Abstract
Organic dust exposure in agricultural environments results in significant airway inflammatory diseases. Gram-positive cell wall components are present in high concentrations in animal farming dusts, but their role in mediating dust-induced airway inflammation is not clear. This study investigated the role of Toll-like receptor (TLR) 2, a pattern recognition receptor for gram-positive cell wall products, in regulating swine facility organic dust extract (DE)–induced airway inflammation in mice. Isolated lung macrophages from TLR2 knockout mice demonstrated reduced TNF-α, IL-6, keratinocyte chemoattractant/CXCL1, but not macrophage inflammatory protein-2/CXCL2 expression, after DE stimulation ex vivo. Next, using an established mouse model of intranasal inhalation challenge, we analyzed bronchoalveolar lavage fluid and lung tissue in TLR2-deficient and wild-type (WT) mice after single and repetitive DE challenge. Neutrophil influx and select cytokines/chemokines were significantly lower in TLR2-deficient mice at 5 and 24 hours after single DE challenge. After daily exposure to DE for 2 weeks, there were significant reductions in total cellularity, neutrophil influx, and TNF-α, IL-6, CXCL1, but not CXCL2 expression, in TLR2-deficient mice as compared with WT animals. Lung pathology revealed that bronchiolar inflammation, but not alveolar inflammation, was reduced in TLR2-deficient mice after repetitive exposure. Airway hyperresponsiveness to methacholine after dust exposure was similar in both groups. Finally, airway inflammatory responses in WT mice after challenge with a TLR2 agonist, peptidoglycan, resembled DE-induced responses. Collectively, these results demonstrate that the TLR2 pathway is important in regulating swine facility organic dust–induced airway inflammation, which suggests the importance of TLR2 agonists in mediating large animal farming–induced airway inflammatory responses.
Keywords: Toll-like receptor 2, swine/pig facility, peptidoglycan, organic dust, lung pathology
Clinical Relevance
This work demonstrates that the Toll-like receptor (TLR) 2 pathway plays an important role in regulating swine facility organic dust–induced airway inflammation in vivo. These findings have important implications for future environmental sampling strategies to focus on, and to reduce exposure of, TLR2 agonists present in agricultural environments. Targeting the TLR2 pathway might also be an important adjunctive therapy when investigating prevention and therapeutic interventions in humans.
Agricultural workers, particularly swine farmers, exhibit a high prevalence of airway diseases, including chronic bronchitis, exacerbation of asthma, and obstructive lung disease, which is thought to be due to repeated exposure to inhaled organic dusts or bioaerosols (1). A challenge in defining mechanisms of organic dust–induced inflammatory responses lies in the inherent complexity of the dust. One established inflammatory component present in organic dust is endotoxin; however, epidemiologic and multiple laboratory-based studies have failed to definitively link endotoxin exposure to disease manifestation (2–8). Consistent with these observations, we and others have found a strong predominance of gram-positive (rather than gram-negative) bacteria in modern swine confinement facility organic dust samples (6, 9). Furthermore, mass spectrometry analysis has demonstrated high concentrations of muramic acid, a component of peptidoglycan (PGN), which originates from the bacterial cell wall of gram-positive bacteria (i.e., 85% of total cell wall) and, to a lesser degree, gram-negative bacteria (i.e., 5% of cell wall) in large animal farming environments (e.g., swine and dairy barns) (6, 10). In addition, prior reports have suggested that nonendotoxin components, such as PGN, are responsible for driving the innate immune inflammatory responses to swine facility animal farming dusts in vitro (6, 10, 11). However, it is not known if targeting innate immune pattern recognition receptors of gram-positive cell wall products will affect complex organic dust–induced airway inflammation, which was the objective of the current study.
