Abstract
We and others reported that Inducible costimulator-deficient (ICOS−/−) mice manifest a defect in Th2-mediated airway inflammation, which was attributed to reduced Th2 differentiation in the absence of ICOS signaling. Interestingly, the number of CD4 T cells present in the airways and lungs after sensitization and challenge is significantly reduced in ICOS−/− mice. We now show that this reduction is not attributable simply to a reduced proliferation of ICOS−/− cells, because significantly more ICOS−/− than wild-type activated CD4 T cells are present in the lymph nodes, suggesting that more ICOS−/− CD4 T cells than wild-type CD4 T cells migrated into the lymph nodes. Further investigation revealed that activated ICOS−/− CD4 T cells express higher concentrations of the lymph node homing receptors, CCR7 and CD62L, than do wild-type CD4 T cells, leading to a preferential return of ICOS−/− cells to the nondraining lymph nodes rather than the lungs. Blocking reentry into the lymph nodes after the initiation of Th2-mediated airway inflammation equalized the levels of CD4 and granulocyte infiltration in the lungs of wild-type and ICOS−/− mice. Our results demonstrate that in wild-type CD4 T cells, co-stimulation with ICOS promotes the down-regulation of CCR7 and CD62L after activation, leading to a reduced return of activated CD4 T cells to the lymph nodes and a more efficient entry into the lungs.
Keywords: ICOS, co-stimulation, Th2, airway inflammation, T-cell migration
Clinical Relevance
Factors that control the migration of T cells to tissues are not fully understood. We describe a novel role of a co-stimulatory receptor, Inducible costimulator (ICOS), in augmenting the migration of T cells to the lungs during inflammation by reducing migration to the lymph nodes. Furthermore, understanding the factors that control the migration of T cells during inflammation will further our ability to promote or inhibit the migration of T cells in the clinical setting.
CD4 T cells can play a key role in initiating and maintaining pulmonary inflammation in diseases as diverse as atopic asthma and influenza (1–5). A significant body of research suggests that these protective and pathological CD4 T cells must first be activated in the lymph nodes. They then leave the lymph nodes and circulate through the blood to reach the lungs (6, 7). The factors that regulate the migration of CD4 T cells from the site of activation (i.e., the lymph nodes) to infected or inflamed lungs remain controversial. In this study, we identify co-stimulation with Inducible costimulator (ICOS) as a novel regulator of CD4 T-cell migration to inflamed lungs.
The ICOS receptor is a member of the CD28 family of co-stimulatory receptors that was first found to enhance the activation of CD4 T cells in vitro and in vivo (8). Since its discovery, ICOS co-stimulation was found to enhance Th2-mediated inflammation in the lungs (9–13) and Th1-mediated protective responses to influenza (14, 15). A deficiency or blockade of ICOS was found to reduce the differentiation of CD4 T cells into Th2 cells (13, 16). However, reduced Th2 differentiation does not provide a mechanism by which ICOS enhances Th1 responses in the lungs and elsewhere, Th17 responses, and Treg responses (14–18). Some studies suggested that co-stimulation with ICOS augments the responses of CD4 T cells by enhancing the proliferation of effector CD4 T cells (8, 13). However, this idea is controversial because other studies found that ICOS plays no role in the proliferation and expansion of CD4 T cells (19, 20). Further research is necessary to understand the mechanisms by which ICOS augments CD4 T cell–mediated inflammation.
In addition to defects in the proliferation and differentiation of CD4 T cells, fewer CD4 T cells are found in inflamed lungs and other tissues in the absence of co-stimulation with ICOS (10, 21, 22). The reduced presence of activated CD4 T cells in tissues in the absence of co-stimulation with ICOS was ascribed to reduced differentiation or proliferation, but in the present study we identified a novel mechanism by which co-stimulation with ICOS enhances the responses of CD4 T cells in vivo, by enhancing the migration of T cells to the lungs. We demonstrate that co-stimulation with ICOS reduces the entry of activated CD4 T cells into the nondraining lymph nodes by promoting the down-regulation of lymph node homing receptors CCR7 and CD62L. We further demonstrate that the migration of ICOS−/− CD4 T cells to the lungs may be restored to wild-type levels by blocking migration to the lymph nodes. These findings reveal a previously unknown (to the best of our knowledge) role for co-stimulation with ICOS in regulating the migration of activated CD4 T cells to lungs by reducing migration to the lymph nodes.
Materials and Methods
Mice
C57Bl/6 ICOS−/− mice were a generous gift of Dr. Richard Flavell (13). Balb/c ICOS−/− mice and C57Bl/6 ICOSL−/− mice (23) were a kind gift of Dr. Andrew Welcher (Amgen, Thousand Oaks, CA). DO11.10 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). This study was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Adoptive Transfer Model
CD4+CD62Lhigh T cells were enriched from spleens and lymph nodes of DO11.10 and ICOS−/− DO11.10 mice by Magnetic-activated cell sorting (MACS) separation (Miltenyi Biotec, Auburn, CA). We injected 1 × 106 CD3+CD4+KJ1–26+ Carboxyfluorescein succinimidyl ester (CFSE)-labeled cells intravenously into Balb/c hosts, which were immunized intraperitoneally with 200 μg ovalbumin323–339 peptide (OVAp) and 2,500 inactivated Schistosoma mansoni eggs (Dr. Joel Weinstock, New England Medical Center, Boston, MA). For some experiments (Figure 5), fewer (1 × 104) CD4 T cells were transferred. For co-transfer experiments, equal numbers of DO11.10 and ICOS−/− DO11.10 cells were mixed, and 1 × 106 cells were injected intravenously into each naive host. Where noted, mice were treated with either 6 μg FTY720 (Cayman Chemical, Ann Arbor, MI) by oral gavage daily (24) or 300 μg of anti-CD62L antibody by intraperitoneal injection on Days 0 and 2 after immunization. As expected according to previous studies, FTY720 inhibited exit from the lymph nodes, augmenting cell numbers in the lymph nodes and reducing cell counts in the spleen and blood (Figure E1A in the online supplement), and Mel-14 blocked entry into the lymph nodes, reducing cell counts in the lymph nodes and augmenting cell counts in the spleen (Figure E1A).
