiated in a blinded fashion by counting at least 300 cells under light microscopy
Histochemistry
Lungs were fixed by inflation (1 ml) and immersion in 10% formalin. For detection of mucus-containing cells in formalin-fixed airway tissue, sections were stained with periodic acid-Schiff (PAS) and H&E and quantitated as previously described (25).
Lung Cell Isolation
Lung cells were isolated as previously described following collagenase digestion (25).
Measurement of Cytokines and Chemokines
Cytokine levels in the BAL fluid, cell culture supernatants and lung homogenates were measured by ELISA as previously described (25). The limits of detection were 4 pg/ml for IL-4, IL-5, IL-13, 5 pg/ml for IL-17, 2 pg/ml for KC, and 1.5 pg/ml for macrophage inflammatory protein (MIP)-2.
Measurement of Total and OVA-Specific Antibody
Serum levels of Ig were measured by ELISA.
Statistical Analysis
All results were expressed as the means ± SEM. ANOVA was used to determine the levels of difference between all groups. Pairs of groups of samples distributed parametrically were compared by unpaired two-tailed Student t test, and samples distributed nonparametrically were compared by Mann-Whitney U test. Significance was assumed at P values of < 0.05.
Results
The Early Asthmatic Response Is Not Abolished by Blocking the LTB4-BLT1 Pathway
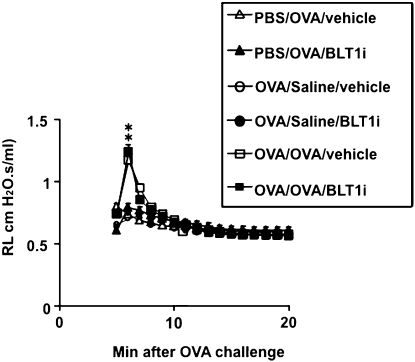
After exposure to 5% OVA, previously (6 wk earlier) sensitized and challenged mice developed an EAR. Increases in RL reached a maximum around 7 minutes after OVA challenge and returned to baseline 20 minutes after challenge (Figure 1). This early increase in RL was seen in mice that were previously sensitized and challenged and then secondarily challenged mice with allergen but was not seen in nonsensitized mice or mice sensitized and challenged but secondarily challenged with saline. Sensitized and challenged mice treated with the BLT1 antagonist showed the same early RL increase as the mice treated with vehicle (Figure 1).
Figure 1.
Altered airway function in the early asthmatic response. All groups were exposed to secondary challenge with 5% ovalbumin (OVA), and changes in lung resistance (RL) were monitored. Administration of the BLT1 antagonist was as described in Materials and Methods. n = 12 in each group. Means ± SEM are shown. *P < 0.05. PBS/OVA/vehicle PBS/OVA/antagonist versus OVA/OVA/vehicle OVA/OVA/antagonist. PBS/OVA = nonsensitized and challenged. OVA/Saline = sensitized and challenged and secondary challenged with saline. OVA/OVA = sensitized and challenged.
The LAR Is Reduced by Blocking the LTB4-BLT1 Pathway 6 Hours after Secondary Challenge
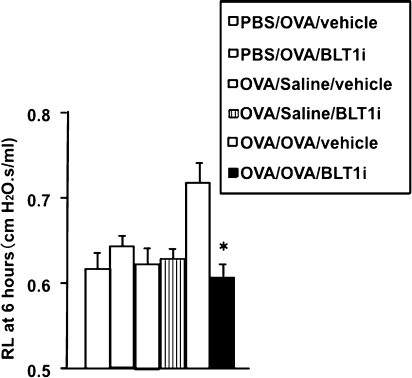
To assess the LAR, mice were rechallenged with OVA. Six hours after OVA challenge, mice previously sensitized and challenged to OVA and treated with vehicle developed allergen-induced alterations in airway function, as shown by increased RL compared with nonsensitized but OVA-challenged mice or sensitized and challenged and secondarily saline-challenged mice (Figure 2). In contrast, sensitized and challenged mice treated with the BLT1 antagonist did not develop an LAR (Figure 2).
Figure 2.
Altered airway function during the late asthmatic response. Previously sensitized and challenged mice were exposed to secondary allergen challenge, and changes in RL were monitored 6 hours later. n = 12 in each group. Means ± SEM are shown. *P < 0.05 compared with OVA/OVA/vehicle group.
