Abstract
Recent reports postulate that the dual oxidase (DUOX) proteins function as part of a multicomponent oxidative pathway used by the respiratory mucosa to kill bacteria. The other components include epithelial ion transporters, which mediate the secretion of the oxidizable anion thiocyanate (SCN−) into airway surface liquid, and lactoperoxidase (LPO), which catalyzes the H2O2-dependent oxidation of the pseudohalide SCN− to yield the antimicrobial molecule hypothiocyanite (OSCN−). We hypothesized that this oxidative host defense system is also active against respiratory viruses. We evaluated the activity of oxidized LPO substrates against encapsidated and enveloped viruses. When tested for antiviral properties, the LPO-dependent production of OSCN− did not inactivate adenovirus or respiratory syncytial virus (RSV). However, substituting SCN− with the alternative LPO substrate iodide (I−) resulted in a marked reduction of both adenovirus transduction and RSV titer. Importantly, well-differentiated primary airway epithelia generated sufficient H2O2 to inactivate adenovirus or RSV when LPO and I− were supplied. The administration of a single dose of 130 mg of oral potassium iodide to human subjects increased serum I− concentrations, and resulted in the accumulation of I− in upper airway secretions. These results suggest that the LPO/I−/H2O2 system can contribute to airway antiviral defenses. Furthermore, the delivery of I− to the airway mucosa may augment innate antiviral immunity.
Keywords: respiratory syncytial virus, adenovirus, lactoperoxidase, thiocyanate, iodide
Clinical Relevance
This study shows that the lactoperoxidase/I−/ H2O2 system can contribute to airway antiviral defenses. Furthermore, the delivery of I− to airway mucosa may augment innate antiviral immunity. The identification of a new prophylactic or therapeutic approach to prevent or ameliorate respiratory viral infections could have broad implications for human health.
Acute viral lower respiratory tract infections represent enormous disease burdens for infants, children, and adults throughout the world. In children, acute lower respiratory infections are the most frequent cause of mortality worldwide (1, 2). Despite the prevalence and severity of these infections, few specific prophylactic or therapeutic interventions are available for many of these common diseases, including effective vaccines or antiviral treatments. For example, Group B and C adenoviruses are frequent causes of bronchiolitis and pneumonia, and occasionally cause postinfectious bronchiolitis obliterans (3, 4). Although respiratory syncytial virus (RSV) is the most common cause of acute lower respiratory infections throughout the world (5), no effective vaccine or specific, widely used antiviral therapy is available.
In evaluating novel approaches to antiviral defenses, we considered the redundant layers of innate immunity at play in the airways. We questioned whether an existing host defense mechanism might be induced or augmented to achieve enhanced antiviral protection in the lung. Both nonoxidative and oxidative host defense systems contribute to the antimicrobial activity of airway surface liquid (ASL) (6). Although the host defense roles of many secreted proteins and peptides are well-characterized, recognition that the airway epithelium orchestrates an oxidative extracellular microbicidal system is recent (7–10). This reactive oxygen species–producing system consists of the airway epithelial proteins dual oxidase (DUOX) 1 and 2, lactoperoxidase (LPO; secreted by submucosal glands), and the pseudohalide ion (thiocyanate, SCN−; secreted by epithelia) (11). However, the efficacy of this LPO/halide/H2O2 airway mucosal defense system has not been investigated for viral respiratory pathogens.
The LPO-catalyzed oxidation of SCN− produces hypothiocyanite (OSCN−) (12), whereas the oxidation of I− produces hypoiodous acid (HOI) (13). Both OSCN− and HOI are short-lived, reactive intermediates that are known to be bactericidal. We hypothesized that this oxidative antimicrobial system might possess functional relevance for preventing respiratory viral infections, through the generation of OSCN− or HOI. Here we show that the delivery of I− to respiratory epithelial cells supports the generation of HOI in ASL. HOI, but not OSCN−, readily inactivates adenovirus and RSV. Supplementation with I− may serve to augment mucosal antiviral defenses.
Materials and Methods
Chemicals and Enzymes
Sodium iodide (catalogue number S324–500; Fisher, Pittsburgh, PA) and sodium thiocyanate (catalogue number 251410–500G; Sigma, St. Louis, MO) were dissolved in deionized, distilled water, sterile-filtered, and stored at −20°C. Thirty percent H2O2 (catalogue number H325–500) was purchased from Fisher. Bovine milk LPO was obtained from Sigma (catalogue number L2005–25MG). The entire sample was resuspended in deionized, distilled water to a final concentration of 1 mg/ml (39 U/ml). Bovine liver catalase was obtained from Sigma (catalogue number C1345).
Culture of Cell Lines
Vero cells (ATCC, Manassas, VA) were grown in Dulbecco's minimum essential medium (DMEM; Invitrogen, Carlsbad, CA), supplemented with 10% FBS and 1% penicillin–streptomycin (PS). A549 and HEp-2 cells (ATCC) were grown in DMEM/F12 with 10% FBS and 1% PS.