Figure 5.
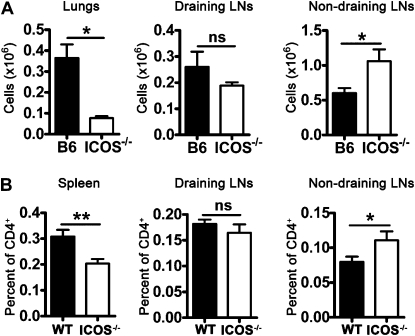
Activated ICOS−/− CD4 T cells preferentially migrate to nondraining lymph nodes (LNs). (A) B6 and ICOS−/− mice were sensitized and challenged with inactivated S. mansonii eggs and SEA, as in Figure 1. On Day 4 after challenge, mice were killed, and their lung (tissue digest), draining lymph node (mediastinal), and nondraining lymph node (inguinal, axial, and brachial) cells were analyzed by flow cytometry for recently activated CD4+ T cells (CD69+). This experiment was replicated twice (n ≥ 4 for each group). (B) Naive hosts received 1 × 104 DO11.10 or ICOS−/− DO11.10 cells, and were then immunized with S. mansonii eggs and OVAp. On Day 4 after immunization, mice were killed, and their spleen, draining lymph nodes (mediastinal and mesenteric), and nondraining lymph nodes (axial and cervical) were analyzed by flow cytometry for the presence of CD4+KJ1–26+ antigen-specific cells. This experiment was replicated twice (n ≥ 4 for each group). *P < 0.05, **P < 0.01, and ***P < 0.001.
In Vitro Activation
We cultured 2 × 105 naive DO11.10 or ICOS−/− DO11.10 T cells with 2 × 105 splenocytes and 1 μg/ml OVAp for 3 days. Cells were washed and resuspended in fresh media at 1 × 106 live cells/ml for 1 day before being harvested and stained for flow cytometry.
Model of Airway Inflammation
As previously described, ICOS−/− or ICOS+/+ (C57Bl/6) mice were sensitized intraperitoneally with 5,000 inactivated S. mansonii eggs, followed by intratracheal challenge 7 days later with 5 μg of soluble egg antigen (SEA) (9). Four days after the challenge, the mice were killed, and bronchoalveolar lavage (BAL) was performed. For competitive migration studies, Ly5.1+Ly5.2+ICOS+/+ and Ly5.2+/+ICOS−/− mice were sensitized and challenged, as already described. Three days after the challenge, the mice were killed, and their draining lymph nodes (dLNs; mediastinal) were harvested. Equal numbers of draining lymph-node cells from ICOS+/+ and from ICOS−/− mice were combined, and 1 × 107 cells were injected intravenously into previously sensitized and challenged Ly5.1+/+ hosts. The next day, dLNs from the hosts were harvested, and the percentages of ICOS+/+ and ICOS−/− donor cells were identified by staining for Ly5.1, Ly5.2, and CD4. Where noted (Figure 6), mice were treated with Mel-14 on Day 1 after challenge, to prevent entry into the lymph nodes.
Figure 6.
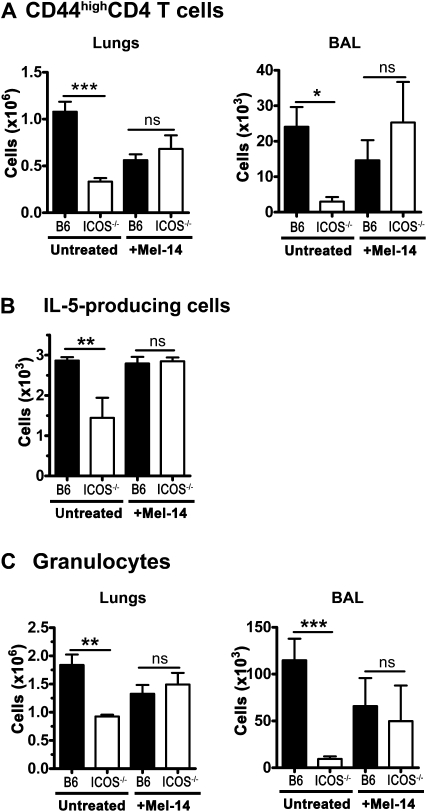
Blocking entry into the lymph nodes permits ICOS−/− CD4 T cells to enter lungs and airways and mediate inflammation, similar to wild-type cells. B6 and ICOS−/− mice were sensitized and challenged with inactivated S. mansonii eggs and SEA, as in Figure 1. On Day 1 after challenge, half of the mice were treated with Mel-14 to block entry into the lymph nodes. On Day 4 after challenge, the mice were killed, and brochoalveolar lavage and lung digests were performed to isolate infiltrating lymphocytes. Lung (A) and BAL (B) cells were analyzed by flow cytometry for Gr-1(Ly6G)+ SSChigh granulocytes and activated CD4+ T cells. (C) Lung cells were restimulated with SEA, and the number of cells producing IL-5 was quantified by Enzyme-linked immunosorbent spot (ELISPOT) assay. This experiment was repeated twice (n ≥ 3 for each group). *P < 0.05, **P < 0.01, and ***P < 0.001.