One family of innate immune receptors responsible for recognizing highly conserved microbial motifs are the Toll-like receptors (TLRs) (12). Of the 10 TLRs, TLR2 has been implicated as a critical receptor of gram-positive bacteria, because TLR2 recognizes PGNs, lipoteichoic acid, and lipoproteins that are associated with the cell wall of gram-positive bacteria (12, 13). TLR2 is highly expressed on antigen-presenting cells, such as macrophages, cells important in mediating the innate immune response to inhaled organic dust (14, 15). On airway epithelial cells, TLR2 expression is up-regulated after swine facility organic dust exposure, and blocking epithelial cell TLR2 results in a dampening of proinflammatory cytokine release after organic dust exposure in vitro (16, 17). In general, the role of TLR2 in mediating airways disease is controversial. In various lung infection models, deficiencies in TLR2 have been associated with increased proinflammatory mediator release and resistance to infection (18), decreased proinflammatory mediator release and failure to control infection (19–21), or no significant effect with lung inflammation indices (22). In comparison, airway inflammatory consequences after inhalation challenge with pure selective TLR2 agonists are reduced in TLR2-deficient mice (23). It is not known if there is a role for TLR2 in modulating airway inflammatory responses to a complex microbial exposure, such as large animal farming dusts. However, these previous observations suggested to us that TLR2 might play an important role in mediating airway inflammatory responses after large animal farming dust exposures, an environment rich in gram-positive cell wall products.
In this study, we hypothesized that TLR2 loss would significantly reduce airway inflammatory responses to swine facility organic dust extract (DE) exposure. To test this hypothesis, we first determined whether there would be a reduction in cytokine/chemokine release from primary lung macrophages isolated from TLR2-deficient mice compared with wild-type (WT) animals. Next, we investigated, in an established in vivo murine model (14), if airway inflammatory responses to intranasal inhalation of organic DE in TLR2-deficient mice would differ as compared with WT animals at various time points and concentrations of DE. Finally, we determined if a TLR2 agonist, PGN, would induce similar airway inflammatory responses as DE. Collectively, we found an important role for TLR2 in mediating airway inflammatory responses to swine facility organic dust in vivo. Namely, the absence of TLR2 signaling resulted in an attenuation of dust-dependent airway inflammation and lung pathology, but not acute airway hyperresponsiveness (AHR) and alveolar inflammation.
Materials and Methods
DE
Swine confinement animal feeding operation facility organic dust was collected and prepared as DE as previously described (7, 8) and as described in the online supplement.
Mice
C57BL/6 WT mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and TLR2 knockout (KO; same C57BL/6 background) mice were provided by S. Akira (Osaka, Japan). Animal studies were approved by the Institutional Animal Care and Use Committee of the Omaha Veterans Affairs Medical Center and the University of Nebraska Medical Center, according to National Institutes of Health guidelines for the use of rodents.
Lung Macrophages
Lung macrophages were isolated as described in the Methods section in the online supplement. After isolation, lung macrophages were stimulated with 1% DE. After 24 hours, cell-free supernatants were collected and stored at −80°C.
Animal Model
Mice received saline or DE by an intranasal exposure method, as previously established (14). A concentration of 12.5% DE is an optimal concentration for eliciting lung inflammation (14). In some experiments, a suboptimal 2.5% DE concentration was also used to determine the importance of TLR2 at a relatively high and low organic dust concentration exposure. Animals also received PGN, LPS, and heat-inactivated DE, which is described in the Methods in the online supplement.
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) fluid was collected as previously described (14). Total cell number was enumerated and differential cell counts were determined on cytospin-prepared slides (Cytopro Cytocentrifuge, Wescor Inc., Logan, UT) stained with DiffQuick (Dade Behring, Newark, DE).
Cytokine/Chemokine Assays
Murine TNF-α, IL-6, keratinocyte chemoattractant (KC; CXCL1), and macrophage inflammatory protein (MIP)–2 (CXCL2) concentrations were determined in BAL fluid according to manufacturer's instructions using commercially available ELISA kits (R&D Systems, Minneapolis, MN).
Nitric Oxide Analysis
Nitric oxide (NO) production in BAL fluid was assessed via the detection of NO by a gas-phase chemiluminescent reaction between NO and ozone (Sievers Instruments Model 280i; GE Analytical Instruments, Boulder, CO) according to previous published procedures, because this is a highly sensitive assay, detecting NO at low levels (24).