Primary Culture of Airway Epithelia
Primary human or porcine airway epithelial cells grown at the air–liquid interface were prepared at the In Vitro Models Core Facility at the University of Iowa, using previously described methods (14). The use of human tissue samples in this study was approved by the Institutional Review Board of the University of Iowa. The use of porcine tissue was approved by the Institutional Animal Care and Use Committee of the University of Iowa. All primary cells used in this study were well-differentiated (> 2 weeks of culture), and were maintained in DMEM/F12 medium containing 1% PS, 50 μg/ml gentamicin sulfate, and 2% Ultroser G (BioSepra, Villeneuve, La Garenne, France).
Adenovirus
Replication-deficient recombinant adenovirus expressing enhanced green fluorescent protein (eGFP) (Ad5–CMV–eGFP) was produced and tittered, using previously described methods (15). Sucrose gradient–purified virus preparations were obtained from the Gene Transfer Vector Core at the University of Iowa. The virus was titered at 1 × 1012 particles/ml (∼ 1 × 1010 transducing units (TU)/ml).
Respiratory Syncytial Virus
RSV strain A2 (provided by Barney Graham at the National Institutes of Health and Dr. Steven Varga at the University of Iowa) was grown in HEp-2 cells and prepared as a clarified crude lysate, with a titer of approximately 2 × 107 plaque forming units (PFU)/ml. Virus was titered via syncytia titration on Vero cells (16). Red fluorescent protein (RFP)–expressing virus (rrRSV), provided by Dr. Steven Varga (University of Iowa), was previously described (17).
In Vitro Virus Inactivation Assays
See the online supplement for details.
Cell-Dependent Viral Inactivation
For experimental details, see the online supplement.
Measurement of HOI
The production of HOI was detected based on the iodination of nicotinamide adenine dinucleotide phosphate reduced (NADPH), as described previously (18). For additional details, see the online supplement.
Cell Toxicity Assay
The release of lactate dehydrogenase (LDH) was measured in well-differentiated human airway epithelia, using a commercially available kit according to the manufacturer's instructions (LDH-Cytotoxicity Assay Kit, catalogue number K311–400; BioVision, Mountain View, CA).
pH Dependence of Oxidative Antiviral Activity
For experimental details, see the online supplement.
RSV Syncytia Titration
RSV was titrated by a variation of the microtiter assay originally described by Trepanier and colleagues (16). For experimental details, see the online supplement.
Administration of Iodide to Human Subjects, and Analysis of Nasal Airway Surface Liquid Composition
For experimental details, see the online supplement.
Results
Oxidized Forms of I−, but Not SCN−, Are Virucidal against Adenovirus
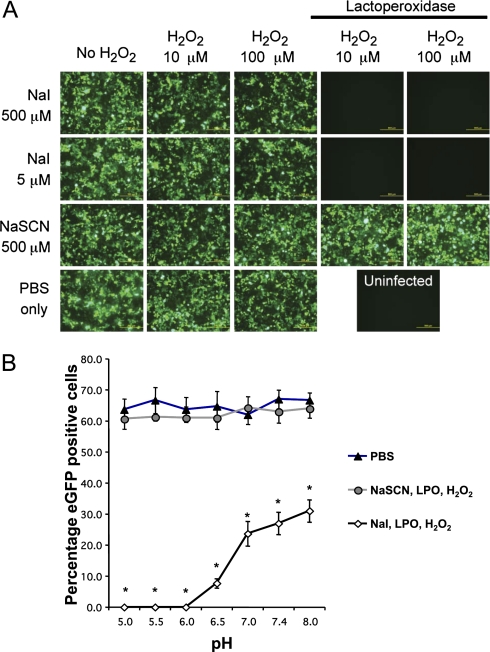
We first asked whether the oxidized halide and pseudohalide substrates of LPO-catalyzed oxidation were active against adenovirus. We selected adenovirus, a protein-encapsidated DNA virus, because it is an important respiratory pathogen. To test whether reactions generating OSCN− or HOI inhibit infection with adenovirus, we mixed both complete and incomplete LPO mixtures with recombinant Ad5–CMV–eGFP under cell-free conditions, and used the expression of eGFP to monitor the virus transduction of target cells. In Figure 1A, we show that the addition of the replete HOI-generating reaction completely abrogated transduction by Ad5–CMV–eGFP. By contrast, a comparable OSCN−-generating reaction failed to block transduction. None of the incomplete LPO reactions possessed discernable antiviral activity. H2O2 alone did not inhibit infection by adenovirus.
Figure 1.