ELISPOT
C57Bl/6 and ICOS−/− cells were isolated from the lungs and restimulated with 5 μg/ml SEA for 48 hours on coated plates. The number of cells producing IL-5 was quantified using an IL-5 ELISPOT kit (BD Biosciences, San Jose, CA).
Statistical Analysis
Unless otherwise indicated, significance was determined according to unpaired Student t tests (GraphPad, San Diego, CA). Error bars represent the SEM (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
Results
Fewer ICOS−/− CD4 T Cells Are in the Lungs, yet More ICOS−/− CD4 T Cells Are Found in the Lymph Nodes
In this study, we sought to determine the mechanism by which co-stimulation with ICOS enhances CD4-mediated inflammation. In our model of airway inflammation, we found that ICOS−/− mice exhibited reduced eosinophilia compared with wild-type mice, indicating reduced CD4-mediated inflammation. However, the most dramatic defect in ICOS−/− mice involved the number of CD4 T cells in the airways (Figure 1A) and the number of activated CD4 T cells in the lungs (Figure 1B). This decrease in CD4 T cells in the lungs may be attributable to the reduced proliferation or reduced trafficking of CD4 T cells to the lungs.
Figure 1.
Fewer activated Inducible costimulator-deficient (ICOS−/−) CD4 T cells are found in inflamed airways and lungs than wild-type cells. C57Bl/6 (B6) and ICOS−/− mice were sensitized and challenged with inactivated Schistosoma mansonii eggs and soluble egg antigen (SEA). On Day 4 after challenge, mice were killed, and airway (bronchoalveolar lavage; BAL) and lung (tissue digest) cells were analyzed by flow cytometry. (A) CCR3+SSChigh eosinophils and CD3+CD4+ T cells from BAL. (B) CD4+ T cells and CD44+CD62L− activated CD4+ T cells from the lungs. This experiment was repeated three times (n ≥ 4 for each group). *P < 0.05, **P < 0.01, and ***P < 0.001.
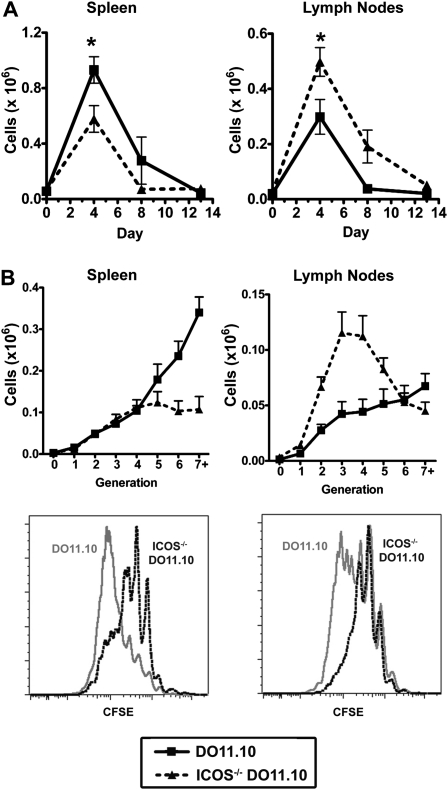
To track the proliferation and location of antigen-specific wild-type and ICOS−/− CD4 T cells, we used an adoptive transfer model, in which naive DO11.10 or ICOS−/− DO11.10 cells were transferred to hosts, followed by immunization. Whereas fewer ICOS−/− than ICOS+/+ DO11.10 cells were found in the spleen (Figure 2A, left), the number of ICOS−/− DO11.10 cells in the lymph nodes was significantly higher than the number of DO11.10 cells (Figure 2A, right). ICOS−/− cells may have been dividing more in the lymph nodes than were wild-type (WT) cells, thereby resulting in the greater number of ICOS−/− cells observed in the lymph nodes. However, ICOS−/− cells divided fewer times than WT DO11.10 cells in both the lymph nodes and spleen (Figure 2B). Differences in proliferation between ICOS−/− and WT T cells, therefore, cannot account for the greater number of ICOS−/− cells in the lymph nodes. ICOS−/− CD4 T cells, as well as exhibiting less proliferation, may manifest defects in survival, as shown previously (20, 25). In that case, the greater number of ICOS−/− CD4 T cells compared with WT CD4 T cells in the lymph nodes may under-represent the total difference between WT and ICOS−/− CD4 T-cell trafficking.
Figure 2.
Activated antigen-specific ICOS−/− CD4 T cells accumulate more in the lymph nodes, yet proliferated less than wild-type cells. Naive hosts received wild-type or ICOS−/− CD4+ KJ1–26+ cells, were subsequently immunized, and 3–5 hosts were killed at each time-point shown for each group (wild-type or ICOS−/−). (A) Splenocytes and lymph node (axial, brachial, and inguinal; draining and nondraining) cells were counted and then analyzed by flow cytometry for CD4+KJ1–26+ cells. The number of CD4+KJ1–26+ cells was calculated by multiplying the percent CD4+KJ1–26+ cells within the organ by the total number of cells in the organ. (B) At the peak of response on Day 4, proliferation was assessed by flow cytometry as CFSE dilution in CD4+KJ1–26+ cells. The number of CD4+KJ1–26+ cells in each generation of division is shown. Lower graphs in B illustrate representative CFSE dilution plots of DO11.10 (gray line) and ICOS−/− DO11.10 (black dotted line) cells. This experiment was repeated twice (n ≥ 4 for each group). *P < 0.05, **P < 0.01, and ***P < 0.001.
