Abstract
The chemokine, CXCL10, and its cognate receptor, CXCR3, are important mediators of the pathobiology of lung fibrosis. Macrophages are a known source of CXCL10, but their specific source in the lung is poorly defined due to incomplete characterization of macrophage subpopulations. We recently developed a novel flow cytometric approach that discriminates resident alveolar macrophages from recruited exudative macrophages (ExMacs) after infectious lung injury. We hypothesized that ExMacs are present after noninfectious lung injury with bleomycin, and are a source of CXCL10. We found that ExMacs are recruited to the lung after injury, peaking at Day 7, then maintained through Day 28. ExMac recruitment was significantly reduced, but not abolished, in CCR2 null mice. ExMacs, but not alveolar macrophages, produce CXCL10, both constitutively and after stimulation with hyaluronan (HA) fragments. Interestingly, ExMac stimulation with LPS resulted in complete suppression of CXCL10. In contrast, ExMacs produced TNF-α and CXCL2/MIP-2 (Macrophage Inflammatory Protein-2) after stimulation with both HA and LPS. ExMacs were present in CXCR3 null mice after bleomycin, but produced minimal CXCL10. This impairment was overcome by administration of exogenous IFN-γ or IFN-γ with HA. Collectively, these data suggest that ExMacs are recruited and maintained in the lung after noninfectious lung injury, are a source of a variety of cytokines, but importantly, are essential for the production of antifibrotic CXCL10. Understanding the contribution of ExMacs to the pathobiology of lung injury and repair could lead to new treatment options for fibrosing lung diseases.
Keywords: macrophage, bleomycin, pulmonary fibrosis, CXCR3, CXCL10
Clinical Relevance
This data identify a macrophage subpopulation with potential relevance to the modification of pulmonary fibrosis after noninfectious lung injury. Further definition and understanding of the role of macrophage subpopulations could lead to new treatment options for fibrosing lung diseases.
Pulmonary fibrosis is a disease entity characterized by chronic interstitial inflammation, extracellular matrix deposition, and the accumulation of fibroblasts and myofibroblasts, leading to collapse of alveoli and loss of lung functional units (1, 2). The disease process is heterogeneous, occurring in a wide variety of clinical settings, both with identifiable causes, as in systemic autoimmune diseases, such as scleroderma, or in settings that are without identifiable cause, termed idiopathic interstitial pneumonias. The pathogenesis of pulmonary fibrosis is unclear, and most likely reflects multiple pathways and responses to injury (3, 4).
One such pathway involves the CXC chemokine, CXCL10, and its cognate receptor, CXCR3 (5). Previous studies have demonstrated the critical importance of CXCL10 and CXCR3 to the development of lung fibrosis (6, 7). CXCL10 is produced by macrophages after stimulation with IFN-γ, and has critical roles in angiogenesis and T-cell trafficking (8, 9). CXCL10 inhibits fibroblast chemotaxis after bleomycin injury, thereby reducing the accumulation of fibroblasts and subsequent development of fibrosis (7). More recently, it has been shown that the inhibition of fibroblast chemotaxis requires the proteoglycan, syndecan-4 (10). CXCR3 null mice exhibit enhanced fibrosis after bleomycin injury and impaired production of endogenous IFN-γ and CXCL10 (6). Collectively, these studies demonstrate that the CXCL10–CXCR3 axis is an important regulatory pathway in pulmonary fibrosis. CXCL10 can be produced by a variety of cells, but several studies suggest that lung macrophages may be an important source after injury (11–13). The specific source of macrophage-derived CXCL10 has not been elucidated, in part due to difficulties in characterizing lung macrophage subpopulations.
Macrophages are important mediators of lung injury, inflammation, and fibrosis (14, 15). There is enormous heterogeneity in macrophage populations. Recent studies have identified distinct macrophage subpopulations with unique functions in multiple tissues (16). In the lung, distinct macrophage subpopulations have been characterized primarily in murine infectious disease and asthma models (17–20). Macrophage heterogeneity is thought to be due to both the heterogeneity of macrophage precursors (i.e., monocytes) and the specific developmental pathways that are stimulated by local conditions at the time of monocyte differentiation (16).