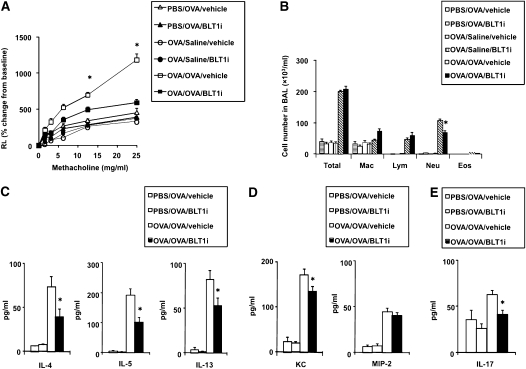
Previously sensitized and challenged mice showed an increase in AHR 6 hours after secondary allergen challenge (Figure 3A). Under these conditions, BLT1 antagonist treatment prevented the increases in AHR at this time point (Figure 3A).
Figure 3.
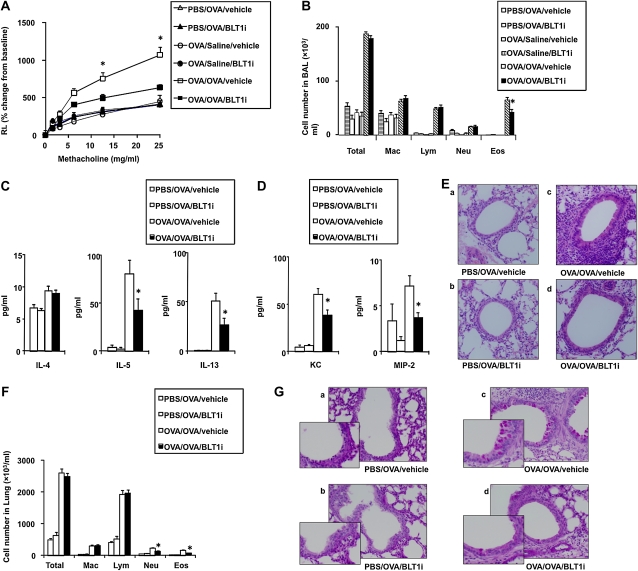
BLT1 antagonist reduces AHR and airway inflammation 6 hours after secondary challenge. (A) RL was measured in sensitized and challenged mice 6 hours after the secondary provocation with OVA. n = 12 in each group. *P < 0.05 compared with all other groups. (B) Cellular composition in bronchoalveolar lavage (BAL) fluid. (C) BAL cytokine levels. (D) BAL chemokine levels. (E) BAL IL-17 levels. *P < 0.05 compared with OVA/OVA/vehicle.
The number of neutrophils has been shown to increase in BAL fluid 6 hours after secondary challenge, whereas eosinophils increase in lung tissue 48 hours after secondary challenge (13, 14). LTB4 is thought to play an important role in the activation and recruitment of leukocytes (18–21). In sensitized mice, inflammatory cell accumulation in the BAL fluid was increased after secondary allergen challenge (Figure 3B). The increase in total cell numbers was largely due to increased numbers of neutrophils and lymphocytes in the BAL fluid; few eosinophils were seen (Figure 3B). Administration of the BLT1 antagonist at the time of secondary challenge led to a significant (P < 0.05) decrease in neutrophil numbers (Figure 3B).
Six hours after secondary allergen challenge, BAL fluid and lung homogenates were obtained to assess levels of cytokines and neutrophil-related chemokines. After secondary challenge, levels of IL-13, IL-4, and IL-5 were increased in previously sensitized and challenged mice treated with vehicle compared with challenged-only mice (Figure 3C). Treatment with the BLT1 antagonist significantly reduced the levels of these cytokines in BAL fluid (Figure 3C).
Levels of KC and MIP-2 in BAL fluid were also increased in vehicle-treated sensitized and challenged mice, and treatment with the antagonist significantly reduced the level of KC but not of MIP-2 (Figure 3D). IL-17 was not detectable in the BAL fluid (data not shown); therefore, IL-17 levels in lung homogenates were assessed. Levels of IL-17 in lung homogenates were increased in sensitized and challenged mice treated with vehicle, and the BLT1 antagonist significantly inhibited IL-17 production (Figure 3E).