ICOS Regulates CD4 T-Cell Trafficking into the Lymph Nodes by a Cell-Intrinsic Mechanism
To determine how co-stimulation with ICOS regulates trafficking, DO11.10 and ICOS−/− DO11.10 cells were co-transferred into hosts that were immunized in conjunction with blocking entry or exit into the lymph nodes. As a control, cells were also co-transferred without immunization, in which case the ratio of ICOS−/− to WT antigen-specific CD4 cells in the lymph nodes was close to 1 (Figure 3A). Strikingly, after immunization, more than twice as many activated, antigen-specific ICOS−/− CD4 cells were present in the lymph nodes, compared with WT cells (Figure 3A).
Figure 3.
ICOS−/− CD4 T cells preferentially migrate into the lymph nodes. (A) Wild-type (WT) and ICOS−/− DO11.10 cells were mixed and co-transferred into Balb/c hosts, which were then immunized. In unimmunized mice (leftmost squares) and on Day 4 after immunization, the ratio of ICOS−/−/wild-type CD4+KJ1–26+ cells was quantified. The mice were injected intraperitoneally with Mel-14 on Days 0 and 2 (right, upside-down triangles), treated with FTY720 daily by oral gavage (middle, triangles), or left untreated after immunization (second from left, open circles). This experiment was repeated three times for Mel-14–treated mice and untreated mice, and twice for FTY720-treated mice (n ≥ 4 for each group). (B) Cells were harvested from the draining lymph nodes (mediastinal) of wild-type and ICOS−/− mice (sensitized and challenged as described in Figure 1), and wild-type and ICOS−/− cells were transferred together into sensitized and challenged recipient wild-type mice. Sixteen hours later, the migration of CD4 T cells back to the draining lymph nodes versus the spleen was assessed. This experiment was replicated twice (n ≥ 3 for each group). ns, no significance. *P < 0.05, **P < 0.01, and ***P < 0.001.
We next investigated whether the increased number of ICOS−/− CD4 T cells in the lymph nodes was attributable to increased entry into the lymph nodes or reduced egress from the lymph nodes by blocking entry or exit. Anti-CD62L (Mel-14) and a pharmacological inhibitor of S1P1 (FTY720) treatment were used to block the entry or exit, respectively, of T cells from the lymph nodes. T cell numbers in the lymph nodes and spleens of Mel-14 or FTY720 treated or untreated mice were compared, and these treatments worked as expected (Figure E1). When the entry of T cells into the lymph nodes was blocked with anti-CD62L antibody (Mel-14) treatment, the ratio of ICOS−/−/WT CD4 T cell numbers in the lymph nodes was significantly decreased (Figure 3A). This result suggests that the increased number of ICOS−/− T cells in the lymph nodes is dependent on the migration of ICOS−/− T cells into the lymph nodes. To determine whether ICOS also regulates the number of CD4 cells in the lymph nodes by controlling the exit of CD4 T cells, the exit of T cells from lymph nodes was inhibited by treatment with FTY720. When exit from the lymph nodes was blocked, the ratio of ICOS−/−/WT cells remained significantly higher in the lymph nodes (Figure 3A). Collectively, these data indicate that co-stimulation with ICOS specifically regulates the migration of CD4 T cells into the lymph nodes. Moreover, our finding that ICOS−/− CD4 cells, more than WT cells, migrate to the lymph nodes in the same host suggests that ICOS regulates migration into the lymph nodes in a cell-intrinsic manner.
Next, we investigated whether ICOS−/− CD4 T cells selectively migrated to the lymph nodes in our model of airway inflammation. To follow the migration of activated T cells, draining lymph node cells from sensitized and challenged WT or ICOS−/− mice were co-transferred into sensitized and challenged congenic mice. Sixteen hours later, ICOS−/− CD4 T cells migrated more to the draining lymph nodes and less to the spleen than did WT CD4 T cells (Figure 3B). These results demonstrate that co-stimulation with ICOS regulates the entry of T cells into the lymph nodes during an airway inflammatory response. Thus, the reduced CD4 T-cell infiltration of the airways in ICOS−/− mice may be partly attributed to a greater migration of ICOS−/− CD4 T cells to the lymph nodes.
ICOS−/− Cells Express Higher Levels of CD62L and CCR7 than Do Wild-Type Cells In Vivo and In Vitro
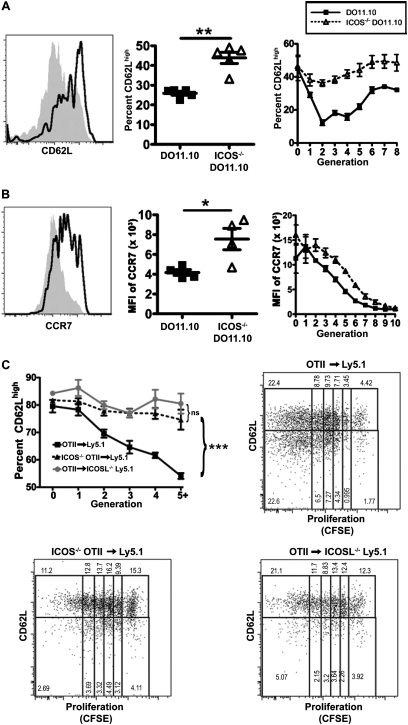
The migration of T cells into the lymph nodes is mediated by two key receptors, CCR7 and CD62L (26, 27). Previous studies demonstrated that activated ICOS−/− CD4 T cells may express higher levels of CD62L than do WT CD4 T cells (13, 25, 28). Therefore, we hypothesized that co-stimulation with ICOS may regulate the entry of T cells into the lymph nodes in our model by controlling the expression of CCR7 and CD62L. We transferred WT or ICOS−/− DO11.10 T cells into recipient mice, immunized the recipients, and assayed the expression of both CD62L and CCR7. At the peak of the response, a greater proportion of ICOS−/− than WT DO11.10 cells remained CD62Lhigh (Figure 4A, middle). This effect was independent of proliferation, because more ICOS−/− T cells were CD62Lhigh compared with WT T cells that had undergone similar numbers of divisions (Figure 4A, right). ICOS−/− CD4 T cells expressed higher concentrations of CD62L throughout the duration of the immune response (Figure E2A). CD4 T cells also down-regulated the expression of CCR7 after activation. Similar to CD62L, activated antigen-specific ICOS−/− CD4 T cells expressed significantly higher concentrations of CCR7 than did WT cells (Figure 4B, middle). The effect of co-stimulation with ICOS on the down-regulation of CCR7 was also independent of cell division (Figure 4B, right). Therefore, co-stimulation with ICOS augments the down-regulation of both CD62L and CCR7 in CD4 T cells after activation.