Two unique populations of monocytes are present in the circulation (21). Constitutive (Gr-1−) monocytes enter tissues under steady-state conditions in the lung, and develop into resident tissue interstitial macrophages and alveolar macrophages (AMs) (21–23). In contrast, inflammatory (Gr-1+) monocytes are recruited to the lung during inflammation in a CCR2-dependent manner, and develop into either an activated macrophage population, known as exudative macrophages (ExMacs), or into monocyte-derived dendritic cells (moDCs) (21, 24). The morphology, phenotype, and effector functions of constitutive monocyte-derived macrophages differ markedly from inflammatory monocyte-derived macrophages (20). Resident lung interstitial and AMs express relatively little major histocompatibility complex (MHC) II and costimulatory molecules. After activation, they produce low levels of inflammatory cytokines, and do not promote T-cell activation. In contrast, ExMacs express high levels of MHCII and costimulatory molecules, stimulate T-cell activation, and are a major source of inflammatory cytokines and chemokines (20). For example, during influenza infection, ExMacs and monocyte-derived DCs, but not resident AMs, are the major source of the TNF-α and nitric oxide synthase 2, and are responsible for the immune-mediated pathology (20). Based on these studies, we hypothesized that ExMacs would be recruited to the lung after noninfectious lung injury and have effector functions distinct from resident macrophages. In addition, in contrast to the critical role of ExMacs in causing lung pathology after infectious inflammation, we sought to determine if ExMacs have a previously unrecognized role in limiting the extent of tissue pathology after noninfectious lung injury by producing CXCL10.
Materials and Methods
Mice and Bleomycin Administration
Age- and sex-matched C57Bl/6, CCR2−/− and CX3CR1GFP/GFP mice were purchased from Jackson Laboratory (Bar Harbor, ME). CX3CR1GFP/GFP mice were crossed with C57Bl/6 mice to produce CX3CR1+/GFP mice. CXCR3−/− mice were described previously (25). All mice are on a C57Bl/6 background. Animal experiments were conducted in accordance with National Institutes of Health guidelines, and protocols approved by the Animal Care and Use Committee at Duke University. Bleomycin (2.5 U/kg; Hospira, Inc., Lake Forest, IL) was administered by intratracheal instillation. Control animals received saline.
Lung Parenchymal Cell Isolation
Mice were killed on Days 0, 3, 7, 14, and 28 after bleomycin. The lungs were perfused with Hanks' balanced salt solution to remove residual red blood cells from the pulmonary circulation. They were minced and digested for 40 minutes at 37°C in Hanks' balanced salt solution with 1 mg/ml collagenase A (Roche, Indianapolis, IN) and 0.2 mg/ml of DNase1 (Sigma, St. Louis, MO). The digestion solution was passed through a 70-um mesh strainer and centrifuged at 535 × g at room temperature over an 18% nicodenz (Accurate Chemical Co., Westbury, NY) cushion. Low-density cells were collected, underwent red cell lysis, and were then washed twice.
Flow Cytometric Analysis
The following antibodies were used: anti–I-A/I-E FITC, anti–I-A/I-E phycoerythrin (PE) (used in experiments with CX3CR1GFP/WT mice), anti–Ly6-G PE, anti–Gr-1 APC, and anti–CD11b APC Cy7 (all from BD Pharmagen, San Jose, CA); and anti-CD11c PE Cy5.5 (eBioscience, San Diego, CA). All staining was performed in PBS with 3% FBS, 10 mM EDTA, 5% normal mouse serum, 5% normal rat serum, and 1% FcBlock (BD Pharmagen). To reduce nonspecific binding, the cells were incubated in staining buffer at 4°C for 10 minutes before the addition of antibodies. After staining, the cells were washed three times and analyzed using a BD Canto II flow cytometer (BD Biosciences, San Jose, CA). Data analysis was performed using FloJo software (version 8.8.6; Ashland, OR). Cell sorting was accomplished using a BD FACSVantage cell sorter (BD Biosciences, San Jose, CA).
Stimulation of Sorted Macrophages
Sorted cells were cultured overnight (50,000 or 150,000 cells/well) in RPMI 1640 plus glutamine plus 10% FBS. The media were then replaced with RPMI without FBS. Cells were stimulated as follows: (1) unstimulated; (2) hyaluronan (HA) fragments (100 μg/ml) (ICN Biomedicals, Inc., Irvine, CA); and (3) LPS (100 ng/ml) (Escherichia Coli 0111:B4 Sigma no. L3137). Polymixin B (20 μg/ml) (Calbiochem, Darmstadt, Germany) was added to non–LPS-stimulated groups to inactivate potential LPS contamination. After 24 hours, the supernatants were collected for cytokine evaluation. Additional experiments were performed which stimulated sorted cells with IFN-γ (10 ng/ml), IFN-γ with HA (100 μg/ml), and IFN-γ with LPS (100 ng/ml).
ELISA of Supernatants from Sorted Cells
Protein levels of CXCL10, TNF-α and CXCL2 from the supernatants of the sorted macrophage populations were measured with commercially available ELISA kits (R&D Systems Inc., Minneapolis, MN) according to the manufacturers' instructions.
Statistical Analysis
Differences in the measured values between the cell populations of the genetically altered mice and control groups were assessed using the Student's t test. The data are expressed as the mean (±SEM). Statistical differences were accepted at a P value less than 0.05. In the flow cytometry experiments, an average of three to four mice was used for each group/time point and analyzed individually. These experiments were then repeated twice.
