Abstract
Activation of β1 integrins of blood eosinophils, assessed by mAb N29, correlates inversely with FEV1 in two paradigms for studying control of human asthma. We asked whether P-selectin causes eosinophil β1 integrin activation and results in increased adhesivity. By dual-label flow cytometry, eosinophils with high levels of surface-associated P-selectin had higher reactivity with the activation-sensitive anti-β1 mAbs N29, 8E3, and 9EG7 than eosinophils with no or with a low-level of surface-associated P-selectin. Among patients with nonsevere asthma, surface P-selectin correlated with N29, 8E3, and 9EG7 signals. By immunofluorescence microscopy, surface-associated P-selectin was present in patches on eosinophils, some of which stained for the platelet marker thrombospondin-1. Activated β1 and P-selectin partially colocalized on eosinophils. Soluble P-selectin added to whole blood enhanced activation of eosinophil β1, but not β2, integrins. In contrast, IL-5 activated eosinophil β2, but not β1, integrins. Eosinophils that did not attach to vascular cell adhesion molecule-1 (VCAM-1) in a static adhesion assay had a lower N29 signal than the original population. Soluble P-selectin added to whole blood enhanced eosinophil adhesion to VCAM-1. These findings are compatible with a scenario whereby P-selectin, on eosinophil-associated activated platelets or acquired from plasma or from prior interactions with endothelial cells or platelets, activates eosinophil α4β1 integrin and stimulates eosinophils to adhere to VCAM-1 and move to the airway in asthma.
Keywords: eosinophils, adhesion molecules, integrins, P-selectin, cell adhesion
Clinical Relevance
We demonstrate that P-selectin is bound to the surface of eosinophils to a varying degree among cells and among human subjects. A high level of surface P-selectin is associated with a high degree of eosinophil β1 integrin activation in vivo. The addition of soluble P-selectin to blood in vitro enhances activation of β1, but not β2, integrins on eosinophils and enhances eosinophil adhesion to vascular cell adhesion molecule-1. Our findings are compatible with a scenario whereby P-selectin on activated platelets, or acquired from plasma or endothelial cells, activates eosinophil α4β1 integrin and stimulates eosinophils to adhere to activated endothelium and move to the airway. Targeting P-selectin–triggered eosinophil β1 integrin activation may represent a new therapeutic approach in asthma.
Airway eosinophilic inflammation is characteristic of asthma, contributes to exacerbations, and regulates airway remodeling (1, 2). Arrest and extravasation of eosinophils, as with other leukocytes, are believed to involve tethering and rolling on endothelium, mediated by selectins, cytokine- or chemokine-mediated activation of integrins, and transmigration in response to chemoattractants (3–5). An essential step in eosinophil arrest is the interaction of α4β1 integrin with vascular cell adhesion molecule-1 (VCAM-1), induced on endothelium in response to T helper cell type 2 (Th2) immunity mediators (1, 4–7) and expressed in bronchial vessels of the asthmatic lung (8).
Integrin-mediated cell adhesion is a function of integrin density, ligand density, and integrin activation state (9–11). In patients with mild asthma, there is enhanced activation of β1 integrins, assessed with activation-sensitive mAb N29 (12), on blood eosinophils after segmental lung antigen challenge, a trend to such enhanced activation after inhaled corticosteroid (ICS) withdrawal, and an inverse correlation of N29 reactivity with FEV1 (13, 14). Activation of β2 integrins, assessed with activation-sensitive mAb24 (11), is increased on bronchoalveolar lavage (BAL) eosinophils but not on blood eosinophils after segmental antigen challenge. The increase on BAL eosinophils correlates with IL-5 concentration in BAL fluid, whereas β1 activation is increased on blood and BAL eosinophils and does not correlate with IL-5 (14). These results suggest that activation of β1 integrins on circulating eosinophils complements induction of VCAM-1 to cause eosinophil recruitment to the airway. The results also indicate that β1 and β2 integrins are activated independently in vivo, which is somewhat surprising given the commonality of pathways that regulate β1 and β2 integrin activation (11). Although IL-5 is the likely in vivo β2 activator (14–17), the results raise the question of which stimulus is responsible for β1 integrin activation on blood eosinophils in vivo.
A variety of observations suggest that this activator is P-selectin. Therefore, we evaluated the hypothesis that P-selectin is responsible for β1 integrin activation on blood eosinophils in asthma in a way that would favor eosinophil movement to the airway. We analyzed (1) P-selectin associated with the eosinophil surface, (2) eosinophil P-selectin glycoprotein ligand (PSGL)-1 expression, and (3) the β1 activation state in blood samples from subjects with mild/moderate (nonsevere) asthma who were enrolled in an observational study. We performed complementary in vitro experiments to learn the effect of added P-selectin on the β1 activation state of blood eosinophils and explored whether β1 activation epitope expression is linked to the capacity of an eosinophil to adhere to VCAM-1 in vitro. The results indicate that P-selectin activates β1, but not β2, integrins of eosinophils and causes increased eosinophil adhesion to VCAM-1.
Materials and Methods
Subjects
Twenty-three subjects with mild or moderate (nonsevere) physician-diagnosed allergic asthma (Table 1) were studied as part of the VIAX (Virus-Induced Asthma Exacerbation) study at the University of Wisconsin-Madison Hospital (18, 19). Subjects were recruited and screened and then asked to return at the onset of an infection. All subjects had a positive skin prick test to at least 1 of 12 aeroallergens, did not have a history of severe episodes of asthma with respiratory infections, did not have an ICS dose of greater than 400 μg fluticasone/d or equivalent or Advair > 250/50, did not have an albuterol use of at least 6 puffs/d, were nonsmokers, and were not pregnant or breastfeeding (18, 19). Other subjects, who were normal, allergic rhinitic (nonasthmatic), and allergic asthmatic volunteers, donated blood for in vitro studies of whole blood and purified eosinophils as before (20). The studies were approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. Informed written consent was obtained from each subject before participation.
TABLE 1.
CHARACTERISTICS OF SUBJECTS WITH NONSEVERE ALLERGIC ASTHMA
| Sex | 16 females, 7 males |
| Age, yr | 22 (20, 30) |
| FEV1, l | 3.5 (2.9, 3.9) |
| FEV1, % pred. | 93 (86, 101) |
| PC20, mg/ml | 8.3 (1.1, 25) |
Definition of abbreviations: FEV1, forced expiratory volume in 1 s; PC20, provocative concentration of methacholine producing a 20% fall in FEV1; % pred., percentage of the predicted value.