Figure 4.
Activated ICOS−/− CD4 T cells express higher concentrations of the lymph node homing molecules CCR7 and CD62L than do wild-type CD4 T cells. DO11.10 and ICOS−/− DO11.10 cells were assessed on Day 4 after adoptive transfer, as described in Figure 2. (A) CD4+KJ1–26+ cells were evaluated for the expression of CD62L, shown as a representative plot (left, wild-type, gray shaded histogram; ICOS−/−, black line), the total percent of CD62Lhigh cells (middle), and the percent of cells in each cell division expressing CD62L (right). (B) The expression of CCR7 was analyzed in CD4+KJ1–26+ cells. A representative plot is shown (left, wild-type, gray shaded histogram; ICOS−/−: black line). The median fluoresence intensity (MFI) of CCR7 of all CD4+KJ1–26+ cells (middle) and the MFI of CCR7 in each cell division (right) were measured. Experimental results in A and B were replicated in two independent experiments (n ≥ 4 for each experiment). (C) OTII and ICOS−/− OTII cells were labeled with CFSE and adoptively transferred to ICOSL−/− or WT congenic hosts, which were immunized with inactivated S. mansonii eggs and ovalbumin323–339 peptide (OVAp). On Day 4, the CD62L expression of T cell receptor-transgenic cells at each cell division was quantified. Representative plots show the CD62Lhigh and CD62Llow fractions of each generation for each condition. The percent CD62Lhigh within a generation is calculated as CD62Lhigh/total in generation (i.e., CD62Lhigh/[CD62Lhigh + CD62Llow]). For A and B, significance was determined by unpaired Student's t tests. For C, significance was calculated by 2-way ANOVA. For all results, *P < 0.05, **P < 0.01, and ***P < 0.001.
To determine whether co-stimulation with ICOS exerts an effect on the down-regulation of CD62L and CCR7 in the absence of in vivo migration, we stimulated WT and ICOS−/− cells in vitro where activated cells would be unable to migrate, regardless of CD62L and CCR7 expression. ICOS−/− cells expressed higher concentrations of CCR7 and CD62L than did WT cells, similar to our findings in vivo (Figure E2B). These data reveal that co-stimulation with ICOS regulates the expression of CCR7 and CD62L, independent of any effects on proliferation in vivo and in vitro.
ICOS−/− CD4 T cells may develop differently than WT T cells, such that intrinsic differences in the cells, rather than ICOS signaling during activation, led to the reduced down-regulation of CCR7 and CD62L in these cells. To differentiate between these possibilities, we adoptively transferred WT OTII T cells into ICOS ligand-deficient (ICOSL−/−) congenic hosts, and compared them with either WT or ICOS−/− OTII cells transferred into WT hosts. After immunization, WT CD4 T cells in wild-type hosts down-regulated CD62L, but CD4 T cells activated in the absence of ICOS or the absence of ICOSL failed to down-regulate CD62L, even after five generations of proliferation (Figure 4C). Accordingly, we concluded that ICOS signaling during activation is responsible for differences in CD62L down-regulation, rather than developmental defects in ICOS−/− CD4 T cells. Collectively these results show that activated ICOS−/− CD4 T cells express higher concentrations of lymph node homing receptors, suggesting that increased migration to the lymph nodes may result in fewer ICOS−/− Th2 cells reaching the airways.
ICOS−/− CD4 Cells Preferentially Recirculate to the Nondraining Lymph Nodes
Next we hypothesized that if co-stimulation with ICOS had increased the entry of activated CD4 T cells into the lymph nodes, as shown in Figure 3, then perhaps activated ICOS−/− CD4 cells were also recirculating into the lymph nodes during airway inflammation. To investigate this hypothesis, we identified the number of recently activated (CD69+) CD4 T cells in the lungs and draining lymph nodes. As indicated previously, ICOS−/− mice exhibited fewer activated CD4 T cells in their lungs (Figure 5A, left). Interestingly, we found no difference in activated CD4 T cells in the draining lymph nodes (Figure 5A, middle). We hypothesized that because the draining lymph nodes comprise the site of primary antigen-specific activation, the reduced proliferation of ICOS−/− cells in the draining lymph nodes may obscure any increase in migration to this site. Therefore, we investigated the nondraining lymph nodes, where proliferation would not compensate for any differences in the migration of activated CD4 T cells. In the nondraining lymph nodes, ICOS−/− mice exhibited significantly more activated CD4 cells than did WT mice (Figure 5A, right). These data indicate that ICOS−/− CD4 T cells can exit the draining lymph nodes after activation, but may recirculate to the nondraining lymph nodes during circulation instead of going to the lungs.