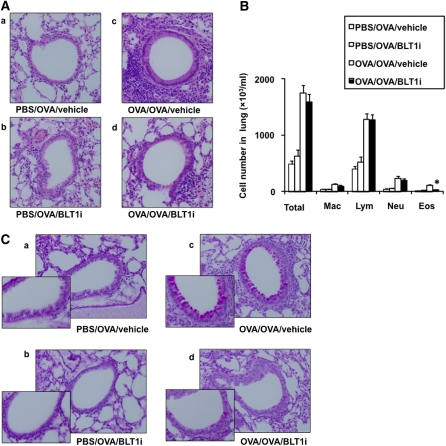
In previous studies, development of the LAR was associated with inflammatory changes in lung tissue (12). To determine if blocking of the LTB4-BLT1 pathway affected inflammatory changes in the lung, we assessed tissue inflammation 6 hours after 1% OVA challenge. Lung tissue was stained with H&E and PAS. To quantify the numbers of leukocytes in the lung, lung tissue was digested, and the number of leukocytes was determined. At 6 hours, there was a slight increase in peribronchial inflammation observed in sensitized and challenged animals compared with the nonsensitized mice, and this inflammatory response was decreased in mice treated with the BLT1 antagonist (Figure 4A). The number of eosinophils was increased after OVA sensitization and challenge, and treatment with the BLT1 antagonist reduced this number (Figure 4B).
Figure 4.
(A) Effect of BLT1 antagonist treatment on tissue inflammation and goblet cell metaplasia in sensitized and challenged mice 6 hours after secondary allergen challenge. (B) Eosinophil quantitation. *P < 0.05 compared with OVA/OVA/vehicle. (C) PAS-stained sections 6 hours after secondary allergen challenge.
In tissue assessed after PAS staining, at 6 hours, challenged-only mice showed no PAS-positive cells, whereas sensitized and challenged mice treated with vehicle showed goblet cell metaplasia (Figure 4C). Few PAS-positive cells were detected in mice treated with the BLT1 antagonist.
Development of Allergic Airway Responses 48 Hours after Secondary Challenge Is Reduced by Blocking the LTB4-BLT1 Pathway
We previously demonstrated that secondary allergen challenge induces a strong inflammatory reaction with development of AHR (13). Secondary allergen challenge in previously sensitized and challenged mice elicited an increase in AHR (Figure 5A). Under these conditions, treatment with the BLT1 antagonist also prevented the increases in AHR. At this time point, increased total cell numbers were largely due to increased numbers of eosinophils and lymphocytes in BAL (Figure 5B). Administration of the BLT1 antagonist at the time of the secondary challenge led to a significant decrease in eosinophil numbers in BAL fluid (Figure 5B). In parallel to the increases in AHR and airway eosinophilia, levels of IL-4, IL-5, and IL-13 were increased, and treatment with the inhibitor significantly reduced the levels of IL-5 and IL-13 (Figure 5C). Similarly, KC and MIP-2 levels in BAL fluid were increased, and treatment with the BLT1 antagonist reduced these levels (Figure 5D).
Figure 5.
BLT1 antagonist reduces AHR and airway inflammation 48 hours after secondary challenge. (A) RL was measured in sensitized and challenged mice 48 hours after secondary OVA provocation. *P < 0.05 compared with all other groups. (B) Cellular composition in BAL fluid. (C) BAL cytokine levels. (D) BAL chemokine levels. (E) H&E staining. (F) Lung cell composition. (G) Periodic acid-Schiff staining. *P < 0.05 compared with OVA/OVA/vehicle. n = 12 in each group. *P < 0.05 compared with OVA/OVA/vehicle.
Increased peribronchial and perivascular inflammatory infiltrates as well as increased numbers of PAS-positive cells were seen in secondary allergen–challenged mice, and treatment with the BLT1 antagonist reduced the numbers of these cells. (Figures 5E–5G).
Blocking BLT1 Does Not Alter Antigen-Specific Antibody Production
Serum Ig levels in mice exposed to secondary allergen challenge were elevated compared with nonsensitized control mice (Table 1), and treatment with the BLT1 receptor antagonist did not significantly alter levels of total IgE, OVA-specific IgE, or IgG1 (Table 1).