Lung Collection
After lung lavage, whole lungs were excised and inflated to 10 cm H2O pressure with 10% formalin (Sigma, St. Louis, MO) solution to preserve pulmonary architecture. Lungs were embedded in paraffin, and sections (4–5 M) were cut and stained with hematoxylin and eosin. Lung slides were reviewed and semiquantitatively assessed for the degree of inflammation as well as the distribution of the inflammation by a reviewer (pathologist [W.W.W.]) blinded to the treatment conditions using a previously published scoring system (14).
Pulmonary Function Measurement
At 3 hours after a single intranasal instillation of 12.5% DE or PGN (14), mice were anesthetized, tracheostomized, and mechanically ventilated at a rate of 160 breaths/min and a tidal volume of 0.15 ml, using a computerized small animal ventilator (Finepoint; Buxco Electronic, Wilmington, NC) (14, 25). Dose responsiveness to aerosolized methacholine (1.5–48.0 mg/ml) was obtained and results reported as total lung resistance.
Statistical Analysis
Data are presented as the mean (±SEM). Statistical significance was assessed by one-way ANOVA and two-tailed unpaired and paired t test, where appropriate, to determine significant changes among treatment groups using GraphPad Prism version 5.0 (GraphPad Inc., La Jolla, CA) software.
Results
Organic Dust–Induced Inflammatory Mediator Production in Primary Lung Macrophages Is Predominately TLR2 Dependent
To define if there were functional roles of TLR2 in mediating lung macrophage response to organic DE, lung macrophages were isolated from TLR2-deficient and WT mice (C57BL/6 background) and ex vivo stimulated with 1% DE for 24 hours. There were significant reductions in TNF-α (−55%), IL-6 (−64%), and CXCL1 (−87%) production in TLR2-deficient lung macrophages; however, there was no significant change in CXCL2 expression (Figure 1; n = 4 mice per group). Trypan blue exclusion analysis demonstrated that the effects of TLR2 loss were not due to differences in cell viability or number (data not shown). Collectively, these results suggest that TLR2 signaling is important for regulating the expression of select cytokines/chemokines in lung macrophages after DE challenge.
Figure 1.
Isolated lung macrophages from Toll-like receptor (TLR) 2 knockout (KO) mice demonstrate dampened TNF-α, IL-6, keratinocyte chemoattractant (KC)/CXCL1, but not macrophage inflammatory protein (MIP)–2/CXCL2, production after ex vivo stimulation with 1% swine facility dust extract (DE) stimulation for 24 hours as compared with lung macrophages from DE-stimulated wild-type (WT) mice. Mean results are presented per 200,000 cells (±SEM); n = 4 mice per group. Statistically significance: *P < 0.05, **P < 0.01.
Acute Dust-Induced Airway Cellular Inflammation and Cytokine/Chemokine Release Is Reduced in TLR2-Deficient Mice
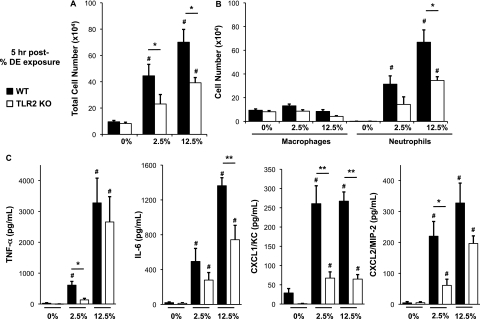
We have previously established that a one-time (single/acute) intranasal inhalation challenge with 12.5% DE resulted in significant increases in cellular influx and lavage fluid cytokine/chemokine release at 5 hours after exposure (14). In the current study, we sought to determine if TLR2 deficiency would dampen airway inflammatory responses after a one-time DE challenge in mice. The increase in total leukocyte counts in the lavage fluid after DE challenge was significantly reduced in TLR2-deficient mice as compared with WT mice at both low (−48%) and high (−55%) DE concentrations (Figure 2A). Consistent with our previous work, DE induced the rapid influx of airway neutrophils (Figure 2B); however, airway neutrophils were significantly reduced (−55%) in TLR2-deficient mice receiving 12.5% DE as compared with WT animals (Figure 2B).