Data are shown as medians (25th, 75th percentiles). FEV1 values are from visit 10 and PC20 values are from visit 8 of the VIAX study. Spirometry and methacholine challenge were performed according to American Thoracic Society guidelines (18, 19).
Antibodies, Flow Cytometry, and Immunofluorescence Microscopy
Antibodies used; flow cytometric analysis of whole, unfractionated blood or purified eosinophils; and immunofluorescence microscopy staining of eosinophils in a cytospun whole leukocyte population are described in the online supplement.
Cells
Eosinophils were purified from peripheral heparinized blood of volunteer donors by negative selection using a cocktail of anti-CD16, anti-CD14, and anti-CD3 magnetic beads in the AutoMACS system (Miltenyi, Auburn, CA) (20). The purity of eosinophils was greater than 99% as determined by Diff-Quik staining (Dade, Düdingen, Switzerland). Viability was greater than 98% as assessed by staining with propidium iodide and annexin V-FITC (BD Biosciences, San Jose, CA). A sample of the human EoL-3 eosinophilic leukemic cell line (21) was from Richard Lynch (University of Iowa, Iowa City, IA) and cultured as described (20).
Addition of Soluble P-Selectin or IL-5
Venous blood drawn into green-top heparin tubes (BD Vacutainer Systems, Franklin Lakes, NJ), or purified blood eosinophils resuspended at 5 × 106/ml in RPMI 1640 medium (Mediatech, Herndon, VA) with 0.2% BSA from volunteer donors were preincubated with or without different concentrations of added recombinant soluble extracellular domain of P-selectin or IL-5 (both from R&D Systems, Minneapolis, MN) for 1 hour at 37°C before being processed for flow cytometry as above.
Adhesion to VCAM-1
Selective adhesion to VCAM-1 and adhesion under static conditions were assayed as described in the online supplement.
Statistics
The Spearman rank correlation test was used to analyze correlations. The means of two populations or conditions from the same subjects were compared using two-tailed paired t test. A level of P ≤ 0.05 was considered significant. Analyses were performed using Prism (GraphPad, San Diego, CA).
Results
Correlation between Eosinophil Surface–Associated P-Selectin and β1 Integrin Activation State in Subjects with Nonsevere Asthma
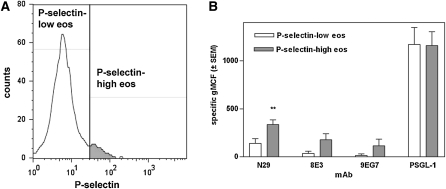
To begin the evaluation of the hypothesis that binding of P-selectin to eosinophils in whole blood causes activation of β1 integrins, we used flow cytometry to quantify the amounts of P-selectin on individual eosinophils in comparison to the densities of the epitopes for three activation-sensitive β1 mAbs, mouse N29 (12), mouse 8E3 (22), and rat 9EG7 (23). N29 is the mAb that we used previously (13, 14). We added mAb 8E3 here with the expectation that it would behave in a similar manner to N29 and strengthen the conclusions based on N29. N29 and 8E3 recognize Glu-4 in the amino-terminal plexin-semaphorin-integrin domain of the human β1 integrin subunit that is lysine in the mouse protein (17, 24). MAb 9EG7 was included to examine whether the β1 integrin activation on eosinophils encompasses regions in addition to the plexin-semaphorin-integrin domain. The 9EG7 epitope is located in the epidermal growth factor–like domains of the β1 “leg” (17, 23). Our first experiments included dual labeling and were done on three subjects with nonsevere asthma, a group in which β1 activation, assessed with N29, correlates with measurement of disease (13). Figure 1A and Figure E1 in the online supplement show the results from a representative subject. Figure 1B summarizes the results from the three subjects. Most eosinophils had no or low amounts of cell-surface–associated P-selectin (the white area of the distribution in Figure 1A, dots in the left part of the plots in Figure E1), but there was a population of eosinophils with higher P-selectin levels (the gray area in Figure 1A, population marked with ellipses in Figure E1). The background signal with isotype control antibodies was similar for the P-selectin–low and –high populations (Figures E1A and E1E). However, staining with N29, 8E3, or 9EG7 showed that the P-selectin–high population had a greater signal for these activation-sensitive β1 mAbs than had the P-selectin–low population (Figures E1B, E1C, and E1F). The P-selectin–high population denoted by the ellipses is located higher up than the P-selectin–low population, indicating that higher density of P-selectin was associated with higher density of activated β1 integrins. Quantifying the specific staining in the three subjects confirmed that the specific signal with the activation β1 mAbs was greater in the P-selectin–high than in the P-selectin–low eosinophil population (Figure 1B). Such a difference due to the P-selectin level was not seen with expression of the P-selectin counterreceptor PSGL-1 (Figures 1B and E1D).
Figure 1.
Expression of epitopes for activation-sensitive β1 integrin mAbs N29, 8E3, and 9EG7 and anti–P-selectin glycoprotein ligand (PSGL)-1 in two populations of eosinophils in whole blood with low or high levels of cell-surface–associated P-selectin. (A) Division of eosinophils into populations with a low (white) and high (gray) level of cell-surface–associated P-selectin. (B) Specific expression of N29, 8E3, or 9EG7 epitopes or anti–PSGL-1 in the P-selectin–low (white bars) and P-selectin–high (gray bars) populations. n = 3 subjects with nonsevere allergic asthma. Error bars = SEM. **P ≤ 0.01 versus the P-selectin–low population.