To follow the migration of antigen-specific CD4 T cells to the draining versus nondraining lymph nodes, we adoptively transferred a small number (to better mimic the small number of antigen-specific cells in a polyclonal model) of naive WT or ICOS−/− DO11.10 CD4 T cells into congenic hosts. Four days after immunization, we analyzed the numbers of antigen-specific CD4 T cells in the draining lymph nodes, nondraining lymph nodes, and spleens. Similar to previous data (Figure 2), fewer ICOS−/− CD4 T cells were found in the spleen (Figure 5B, left). As before, similar numbers of ICOS−/− and WT CD4 T cells were present in the draining lymph nodes, but more ICOS−/− than WT DO11.10 CD4 T cells were present in the nondraining lymph nodes (Figure 5B, right two graphs). Together, these results demonstrate that activated ICOS−/− CD4 T cells preferentially recirculate to the nondraining lymph nodes after activation, instead of migrating to the tissue.
Migration of ICOS−/− CD4 T Cells to the Airways and Lungs Is Restored When Entry into the Lymph Nodes Is Blocked
We hypothesized that if ICOS−/− CD4 T cells could be prevented from reentering the lymph nodes after activation, they might stand a better chance of migrating to the tissue and mediating inflammation. To test this hypothesis, B6 and ICOS−/− mice were sensitized and challenged, as described in Figure 1. One day after challenge, half of the B6 and ICOS−/− mice were treated with Mel-14 to prevent the entry of CD4 T cells into the lymph nodes. On Day 4 after the challenge, CD4 and granulocyte infiltration into the lungs and airways was assessed in Mel-14–treated and untreated control B6 and ICOS−/− mice (Figures 6A and 6C). As seen previously, fewer activated CD4 T cells infiltrated the airways and lungs of untreated ICOS−/− mice than WT mice (Figure 6A, Untreated). Although blocking into the lymph nodes consistently dampened the immune response, CD4 infiltration into the lungs and airways was equalized in B6 and ICOS−/− mice after treatment with Mel-14 (Figure 6A). Because the lungs contain a significant resident population of naive and memory CD4 T cells, we further investigated the effects of Mel-14 treatment on the population of SEA-responsive cells in the lungs (Figure 6B). Untreated ICOS−/− mice demonstrated significantly fewer IL-5–producing cells than WT mice, but after with Mel-14, ICOS−/− and WT mice demonstrated similar numbers of cells producing IL-5 (Figure 6B). Finally, untreated ICOS−/− mice exhibited significantly fewer granulocytes in their lungs and BAL, but after treatment with Mel-14, these numbers equalized between ICOS−/− and WT mice (Figure 6C). These data indicate that blocking entry into the lymph nodes with Mel-14 is sufficient to restore the migration of activated ICOS−/− CD4 T cells to the lung tissue and airways, and to restore the inflammation induced by these activated CD4 T cells.
Discussion
In this study, we establish a novel role for the ICOS receptor in regulating the recirculation of activated CD4 T cells to the lymph nodes. Collectively, our results demonstrate that the ICOS receptor regulates the migration of CD4 T cells to the lungs by enhancing CCR7 and CD62L down-regulation and reducing the recirculation of activated CD4 T cells to the lymph nodes. Blocking this migration into the lymph nodes was sufficient to equalize CD4 T cell infiltration and CD4-mediated granulocyte infiltration into the lungs of ICOS−/− and WT mice. Interestingly, several models showed that activated ICOS−/− CD4 T cells express higher concentrations of CD62L than do WT CD4 T cells (13, 25, 28). Our present study demonstrates that the differences of expression in CD62L and CCR7 on activated ICOS−/− CD4 T cells lead to altered migration in vivo, with specifically more migration of activated CD4 T cells to the lymph nodes and reduced migration to inflamed lungs.
Odegard and colleagues found no differences in trafficking receptor expression in ICOS−/− compared with WT CD4 T cells in a murine model of lupus (28). Specifically, the expression of lupus-associated kidney homing receptors was characterized within the population of CD62Llow CD4 T cells. The authors found no differences in CXCR3 and P-selectin glycoprotein ligand-1 (PSGL-1) expression in CD62Llow CD4 T cells in WT and ICOS−/− mice, and concluded that ICOS−/− CD4 T cells manifested no defect in the up-regulation of tissue-homing receptors. Interestingly, Odegard and colleagues did observe (as we also did) that ICOS−/− mice had fewer CD62Llow CD4 T cells, but attributed this finding to the reduced activation or expansion of CD4 T cells in ICOS−/− mice. In contrast, our study demonstrated that even after similar levels of proliferation, ICOS−/− CD4 cells express higher concentrations of CD62L and CCR7, and that the higher expression of these receptors leads to reduced trafficking to the lungs and increased trafficking to the nondraining lymph nodes. Our study further supports previous findings associating the down-regulation of CD62L and CCR7 with the successful migration of activated T cells into inflamed lungs (29–31).
Previous findings by our laboratory highlight a dual role for ICOS in the migration of CD4 T cells to lymph nodes. Tesciuba and colleagues found that ICOS costimulation enhanced the recruitment of naive cells into the lymph nodes by enhancing the production of chemokines CCL21 and CXCL13 (32). The present study demonstrates that co-stimulation with ICOS reduces the recirculation of activated cells into the lymph nodes by down-regulating the expression of CD62L and CCR7 in activated cells. The studies are not directly comparable, because the previous study examined the effects of a super-induction of ICOS co-stimulation, using large doses of B7RP-1–Fc (a soluble form of ICOSL) in the absence of T-cell receptor signaling, whereas in our study, WT and ICOS−/− cells were investigated in the context of an antigen-specific immune response. Together, this study and our previous work indicate that co-stimulation with ICOS enhances the recruitment of naive CD4 T cells into the lymph nodes, while promoting activated CD4 T cell trafficking to the tissues.