Results
Noninfectious Lung Injury Results in the Influx of Monocyte-Derived DCs and ExMacs
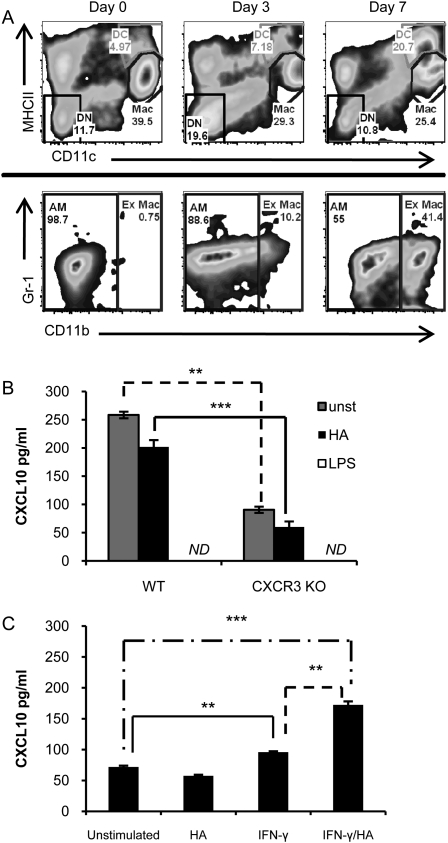
We performed flow cytometric analysis of lung inflammatory cells after intratracheal bleomycin, as previously described (20). We have previously shown that, after influenza infection, inflammatory monocytes (MHCII− CD11c− CD11b+ Gr-1hi) enter the lungs and up-regulate CD11c and MHCII expression to become a transitional cell type, referred to as double intermediate (DI) cells (MHCIIint CD11cint CD11b+ Gr-1hi), and then subsequently matured into either moDCs (MHCIIhi CD11cint CD11b+ Gr-1+) or ExMacs (autofluorescent+ MHCIIint CD11chi CD11b+). ExMacs can be distinguished from AMs (autofluorescent+ MHCIIint CD11chi CD11b−) by the expression of CD11b or CX3CR1 when CX3CR1GFP/+ mice are used. In CX3CR1GFP/+ mice, monocytes, DI cells, moDCs, and ExMacs are all GFP+, whereas neutrophils, AMs, and lymphocytes are GFP− (20, 26). Before injury, AMs and monocytes were the primary cell types identified in lung digests, representing resident populations (Figure 1, Day 0). After injury, there was an accumulation of DCs and ExMacs (Figures 1C and 1D). Subgate analysis of the DCs (Figure 1C) at Day 3 revealed a portion of the cells to be CD11b+ Gr-1+, consistent with previous descriptions of moDCs (20, 27). This early Gr-1 expression by DCs was believed to reflect their recent development from Gr-1+ monocytes. The peak number of total DCs occurred by Day 7 after bleomycin, and then declined, but did not return to baseline by Day 28 (Figure 1C). The appearance of ExMacs began at Day 3, peaked at Day 7, and then declined, but remained present, through Day 28 (Figure 1D). ExMacs were uniformly CD11b+ Gr-1int at all time points after bleomycin. This cell surface expression pattern was distinct from influenza, where ExMacs initially expressed high Gr-1, but then later became Gr-1low (20). This may reflect the fact that ExMacs after noninfectious injury were either not derived from a Gr-1+ precursor, or that the inflammatory stimulus of bleomycin was distinct from influenza, resulting in altered cell surface expression. ExMacs exhibited enhanced CX3CR1 expression compared with AMs after bleomycin (Figure 1E), and were F4/80+ to delineate them from DCs that are also CD11b+ (Figure 1F). We also found an influx of cells demonstrating intermediate expression of MHCII and CD11c in addition to the expression of CD11b and CX3CR1. These cells are consistent with previously described DI cells (20). DI cells were only minimally present in unchallenged lung, and had peak influx at Day 3 after bleomycin treatment (data not shown). Total cell counts from the flow analysis demonstrated that there was little change in the overall numbers of monocytes (which were CX3CR1 positive [Figure 1G]) or AMs after bleomycin injury (Figures 2A and 2B). However, there was a marked influx of DCs and ExMacs (Figures 2A and 2B). DC and ExMac numbers persisted at much later time points after bleomycin than has been routinely demonstrated in standard monocyte/macrophage cell count differentials (Figures 2A and 2B).
Figure 1.