(A) Cell-free inactivation of adenovirus by hypoiodous acid. Replication-deficient recombinant adenovirus expressing enhanced green fluorescent protein (eGFP) (Ad5–CMV–eGFP) was mixed with combinations of H2O2, NaSCN, NaI, and lactoperoxidase (LPO) (20 μg/ml), as described in the text, and incubated for 10 minutes. The virus was then applied to monolayers of A549 cells. Cell monolayers were imaged by fluorescence microscopy at 48 hours after transduction to monitor the expression of GFP, and photomicrographs were captured. Representative en face views are shown. The LPO-catalyzed oxidation of I− resulted in a complete block of adenovirus transduction. Scale bars, 500 μm. Results are representative of three independent experiments. (B) Dependence on pH of adenovirus inactivation by HOI. Cell-free inactivation reactions for Ad5–CMV–eGFP were prepared with incomplete or complete (5 μM NaI, 20 μg/ml LPO, and 100 μM H2O2) LPO reactions at different pH conditions and incubated for 10 minutes. Samples were then diluted and applied to A549 cells at approximately 50 multiplicity of infection (MOI), and 48 hours later, numbers of eGFP-positive cells were counted by fluorescence microscopy. The percentage of eGFP-positive cells was the average counted from three separate fields. Results are representative of three independent experiments. Values shown represent the mean ± SE. *P < 0.0001, two-way ANOVA with Tukey post hoc test.
Inactivation of Adenovirus by Hypoiodous Acid Is pH-Dependent
The pH of airway surface liquid is slightly acidic, with pH values ranging from 6.57–7.18 (19–21). To determine whether or not the inactivation of adenovirus by HOI was pH-dependent, we monitored adenovirus titers after exposure to HOI across pH ranges from 5–8. At a pH between 6 and 7, the application of LPO, I−, and H2O2 was significantly more virucidal than at physiologic pH (Figure 1B). At pH 8, the oxidation of I− also significantly reduced the adenovirus titer, compared with the PBS control.
Airway Epithelia Produce Hypoiodous Acid in an Iodide Concentration–Dependent Manner
We previously reported that well-differentiated airway epithelia generate H2O2 apically, in a DUOX-dependent manner (8). Here we asked whether airway epithelia produced sufficient H2O2 to support the production of HOI in the presence of the physiological LPO concentration of airway surface liquid and I−. Well-differentiated primary airway epithelia were cultured at the air–liquid interface and stimulated by the apical addition of ATP, which maximizes the DUOX-mediated production of H2O2 (22). The apical buffer (PBS) also contained a range of I− concentrations and LPO. Although a high concentration of LPO is maintained in the airway surface fluid by the LPO-secreting submucosal glands (23), the cultured surface airway epithelia do not secrete LPO (data not shown) (9). The production of HOI by airway epithelia was determined according to the iodination of extracellular NADPH, as previously described (18). As shown in Figure 2A, under these conditions, well-differentiated porcine airway epithelia supported the generation of HOI at a rate similar to that of previously reported H2O2 production (8). Similar results were observed using primary human airway epithelia (data not shown). The addition of the H2O2 scavenger catalase to the apical buffer, or the omission of either LPO or I−, inhibited the generation of HOI, confirming that the production of HOI was dependent on apical H2O2, LPO, and I−.
Figure 2.
(A) Rates of hypoiodous acid (HOI) production by ATP-stimulated (100 μM) porcine airway epithelial cells in the presence of apical LPO (6.5 μg/ml) and the indicated I− concentrations (open squares, mean ± SE; n = 3). The production of HOI was determined by adding nicotinamide adenine dinucleotide phosphate reduced (NADPH) (100 μM) to the apical buffer, and measuring changes in absorbance at 282 and 340 nm. The apical buffer of negative control cultures lacked LPO (solid square), or was supplemented with 200 U/ml of catalase (solid triangle). (B) Cell-dependent inactivation of adenovirus by the generation of HOI. Porcine airway epithelia were stimulated with ATP to generate H2O2 and were supplemented with 0 μM, 5 μM, or 500 μM NaI, as indicated. LPO and Ad5–CMV–eGFP were added to the apical surface after 30 minutes of stimulation, and were allowed to adsorb overnight. Representative micrographs from three independent specimens are presented from 48 hours after transduction. The addition of 5 μM or 500 μM I− reduced the transduction of adenovirus, compared with no I−. Scale bar = 500 μm. (C) Cell-dependent inactivation of adenovirus by the generation of HOI (quantitation of data presented in B). Porcine airway epithelia were stimulated with ATP and supplemented with 0 μM, 5 μM, or 500 μM NaI. LPO and Ad5–CMV–eGFP were added to the apical surface after 30 minutes of stimulation, and allowed to adsorb overnight. Error bars represent standard error for three independent porcine airway epithelial cell cultures. *P < 0.05, **P < 0.005, Student t test with Bonferroni correction for multiple comparisons.
Production of HOI by Well-Differentiated Airway Epithelia Inhibits Adenovirus Transduction without Evidence of Cytotoxicity
To test whether airway epithelia could generate enough reactive product to inactivate adenovirus, Ad5–CMV–eGFP (multiplicity of infection [MOI], 12.5 TU/cell) was inoculated apically onto well-differentiated primary porcine airway epithelial cells. Because the Coxsackie virus and adenovirus receptor is expressed basolaterally (24), adenovirus requires prolonged incubation to enter when applied to the apical surface (25). Therefore, the virus was allowed to adsorb overnight to allow for transduction. As shown in Figure 2B, supplementing epithelia with LPO and I− at 5 μM or 500 μM significantly reduced the number of eGFP-expressing cells compared with no I− controls, indicating that viral transduction was inhibited. Quantification of the foci of infection indicated that the inactivation of adenovirus was I− dose–dependent (Figure 2C). The addition of 5 μM I− was sufficient to decrease the foci of infection significantly.