TABLE 1.
CONCENTRATIONS OF TOTAL IMMUNOGLOBULIN E AND OVALBUMIN-SPECIFIC ANTIBODIES IN THE SERUM*
| PBS/OVA/vehicle | PBS/OVA/ BLT1i | OVA/OVA/vehicle | OVA/OVA/ BLT1i6 | |
| Total IgE, ng/ml | 1814 ± 390† | 1618 ± 483 | 4101 ± 358‡ | 4167 ± 565‡ |
| OVA-specific IgE, mU/ml | <10 | <10 | 30.6 ± 9.0‡ | 26.8 ± 8.8‡ |
| OVA-specific IgG1, EU/ml | <10 | <10 | 1681 ± 119‡ | 1524 ± 97‡ |
Definition of abbreviations: mU/ml = ELISA mUnits/ml; OVA/OVA/BLT1i = sensitized and challenged mice followed by treatment with BLT1 antagonist; OVA/OVA/vehicle = sensitized and challenged mice followed by vehicle treatment; PBS/OVA/BLT1i = nonsensitized but challenged mice followed by treatment with BLT1 antagonist; PBS/OVA/vehicle = nonsensitized but challenged mice followed by treatment with vehicle.
Mice were sensitized and challenged as described in Materials and Methods. Serum levels of immunoglobulins were assessed 48 h after the last challenge.
Mean ± SEM.
P < 0.05. PBS/OVA/vehicle PBS/OVA/BLT1i vs. OVA/OVA/vehicle OVA/OVA/BLT1i (n = 12 in each group).
Discussion
In humans, the measurement of early- and late-phase airway responses after allergen challenge is often used to assess the effectiveness of treatment interventions (33, 34). Experimental models of asthma in mice can also demonstrate early- and late-phase airway responses to inhaled allergen and can be used to assess mechanisms and interventions (11, 35). In the present study, we evaluated the role of the LTB4-BLT1 pathway in the development of allergen-induced EAR and LAR in previously sensitized and challenged mice after secondary allergen challenge. We demonstrated that oral treatment with a specific antagonist of the high-affinity LTB4 receptor, BLT1, effectively prevented development of the LAR but not the EAR after provocative (secondary) allergen challenge.
Blockade of BLT1 was achieved after systemic administration of a specific antagonist that was previously shown to be effective in preventing the development of AHR in a similar model (28) as well as in a primate model (36). Using BLT1-deficient mice, we and others have also shown that the absence of expression of BLT1 prevents the development of AHR and Th2-type responses (25, 26). We have also shown that recruitment of antigen-specific effector CD8+ T cells to the airway requires an intact LTB4-BLT1 pathway (24, 28). These findings clearly demonstrated that BLT1 was involved in the initial development of asthma. However, the role of the LTB4-BLT1 pathway in established asthma has not been defined. Here, we examined the importance of LTB4 in the development of the EAR and LAR and the associated neutrophilic and eosinophilic airway inflammation in established asthma and showed for the first time that blocking the LTB4-BLT1 pathway significantly suppressed allergic airway responses in established airway disease. Although the EAR was unaffected, blockade of BLT1 significantly decreased the number of neutrophils in the BAL fluid and eosinophils in the lung 6 hours after secondary challenge, and this was associated with decreases in the neutrophil-related chemokines KC, MIP-2, and IL-17, as well as the Th2-type cytokines IL-5 and IL-13. Thus, interfering with the LTB4-BLT1 pathway may have important treatment implications in established disease.