Analysis of inflammatory cell types in the lungs of CX3CR1GFP/+ mice after injury with bleomycin. (A) Flow profile of total lung cells isolated on various days after bleomycin injury are analyzed by CD11c versus major histocompatibility complex (MHC) II expression to identify individual cell populations. Double negative (DN) cells are MHCII- and CD11c-negative (black border), dendritic cells (DCs) are MHCIIhigh and CD11c+ (green border), and macrophages (Mac) are MHCIIint and CD11c+ (purple border). The numbers represent the percentages of these various cell groups. (B–D) Subgating is performed on the DN (B), DC (C), and Mac (D) populations identified in (A). Expression in these subgates is based on CD11b versus Gr-1 cell surface expression. CD11b+ Gr-1high cells are identified from the DN gate (R1, black border). The majority of DCs are CD11b+ Gr-1low. A Gr-1high DC population exists at Day 3 after bleomycin. Alveolar macrophages (AMs) and exudative macrophages (ExMacs) are identified from the Mac gate. These cell types are discriminated based on CD11b expression, AM CD11blow (blue border) and ExMac CD11bhigh (red border). (E) Histograms demonstrating fluorescent intensity of CX3CR1-GFP expression from the macrophage populations (AM, blue; ExMac, red) over time after bleomycin instillation. Shift of the histogram to the right denotes enhanced GFP expression. Basal autofluorescence of macrophages for FITC/GFP is shown in the black histogram (using C57B6 wild-type [WT] mice). (F) ExMacs demonstrate enhanced expression of F4/80 as compared with DCs at Days 3, 7, and 14 after bleomycin injury (DC, green gate; ExMac, red gate). (G) R1 population is analyzed for CX3CR1– GFP expression, which separates monocytes (black box) from neutrophils (PMN, purple box). The flow plots and the histograms are from a representative sample of bleomycin-injured CX3CR1GFP/+ mice. Data were derived from three to four mice per time point, and are representative of two separate experiments.
Figure 2.
Total inflammatory cell influx after bleomycin identified by flow cytometry. (A) A comparison of the influx of monocytes, DCs, and macrophages reveals only a small influx of monocytes and DCs occurring at Day 3 after bleomycin injury. DC influx peaks at Day 7, at which time there is a significant influx of macrophages. (B) Comparison of macrophage subpopulation influx after bleomycin injury; AMs (blue); and ExMacs (red). The data represents total cell counts and demonstrates that AMs are the predominant macrophage cell type in whole lung before injury. They remain relatively constant after injury. ExMacs are recruited starting at Day 3, peaking at Day 7, and then persisting at a low level through Day 28. The data are from one time course experiment with three to five mice at each time point, with one repeat.
Influx of Monocytes, DCs, and ExMacs Is Dependent on CCR2
Prior work suggested that monocyte-derived DCs and ExMacs were derived from inflammatory monocytes recruited from the circulation in a CCR2-dependent manner (20). To determine the role of CCR2 in noninfectious injury, we compared the influx of monocytes, DCs, and ExMacs in CCR2 null mice and C57Bl/6 wild-type (WT) mice after bleomycin treatment. We focused this analysis on Days 3 and 7 based on our characterization of the cellular influx after bleomycin. Flow plot analysis revealed similar cell surface marker identification of monocytes, DCs, AMs, and ExMacs in CCR2 null and WT mice after injury (Figures 3A–3C). As this analysis was not performed on CX3CR1GFP/+ mice, monocytes in CCR2 null mice were separated from neutrophils by anti-Ly6G antibody staining (Figure 3D). The peak influx of cells in WT mice occurred at Day 7, but was not observed in CCR2 null mice until Day 14 (Figure 3E). No difference was noted in the total cell numbers between WT and CCR2 null mice at Days 0, 3, or 14. Using the total cell counts, the subpopulation differences between CCR2 null and WT mice were assessed. At Day 3, monocytes and DCs demonstrated impaired influx in CCR2 null mice (Figure 3F). Although not statistically significant, we found a trend toward decreased numbers of ExMacs at Day 3. At Day 7, all three inflammatory cells types in the CCR2 null mice had impaired recruitment to the lung (Figure 3G). These data support an important role for CCR2 in the recruitment of monocytes, DCs, and ExMacs after injury with bleomycin. The result was most pronounced at Day 7 after injury. This finding correlated well with an overall reduction in total cell numbers, as quantified by a hemocytometer count (Figure 3E).
Figure 3.
Flow plot analysis of CCR2 null mice after bleomycin. (A) Lung single-cell homogenates were analyzed against MHCII and CD11c cell surface expression and DNs, DCs, and Macs were identified. (B–C) Subgate analysis is performed from the DN (B) and Mac (C) populations. R1 cells are identified again as CD11b+ Gr-1high. AMs are CD11b−, whereas ExMacs are CD11b+. There is an influx of ExMacs occurring at Day 3, peaking at Day 7, and then persisting through Day 14. (D) Subgating of the R1 gate (from [B]) reveals that the cells are Ly6G-negative, consistent with monocytes. (E) Total cells were counted by a hemocytometer from CCR2 null and C57B6 WT mice after bleomycin (WT, open box; CCR2, closed box). (F–G) Individual cell monocyte-derived populations are determined at Day 3 (F) and Day 7 (G) after bleomycin. Graphs are representative of pooled data from three different experiments performed at each time point (n = 3–4 mice at each time point; *P < 0.05, **P < 0.005, ***P < 0.0005 between CCR2 null and WT mice).