We previously showed that OSCN− was not toxic to airway epithelia (8). We asked whether the production of HOI by airway epithelia was associated with evidence of cell injury by measuring the release of LDH. The addition of a complete mixture of LPO, NaI, and H2O2 to well-differentiated epithelia did not result in significant cell toxicity (Figure 3).
Figure 3.
Airway epithelium–dependent production of HOI is not toxic to epithelial cells. Cytotoxicity was monitored by lactate dehydrogenase release assay, as described in Materials and Methods. Well-differentiated primary human airway epithelial cells were treated overnight under the indicated conditions. These included assay buffer alone (PBS, pH 6.6), and assay buffer containing the indicated combinations of catalase (750 U/ml), ATP (100 μM), LPO (20 μg/ml), and I− (400 μM). Media from both apical and basolateral cell surfaces were harvested and pooled. The positive control represents cells exposed to 1% Triton X-100 for 30 minutes at 37°C. Results shown represent mean ± SE, and are from three independent experiments with cells from three different human donors. *P < 0.05, Student t test.
Oxidized Forms of I−, but Not SCN−, Are Virucidal against RSV
We extended the observations with adenovirus to an enveloped respiratory virus, RSV. We hypothesized that OSCN− and HOI might exhibit antiviral activity against RSV. We incubated wild-type RSV for up to 2 hours with 100 μM H2O2 at pH 7.4. Under these conditions, the virus titer was unchanged compared with control PBS (Figure 4A). To test whether oxidized or unoxidized LPO substrates are virucidal, we assembled various mixtures of LPO, I−, SCN−, H2O2, and approximately 1 × 105 PFU/ml of RSV (Figure 4A). We found that neither SCN− and I− alone, nor LPO delivered with either I-− or SCN−, decreased the recovery of RSV, compared with PBS. These controls demonstrated that neither LPO nor its substrates were virucidal under these conditions. When a complete reaction mixture of LPO, I−, and H2O2 was assembled to yield HOI, the RSV titer was rapidly reduced to undetectable levels. Therefore, the generation of HOI is significantly virucidal against RSV, whereas the oxidation of SCN− to OSCN− failed to reduce the RSV titer.
Figure 4.
(A) Cell-free inactivation of respiratory syncytial virus (RSV) by the generation of HOI. RSV (1.7 × 105 plaque forming units [PFU]/ml) was incubated for 5 minutes with various combinations of H2O2 (100 μM), NaSCN (500 μM), NaI (500 nM, 5 μM, and 500 μM), and LPO (20 μg/ml). Treatment categories are indicated on the x-axis, and the resultant virus titer is presented on the y-axis. SFU, syncytia-forming units. The limit of detection for this assay is 10 SFU/ml. Values represent mean ± SD for one of three independent experiments. *P < 0.001, one-way ANOVA with Tukey post hoc test. (B) Inactivation of RSV by HOI is dependent on pH. RSV was mixed with complete or incomplete LPO reactions (5 μM NaI, 100 μM H2O2) and incubated for 10 minutes. Samples were then diluted and applied to Vero cell monolayers for syncytia titration. At a pH less than 7, the I−-dependent inactivation of virus was significantly greater than at physiologic pH (open diamonds). No other conditions caused dramatic shifts in RSV titer, including buffer pH, hydrogen peroxide, or LPO substrates alone. Values represent the mean ± SD for n = 3 replicate wells. Results are representative of three independent experiments. *P < 0.001, two-way ANOVA with Tukey post hoc test.
Inactivation of RSV by Hypoiodous Acid Is Most Effective at Low pH
We evaluated the pH dependence of RSV inactivation by applying either 5 μM NaI or 500 μM NaSCN in cell-free LPO reactions. Because the application of 5 μM NaI results in a suboptimal reduction in RSV titer at pH 7.4 (Figure 4A), we used this concentration to monitor the dependence of buffer pH on virus inactivation in hypoiodous acid–generating reactions. We found that at a pH between 6 and 6.5, the application of LPO, I−, and H2O2 was significantly more virucidal than at physiologic pH, with at least a 10-fold reduction in RSV titer compared with pH 7 (Figure 4B). Under high pH conditions (pH 8.0), the oxidation of I− did not substantially reduce the virus titer. The oxidation products of SCN− failed to show significant antiviral activity across a range of pH conditions.