Figure 2.
(A–C). Lung lavage cellularity and cytokine/chemokine release is acutely reduced in TLR2-deficient mice after a one-time challenge with low- and high-concentration DE. Lung lavage fluid mean concentration of total cells (A) and cell differential (B) 5 hours after single intranasal inhalation exposure of DE (0% [saline], 2.5%, and 12.5%) in WT and TLR2 KO mice. (C) Cell-free mean levels of lung lavage fluid supernatant cytokines/chemokines collected 5 hours after exposure to DE. Error bars represent SE (n = 4–6 mice per group). #Statistically significant between respective DE-treated and saline-treated group. *P < 0.05 and **P < 0.01 indicate statistical significance between WT and KO DE-treated mice.
Organic dust–induced airway disease has also been associated with increases in TNF-α, IL-6, and neutrophil chemoattractants (human IL-8, murine KC/CXCL1, and MIP-2/CXCL2), which are particularly important after an acute (single) exposure in naive humans and mice (14, 26, 27). At 5 hours after a 2.5% DE exposure, there was a significant reduction in TNF-α, CXCL2, and CXCL1, but not IL-6, in TLR2-deficient mice (Figure 2C). In comparison, 5 hours after a 12.5% DE exposure, there was a significant reduction in IL-6 and CXCL1, but not TNF-α and CXCL2 (Figure 2C). Collectively, these data demonstrate that TLR2-dependent pathways are involved in rapidly responding to swine facility organic dust exposure in the airway.
Airway Cellular Influx and Cytokine/Chemokine Release Remains Dampened over Time after a Single DE Challenge in TLR2-Deficient Mice
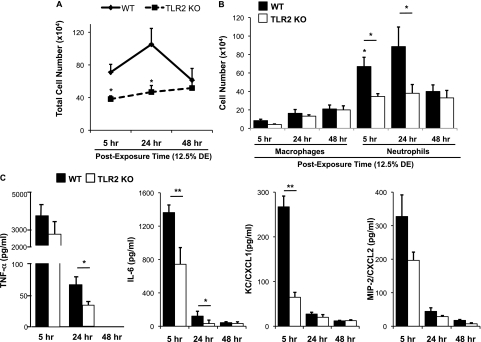
Because the cytokine/chemokine response to DE was impaired acutely (at 5 h) in the TLR2-deficient mice, we hypothesized that there could be a delayed, compensatory increase in airway cellularity influx and cytokine/chemokine release in the TLR2-deficient mice after DE challenge. However, we found no delayed compensatory increase in these proinflammatory outcomes. Using a high concentration of DE (12.5%), TLR2-deficient and WT mice were killed at 24 and 48 hours after single intranasal inhalation DE challenge. There was no significant delayed increase of inflammatory cellular influx in TLR2-deficient mice at 24 hours after DE exposure. Total lavage cellularity (Figure 3A) and airway neutrophils (Figure 3B) remained significantly reduced in TLR2 KO mice as compared with WT mice. At 48 hours after DE exposure, there was no significant difference between groups, which may be explained by a clearing of the cells from the airway in the WT mice. At all times and dose concentrations, the percentage of lymphocytes and/or eosinophils ranged from 0 to 1% in all animals (data not shown).
Figure 3.
(A–C). Lung lavage cellular influx and selective cytokine/chemokine release remains dampened over time after a single DE challenge in TLR2-deficient mice. Lung lavage fluid mean total cells (A) and cell differential (B) after 12.5% DE at 5, 24, and 48 hours after intranasal inhalation challenge. (C) Cell-free mean levels of lung lavage fluid supernatant cytokines/chemokines collected at 5, 24, and 48 hours after exposure to 12.5% DE. Error bars are SE (n = 6–8 mice per group). *P < 0.05 and **P < 0.01 indicate statistical significance between WT and KO DE-treated mice.