In humans, similar to the mouse, the LAR to inhaled antigen is associated with airway inflammation and increased AHR (37). The cellular response is characterized by increased migration of eosinophils to the lung tissue and neutrophils in the BAL fluid (12, 14, 38). Eosinophils are thought to play an important role in the development of the LAR, and the altered airway function that develops in the LAR together with eosinophilic inflammation can be suppressed by treatment with neutralization of IL-5 or IL-13, cromoglycates, or corticosteroids (12, 39). Neutrophils may also contribute to the development of asthma. In human asthma, increased numbers of neutrophils were found in the lungs of patients with fatal asthma (40, 41). Nagata and colleagues have shown that neutrophils enhance the transbasement membrane migration of eosinophils in vitro; therefore, activation of neutrophils may enhance the accumulation of eosinophils in the airways, sustaining allergic inflammation (42–44). LTB4 is thought to play an important role in the activation and recruitment of leukocytes, including neutrophils (45). Based on these findings and the results of our studies, chemoattraction and activation of neutrophils through LTB4-BLT1 may contribute at least in part to allergic airway inflammation in established asthma. Mast cells are a source of LTB4 in models of mast cell–dependent allergic airway responses (46). However, in mast cell–independent models and in models of established disease, the source of LTB4 is not established. Because granulocytes are a major source of LTB4 (47) and LTB4 may further stimulate 5-lipoxygenase activity in these cells (48), it thus is possible that increased neutrophil recruitment into the airways amplifies LTB4 production and cellular activation.
Th17 cells producing IL-17 have been shown to mediate a neutrophil-dominant, steroid-resistant airway response (49). In some studies, IL-13 induced neutrophil recruitment (9) and activation (50) in the airways, and neutrophil recruitment may be directly associated with goblet cell metaplasia (50). In the present study, IL-13 levels in BAL fluid and IL-17 levels in lung homogenates were significantly decreased after administration of the BLT1 antagonist before secondary allergen challenge.
It has been reported that blockade of LTB4 did not suppress AHR in human asthma, although it suppressed neutrophilic inflammation in the BAL fluid (51). In this clinical study, the number of patients enrolled was small, and the LTB4 antagonist used was different from that in the current study. The level of disease severity in the treated patients and the extent to which BLT1 blockade was achieved in vivo were not defined. We have recently shown that the LTB4-BLT1 pathway may contribute to severe asthma, including steroid-resistant asthma with increased numbers of BLT1-expressing CD8+ T cells (52).
We have shown that not only CD4+ T cells but also CD8+ T cells play a pivotal role in the development of AHR and airway inflammation (7) and that BLT1 expression on effector memory CD8+ T cells was essential to development of these lung allergic responses (24, 26). ERK1/2 controls effector CD8+ T-cell–mediated allergic responses (53). Migration of neutrophils was also dependent on ERK1/2 (54). Therefore, control of ERK1/2 may suppress not only BLT1-effecor CD8+ T-cell–mediated allergic airway responses but also BLT1-mediated neutrophilic responses in the airways. Controlling LTB4-BLT1 activation through ERK1/2 signaling may provide an approach to control BLT1 expression.
In the present study, blockade of LTB4 had no effect on the early airway response. Previous studies have shown that the early response after allergen challenge was dependent on the presence of allergen-specific IgG (35) and could be abolished using β2-adrenoceptor antagonists or cromoglycates (12, 39). Blockade of the LTB4-BLT1 pathway did not affect serum levels of allergen-specific antibodies because sensitization was completed before intervention, which may explain the failure to alter development of the EAR.
In summary, our results demonstrate that the LTB4-BLT1 pathway is important to the development of the LAR after provocative allergen challenge in mice with established allergic disease. In addition, we show the importance of LTB4 in the accumulation of neutrophils and eosinophils in the airways during the LAR. The data suggest that manipulating the LTB4-BLT1 pathway may be beneficial in the treatment of established asthma.
Supplementary Material
Acknowledgments
The authors thank Diana Nabighian (National Jewish Health) for assistance in preparation of the manuscript.