The mechanism by which ICOS affects the down-regulation of CD62L and CCR7 remains unknown. The PI3K signaling pathway was implicated in the down-regulation of CCR7 and CD62L in T cells (33–40). Co-stimulation with ICOS was found to signal through PI3K, suggesting a possible mechanism by which ICOS might augment the down-regulation of CCR7 and CD62L (41, 42). However, other signaling pathways and molecules, including Forkhead box protein O1 (FoxO1), mammalian target of rapamycin (mTOR), and Kruppel-like factor 2 (KLF2), were also implicated in the control of CD62L and CCR7 expression in T cells (33–40). Further research is required to elucidate the mechanism by which co-stimulation with ICOS regulates the expression of CCR7 and CD62L after activation, leading to reduced CD4 T-cell trafficking to the lymph nodes and more efficient entry into the lungs.
Co-stimulation with ICOS was previously found to enhance lung inflammation in multiple in vivo murine models (12, 15, 17, 41, 43). Interestingly, CD4 T cell–mediated lung inflammation is associated with the down-regulation of CCR7 and CD62L (26, 29–31). This study suggests that co-stimulation with ICOS can augment lung inflammation by promoting the down-regulation of CCR7 and CD62L in effector CD4 T cells. Specifically, we found that the selective migration of activated ICOS−/− CD4 T cells into the lymph nodes was associated with a reduced presence of effector CD4 T cells in the lungs and reduced CD4-mediated lung inflammation. However, in some models of disease, ICOS−/− mice do not manifest decreased tissue inflammation. For example, Dong and colleagues found that ICOS−/− mice unexpectedly exhibited more severe neurological inflammation than WT mice after the induction of Experimental Autoimmune Encephalomyelitis (EAE) (13). Interestingly, high levels of CCR7 expression were recently associated with enhanced migration to the brain (44). Thus, the greater expression of CCR7 in ICOS−/− CD4 T cells may permit T cells to migrate to the brain more than WT cells.
Blocking co-stimulation with ICOS is under consideration as a possible treatment for asthma, whereas enhancing co-stimulation with ICOS is being considered in the therapeutic induction of antitumor responses by T cells (45, 46). Our study demonstrates that in addition to augmenting Th2 and Th17 differentiation and the proliferation of T cells, co-stimulation with ICOS also directs the migration of CD4 T cells by reducing the recirculation of activated CD4 T cells to the lymph nodes, thereby augmenting the migration of T cells to the lungs. Our findings indicate that further study of the effects of co-stimulation with ICOS on the migration of CD4 T cells in vivo may provide a better understanding of the potential benefits and pitfalls of developing therapeutic treatments for asthma and cancer.
Supplementary Material
Acknowledgments
The authors thank Kelly Blaine, Donna Decker, Tiffany Lu, Jesse Williams, Caroline Ferreira, and Elizabeth Berry for their assistance during these studies. The authors also thank Dr. Joel Weinstock for inactivated S. mansonii eggs and soluble egg antigen, Dr. Andrew Welcher for Balb/c.ICOS−/− and B6.ICOSL−/− mice, and Dr. Richard Flavell for B6.ICOS−/− mice.
Footnotes
This work was supported by National Institutes of Health grant R01 AI67697 (A.I.S.) and by a grant from the Blowitz–Ridgeway Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0466OC on March 18, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304 [DOI] [PubMed] [Google Scholar]
- 2.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity 2009;31:425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med 1997;186:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodland DL, Hogan RJ, Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol Res 2001;24:53–67 [DOI] [PubMed] [Google Scholar]
- 5.Swain SL, Dutton RW, Woodland DL. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol 2004;17:197–209 [DOI] [PubMed] [Google Scholar]
- 6.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol 2008;8:675–684 [DOI] [PubMed] [Google Scholar]
- 7.Curtis JL. Cell-mediated adaptive immune defense of the lungs. Proc Am Thorac Soc 2005;2:412–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999;397:263–266 [DOI] [PubMed] [Google Scholar]
- 9.Tesciuba AG, Subudhi S, Rother RP, Faas SJ, Frantz AM, Elliot D, Weinstock J, Matis LA, Bluestone JA, Sperling AI. Inducible co-stimulator regulates Th2-mediated inflammation, but not Th2 differentiation, in a model of allergic airway disease. J Immunol 2001;167:1996–2003 [DOI] [PubMed] [Google Scholar]
- 10.Clay BS, Shilling RA, Bandukwala HS, Moore TV, Cannon JL, Welcher AA, Weinstock JV, Sperling AI. Inducible costimulator expression regulates the magnitude of Th2-mediated airway inflammation by regulating the number of Th2 cells. PLoS ONE 2009;4:e7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shilling RA, Clay BS, Tesciuba AG, Berry EL, Lu T, Moore TV, Bandukwala HS, Tong J, Weinstock JV, Flavell RA, et al. CD28 and ICOS play complementary non-overlapping roles in the development of Th2 immunity in vivo. Cell Immunol 2009;259:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, Al-Garawi A, Kroczek R, Gutierrez-Ramos JC, Coyle AJ. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol 2001;2:597–604 [DOI] [PubMed] [Google Scholar]
- 13.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 2001;409:97–101 [DOI] [PubMed] [Google Scholar]
- 14.Bertram EM, Tafuri A, Shahinian A, Chan VS, Hunziker L, Recher M, Ohashi PS, Mak TW, Watts TH. Role of ICOS versus CD28 in antiviral immunity. Eur J Immunol 2002;32:3376–3385 [DOI] [PubMed] [Google Scholar]
- 15.Humphreys IR, Edwards L, Snelgrove RJ, Rae AJ, Coyle AJ, Hussell T. A critical role for ICOS co-stimulation in immune containment of pulmonary influenza virus infection. Eur J Immunol 2006;36:2928–2938 [DOI] [PubMed] [Google Scholar]