ExMacs Are the Macrophage Source of CXCL10
The flow cytometric evaluation of inflammatory cell types after bleomycin administration demonstrated the presence of ExMacs. The functions of ExMacs have not been well described in the lung after bleomycin treatment. We sought to determine if unique functional characteristics distinguished ExMacs from AMs beyond their differences in cell surface expression. We therefore evaluated the production of cytokines previously reported to be important for the development of pulmonary fibrosis. We found no evidence for constitutive production of transforming growth factor–β, insulin-like growth factor–1, or IL-13 in the cultured supernatants of AMs or ExMacs (data not shown). ExMacs isolated at both Days 7 and 14 after bleomycin administration were the major source of macrophage-derived CXCL10 (Figures 4A and 4B). AMs produced no significant CXCL10 at Day 7. At Day 14, AMs produced minimal CXCL10 compared with ExMacs. CXCL10 production was higher from ExMacs at Day 7 than at Day 14. To test if the lack of CXCL10 production by AMs was due to the ex vivo environment lacking appropriate stimulation, we treated AMs and ExMacs with either low–molecular weight HA fragments or LPS, and analyzed cytokine production. HA fragments have been previously shown to stimulate CXCL10 in mouse peritoneal macrophages (28). Stimulation of AMs with HA and LPS resulted in no detectable CXCL10 production (Figures 4A and 4B). Interestingly, HA and LPS had differing effects on ExMac CXCL10 production. Whereas HA treatment did not alter CXCL10 production by ExMacs, LPS stimulation resulted in complete suppression of CXCL10 production. We determined whether this inability to produce CXCL10 by AMs extended to other macrophage-derived cytokines, such as CXCL2 and TNF-α. Without stimulation, neither AMs nor ExMacs produced detectable CXCL2 or TNF-α at Day 7 after bleomycin injury. HA and LPS stimulated both AMs and ExMacs to produce cytokines (Figures 4C and 4D). ExMacs were the predominant source of both CXCL2 and TNF-α. These data suggest that AMs and ExMacs represent distinct macrophage populations after noninfectious fibrotic lung injury, both by cell surface expression and cytokine production.
Figure 4.
Cytokine expression from flow sorted macrophage populations. C57Bl/6 WT mice were given 2.5 U/kg of bleomycin and then the lungs were harvested at Days 7 and 14 after exposure. After digestion and centrifugation over an 18% Nicodenz cushion, the cells were sorted by flow cytometry for AMs and ExMacs, and then cultured overnight to allow cells to adhere to the tissue culture plate. After adherence, the cells were either given no stimulation, or were stimulated with hyaluronan (HA) (100 μg/ml) or LPS (100 ng/ml) for an additional 24 hours. The supernatants were collected and evaluated for cytokine expression by ELISA. (A and B) These figures represent CXCL10 expression by ELISA at Day 7 (A) and Day 14 (B) for AMs (open bar) and ExMac (closed bar). (C and D) Figures demonstrate TNF-α (C) and CXCL2 (D) production in AMs and ExMacs at Day 7 (n = 6–9 pooled mice per experiment; 50,000 sorted macrophages were used per culture well in the Day-7 experiments; 150,000 cells/well were used in the Day-14 experiments; *P < 0.05, **P < 0.005, P < 0.0005 between AMs and ExMacs). This is representative of two experiments at each time point.
CXCL10 Production by ExMacs Is Decreased in CXCR3 Null Mice
We then explored the relationship between CXCL10 production by ExMacs and the in vivo cytokine environment produced in a profibrotic milieu. We hypothesized that the alteration in the cytokine milieu in the CXCR3 null mice would lead to a reduction in CXCL10 production by ExMacs. First, we determined if CXCR3 deficiency altered ExMac recruitment. Time course studies revealed that ExMacs are recruited to the lung after bleomycin injury in the absence of CXCR3 in a manner similar to WT mice (Figure 5A). We then examined the cytokine profiles in response to exogenous stimuli. We focused on macrophages harvested at Day 7 after bleomycin, as this was the time of peak CXCL10 production noted in the prior experiments. As seen with the WT sorted macrophages, ExMacs from CXCR3 null mice were the prime source of CXCL10 (Figure 5B). AMs from CXCR3 null mice produced no detectable CXCL10 (data not shown). CXCL10 production in ExMacs was completely suppressed by stimulation with LPS. Interestingly, CXCR3 null ExMacs demonstrated decreased production of CXCL10 compared with WT ExMacs (Figure 5B). The data suggested that the cytokine environment in the CXCR3 null mice altered the ability of ExMacs to elaborate CXCL10. To confirm this finding, we undertook experiments to see if in vitro stimulation could recover the production of CXCL10 by CXCR3 ExMacs. Stimulation with IFN-γ resulted in an increase in CXCL10 production from CXCR3 null ExMacs as compared with the unstimulated group (Figure 5C). IFN-γ and HA in combination caused an even greater increase in CXCL10 production from CXCR3 null ExMacs (Figure 5C). This demonstrated that exogenous IFN-γ could restore the production of CXCL10 from CXCR3 null ExMacs, and that the combination of IFN-γ and HA had an additional synergistic effect on this production of CXCL10.