Airway Epithelia Produce Sufficient Hypoiodous Acid for the Inactivation of RSV
We next asked whether airway epithelia produce enough HOI to inactivate viruses in situ via the LPO and I− antimicrobial system. Well-differentiated primary human airway epithelia, cultured at the air–liquid interface, were stimulated to produce H2O2 by an apical addition of ATP, and I− was supplied to the cells by addition to the basolateral and apical media. LPO was added to the apical surface at a concentration of 6.5 μg/ml (8). We added rrRSV to the apical surface in a total volume of 50 μl, with combinations of ATP, LPO, catalase, and varying concentrations of I−. If the epithelia produced sufficient H2O2, a significant loss of RFP expression should occur. Indeed, when I− was present in the reaction, a dose-dependent decrease in foci of infection was evident, 48 hours after infection (Figure 5). When I− was present at a concentration of 5 μM, the number of foci of infection decreased, but this trend did not reach statistical significance. Increasing the concentration of I− to 500 μM resulted in a marked and significant decrease in the number of foci of infection. The addition of the enzyme catalase rescued RSV infection. Based on these results, we conclude that human airway epithelia can generate sufficient H2O2 to inactivate RSV when supplemented with pharmacologic concentrations of I− in the presence of physiologic concentrations of LPO.
Figure 5.
Cell-dependent inactivation of RSV by the generation of HOI. Human airway epithelia were stimulated with ATP and supplemented with 0 μM, 5 μM, or 500 μM NaI, as indicated. LPO and red fluorescent protein–expressing virus were added to the apical surface after 30 minutes of stimulation, and allowed to adsorb overnight. Foci of infection were identified by the expression of red fluorescent protein. Data are presented as the percentage of foci formed in the absence of I−. Values shown represent mean ± SE for results from three separate human donor cultures. * P < 0.05, ** P < 0.005, one-way ANOVA with Tukey post hoc test.
After Bolus Intake of I−, the Concentration of I− in ASL Exceeds That of SCN− in Humans
The secretion of SCN− by the airway epithelium is thought to be mediated by the sodium–iodide symporter (NIS) in the basolateral plasma membrane, and by cystic fibrosis transmembrane conductance regulator (CFTR) and pendrin in the apical plasma membrane (9, 10). Because these SCN−-transporting proteins are also capable of I− transport, we hypothesized that I− is absent from ASL only because of its low serum concentration (10–100 nM). To examine the I−-secreting capacity of the airway, we performed a study with nine human subjects. After the verification of normal thyroid function (based on blood thyroid-stimulating hormone [TSH] and free thyroxine [T4] levels; data not shown), subjects took a potassium iodide tablet orally (130 mg KI, Iosat; Anbex Inc., Williamsburg, VA). Twenty-four hours before the intake of KI, and 2, 6, and 24 hours later, nasal ASL was harvested using microsampling probes (26), and blood was drawn from arm veins for inorganic ion analysis by anion-exchange chromatography (27). Before the intake of KI, the I− content of ASL was below the detection limit of our assay (< 0.25 μM in 50-fold diluted ASL). However, 2 hours after the intake of KI, I− accumulated in ASL at approximately 500 μM (Figures 6A and 6B). The concentration of I− in ASL remained in the range of several hundred micromolar for many hours, and exceeded the concentration of serum I− by more than 50-fold at 6 hours (Figures 6B). Interestingly, the concentration of SCN− was reduced in ASL after the intake of KI (Figures 6A and 6C), whereas the serum concentration of SCN− remained stable. These results suggests that I− and SCN− compete for transepithelial secretion in the respiratory tract. Our data also show that the oral intake of a single KI dose (130 mg) leads to the accumulation of I− in ASL at concentrations that (based on our cell-culture assays) can support antiviral activities.
Figure 6.
Accumulation of I− in airway surface liquid (ASL) after bolus intake of KI. (A) A representative result of nasal ASL analysis before (blue trace) and 2 hours after (red trace) intake of KI (130 mg) by a human subject. (B and C) Summarized data show concentrations of I− (B) and oxidizable anion thiocyanate (SCN−) (C) in nasal ASL (triangles) and serum (closed squares) at indicated time points before and after intake of KI (0 hour). Results are presented as mean ± SE (n = 9). For ASL SCN−, repeated-measures ANOVA, P = 0.0032; post hoc Dunnett test, control time point at −24 hours, *P < 0.05 and **P < 0.01. For ASL I−, repeated-measures ANOVA, P < 0.0001; post hoc Dunnett test, control time point at −24 hours, *P < 0.05 and **P < 0.01.
Discussion
Viral respiratory-tract infections are significant causes of morbidity and mortality throughout the world (1). Here we show that the LPO/I−/H2O2 oxidative host defense system demonstrated robust activity against two major respiratory viral pathogens, adenovirus and RSV. Although OSCN− was not effective against either adenovirus or RSV, we discovered that the delivery of the alternative halide substrate I− to the airway epithelium converted the DUOX/LPO enzymes into a HOI-producing antiviral system. Remarkably, HOI exhibited virucidal activity against both encapsidated and enveloped respiratory viruses. Importantly, the oral administration of KI in human subjects increased serum I− concentrations and yielded I− concentrations in the upper respiratory secretions that supported the antiviral activity of airway epithelial cells ex vivo. Because the concentration of LPO in our cell-based study was similar to that of in vivo airways (28), we speculate that a topical or systemic supplementation of I− alone might be sufficient to inactivate virus.