Cytokine/chemokine levels in BAL fluid were also measured at 24 and 48 hours after a single DE (12.5%) challenge, with results depicted in Figure 3C. Overall, levels of mediators were less at 24 and 48 hours as compared with 5 hours after DE challenge in WT and TLR2 KO mice, which is consistent with the kinetics of these mediators (28, 29). Compared with WT mice, TNF-α and IL-6, but not neutrophil chemoattractants (CXCL1, CXCL2), were significantly reduced in TLR2 KO mice after 12.5% DE at 24 hours after challenge. At 48 hours after DE (12.5%) inhalation challenge, all mediators were either undetected or at the lower limit of assay sensitivity/detection. Together, these studies demonstrate that TLR2-deficient mice have reduced inflammatory cellular influx and select cytokine/chemokine release in the lavage fluid after a one-time challenge with organic dust, and that there is no delayed or compensatory recovery in these inflammatory indices.
TLR2 Impacts Dust-Induced NO Production in a Time-Dependent Manner
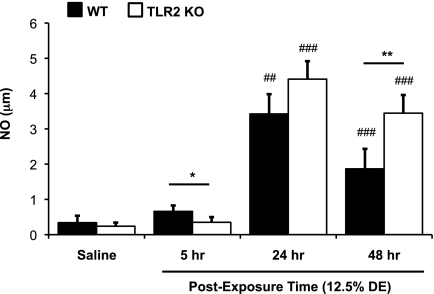
In these experiments, NO levels in lavage fluid were investigated because NO is known to modify airway inflammation (30), and there are subtle, but statistically significant, increases in exhaled NO in normal subjects and workers exposed to swine confinement facility dusts (26, 27). There was an elevation in lavage fluid NO in WT mice after single DE exposure as compared with saline, which was only significant at the 24 and 48 hours after exposure time point (Figure 4). Although there was a statistically significant decrease (P = 0.041) at 5 hours after DE challenge in the TLR2-deficient mice as compared with WT mice, our main observation was that, at 48 hours, but not 24 hours, after exposure there was a significant increase (P = 0.003) in NO production in the TLR2-deficient mice as compared with WT mice (Figure 4). These findings suggest a time-dependent and potential compensatory role for the TLR2 pathway in mediating ODE-induced NO production.
Figure 4.
Increases in swine facility organic DE-induced nitric oxide (NO) is dependent on the TLR2 pathway. Lung lavage fluid mean NO concentrations 5, 24, and 48 hours after saline and 12.5% DE in WT and TLR2 KO mice (n = 4–8 mice per group). Error bars represent SE. *P < 0.05 and **P < 0.01 are statistically significant between WT and KO 12.5% DE–treated mice; ##P < 0.01 and ###P < 0.001 are statistically significant between respective saline and DE-treated groups.
Repetitive Dust-Induced Inflammatory Cell and Mediator Release Is Dependent on the TLR2 Pathway
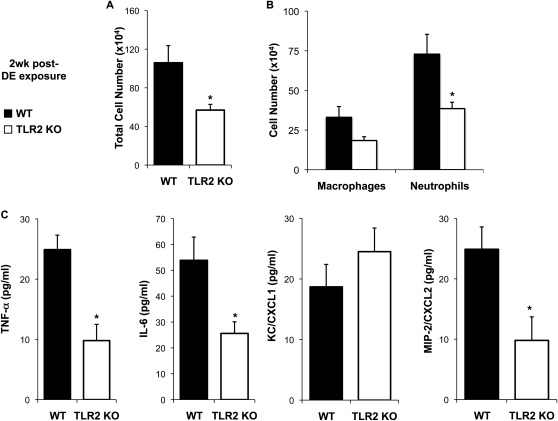
Because the overall acute airway inflammatory response to DE was diminished in TLR2 KO mice, we next hypothesized that the airway inflammation would be reduced after daily, repetitive exposure to DE in the TLR2-deficient animals. To test this hypothesis, TLR2 KO and WT mice received an intranasal inhalation of 12.5% DE daily for 2 weeks, and, at 24 hours after final exposure, mice were killed and BAL fluid was collected for inflammatory cell and mediator analysis. There was a significant reduction in total cellularity in the lavage fluid of TLR2-deficient mice as compared with WT mice (Figure 5A). Whereas the influx of macrophages trended to be lower in TLR2-deficient mice, there was a significant reduction in the influx of neutrophils in TLR2 KO as compared with WT animals (Figure 5B). The percentage of lymphocytes or eosinophils ranged from 0 to 1% in all animals (data not shown). There was also significant reduction in TNF-α, IL-6, MIP-2/CXCL2, but not KC/CXCL1, in TLR2-deficient mice as compared with WT animals (Figure 5C).