Figure 5.
ExMacs from CXCR3 null mice produce less CXCL10. (A) Flow plot analysis from CXCR3 null mice identifies that ExMacs are produced after bleomycin instillation. Flow analysis identifies macrophages using MHCII and CD11c cell surface expression. Subgating on the macrophage population distinguishes CD11blow AMs from CD11bhigh ExMacs. (B) Comparison of CXCL10 production of ExMacs from C57Bl/6 WT and CXCR3−/− mice at Day 7 after bleomycin. (C) CXCL10 production by CXCR3−/– ExMacs was increased after stimulation with IFN-γ and a combination of IFN-γ and HA. These data are representative of two experiments with pooled samples from six to nine mice (**P < 0.005, ***P < 0.0005).
Discussion
Our results suggest that ExMacs are recruited to the lung after noninfectious injury, and are the major source of macrophage-derived CXCL10. We identified an influx of ExMacs, which peaked at Day 7 after bleomycin injury. ExMacs were CD11c+ MHCIIInt Gr-1Int and were separated from resident AMs by high expression of both CD11b and CX3CR1. In addition to the role of ExMacs as the macrophage source of CXCL10 in the lung, we also identified them as a primary source of macrophage-derived cytokines, TNF-α and CXCL2. These data suggest a role for ExMacs in the modulation of the fibrotic response to noninfectious lung injury, and highlight the need for careful identification of macrophage subpopulations as critical to understanding effector functions.
Macrophages accumulate in areas of fibrotic injury, but their role remains incompletely understood, in large part due to the paucity of tools to characterize phenotypic subsets (1, 29). Several studies have attempted to characterize macrophage subsets after bleomycin injury. Everson and colleagues (15) separated AMs into 18 density-defined subpopulations. Bleomycin altered the proportions of these subpopulations, and TNF-α production was only enhanced in the specific subpopulations present after bleomycin administration. Work from our laboratory determined that inducible nitric oxide synthase was increased after stimulation with IFN-γ only in macrophages obtained from bronchoalveolar lavage after bleomycin injury, and not in macrophages lavaged before the injury (30). This demonstration of altered morphologic and functional activities of macrophages after noninfectious injury suggests either recruitment of specific macrophage subsets with unique functional capabilities, or maturation of local resident cells after injury. The present study adds to this body of data by providing evidence that these uniquely functioning macrophages are ExMacs recruited to the lung after bleomycin injury. ExMac function has been described in infectious lung injury models, but these are the first data in noninfectious models.
The major observation of this study is that ExMacs are the primary source of the antifibrotic cytokine, CXCL10, and that there are important differences in how these cells respond to exogenous stimuli. Tager and colleagues (7) demonstrated that CXCL10 inhibited the chemotaxis of fibroblasts to bronchoalveolar lavage from injured lung tissue and prevented the development of fibrosis. We recently demonstrated that these antifibrotic effects of CXCL10 require the proteoglycan, syndecan-4, expressed on fibroblasts (10). The relevant sources of CXCL10 expression in the lung after bleomycin have been unclear, because a variety of cell types are candidates for sources of chemokine production. The identification of CXCL10 production by ExMacs, but not resident alveolar or interstitial macrophages, provides new insights into understanding the mechanisms of CXCL10 production in the lung.
The ability to sort individual cell populations allowed us to study the effect of exogenous ligands on CXCL10 production by lung macrophages. Based on prior work in our laboratory and by others, we examined the effect of HA and LPS stimulation on cytokine production (28, 31, 32). HA is an extracellular matrix glycosaminoglycan, which is degraded in the setting of inflammation to lower molecular weight fragments with immunostimulatory potential (33, 34). These lower molecular weight fragments accumulate in the lung after bleomycin injury, and must be cleared for effective resolution of inflammation (35–37). Surprisingly, CXCL10 production by ExMacs was unchanged after HA stimulation, which differed from our previous findings with peritoneal macrophages (28). Whereas HA administration had no effect on CXCL10 release, surprisingly, LPS stimulation resulted in complete suppression of CXCL10 production from ExMacs. LPS is the prototypical pathogen-associated molecular pattern representing an infectious stimulus. LPS administration to peritoneal macrophages stimulates CXCL10 both in vivo and in vitro (31). LPS stimulation of MH-S cells, an immortalized AM cell line, also resulted in CXCL10 production (32). This finding of CXCL10 suppression by LPS stimulation was specific to CXCL10, as LPS stimulation caused robust TNF-α and CXCL2 production by ExMacs. These data suggest that ExMacs recruited to the lung after noninfectious injury respond differently to endogenous matrix and exogenous pathogen-associated ligands, and highlight the importance of local matrix–cell interactions in regulating the extent of fibroproliferation.