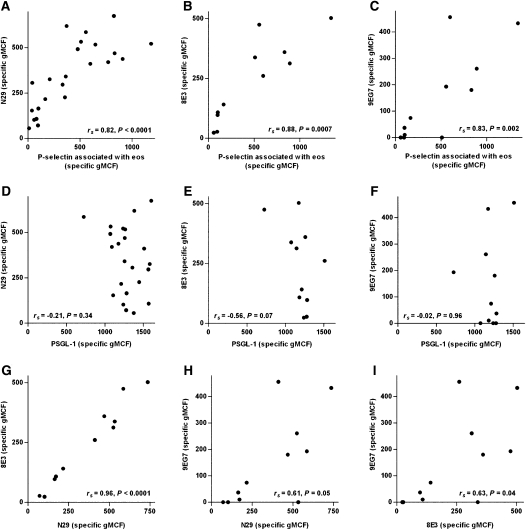
We examined whole blood from 23 subjects with nonsevere asthma (Table 1) for N29 epitope expression on eosinophils and a subset of the subjects with 8E3 and 9EG7. We determined whether there were any correlations between β1 integrin activation (reported by N29, 8E3, or 9EG7) and cell-surface–associated P-selectin or PSGL-1 and analyzed the correlations among the N29, 8E3, and 9EG7 epitopes. We found that there was a tight correlation (Spearman rank correlation coefficient [rs] > 0.8) between specific reactivity (adjusted for isotype control) with N29, 8E3, or 9EG7 and the specific level of P-selectin associated with eosinophil surface (Figures 2A–2C). In contrast, N29, 8E3, or 9EG7 reactivity did not correlate with PSGL-1 (Figures 2D–2F). Reactivities with 8E3 and N29 correlated with rs = 0.96, presumably the maximum correlation that can be achieved by flow cytometry (Figure 2G). Reactivity with 9EG7 tracked N29 or 8E3 but with lower correlation coefficients of 0.61 and 0.63 (Figures 2H and 2I).
Figure 2.
Correlations among anti–P-selectin, anti–PSGL-1, and epitopes for activation-sensitive anti-β1 integrin mAbs N29, 8E3, and 9EG7 on eosinophils in whole blood. Samples were from subjects with nonsevere allergic asthma. Data are expressed as specific geometric mean channel fluorescence (i.e., adjusted for isotype control; see Materials and Methods). N29 (A), 8E3 (B), or 9EG7 (C) versus anti–P-selectin; N29 (D), 8E3 (E), or 9EG7 (F) versus anti–PSGL-1; 8E3 (G) or 9EG7 (H) versus N29; and 9EG7 versus 8E3 (I). Flow cytometry data are from one randomly chosen visit (no. 3 or 11 in the VIAX study) per subject. n = 23 (in A and D) or a subset of 11 (in B, C, and E–I).
Cellular Staining for Activated β1 Integrin and P-Selectin
Eosinophils do not contain mRNA for P-selectin, as assessed by transcriptional array analysis (25). Possible in vivo sources of the eosinophil-bound P-selectin protein are platelets, endothelial cells, and plasma (26–28). To study the association between activated β1 integrin and P-selectin on circulating eosinophils and to determine possible sources of P-selectin, we performed double immunofluorescence microscopy staining of cytospins of mixed leukocytes from seven subjects with nonsevere asthma. We used thrombospondin (TSP)-1, a protein present in platelet α granules (29), and the αIIb subunit of αIIbβ3 integrin, an abundant platelet surface protein (30), as platelet markers. Eosinophils were identified by morphology (Figures E2C and E2E) and positive staining for eosinophil major basic protein (Figure E2B) or eosinophil peroxidase (EPO).
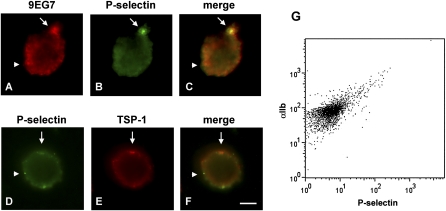
The proportion of cells positive for eosinophil major basic protein (Figure E2B) or EPO (i.e., eosinophils) in the cytospun leukocyte population from these subjects was 4 to 8%. Although there was heterogeneity among the subjects, general patterns discerned by immunofluorescence microscopic staining of eosinophils are shown in Figures 3 and E2 and summarized in Table 2. The eosinophils stained only weakly with N29 and 8E3 (not shown). Staining with activation-sensitive anti-β1 mAb 9EG7 was brighter and present in patchy and in diffuse patterns (Figure 3A). MAb 9EG7 staining was a subset of staining for total β1 with mAb13 or MAR4 (not shown). P-selectin staining was mostly punctate or patchy (Figures 3B and 3D). Stainings for 9EG7 and P-selectin partly overlapped (Figure 3C), but 9EG7-positive/P-selectin–negative areas were found (Figures 3A and 3C), indicating that some but not all sites of activated β1 on the eosinophil surface are at or close to sites where P-selectin has bound. TSP-1 staining was patchy (Figures 3E and E2A), with 5 to 25% of eosinophils being positive. αIIb staining was similar to that of TSP-1 (Figure E2D). P-selectin and TSP-1 stainings partly overlapped (Figure 3F), but there were sites that were P-selectin–positive and TSP-1–negative (Figures 3D and 3F), indicating that some but not all sites of P-selectin binding to the eosinophil surface are due to the association of platelets or platelet fragments with the eosinophil surface. Flow cytometric analysis showed that αIIb was higher on eosinophils with a high level of P-selectin (Figure 3G), indicating that most eosinophils with high surface P-selectin also had platelet satellitism.
Figure 3.
Double immunofluorescence microscopic localization of epitopes for activation-sensitive anti-β1 integrin mAb 9EG7, anti–P-selectin, and anti–TSP-1 and flow cytometric analysis of anti-αIIb integrin versus anti–P-selectin on eosinophils. Representative results of experiments on cells from seven subjects with nonsevere allergic asthma. (A–F) Eosinophils in cytospun whole leukocytes were identified by morphology or by positive staining for antibodies to eosinophil major basic protein or eosinophil peroxidase (see Figure E2 in the online supplement). Micrographs of eosinophils analyzed by staining with 9EG7 and rhodamine-conjugated anti-rat secondary antibody (A, C), mAb to P-selectin and FITC-conjugated anti-mouse secondary antibody (B, C, D, F), and biotinylated mAb to TSP-1 and rhodamine-avidin (E, F). Arrows, areas with colocalization. Arrowheads, areas with no colocalization. Note partial colocalization between 9EG7 epitope (arrow in A and C) and P-selectin (arrow in B and C) and between P-selectin (arrow in D and F) and TSP-1 (arrow in E and F). Also note 9EG7-positive/P-selectin–negative areas (arrowhead in A and C) and P-selectin–positive/TSP-1–negative areas (arrowhead in D and F). Scale bar = 5 μm. (G) Flow cytometry of αIIb (mAb HIP8) versus P-selectin on eosinophils in whole blood.
TABLE 2.