- 16.Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med 2000;192:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation 2006;114:2047–2055 [DOI] [PubMed] [Google Scholar]
- 19.Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, Yu XZ, Dong C. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J 2006;25:2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahajan S, Cervera A, MacLeod M, Fillatreau S, Perona-Wright G, Meek S, Smith A, MacDonald A, Gray D. The role of ICOS in the development of CD4 T cell help and the reactivation of memory T cells. Eur J Immunol 2007;37:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson EH, Zaph C, Mohrs M, Welcher A, Siu J, Artis D, Hunter CA. B7RP-1–ICOS interactions are required for optimal infection-induced expansion of CD4+ Th1 and Th2 responses. J Immunol 2006;177:2365–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS–B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol 2001;2:591–596 [DOI] [PubMed] [Google Scholar]
- 23.Mak TW, Shahinian A, Yoshinaga SK, Wakeham A, Boucher LM, Pintilie M, Duncan G, Gajewska BU, Gronski M, Eriksson U, et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell–dependent B cell responses. Nat Immunol 2003;4:765–772 [DOI] [PubMed] [Google Scholar]
- 24.Matloubian M, Lo CG, Cinamon G, Lesnesk MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355–360 [DOI] [PubMed] [Google Scholar]
- 25.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector–memory and regulatory T cells. J Immunol 2008;180:774–782 [DOI] [PubMed] [Google Scholar]
- 26.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol 2005;6:895–901 [DOI] [PubMed] [Google Scholar]
- 27.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol 2005;6:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odegard JM, DiPlacido LD, Greenwald L, Kashgarian M, Kono DH, Dong C, Flavell RA, Craft J. ICOS controls effector function but not trafficking receptor expression of kidney-infiltrating effector T cells in murine lupus. J Immunol 2009;182:4076–4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagome K, Dohi M, Okunishi K, To Y, Sato A, Komagata Y, Nagatani K, Tanaka R, Yamamoto K. Antigen-sensitized CD4+CD62Llow memory/effector T helper 2 cells can induce airway hyperresponsiveness in an antigen free setting. Respir Res 2005;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grinnan D, Sung SS, Dougherty JA, Knowles AR, Allen MB, Rose CE. III, Nakano H, Gunn MD, Fu SM, Rose CE Jr. Enhanced allergen-induced airway inflammation in paucity of lymph node T cell (PLT) mutant mice. J Allergy Clin Immunol 2006;118:1234–1241 [DOI] [PubMed] [Google Scholar]
- 31.Xu B, Aoyama K, Kusumoto M, Matsuzawa A, Butcher EC, Michie SA, Matsuyama T, Takeuchi T. Lack of lymphoid chemokines CCL19 and CCL21 enhances allergic airway inflammation in mice. Int Immunol 2007;19:775–784 [DOI] [PubMed] [Google Scholar]
- 32.Tesciuba AG, Shilling RA, Agarwal MD, Bandukwala HS, Clay BS, Moore TV, Weinstock JV, Welcher AA, Sperling AI. ICOS costimulation expands Th2 immunity by augmenting migration of lymphocytes to draining lymph nodes. J Immunol 2008;181:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3–OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol 2008;9:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarmin SJ, David R, Ma L, Chai JG, Dewchand H, Takesono A, Ridley AJ, Okkenhaug K, Marelli-Berg FM. T cell receptor–induced phosphoinositide-3–kinase p110delta activity is required for T cell localization to antigenic tissue in mice. J Clin Invest 2008;118:1154–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AL, Schwartz MD, Jameson SC, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110 gamma isoform of phosphatidylinositol 3–kinase. J Immunol 2008;180:2081–2088 [DOI] [PubMed] [Google Scholar]
- 36.Biggs WH. III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt–mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 1999;96:7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, et al. FOXO1 regulates L-selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3–kinase. J Immunol 2008;181:2980–2989 [DOI] [PubMed] [Google Scholar]
- 38.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. FOXO1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol 2009;10:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 2006;442:299–302 [DOI] [PubMed] [Google Scholar]
- 40.Cantrell DA. T cell antigen receptor signal transduction pathways. Cancer Surv 1996;27:165–175 [PubMed] [Google Scholar]
- 41.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, et al. The CD28-related molecule ICOS is required for effective T cell–dependent immune responses. Immunity 2000;13:95–105 [DOI] [PubMed] [Google Scholar]
- 42.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh WK. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3–kinase. Proc Natl Acad Sci USA 2009;106:20371–20376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beier KC, Hutloff A, Lohning M, Kallinich T, Kroczek RA, Hamelmann E. Inducible costimulator-positive T cells are required for allergen-induced local B-cell infiltration and antigen-specific IgE production in lung tissue. J Allergy Clin Immunol 2004;114:775–782 [DOI] [PubMed] [Google Scholar]
- 44.Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, Klinakis A, Lukyanov Y, Tseng JC, Sen F, et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 2009;459:1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroczek R, Hamelmann E. T-cell costimulatory molecules: optimal targets for the treatment of allergic airway disease with monoclonal antibodies. J Allergy Clin Immunol 2005;116:906–909 [DOI] [PubMed] [Google Scholar]
- 46.Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, Carroll RG, Riley JL, June CH. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Science Translat Med 2010;2:55–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.