CXCR3 deficiency markedly enhances the profibrotic milieu. We explored the role of ExMacs in this context. The recruitment of ExMacs did not require CXCR3, but the production of CXCL10 by ExMacs was markedly reduced in the absence of CXCR3. The profibrotic environment in CXCR3 null mice results, in part, from impaired endogenous production of IFN-γ and CXCL10 (6). The data presented in the study suggests that the CXCL10 deficiency observed in the absence of CXCR3 is due to impaired release by ExMacs. To determine if the impaired production of CXCL10 by ExMacs was due to an intrinsic defect or an altered external milieu, we administered exogenous IFN-γ to CXCR3 null ExMacs, and found that CXCL10 production was partially restored. Full restoration of CXCL10 production required both IFN-γ and HA fragments. These data further support an important role for ExMacs in limiting the extent of lung fibrosis by producing CXCL10 in response to endogenous HA fragments and IFN-γ. Collectively, these data suggest a previously unrecognized role for ExMacs in the exacerbated fibrosis of CXCR3 null mice after bleomycin injury.
Several groups have examined the role of CCR2 in the pathobiology of lung fibrosis. CCR2 affords some protection against experimental lung fibrosis, but there are differing reports on the mechanisms (38–40). Several studies have shown that CCR2 is critical to the recruitment of monocytes to sites of injury (20, 41, 42). The role of CCR2 after fibrotic lung injury has been explored, but the findings are divergent with respect to the impact of CCR2 deficiency on subsets of mononuclear cells (38, 39). Moore and colleagues (38) demonstrated that CCR2 null mice were protected from lung fibrosis using both bleomycin and FITC-induced fibrotic injury models. They did not appreciate alterations in macrophage cellular influx after FITC exposure by both cell counts and flow cytometry analysis. A subsequent study from the same group attributes the reduction in fibrosis to a decrease in the number fibrocytes recruited to the lung from the bone marrow in a CCR2-dependent manner (40). Okuma and colleagues (39) also reported that CCR2 null mice were protected from bleomycin-induced lung fibrosis. However, they found that macrophage numbers were decreased, but they did not examine subpopulations as performed in the present study. We have confirmed the findings of Okuma and colleagues that macrophage recruitment is impaired in the absence of CCR2, and further defined the relevant populations. Interestingly, at no time point after bleomycin injury was ExMac influx completely abolished. This is in contrast to the role of CCR2 in protection against influenza infection, where ExMac recruitment was completely abolished (20). In fact, the most striking reduction in specific cell populations was in the monocyte and DC compartments. Recent data suggest that DC function is critical to the development of fibrosis after bleomycin injury (43). This finding suggests that the reductions in DCs in CCR2 null mice are relevant to the antifibrotic phenotype. Further investigations are needed to determine the exact role of monocytes and DCs in the fibrotic phenotype of CCR2 null mice.
In summary, we have provided a detailed in vivo analysis of total lung monocyte and monocyte-derived cell recruitment after injury with bleomycin. We highlight that these cells are recruited in a CCR2-dependent fashion, albeit less completely than in infectious models. We have defined the role of ExMacs, a macrophage subpopulation that is distinct from AMs, both by cell surface marker expression, but also by cytokine production, both constitutively and in response to exogenous stimuli. In particular, we show that ExMacs are the major macrophage source of the antifibrotic chemokine, CXCL10. Furthermore, we provide data suggesting that the response of ExMacs to infectious and noninfectious stimuli may be quite different. These data suggest that ExMacs are important modulators of lung fibrosis. Improved understanding of the mechanisms that regulate macrophage subpopulations could lead to new insights into the pathogenesis of fibrotic diseases.
Footnotes
This work was supported by National Institutes of Health grants R01 HL77291-02 and T32 HL007538-23.