Figure 5.
(A–C). TLR2-deficient mice demonstrate reduced lavage inflammatory cellular influx and TNF-α, IL-6, and CXCL2 after repetitive daily exposure for 2 weeks to 12.5% DE. Lung lavage fluid mean concentration of total cells (A) and cell differential (B) 24 hours after final exposure of 12.5% DE in WT and TLR2 KO mice. (C) Mean level of cell-free lung lavage supernatant release of cytokines/chemokines in WT and TLR2 KO of 2-week repetitively exposed mice, 24 hours after final exposure. Error bars represent SE (n = 6 mice per group). *P < 0.05 is statistically significant between WT and KO DE-treated mice.
Dust-Induced Bronchiolar Inflammation Is Reduced in TLR2-Deficient Mice after Repetitive Exposure
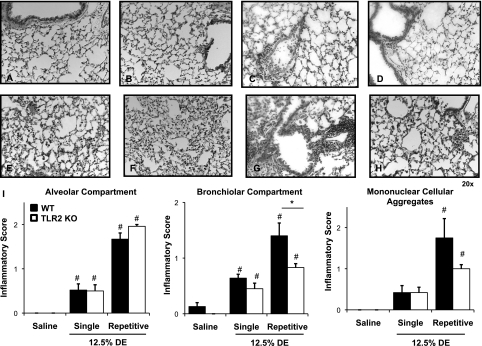
To determine if TLR2 loss would affect DE-induced lung parenchymal inflammation, we examined formalin-fixed, paraffin-embedded whole lungs of WT and TLR2-deficient mice (Figures 6A–6H). In microscopic review of the lung tissue, there were lung parenchymal differences between TLR2-deficient and WT mice after repetitive challenge with DE. To assess and compare semiquantitatively the ranges of DE-induced histopathologic changes, pathology inflammatory scores were determined (14). There was a significant decrease in the bronchiolar compartment inflammatory score, but not alveolar inflammatory compartment and mononuclear cellular aggregates in TLR2 KO mice as compared with WT mice after repetitive inhalation challenge to DE daily for 2 weeks (Figure 6I).
Figure 6.
Reduced lung inflammation in TLR2-deficient mice after intranasal inhalation of DE. Saline-treated WT mice (A) and saline-treated TLR2 KO mice (B) are compared, with mice exposed once ([C] WT and [D] TLR2 KO) and mice exposed repeatedly for 2 weeks ([E and G] WT; [F and H] TLR2 KO) to DE (12.5%). A representative 4- to 5-μm-thick section of one of four to six mice per treatment group is shown at 20× magnification. (E and F) Alveolar compartment inflammation; (G and H) Bronchiolar compartment inflammation after repetitive (2-wk) DE challenge in WT and TLR2 KO mice, respectively. (I) Semiquantitative inflammatory score of lungs after intranasal inhalation of saline and DE (12.5%) after single exposure or repetitive (2-wk) exposure of DE. Semiquantitative distribution of lung alveolar inflammation, bronchiolar inflammation, and mononuclear cellular aggregates in mice (n = 4–6 mice per group). Error bars represent SE. #P < 0.05, significant differences between saline and DE treated; (*P < 0.05, differences between TLR2 KO and WT.