Footnotes
This work was supported by NIH grants HL-36577, HL-61005, and AI-77609 and EPA grant R825702 (E.W.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NHLBI. N.M. was supported in part by a grant from the Ministry of Education, Science and Culture of Japan, and NOVARTIS Foundation for the Promotion of Science.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0455OC on March 18, 2011
Author Disclosure: N.M. received sponsored grants from Novartis and Takeda Science Foundation. A.K. received lecture fees from AstraZeneca, GlaxoSmithKline, Merck, and Boehringer Ingelheim. E.G. received consultancy fees from Kalypsys, lecture fees from Merck and serves on the advisory board for Merck and Sepracor. E.G. received sponsored grants from Kalypsys, Chemizon, and Merck. K.W., G.I., H.K., Y.F., E.K., Y.T., M.K., and M.T. have no financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med 2001;344:350–362 [DOI] [PubMed] [Google Scholar]
- 2.Lee NA, Gelfand EW, Lee JJ. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J Allergy Clin Immunol 2001;107:945–957 [DOI] [PubMed] [Google Scholar]
- 3.De Sanctis GT, Itoh A, Green FH, Qin S, Kimura T, Grobholz JK, Martin TR, Maki T, Drazen JM. T-lymphocytes regulate genetically determined airway hyperresponsiveness in mice. Nat Med 1997;3:460–462 [DOI] [PubMed] [Google Scholar]
- 4.Robinson DS, Hamid QA, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304. [DOI] [PubMed] [Google Scholar]
- 5.van Rensen EL, Sont JK, Evertse CE, Willems LN, Mauad T, Hiemstra PS. Sterk PJ, and the AMPUL Study Group. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am J Respir Crit Care Med 2005;172:837–841 [DOI] [PubMed] [Google Scholar]
- 6.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, Miyahara S, Balhorn A, Dakhama A, Gelfand EW. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol 2004;172:2549–2558 [DOI] [PubMed] [Google Scholar]
- 7.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8(+) T cells mediate inflammation and airway hyper-responsiveness. Nat Med 2004;10:865–869 [DOI] [PubMed] [Google Scholar]
- 8.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261 [DOI] [PubMed] [Google Scholar]
- 9.Grunig G, Wamock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma.Science 1998;282:2261–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Ruffin RE, Dolovich J, Hargreave FE. Allergen-induced increase in non-allergic bronchial reactivity. Clin Allergy 1977;7:503–513 [DOI] [PubMed] [Google Scholar]
- 11.Durham SR. The significance of late responses in asthma. Clin Exp Allergy 1991;21:3–7 [DOI] [PubMed] [Google Scholar]
- 12.Cieslewicz G, Tomkinson A, Adler A, Duez C, Schwarze J, Takeda K, Larson KA, Lee JJ, Irvin CG, Gelfand EW. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest 1999;104:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanehiro A, Ikemura T, Makela MJ, Lahn M, Joetham A, Dakhama A, Gelfand EW. Inhibition of phosphodiesterase 4 attenuates airway hyperresponsiveness and airway inflammation in a model of secondary allergen challenge. Am J Respir Crit Care Med 2001;163:173–184 [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Miyahara N, Kodama T, Taube C, Balhorn A, Dakhama A, Kitamura K, Hirano A, Tanimoto M, Gelfand EW. S-carboxymethylcysteine normalises airway responses in sensitized and challenged mice. Eur Respir J 2005;26:577–585 [DOI] [PubMed] [Google Scholar]
- 15.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human diseases. N Engl J Med 1990;323:645–655 [DOI] [PubMed] [Google Scholar]
- 16.Miyahara N, Miyahara S, Takeda K, Gelfand EW. Role of the LTB4/BLT1 pathway in allergen-induced airway hyperresponsiveness and inflammation. Allergol Int 2006;55:91–97 [DOI] [PubMed] [Google Scholar]
- 17.Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 1980;286:264–265 [DOI] [PubMed] [Google Scholar]
- 18.Serhan CN, Prescott SM. The scent of a phagocyte: advances on leukotriene B4 receptors. J Exp Med 2000;192:F5–F8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang WW, Garcia-Zepeda EA, Sauty A, Oettgen HC, Rothenberg ME, Luster AD. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J Exp Med 1998;188:1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids 2003;69:123–134 [DOI] [PubMed] [Google Scholar]