The LPO/halide/H2O2 antimicrobial system is a host defense mechanism previously proposed to serve as a nonspecific antiviral defense (12, 29), particularly in the saliva (30, 31), tears (32), and milk (33). The discovery of DUOX gene products revealed their distribution in human airways (34). The expression of DUOX in epithelia stimulated interest in the role of the LPO/halide/H2O2 system in airway mucosal immunity, because it identified a mechanism for the production of epithelial H2O2 (11). The two DUOX isoforms, DUOX1 and DUOX2, are encoded by closely spaced genes on human chromosome 15, and are transcribed in opposite directions (35, 36). DUOX uses cytoplasmic NADPH as an electron donor to transfer two electrons to oxygen, which is reduced to H2O2 and released extracellularly. The functions of DUOX1 and DUOX2 are also dependent on the co-expression of the regulatory proteins DUOXa1 and DUOXa2, respectively (37). Although they are functionally indistinguishable, DUOX1 and DUOX2 are differentially regulated. Harper and colleagues previously reported that DUOX2 is inducible by IFN-γ, as well as by rhinovirus infection and polyinosinic:polycytidylic acid (poly[I:C]) (38). DUOX1 is induced by IL-4 and IL-13 (10, 38), as well as by bacterial products such as Pseudomonas aeruginosa endotoxin, flagellum, and Type III secretion system products (39).
Three reports showed that airway epithelial cells supplemented with both SCN− and LPO can kill two types of bacteria, Pseudomonas aeruginosa and Staphylococcus aureus (8, 9, 40). This provides evidence that the airway LPO/halide/H2O2 system is active against bacteria. The recognition that the CFTR anion channel conducts SCN− raised the possibility that patients with cystic fibrosis (CF) might manifest impaired LPO/halide/H2O2 microbicidal activity. Indeed, airway epithelia from patients with CF exhibited defects in CFTR-dependent SCN− transport and defective bacterial killing (8–10). These studies suggest a possible mechanistic link between CF lung disease and altered function of the LPO/halide/H2O2 system.
In vitro, LPO has a high affinity for both SCN− and I−. Before this study, the I− concentration in ASL had not (to the best of our knowledge) been reported, but SCN− is present in ASL at approximately 450 μM (41). Therefore, SCN− is thought to be the physiologic LPO substrate in the respiratory tract. What mechanism favors the accumulation of SCN− over I− in the airways? Animal cells cannot synthesize SCN−. Thus, the diet is the only source of both SCN− and I− (8, 11, 42, 43). The average concentration of SCN− in serum is approximately 20 μM (44), whereas the average concentration of I− in serum is only 10–100 nM (45). With a serum concentration of SCN− 200–2,000-fold higher than that of I−, the secretion of SCN− predominates. Airway epithelial cells generate an approximately 20-fold serosal-to-mucosal concentration gradient for the secretion of SCN− by importing SCN− on the basolateral side through NIS (46), and exporting it on the apical side via CFTR and perhaps the anion transporter pendrin (SLC26A4) (9, 10). I− can follow the same secretory pathways. In fact, the NIS favors I− threefold over SCN− (47). Thus, if serum I− reaches micromolar concentrations, I− can also be secreted into the respiratory tract. The data from our investigations of human subjects (Figure 6) support this concept. Therefore, supplementation with I− may allow sufficient I− concentrations in ASL to support the production of HOI. Although chronic treatment with I− may be associated with toxicity, short-term daily administration to humans is safe (48, 49). The topical delivery of I− may further reduce any risk of systemic toxicity.
The mechanism by which hypohalides eliminate bacteria is not yet known, but OSCN− can oxidize thiol groups in surface proteins, thereby mediating conformational changes (13, 50). Although we do not yet know how HOI inactivates adenovirus and RSV, similar mechanisms are likely in play. Adenovirus and RSV represent two different classes of viruses. Adenovirus is a nonenveloped, protein-encapsidated double-stranded DNA virus that enters through endocytosis, whereas RSV is an enveloped, single-stranded RNA virus that enters the cell through receptor-mediated membrane fusion. The results of our cell-free virus inactivation assays are consistent with observations by Belding and colleagues that the peroxidase-mediated oxidation of I− is strongly antiviral (29). Belding and colleagues further reported that HOI was active against polio and vaccinia viruses, particularly at a low pH (29). Interestingly, the LPO/I−/H2O2 system was most virucidal against both adenovirus and RSV at slightly acidic pH. Because the pH of ASL is slightly acidic (19–21), these conditions are expected to favor HOI-dependent virucidal activity. We speculate that HOI modifies viral surface proteins and prevents the binding or the entry of adenovirus and RSV into the epithelium. Alternatively, HOI may inhibit the synthesis or assembly of viral nucleic acids and proteins, or prevent the release of virus from cells. Our results suggest that the innate oxidative antiviral system present in the airways is a nonspecific mechanism to inactivate invading viruses.