SUMMARY OF IMMUNOFLUORESCENT MICROSCOPIC STAINING OF EOSINOPHILS FOR ACTIVATED β1 INTEGRIN, P-SELECTIN, AND PLATELET MARKERS
| Antibody | Pattern | Colocalization with others | Figure |
| Anti-total β1 | Patchy and diffuse, in all eos | More extensive than 9EG7 | NS |
| Anti-active β1 (9EG7) | Patchy and diffuse, in many but not all eos | Less extensive than total β1 | NS |
| Partly overlapping with P-selectin, also P-selectin-negative areas | 3A,C | ||
| 3A,C | |||
| Anti-P-selectin | Punctate or patchy, in many but not all eos | Partly overlapping with 9EG7 | 3B,C |
| Partly overlapping with TSP-1, also TSP-1-negative areas | 3D,F | ||
| 3D,F | |||
| Anti-TSP-1 | Patchy, in some but not all eos | Partly overlapping with 9EG7, similar to αIIb | 3E,F |
| E2A | |||
| Anti-αIIb | Similar to TSP-1 | Similar to TSP-1 | E2D |
| Anti-eos MBP | Granules, in all eos | Not overlapping with the others | E2B |
Definition of abbreviations: eos, eosinophils; EPO, eosinophil peroxidase; MBP, major basic protein; NS, not shown; TSP, thrombospondin.
This table summarizes the data shown in Figures 3 and E2 (in the online supplement) as well as data not shown. Immunofluorescence microscopic staining of cytospun whole leukocytes from seven subjects with nonsevere allergic asthma was performed as described in Materials and Methods in the online supplement. Eosinophils were identified by morphology (see Figure E2C, E) and positive staining for eosinophil MBP (Figure E2B) or EPO (not shown).
Compared with these results with cytospun whole blood leukocytes, in which 5 to 25% of eosinophils had platelets or platelet fragments associated with the cell surface as judged by TSP-1 or αIIb staining, in similar experiments on cytospun purified eosinophils, we found 15 to 25% of the eosinophils to be TSP-1 or αIIb positive (not shown). The leukemic eosinophilic cell line EoL-3, which has constitutive activation of β1 integrins (16), was negative for αIIb (not shown).
Enhancement of Eosinophil β1 Integrin, but Not β2 Integrin, Activation by Added Soluble P-Selectin
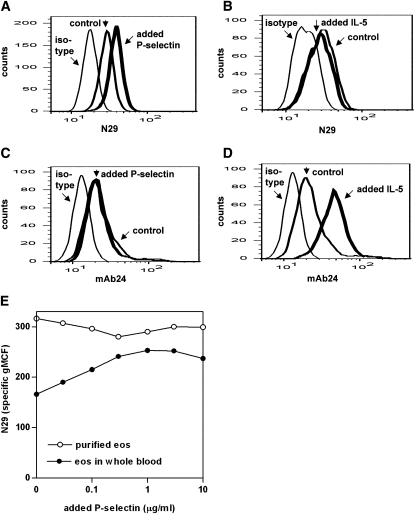
Soluble extracellular domain of P-selectin was added to whole blood or purified eosinophils (Figure 4E), the latter conditions mimicking previous studies done on purified monocytes (31, 32). For the subject shown in Figures 4A and 4E, N29 epitope expression was higher on purified eosinophils than on eosinophils in whole blood in the absence of P-selectin. When we compared the effects of a 1-hour preincubation with added P-selectin on eosinophils in whole blood versus purified eosinophils, N29 reactivity increased (i.e., the N29 distribution shifted to the right) on eosinophils in whole blood (Figures 4A and 4E) but not on purified eosinophils (Figure 4E). The increase in N29 reactivity on eosinophils in whole blood in response to P-selectin was observed in four of five donors from whom paired samples of whole blood and purified eosinophils were obtained, whereas in no sample of purified eosinophils did P-selectin increase N29. Total β1 did not increase after addition of P-selectin in whole blood or on purified eosinophils (not shown). Maximal N29 epitope expression was achieved at concentrations of added P-selectin of 100 to 1,000 ng/ml (Figure 4E). Thus, although P-selectin can increase β1 integrin activation on eosinophils in whole blood, it does not increase activated β1 on purified eosinophils from the same donors, which seem to be maximally stimulated, probably during the purification process.
Figure 4.
Effect of added soluble P-selectin or IL-5 on expression of epitopes for activation-sensitive anti-β1 integrin mAb N29 or activation-sensitive anti-β2 integrin mAb24. N29 (A, B) or mAb24 (C, D) epitope expression on eosinophils in whole blood after preincubation with (thick line) or without (normal line) added soluble P-selectin (0.1 μg/ml) (A, C) or IL-5 (50 ng/ml) (B, D). The mouse IgG1 isotype is shown as a thin line. (E) N29 epitope expression on eosinophils in matched whole blood (closed symbols) and purified blood eosinophils (open symbols) from a normal subject after preincubation with different concentrations of added soluble P-selectin. Representative results of experiments with five donors (see text and Figure E4 in the online supplement).
In studies of subjects with mild asthma undergoing ICS withdrawal, N29 reactivity correlated with FEV1 (13). Experiments on additional subjects indicated that baseline N29 reactivity in whole blood was higher in subjects with asthma than in normal donors (not shown). Such observations prompted us to ask whether the magnitude of the P-selectin–stimulated increase in the N29 signal differ among subject groups due to their status. Enhanced N29 epitope expression after P-selectin preincubation compared with after control preincubation was found in 3 of 3 normal subjects, 8 of 14 nonasthmatic subjects with allergic rhinitis, and 5 of 13 subjects with asthma. The increase was significant in the normal group (P = 0.004) (Figure E3). Baseline N29 reactivity (in the absence of added P-selectin) in the present groups was in the order normal < allergic nonasthmatic < allergic asthmatic donors (Figure E3). These results indicate that whether β1 integrin activation on eosinophils in whole blood is enhanced by the addition of P-selectin depends on the level of baseline activation that is present in vivo compared with maximal possible activation.
We compared the effect of P-selectin with that of IL-5, a known activator of eosinophil αMβ2 integrin (15), in five donors who responded to P-selectin by enhanced N29 reactivity (Figure E4A). IL-5 significantly enhanced reactivity with mAb24, which detects a β2 activation epitope (11) (Figures 4D and E4B), but did not increase N29 reactivity (Figures 4B and E4A). Conversely, mAb24 reactivity was not changed after the addition of P-selectin (Figures 4C and E4B).