- 21.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997;387:620–624 [DOI] [PubMed] [Google Scholar]
- 22.Goodarzi K, Goodarzi M, Tager AM, Luster AD. von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol 2003;4:965–973 [DOI] [PubMed] [Google Scholar]
- 23.Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol 2003;4:982–990 [DOI] [PubMed] [Google Scholar]
- 24.Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, Matsubara S, Dakhama A, Tager AM, Luster AD. et al. Leukotriene B4 (BLT1) is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness.J Immunol 2005;174:4979–4984 [DOI] [PubMed] [Google Scholar]
- 25.Miyahara N, Takeda K, Miyahara S, Matsubara S, Koya T, Matsubara S, Joetham A, Krishnan E, Dakhama A, Haribabu B. et al. Requirement for the leukotriene B4 receptor-1 in allergen-induced airway hyperresponsiveness.Am J Respir Crit Care Med 2005;172:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terawaki K, Yokomizo T, Nagase T, Toda A, Taniguchi M, Hashizume K, Yagi T, Shimizu T. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol 2005;175:4217–4225 [DOI] [PubMed] [Google Scholar]
- 27.Medoff BD, Seung E, Wain JC, Means TK, Campanella GS, Islam SA, Thomas SY, Ginns LC, Grabie N, Lichtman AH, et al. Antibody-antigen interaction in the airway drives early granulocyte recruitment through BLT1. Am J Physiol Lung Cell Mol Physiol 2006;290:L170–L178 [DOI] [PubMed] [Google Scholar]
- 28.Taube C, Miyahara N, Ott V, Swanson B, Takeda K, Loader L, Shultz LD, Tager AM, Luster AD, Dakhama A, et al. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol 2006;176:3157–3164 [DOI] [PubMed] [Google Scholar]
- 29.Miyahara N, Ohnishi H, Matsuda H, Miyahara S, Takeda K, Koya T, Matsubara S, Okamoto M, Dakhama A, Haribabu B, et al. Leukotriene B4 receptor-1 (BLT1) expression on dendritic cells is required for the development of Th2 responses and allergen-induced airway hyperresponsiveness. J Immunol 2008;181:1170–1178 [DOI] [PubMed] [Google Scholar]
- 30.Koch K, Melvin LS, Jr., Reiter LA, Biggers MS, Showell HJ, Griffiths RJ, Pettipher ER, Cheng JB, Milici AJ, Breslow R. (+)-1-(3S,4R)-[3-(4-phenylbenzyl)-4-hydroxychroman-7-yl]cyclopentane carboxylic acid, a highly potent, selective leukotriene B4 antagonist with oral activity in the murine collagen-induced arthritis model. J Med Chem 1994;37:3197–3199 [DOI] [PubMed] [Google Scholar]
- 31.Lee YM, Miyahara N, Takeda K, Prpich J, Oh A, Balhorn A, Joetham A, Gelfand EW, Dakhama A. IFN-[gamma] production during initial infection determines the outcome of re-infection with RSV. Am J Respir Crit Care Med 2007;177:208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JW, Taube C, Yang ES, Joetham A, Balhorn A, Takeda K, Miyahara N, Dakhama A, Donaldson DD, Gelfand EW. Respiratory syncytial virus-induced airway hyperresponsiveness is independent of IL-13 compared with that induced by allergen. J Allergy Clin Immunol 2003;112:1078–1087 [DOI] [PubMed] [Google Scholar]
- 33.Bryan SA, O'Connor BJ, Matti S, Leckie MJ, Kanabar V, Khan J, Warrington SJ, Renzetti L, Rames A, Bock JA. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000;356:2149–2153 [DOI] [PubMed] [Google Scholar]
- 34.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyperresponsiveness, and the late asthmatic response. Lancet 2000;356:2144–2148 [DOI] [PubMed] [Google Scholar]
- 35.Crosby JR, Cieslewicz G, Borchers M, Hines E, Carrigan P, Lee JJ, Lee NA. Early phase bronchoconstriction in the mouse requires allergenspecific IgG. J Immunol 2002;168:4050–4054 [DOI] [PubMed] [Google Scholar]
- 36.Turner CR, Breslow R, Conklyn MJ, Andresen CJ, Patterson DK, Lopez-Anaya A, Owens B, Lee P, Watson JW, Showell HJ. In vitro and in vivo effects of leukotriene B4 antagonism in a primate model of asthma. J Clin Invest 1996;97:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durham SR. Late asthmatic responses. Respir Med 1990;84:263–268 [DOI] [PubMed] [Google Scholar]