To test the in vivo efficacy of the antiviral activity of this HOI-generating mechanism, large animal models of viral infections are needed, because murine airways lack both LPO and DUOX (8), and rat airways contain few LPO-secreting cells (51). In contrast, the respiratory tracts of larger mammals such as sheep and pigs are similar to those of humans in regard to the expression of LPO and DUOX, anatomy, physiology, innate and adaptive immunity, and susceptibility to viral infection (23, 52). An important question for future study involves whether the inactivation of virus in vivo is limited by the generation of H2O2, the concentration of I−, or both.
In conclusion, the airway LPO/I−/H2O2 system exhibits robust antiviral activity against relevant human pathogens. The identification of a new prophylactic or therapeutic approach to prevent or ameliorate respiratory viral infections could have broad implications for human health.
Supplementary Material
Acknowledgments
The authors thank Jennifer Bartlett and Jeydith Gutierrez for critically reviewing and commenting on the manuscript, and Dr. Steven Varga for providing stocks of RSV and technical assistance.
Footnotes
This study was supported by National Institutes of Health grants P50 HL-61234 (P.B.M.), PO1 HL-091842 (P.B.M. and B.B.), N01 AI-30040 (P.B.M.), and R01 HL-090830 (B.B.), the Roy J. Carver Charitable Trust (P.B.M.), and Cystic Fibrosis Foundation Therapeutics Grant BANFI07A0 (B.B.). This study was also supported by the Cell and Tissues and Cell Morphology Cores, which is partly supported by the Center for Gene Therapy for Cystic Fibrosis (through National Institutes of Health grant P30 DK-54759) and the Cystic Fibrosis Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0329OC on March 25, 2011
Author Disclosure: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet 2005;365:1147–1152 [DOI] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008;86:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt CD, Kim HW, Vargosko AJ, Jeffries BC, Arrobio JO, Rindge B, Parrott RH, Chanock RM. Infections in 18,000 infants and children in a controlled study of respiratory tract disease: I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am J Epidemiol 1969;90:484–500 [DOI] [PubMed] [Google Scholar]
- 4.Hayashi S, Hogg JC. Adenovirus infections and lung disease. Curr Opin Pharmacol 2007;7:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib Microbiol 2008;15:147–163 [DOI] [PubMed] [Google Scholar]
- 7.Conner GE, Salathe M, Forteza R. Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med 2002;166:S57–S61 [DOI] [PubMed] [Google Scholar]
- 8.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med 2007;175:174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett 2007;581:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, et al. Thiocyanate transport in resting and IL-4–stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol 2007;178:5144–5153 [DOI] [PubMed] [Google Scholar]
- 11.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal 2009;11:2453–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klebanoff SJ, Clem WH, Luebke RG. The peroxidase–thiocyanate–hydrogen peroxide antimicrobial system. Biochim Biophys Acta 1966;117:63–72 [DOI] [PubMed] [Google Scholar]
- 13.Klebanoff SJ. Iodination of bacteria: a bactericidal mechanism. J Exp Med 1967;126:1063–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia: methods for establishing primary cultures. Methods Mol Biol 2002;188:115–137 [DOI] [PubMed] [Google Scholar]
- 15.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther 2000;7:1034–1038 [DOI] [PubMed] [Google Scholar]
- 16.Trepanier P, Payment P, Trudel M. A simple and rapid microassay for the titration of human respiratory syncytial virus. J Virol Methods 1980;1:343–347 [DOI] [PubMed] [Google Scholar]
- 17.Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol 2006;34:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virion A, Michot JL, Deme D, Pommier J. NAPDH oxidation catalyzed by the peroxidase/H2O2 system: iodide-mediated oxidation of NAPDH to iodinated NADP. Eur J Biochem 1985;148:239–243 [DOI] [PubMed] [Google Scholar]
- 19.Jayaraman S, Song Y, Verkman AS. Airway surface liquid pH in well-differentiated airway epithelial cell cultures and mouse trachea. Am J Physiol Cell Physiol 2001;281:C1504–C1511 [DOI] [PubMed] [Google Scholar]
- 20.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O'Neal WK, Boucher RC. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA 2003;100:16083–16088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol 2006;290:C741–C749 [DOI] [PubMed] [Google Scholar]
- 22.Forteza R, Salathe M, Miot F, Conner GE. Regulated hydrogen peroxide production by DUOX in human airway epithelial cells. Am J Respir Cell Mol Biol 2005;32:462–469 [DOI] [PubMed] [Google Scholar]
- 23.Salathe M, Holderby M, Forteza R, Abraham WM, Wanner A, Conner GE. Isolation and characterization of a peroxidase from the airway. Am J Respir Cell Mol Biol 1997;17:97–105 [DOI] [PubMed] [Google Scholar]
- 24.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem 1999;274:10219–10226 [DOI] [PubMed] [Google Scholar]