Because P-selectin has been reported to activate αMβ2 integrin on purified neutrophils (33–35) and monocytes (32), we analyzed the same data sets for integrin activation on these leukocytes after P-selectin or IL-5 addition to whole blood. Baseline reactivities of N29 and mAb24 on the leukocyte populations were in the order eosinophils < neutrophils < monocytes (Figure E4), as previously reported for N29 (14). Whereas P-selectin enhanced the N29 reactivity on eosinophils (Figure E4A), there were only trends to small increases in the N29 signals on neutrophils (Figure E4C) and monocytes (Figure E4E) that did not reach significance. IL-5 did not increase the mAb24 signal on neutrophils (Figure E4D) or monocytes (Figure E4F), which lack the IL-5 receptor α subunit (30). P-selectin (100 ng/ml) also did not enhance mAb24 reactivity on neutrophils (Figure E4D) and monocytes (Figure E4F), which is different from what would be expected from the literature (32, 34). We have not attempted to use the conditions that were used in published studies (32, 34), such as purified neutrophils or monocytes, the higher P-selectin concentrations of 150 ng/ml to 10 μg/ml, or the activation-sensitive anti-αM mAb CBRM1/5.
Decreased β1 Integrin Activation State and Surface-Associated P-Selectin on Cells Nonadherent to VCAM-1
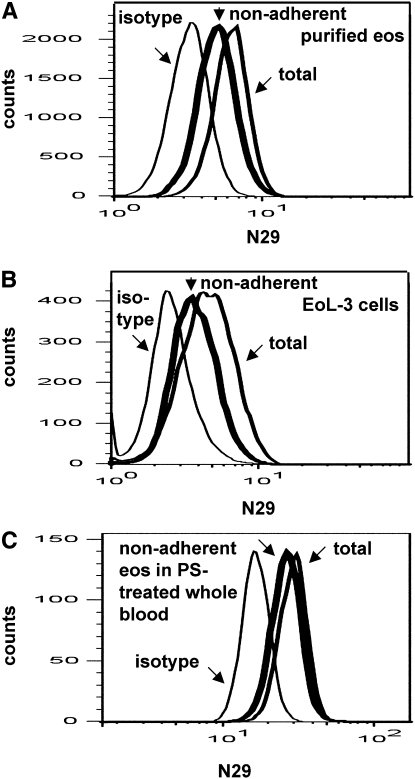
The reactivity of activation-sensitive, antiintegrin mAbs is expected to be linked to a cell's ability to interact with ligand (10). To test whether N29 reactivity reports a cell's capacity to adhere to VCAM-1, purified blood eosinophils were plated in VCAM-1–coated wells, and the cells that did not adhere to the substrate were compared with the starting population and with nonadherent cells in wells coated only with blocking protein. Studies were done in parallel with EoL-3 cells, which have the N29 epitope (16) but no platelet satellitism. Different coating concentrations of VCAM-1 were used for the different cells because EoL-3 cells require less VCAM-1 to adhere than eosinophils (16). The distribution of N29 epitope expression of purified eosinophils and EoL-3 cells nonadherent to VCAM-1 was shifted to the left (Figures 5A and 5B). Results from three experiments with EoL-3 cells showed that nonadherent cells had significantly decreased N29 reactivity compared with the original or total population, whereas total β1 was not significantly decreased (not shown). N29 epitope expression of cells that did not adhere to the control substrate (gelatin for eosinophils, BSA for EoL-3 cells) was not significantly different from that of the original population (not shown).
Figure 5.
N29 epitope expression on blood eosinophils or eosinophilic leukemic EoL-3 cells in suspension before and after adhesion to vascular cell adhesion molecule (VCAM)-1. Representative of results with four donors (eosinophils) or three experiments (EoL-3 cells). N29 epitope expression was measured on purified blood eosinophils from an allergic asthmatic donor (A), EoL-3 cells (B), or eosinophils in whole blood from an allergic asthmatic donor to which soluble P-selectin (0.1 μg/ml) had been added (C). Measurements were made on the original cell population before adhesion to VCAM-1 (“total,” normal line) or on cells nonadherent to coated soluble 7-domain VCAM-1 (thick line). The mouse IgG1 isotype is shown as a thin line. Coating concentrations of VCAM-1 were 10, 1, and 30 μg/ml in A, B, and C, respectively. The fact that all distributions in (C) as well as in Figures 4A–4D are to the right compared with those in A and B is due to different cytometer settings for whole blood to accommodate neutrophils and monocyte distributions compared with for purified cells or cell lines.
Enhancement of Eosinophil Adhesion to VCAM-1 by Added Soluble P-Selectin
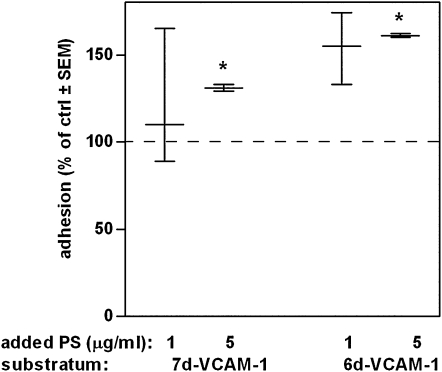
A similar assay to that described above was done with whole blood from four donors to which P-selectin had been added. The N29 epitope distribution of eosinophils in P-selectin–treated whole blood nonadherent to VCAM-1 was different from the original population (Figure 5C) and from cells nonadherent to gelatin (not shown), with a small shift to the left. Eosinophils in whole blood nonadherent to VCAM-1 did not have lower total β1 than the original population or than cells nonadherent to gelatin (not shown). These results indicate that eosinophils with β1 activated in response to P-selectin bind selectively to VCAM-1. To demonstrate this more directly, eosinophil adhesion in whole blood to the alternatively spliced 7- and 6-domain forms of VCAM-1 under static conditions was quantified with the EPO assay. The 7-domain form of VCAM-1 contains two integrin-binding domains, domain 1 and domain 4, whereas the 6-domain form only contains one integrin-binding domain, domain 1 (16, 17). Domain 1 interacts with α4β1 integrin, whereas domain 4 can interact with α4β1 and αMβ2 (16, 17). Thus, 6-domain VCAM-1 supports α4β1–mediated adhesion, whereas 7-domain VCAM-1 primarily supports α4β1–mediated adhesion but can also support αMβ2–mediated adhesion (16). Added P-selectin significantly enhanced adhesion to the 6- and 7-domain forms of VCAM-1 (Figure 6). The P-selectin effect was specific for VCAM-1 as a substratum because P-selectin did not cause any significant increased background adhesion to gelatin (not shown).
Figure 6.