Originally Published in Press as DOI: 10.1165/rcmb.2010-0471OC on February 17, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammar SP, Winterbauer RH, Bockus D, Remington F, Friedman S. Idiopathic fibrosing alveolitis: a review with emphasis on ultrastructural and immunohistochemical features. Ultrastruct Pathol 1985;9:345–372 [DOI] [PubMed] [Google Scholar]
- 3.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 2009;175:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest 2009;136:1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest 2007;117:549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest 2004;114:291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol 2004;31:395–404 [DOI] [PubMed] [Google Scholar]
- 8.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma–inducible protein 10 (IP-10; CXCL10)–deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 2002;168:3195–3204 [DOI] [PubMed] [Google Scholar]
- 9.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon gamma–inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun 1995;210:51–57 [DOI] [PubMed] [Google Scholar]
- 10.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest 2010;120:2049–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malur A, McCoy AJ, Arce S, Barna BP, Kavuru MS, Malur AG, Thomassen MJ. Deletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol 2009;182:5816–5822 [DOI] [PubMed] [Google Scholar]
- 12.Dixon AE, Mandac JB, Madtes DK, Martin PJ, Clark JG. Chemokine expression in Th1 cell–induced lung injury: prominence of IFN-gamma inducible chemokines. Am J Physiol Lung Cell Mol Physiol 2000;279:L592–L599 [DOI] [PubMed] [Google Scholar]
- 13.Korpi-Steiner NL, Bates ME, Lee WM, Hall DJ, Bertics PJ. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J Leukoc Biol 2006;80:1364–1374 [DOI] [PubMed] [Google Scholar]
- 14.Chandler DB, Hyde DM, Giri SN. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol 1983;112:170–177 [PMC free article] [PubMed] [Google Scholar]
- 15.Everson MP, Chandler DB. Changes in distribution, morphology, and tumor necrosis factor–alpha secretion of alveolar macrophage subpopulations during the development of bleomycin-induced pulmonary fibrosis. Am J Pathol 1992;140:503–512 [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964 [DOI] [PubMed] [Google Scholar]
- 17.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 2004;31:22–27 [DOI] [PubMed] [Google Scholar]
- 18.Maus U, Huwe J, Maus R, Seeger W, Lohmeyer J. Alveolar JE/MCP-1 and endotoxin synergize to provoke lung cytokine upregulation, sequential neutrophil and monocyte influx, and vascular leakage in mice. Am J Respir Crit Care Med 2001;164:406–411 [DOI] [PubMed] [Google Scholar]
- 19.Taut K, Winter C, Briles DE, Paton JC, Christman JW, Maus R, Baumann R, Welte T, Maus UA. Macrophage turnover kinetics in the lungs of mice infected with Streptococcus pneumoniae. Am J Respir Cell Mol Biol 2008;38:105–113 [DOI] [PubMed] [Google Scholar]
- 20.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte–derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 2008;180:2562–2572 [DOI] [PubMed] [Google Scholar]
- 21.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82 [DOI] [PubMed] [Google Scholar]
- 22.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol 2007;178:2000–2007 [DOI] [PubMed] [Google Scholar]
- 23.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol 2007;179:3488–3494 [DOI] [PubMed] [Google Scholar]
- 24.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007;117:902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med 2000;192:1515–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McComb JG, Ranganathan M, Liu XH, Pilewski JM, Ray P, Watkins SC, Choi AM, Lee JS. Cx3cl1 up-regulation is associated with recruitment of CX3CR1+ mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke–induced emphysema. Am J Pathol 2008;173:949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tager AM, Luster AD, Leary CP, Sakamoto H, Zhao LH, Preffer F, Kradin RL. Accessory cells with immunophenotypic and functional features of monocyte-derived dendritic cells are recruited to the lung during pulmonary inflammation. J Leukoc Biol 1999;66:901–908 [DOI] [PubMed] [Google Scholar]
- 28.Horton MR, McKee CM, Bao C, Liao F, Farber JM, Hodge-DuFour J, Pure E, Oliver BL, Wright TM, Noble PW. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines MIG and interferon-inducible protein–10 in mouse macrophages. J Biol Chem 1998;273:35088–35094 [DOI] [PubMed] [Google Scholar]
- 29.Bowden DH, Adamson IY. The role of cell injury and the continuing inflammatory response in the generation of silicotic pulmonary fibrosis. J Pathol 1984;144:149–161 [DOI] [PubMed] [Google Scholar]
- 30.McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, Choi AM, Noble PW. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor kappaB–dependent mechanism. J Biol Chem 1997;272:8013–8018 [DOI] [PubMed] [Google Scholar]
- 31.Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, Hamilton TA, Vogel SN. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol 1999;163:1537–1544 [PubMed] [Google Scholar]
- 32.Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol 2007;41:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992;6:2397–2404 [PubMed] [Google Scholar]
- 34.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 2007;23:435–461 [DOI] [PubMed] [Google Scholar]
- 35.Nettelbladt O, Bergh J, Schenholm M, Tengblad A, Hallgren R. Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am Rev Respir Dis 1989;139:759–762 [DOI] [PubMed] [Google Scholar]
- 36.Nettelbladt O, Hallgren R. Hyaluronan (hyaluronic acid) in bronchoalveolar lavage fluid during the development of bleomycin-induced alveolitis in the rat. Am Rev Respir Dis 1989;140:1028–1032 [DOI] [PubMed] [Google Scholar]
- 37.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 2002;296:155–158 [DOI] [PubMed] [Google Scholar]
- 38.Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 2001;167:4368–4377 [DOI] [PubMed] [Google Scholar]
- 39.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol 2004;204:594–604 [DOI] [PubMed] [Google Scholar]
- 40.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 1997;186:1757–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlondorff D, Seeger W, et al. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 2002;166:268–273 [DOI] [PubMed] [Google Scholar]
- 43.Bantsimba-Malanda C, Marchal-Somme J, Goven D, Freynet O, Michel L, Crestani B, Soler P. A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice? Am J Respir Crit Care Med 2010;182:385–395 [DOI] [PubMed] [Google Scholar]