- 25.Zabner J, Zeiher BG, Friedman E, Welsh MJ. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol 1996;70:6994–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki K, Ogura S, Ishizaka A, Oh-hara T, Nishimura M. Bronchoscopic microsampling method for measuring drug concentration in epithelial lining fluid. Am J Respir Crit Care Med 2003;168:1304–1307 [DOI] [PubMed] [Google Scholar]
- 27.Lorentzen D, Durairaj L, Pezzulo AA, Nakano Y, Launspach J, Stoltz DA, Zamba G, McCray PB, Jr, Zabner J, Welsh MJ, et al. Concentration of the antimicrobial precursor thiocyanate in cystic fibrosis airway secretions. Free Radic Biol Med 2011;50:1144–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerson C, Sabater J, Scuri M, Torbati A, Coffey R, Abraham JW, Lauredo I, Forteza R, Wanner A, Salathe M, et al. The lactoperoxidase system functions in bacterial clearance of airways. Am J Respir Cell Mol Biol 2000;22:665–671 [DOI] [PubMed] [Google Scholar]
- 29.Belding ME, Klebanoff SJ, Ray CG. Peroxidase-mediated virucidal systems. Science 1970;167:195–196 [DOI] [PubMed] [Google Scholar]
- 30.Morrison M, Allen PZ, Bright J, Jayasinghe W. Lactoperoxidase: V. Identification and isolation of lactoperoxidase from salivary gland. Arch Biochem Biophys 1965;111:126–133 [DOI] [PubMed] [Google Scholar]
- 31.Thomas EL, Jefferson MM, Joyner RE, Cook GS, King CC. Leukocyte myeloperoxidase and salivary lactoperoxidase: identification and quantitation in human mixed saliva. J Dent Res 1994;73:544–555 [DOI] [PubMed] [Google Scholar]
- 32.Morrison M, Allen PZ. Lactoperoxidase: identification and isolation from harderian and lacrimal glands. Science 1966;152:1626–1628 [DOI] [PubMed] [Google Scholar]
- 33.Allen PZ, Morrison M. Lactoperoxidase: VI. Immunochemical studies on lactoperoxidase from the milk of several species. Arch Biochem Biophys 1966;113:540–547 [DOI] [PubMed] [Google Scholar]
- 34.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 2003;17:1502–1504 [DOI] [PubMed] [Google Scholar]
- 35.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 2000;275:23227–23233 [DOI] [PubMed] [Google Scholar]
- 36.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase: cloning of the porcine and human cDNAs. J Biol Chem 1999;274:37265–37269 [DOI] [PubMed] [Google Scholar]
- 37.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase: evolution of an eukaryotic operon equivalent. J Biol Chem 2006;281:18269–18272 [DOI] [PubMed] [Google Scholar]
- 38.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, DUOX1 and DUOX2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 2005;579:4911–4917 [DOI] [PubMed] [Google Scholar]
- 39.Rada B, Leto TL. Characterization of hydrogen peroxide production by DUOX in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett 2010;584:917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase–based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol 2008;181:4883–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol 2003;29:206–212 [DOI] [PubMed] [Google Scholar]
- 42.Arnhold J, Monzani E, Furtmuller P, Zederbauer M, Casella L, Obinger C. Kinetics and thermodynamics of halide and nitrite oxidation by mammalian heme peroxidases. Eur J Inorg Chem 2006;19:3801–3811 [Google Scholar]
- 43.Aune TM, Thomas EL. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem 1977;80:209–214 [DOI] [PubMed] [Google Scholar]
- 44.Lundquist P, Kagedal B, Nilsson L. An improved method for determination of thiocyanate in plasma and urine. Eur J Clin Chem Clin Biochem 1995;33:343–349 [DOI] [PubMed] [Google Scholar]
- 45.Pantuckova P, Krivankova L. Fast and simple method for determination of iodide in human urine, serum, sea water, and cooking salt by capillary zone electrophoresis. Electrophoresis 2004;25:1102–1110 [DOI] [PubMed] [Google Scholar]
- 46.Fragoso MA, Fernandez V, Forteza R, Randell SH, Salathe M, Conner GE. Transcellular thiocyanate transport by human airway epithelia. J Physiol 2004;561:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 2003;24:48–77 [DOI] [PubMed] [Google Scholar]
- 48.Feek CM, Sawers JS, Irvine WJ, Beckett GJ, Ratcliffe WA, Toft AD. Combination of potassium iodide and propranolol in preparation of patients with Graves' disease for thyroid surgery. N Engl J Med 1980;302:883–885 [DOI] [PubMed] [Google Scholar]
- 49.Takata K, Amino N, Kubota S, Sasaki I, Nishihara E, Kudo T, Ito M, Fukata S, Miyauchi A. Benefit of short-term iodide supplementation to antithyroid drug treatment of thyrotoxicosis due to Graves' disease. Clin Endocrinol (Oxf) 2010;72:845–850 [DOI] [PubMed] [Google Scholar]
- 50.Morrison M, Bayse GS. Catalysis of iodination by lactoperoxidase. Biochemistry 1970;9:2995–3000 [DOI] [PubMed] [Google Scholar]
- 51.Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat 2000;197:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB, Jr, McLennan G, Meyerholz DK, Namati E, Ostedgaard LS, et al. The porcine lung as a potential model for cystic fibrosis.Am J Physiol Lung Cell Mol Physiol 2008;295:L240–L263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.