- 38.Metzger WJ, Zavala D, Richerson HB, Moseley P, Iwamota P, Monick M, Sjoerdsma K, Hunninghake GW. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs: description of the model and local airway inflammation. Am Rev Respir Dis 1987;135:433–440 [DOI] [PubMed] [Google Scholar]
- 39.Taube C, Duez C, Cui ZH, Takeda K, Rha YH, Park JW, Balhorn A, Donaldson DD, Dakhama A, Gelfand EW. The role of IL-13 in established allergic airway disease. J Immunol 2002;169:6482–6489 [DOI] [PubMed] [Google Scholar]
- 40.Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, Reed CE, Hunt LW, Gleich GJ. Sudden-onset fatal asthma: a distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis 1993;148:713–719 [DOI] [PubMed] [Google Scholar]
- 41.James AL, Elliot JG, Abramson MJ, Walters EH. Time to death, airway wall inflammation and remodelling in fatal asthma. Eur Respir J 2005;26:429–434 [DOI] [PubMed] [Google Scholar]
- 42.Kikuchi S, Nagata M, Kikuchi I, Hagiwara K, Kanazawa M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. Int Arch Allergy Immunol 2005;137:7–11 [DOI] [PubMed] [Google Scholar]
- 43.Kikuchi I, Kikuchi S, Kobayashi T, Hagiwara K, Sakamoto Y, Kanazawa M, Nagata M. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. Am J Respir Cell Mol Biol 2006;34:760–765 [DOI] [PubMed] [Google Scholar]
- 44.Kikuchi I, Kikuchi S, Kobayashi T, Takaku Y, Hagiwara K, Kanazawa M, Nagata M. Theophylline attenuates the neutrophil-dependent augmentation of eosinophil trans-basement membrane migration. Int Arch Allergy Immunol 2007;143:44–49 [DOI] [PubMed] [Google Scholar]
- 45.Ohnishi H, Miyahara N, Gelfand EW. The role of leukotriene B4 in allergic diseases. Allergol Int 2008;57:291–298 [DOI] [PubMed] [Google Scholar]
- 46.Miyahara N, Ohnishi H, Miyahara S, Takeda K, Matsubara S, Matsuda H, Okamoto M, Loader JE, Joetham A, Tanimoto M, et al. Leukotriene B4 release from mast cells in IgE-mediated airway hyperresponsiveness and inflammation. Am J Respir Cell Mol Biol 2009;40:672–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou EM, Kisil FT. Antibody responses to allergen Lol pIV are suppressed following adoptive transfer of B lymphocytes from the internal image anti-idiotypic antibody-treated mice. Cell Immunol 1995;165:77–83 [DOI] [PubMed] [Google Scholar]
- 48.Serio KJ, Baker JR, Ring WL, Riddick CA, Bigby TD. Leukotriene B4 costimulates 5-lipoxygenase activity in neutrophils via increased 5-lipoxygenase translocation. Am J Physiol Cell Physiol 1997;272:C1329–C1334 [DOI] [PubMed] [Google Scholar]
- 49.McKinley L, Alcorn JF, Peterson A, DuPont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008;181:4089–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shim JJ, Dabbagh K, Ueki IF, Dao-Pick T, Burgel PR, Takeyama K, Tam DC, Nadel JA. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol 2001;280:L134–L140 [DOI] [PubMed] [Google Scholar]
- 51.Evans DJ, Barnes PJ, Spaethe SM, van Alstyne EL, Mitchell MI, O'Connor BJ. Effect of a leukotriene B4 receptor antagonist, LY293111, on allergen induced responses in asthma. Thorax 1996;51:1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohnishi H, Miyahara N, Dakhama A, Takeda K, Mathis S, Haribabu B, Erwin W, Gelfand EW. Corticosteroids enhance CD8+ T cell-mediated airway hyperresponsiveness and allergic inflammation by upregulating leukotriene B4 receptor-1. J Allergy Clin Immunol 2008;121:864–871 [DOI] [PubMed] [Google Scholar]
- 53.Ohnishi H, Takeda K, Domenico J, Lucas JJ, Miyahara N, Swasy CH, Dakhama A, Gelfand EW. Mitogen-activated protein kinase/extracellular signal-regulated kinase kinases 1/2-dependent pathways are essential for CD8+ T cell-mediated airway hyperresponsiveness and inflammation. J Allergy Clin Immunol 2009;123:249–257 [DOI] [PubMed] [Google Scholar]
- 54.Woo CH, Yoo MH, You HJ, Cho SH, Mun YC, Seong CM, Kim JH. Transepithelial migration of neutrophils in response to leukotriene B4 is mediated by a reactive oxygen species-extracellular signal-regulated kinase-linked cascade. J Immunol 2003;170:6273–6279 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.