Effect of added soluble P-selectin on blood eosinophil adhesion to VCAM-1. Static adhesion of eosinophils in whole blood, after preincubation without (= 100% of control, dashed line) or with added soluble P-selectin (1 or 5 μg/ml) to soluble 7-domain (7 d) or 6-domain (6 d) VCAM-1, coated at 1 μg/ml. n = 4 experiments with 7 d, 3 experiments with 6 d VCAM-1. Median with interquartile range. *P ≤ 0.05 versus no-addition control. Adhesion was quantified by an EPO assay, which measures the optical density of the colored enzyme product at 490 nm.
Discussion
We previously reported that activation of β1 integrins on blood eosinophils can occur in patients with mild asthma, varies among subjects, and correlates with disease activity (13, 14). Several observations in the literature suggested that the stimulus for this activation may be interaction with P-selectin. P-selectin is a counterreceptor for PSGL-1, which is expressed constitutively on eosinophils and other leukocytes (36). P-selectin is sequestered in α granules of platelets and Weibel-Palade bodies of endothelial cells and translocated to the surface in response to various mediators, such as thrombin, histamine, epinephrine, ADP, or vascular endothelial growth factor (26–28, 37, 38). Activated platelets bind to leukocytes via P-selectin (39). An in vitro study indicated that P-selectin–bearing platelets contributes to enhanced tethering of eosinophils to activated endothelium (40). Genetic inactivation in mice has implicated P-selectin as a critical mediator in a model of eosinophil recruitment or asthma (41). Transfusion of platelet-depleted mice with activated wild-type, but not P-selectin–deficient, platelets in a lung antigen challenge model resulted in eosinophil recruitment to the airway (42). The addition of purified soluble P-selectin in vitro to monocytes activated β1 integrins and enhanced adhesion to VCAM-1 (31, 32).
Thus, we evaluated the hypothesis that P-selectin is the stimulus responsible for activation of β1 integrins on blood eosinophils in vivo. This evaluation was not straightforward because of the need to work with eosinophils in unfractionated blood and the variability in baseline β1 activation among subjects. Therefore, we pursued two experimental paradigms, the first exploring the variability among donors and the second testing the effect of adding P-selectin to blood eosinophils, recognizing that individual variability would complicate the interpretation of the results.
By flow cytometry of blood samples from subjects with nonsevere asthma, P-selectin was detected on the eosinophil surface to a variable degree among subjects and among eosinophils within the same subject. Reactivity with activation-sensitive β1 mAb N29, 8E3, or 9EG7 correlated with eosinophil-bound P-selectin among an individual's eosinophils and for the whole eosinophil populations among the subjects. The correlations support the hypothesis that an interaction between P-selectin and eosinophils is responsible for an enhanced eosinophil β1 activation state in vivo.
Immunofluorescence microscopy staining of whole leukocytes from subjects with nonsevere asthma was consistent with the flow cytometry data in that there was variable staining for the β1 activation epitopes and P-selectin among subjects and among eosinophils within a subject. Staining for the platelet markers TSP-1 and αIIb integrin subunit indicated that platelets or platelet fragments were associated with a fraction of eosinophils. This is in agreement with an earlier report of a heterogeneous, moderate level of “expression” of the platelet-specific αIIb integrin subunit on eosinophils by flow cytometry and demonstration by electron microscopy and immunogold that the αIIb staining is due to platelets adhering to or associated with the eosinophil membrane (43). Our experiments showed that there was partial but not complete overlap among eosinophil stainings for β1 activation epitopes, P-selectin, and TSP-1. The results, therefore, indicate that P-selectin associated with the eosinophil surface is partly due to “satellitism” of activated platelets or platelet fragments and partly due to P-selectin binding from other sources, presumably endothelium and/or plasma, and indicate that eosinophil β1 is activated at sites of P-selectin binding and at other sites.
In in vitro experiments, eosinophil N29 reactivity was enhanced by the addition of exogenous soluble P-selectin to blood of many but not all donors. Maximal effect on eosinophil N29 epitope expression was obtained with 100 to 1,000 ng/ml of added P-selectin. The concentration of soluble plasma P-selectin in normal subjects is 20 to 40 ng/ml (27), but the concentration can be increased severalfold in disease states, such as hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and acute myocardial infarction (44). Concentrations up to 70 ng/ml have been measured repeatedly in patients with asthma, and the extent of this elevation has been correlated with measures of disease (45–47). Up to 170 ng/ml has been recorded after exercise in subjects with exercise-induced asthma (47). Thus, the added P-selectin concentration (100 ng/ml) found here to be active in inducing β1 integrin activation in vitro corresponds to a 2.5 to 5-fold increase over the concentration in healthy individuals, is within the range that has been measured in plasma of patients with asthma, and, consequently, is pathophysiologically relevant.
To explore the functional significance of β1 integrin activation, cells nonadherent to VCAM-1 were analyzed. Compared with the original population, the nonadherent population was depleted of the cells with the highest N29 signal. The results indicate that VCAM-1 preferentially supports adhesion of those eosinophils with higher N29 reactivity. Furthermore, the addition of soluble P-selectin enhanced eosinophil adhesion to VCAM-1. This result is in partial accord with other in vitro studies, in which the addition of soluble P-selectin to purified monocytes enhanced reactivity with the activation-sensitive mAbs HUTS-21 to β1 and CBRM1/5 (to αM) and adhesion to VCAM-1, fibronectin, and ICAM-1 (31, 32). Similarly, addition of soluble P-selectin to purified neutrophils in vitro enhanced reactivity with CBRM1/5 and adhesion to fibrinogen and ICAM-1 (33–35). In contrast to the reports that P-selectin activates α4β1 and αMβ2 integrins on purified monocytes and αMβ2 on purified neutrophils, however, we found activation of only β1, and not β2, integrins on eosinophils. The addition of IL-5, conversely, significantly increased eosinophil mAb24 but not N29 epitope expression. Thus, there is specificity of P-selectin–triggered integrin activation on eosinophils (only β1), compared with the reported situation on monocytes (β1 and β2) (32). However, we did not detect a P-selectin–triggered increase in β2 activation on monocytes or neutrophils at the P-selectin concentration used (100 ng/ml) in our assay on whole blood. In any case, in addition to general mechanisms for inside-out integrin activation (11), specific mechanisms for activation of the different β integrin subfamilies must exist for eosinophils.
The present data are compatible with a scenario that P-selectin activates eosinophil β1 integrins, resulting in stimulation of eosinophil adhesion to VCAM-1, egress, and recruitment to the airway. P-selectin may be picked up from activated platelets, encounters with activated endothelial cells, or plasma. Microscopy indicated that some activated β1 is located on the eosinophil surface per se and not on the surface of eosinophil-associated platelets. Thus, the observed activated β1 likely is, in part, the result of triggered intracellular signaling and inside-out activation of eosinophil integrins. In neutrophils or leukemic cell lines, the PSGL-1 cytoplasmic domain binds Nef-associated factor 1, which becomes phosphorylated by Src family kinases and recruits phosphoinositide-3-OH kinase, resulting in αMβ2 integrin activation (35). Evidence exists that the activation level of α4β1 integrin on purified blood eosinophils, judged by anti-α4–inhibitable cell affinity for VCAM-1–coated beads, is maintained by a pathway involving homeostatic intracellular free Ca2+, phospholipase C, and Rap1 (48), consistent with current models for integrin activation (11). Further investigation is needed to determine whether these signaling pathways are responsible for the activation of β1 integrins as a consequence of P-selectin binding to eosinophils.
Supplementary Material
Acknowledgments
The authors thank Nizar Jarjour and William Busse for many helpful discussions and steadfast support; Mary Jo Jackson for patient recruitment, screening, and assessments; Gina Crisafi and Katie Gaworski for providing blood samples from the VIAX study; Anne Brooks, Sarah Petrie, Julie Sedgwick, and Elizabeth Schwantes for blood samples from volunteer donors and for eosinophil purification; Richard Lynch for EoL-3 cells; Martin Humphries for mAb 8E3 and discussion on activation-sensitive anti-β1 integrin mAbs; Nancy Hogg for mAb24; Douglas Annis for biotinylated anti-TSP-1; Kathleen Schell and Joel Puchalski for help with flow cytometry; Kristin Gunderson and Lindsay Hill for help with in vitro experiments; Nizar Jarjour, Ronald Sorkness, Cheri Swenson, Erin Billmeyer, Gina Crisafi, Evelyn Failbene, Margaret McWilliams, and Michael Evans for information and assessment data on the VIAX subjects; and Michael Evans for advice on statistics.
Footnotes
This work was supported by Specialized Center of Research grant P50 HL56396 (W.W.B. and D.F.M.), Program Project grant P01 HL88594 and ARRA supplement grant P01 HL88594–02S1 (N.N.J. and D.F.M.), grant R01 HL80412 (N.N.J.), General Clinical Research Center grant M01 RR03186 (R.N.G.), and Clinical and Translational Science Award grant UL1 RR25011 (M.K.D.) from the National Institutes of Health and by Robert Draper Technology Innovation Funding (M.W.J.) from the Graduate School, University of Wisconsin-Madison.
Originally Published in Press as DOI: 10.1165/rcmb.2010-0402OC on March 25, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wills-Karp M, Karp CL. Eosinophils in asthma: remodeling a tangled tale. Science 2004;305:1726–1729 [DOI] [PubMed] [Google Scholar]
- 2.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009;360:973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994;76:301–314 [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J Allergy Clin Immunol 1999;104:917–926 [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol 2007;119:1303–1310 [DOI] [PubMed] [Google Scholar]
- 6.Schleimer RP, Sterbinsky SA, Kaiser J, Bickel CA, Klunk DA, Tomioka K, Newman W, Luscinskas FW, Gimbrone MA, Jr, McIntyre BW, et al. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium: association with expression of VCAM-1. J Immunol 1992;148:1086–1092 [PubMed] [Google Scholar]
- 7.Banerjee ER, Jiang Y, Henderson WR, Jr, Scott LM, Papayannopoulou T. Alpha4 and beta2 integrins have nonredundant roles for asthma development, but for optimal allergen sensitization only alpha4 is critical. Exp Hematol 2007;35:605–617 [DOI] [PubMed] [Google Scholar]
- 8.ten Hacken NH, Postma DS, Bosma F, Drok G, Rutgers B, Kraan J, Timens W. Vascular adhesion molecules in nocturnal asthma: a possible role for VCAM-1 in ongoing airway wall inflammation. Clin Exp Allergy 1998;28:1518–1525 [DOI] [PubMed] [Google Scholar]
- 9.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 1997;385:537–540 [DOI] [PubMed] [Google Scholar]
- 10.Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci 2009;122:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci 2009;122:215–225 [DOI] [PubMed] [Google Scholar]
- 12.Wilkins JA, Li A, Ni H, Stupack DG, Shen C. Control of beta1 integrin function: localization of stimulatory epitopes. J Biol Chem 1996;271:3046–3051 [PubMed] [Google Scholar]
- 13.Johansson MW, Barthel SR, Swenson CA, Evans MD, Jarjour NN, Mosher DF, Busse WW. Eosinophil beta(1) integrin activation state correlates with asthma activity in a blind study of inhaled corticosteroid withdrawal. J Allergy Clin Immunol 2006;117:1502–1504 [DOI] [PubMed] [Google Scholar]
- 14.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol 2008;180:7622–7635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol 2006;35:378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barthel SR, Annis DS, Mosher DF, Johansson MW. Differential engagement of modules 1 and 4 of vascular cell adhesion molecule-1 (CD106) by integrins alpha4beta1 (CD49d/29) and alphaMbeta2 (CD11b/18) of eosinophils. J Biol Chem 2006;281:32175–32187 [DOI] [PubMed] [Google Scholar]
- 17.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol 2008;83:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorkness RL, Gonzalez-Fernandez G, Billmeyer EE, Evans MD, Gern JE, Jarjour NN. The asthma index: a continuous variable to characterize exacerbations of asthma. J Allergy Clin Immunol 2008;122:838–840 [DOI] [PubMed] [Google Scholar]
- 19.Denlinger LC, Shi L, Guadarrama A, Schell K, Green D, Morrin A, Hogan K, Sorkness RL, Busse WW. Gern JE. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med 2009;179:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson MW, Lye MH, Barthel SR, Duffy AK, Annis DS, Mosher DF. Eosinophils adhere to vascular cell adhesion molecule-1 via podosomes. Am J Respir Cell Mol Biol 2004;31:413–422 [DOI] [PubMed] [Google Scholar]
- 21.Saito H, Bourinbaiar A, Ginsburg M, Minato K, Ceresi E, Yamada K, Machover D, Breard J, Mathe G. Establishment and characterization of a new human eosinophilic leukemia cell line. Blood 1985;66:1233–1240 [PubMed] [Google Scholar]
- 22.Coe AP, Askari JA, Kline AD, Robinson MK, Kirby H, Stephens PE, Humphries MJ. Generation of a minimal alpha5beta1 integrin-Fc fragment. J Biol Chem 2001;276:35854–35866 [DOI] [PubMed] [Google Scholar]
- 23.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem 1995;270:25570–25577 [DOI] [PubMed] [Google Scholar]
- 24.Mould AP, Travis MA, Barton SJ, Hamilton JA, Askari JA, Craig SE, Macdonald PR, Kammerer RA, Buckley PA, Humphries MJ. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J Biol Chem 2005;280:4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bates ME, Liu LY, Esnault S, Stout BA, Fonkem E, Kung V, Sedgwick JB, Kelly EA, Bates DM, Malter JS, et al. Expression of interleukin-5- and granulocyte macrophage-colony-stimulating factor-responsive genes in blood and airway eosinophils. Am J Respir Cell Mol Biol 2004;30:736–743 [DOI] [PubMed] [Google Scholar]
- 26.Andre P. P-selectin in haemostasis. Br J Haematol 2004;126:298–306 [DOI] [PubMed] [Google Scholar]
- 27.Kappelmayer J, Nagy B, Jr, Miszti-Blasius K, Hevessy Z, Setiadi H. The emerging value of P-selectin as a disease marker. Clin Chem Lab Med 2004;42:475–486 [DOI] [PubMed] [Google Scholar]
- 28.Kneuer C, Ehrhardt C, Radomski MW, Bakowsky U. Selectins: potential pharmacological targets?. Drug Discov Today 2006;11:1034–1040 [DOI] [PubMed] [Google Scholar]
- 29.Mosher DF. Physiology of thrombospondin. Annu Rev Med 1990;41:85–97 [DOI] [PubMed] [Google Scholar]
- 30.Kishimoto T, Kikutani H, von dem Borne AEGK, Goyert SM, Mason DY, Miyasaka M, Moretta L, Okumura K, Shaw S, Springer TA, et al. Leucocyte typing VI: white cell differentiation antigens. New York: Garland Publishing, Inc.; 1997 [Google Scholar]
- 31.Yago T, Tsukuda M, Minami M. P-selectin binding promotes the adhesion of monocytes to VCAM-1 under flow conditions. J Immunol 1999;163:367–373 [PubMed] [Google Scholar]
- 32.da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol 2006;79:499–507 [DOI] [PubMed] [Google Scholar]
- 33.Ma YQ, Plow EF, Geng JG. P-selectin binding to P-selectin glycoprotein ligand-1 induces an intermediate state of alphaMbeta2 activation and acts cooperatively with extracellular stimuli to support maximal adhesion of human neutrophils. Blood 2004;104:2549–2556 [DOI] [PubMed] [Google Scholar]
- 34.Woollard KJ, Kling D, Kulkarni S, Dart AM, Jackson S, Chin-Dusting J. Raised plasma soluble P-selectin in peripheral arterial occlusive disease enhances leukocyte adhesion. Circ Res 2006;98:149–156 [DOI] [PubMed] [Google Scholar]
- 35.Wang HB, Wang JT, Zhang L, Geng ZH, Xu WL, Xu T, Huo Y, Zhu X, Plow EF, Chen M, et al. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol 2007;8:882–892 [DOI] [PubMed] [Google Scholar]
- 36.Symon FA, Lawrence MB, Williamson ML, Walsh GM, Watson SR, Wardlaw AJ. Functional and structural characterization of the eosinophil P-selectin ligand. J Immunol 1996;157:1711–1719 [PubMed] [Google Scholar]
- 37.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest 1989;84:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hippenstiel S, Krull M, Ikemann A, Risau W, Clauss M, Suttorp N. VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol 1998;274:L678–L684 [DOI] [PubMed] [Google Scholar]
- 39.de Bruijne-Admiraal LG, Modderman PW, Von dem Borne AE, Sonnenberg A. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood 1992;80:134–142 [PubMed] [Google Scholar]
- 40.Ulfman LH, Joosten DP, van Aalst CW, Lammers JW, van de Graaf EA, Koenderman L, Zwaginga JJ. Platelets promote eosinophil adhesion of patients with asthma to endothelium under flow conditions. Am J Respir Cell Mol Biol 2003;28:512–519 [DOI] [PubMed] [Google Scholar]
- 41.De Sanctis GT, Wolyniec WW, Green FH, Qin S, Jiao A, Finn PW, Noonan T, Joetham AA, Gelfand E, Doerschuk CM, et al. Reduction of allergic airway responses in P-selectin-deficient mice. J Appl Physiol 1997;83:681–687 [DOI] [PubMed] [Google Scholar]
- 42.Pitchford SC, Momi S, Giannini S, Casali L, Spina D, Page CP, Gresele P. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood 2005;105:2074–2081 [DOI] [PubMed] [Google Scholar]
- 43.Wardlaw AJ, Jeffrey PK, Majumdar S, Dewar A, Anwar ARE, Walsh G, Kay AB. Platelet adhesion to eosinophils. Am Rev Respir Dis 1992;145:A664 [Google Scholar]
- 44.Gearing AJ, Newman W. Circulating adhesion molecules in disease. Immunol Today 1993;14:506–512 [DOI] [PubMed] [Google Scholar]
- 45.Yu H, Ren J, Wang F, Liu L, Wu S, Liu Y. (P-selectin and tachykinins in bronchial hyperresponsiveness of asthma.). Zhonghua Nei Ke Za Zhi 1999;38:228–230 [PubMed] [Google Scholar]
- 46.Kowal K, Pampuch A, Kowal-Bielecka O, DuBuske LM, Bodzenta-Lukaszyk A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin Exp Allergy 2006;36:426–432 [DOI] [PubMed] [Google Scholar]
- 47.Zietkowski Z, Skiepko R, Tomasiak MM. Bodzenta-Lukaszyk A. Soluble CD40 ligand and soluble P-selectin in allergic asthma patients during exercise-induced bronchoconstriction. J Investig Allergol Clin Immunol 2008;18:272–278 [PubMed] [Google Scholar]
- 48.Ulfman LH, Kamp VM, van Aalst CW, Verhagen LP, Sanders ME, Reedquist KA, Buitenhuis M, Koenderman L. Homeostatic intracellular-free Ca2+ is permissive for Rap1-mediated constitutive activation of alpha4 integrins on eosinophils. J Immunol 2008;180:5512–5519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.