Dust-Induced AHR Is TLR2 Independent
AHR to swine facility organic dust occurs in humans, and is modeled in mice after a single exposure with an adaptation response marked by resolution of AHR occurring with repetitive exposures (14, 31, 32). For this reason, we examined AHR in the DE mice after a one-time exposure to 12.5% DE. There was no reduction in AHR in TLR2 KO mice challenged with DE as compared with the WT animals after DE (mean ± SEM total lung resistance at 48 mg/ml methacholine [maximum concentration] was: WT, 9.0 ± 0.4 versus TLR2 KO, 8.2 ± 0.5; n = 8 mice per group; P > 0.05). Therefore, although TLR2 KO animals demonstrate an overall impairment in inflammatory parameters after DE challenge, this does not significantly dampen AHR.
A TLR2 Agonist, PGN, Induces Airway Inflammatory Responses Similar to DE
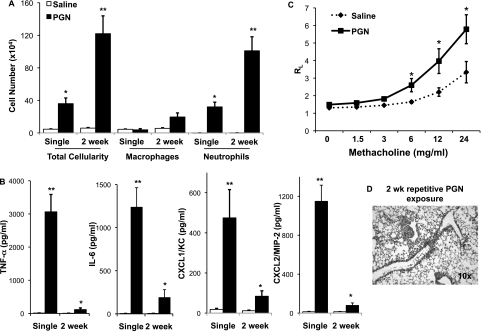
To determine if a TLR2-agonist would induce similar airway inflammatory responses as that of DE, and to remain consistent with our previous published in vitro studies (6, 7, 11), WT mice were intranasally challenged with PGN (100 μg) or saline once (single exposure) and once daily for 2 weeks (repetitive exposure) and subsequently killed at 5 hours after final exposure, as described in the online supplement. As compared with saline, PGN induced a significant increase in total leukocyte counts, predominately due to an expansion of neutrophils, after single and repetitive exposure (Figure 7A). There were also significant increases in TNF-α, IL-6, CXCL1, and CXCL2 after PGN challenge after single and repetitive exposures as compared with saline (Figure 7B). AHR to PGN occurred after single exposure (Figure 7C), with an adaptation response (resolution of AHR) after repetitive exposure (data not shown), as determined by invasive pulmonary function measurements. Finally, microscopic review of lung tissue revealed increased bronchiolar and alveolar compartment inflammation and presence of mononuclear cellular aggregates in WT mice after daily PGN exposure for 2 weeks (Figure 7D). Collectively, these findings demonstrate that PGN, a TLR2 agonist, at high concentration, elicits airway inflammatory responses similar to high-concentration DE.
Figure 7.
Lung lavage cellularity, cytokine/chemokine release, and lung inflammation is increased in WT mice after single and repetitive intranasal challenge(s) with peptidoglycan (PGN) as compared with saline. Lung lavage fluid mean concentration of total cells and cell differential (A) and cytokine/chemokine release (B) 5 hours after single and repetitive (daily instillation for 2 wk) intranasal inhalation of PGN (100 μg) or saline in WT mice (n = 3–4 mice per group). (C) Methacholine (MCh)-induced airway hyperresponsiveness (AHR) after single intranasal inhalation of PGN as compared with saline in mice. Total lung resistance (RL) was directly measured using a mechanically ventilated mouse system. Data are expressed as total RL with SE bars (n = 6–7 mice per group). (D) A representative 4- to -5-μm-thick section after 2-week repetitive exposure to PGN at 10× magnification. *P < 0.05 and **P < 0.01 indicate statistical significance between saline- and PGN-treated mice.
Discussion
Because recent studies of environmental dusts from animal farming environments reveal a diverse population of gram-positive bacteria and high concentrations of PGN (6, 10), we focused on TLR2 as a likely key receptor in regulating dust-induced airway inflammation, because TLR2 recognizes cell wall components of gram-positive bacteria (12, 13). We found that organic dust–induced airway inflammation was highly dependent on the TLR2 pathway in mice after single and repetitive challenge(s) to DE. This dependence was demonstrated by a reduction in cellular influx, airway cytokine/chemokine, and bronchiolar inflammation in TLR2-deficient mice as compared with control mice. However, AHR and alveolar inflammation was not altered in the TLR2-deficient animals, suggesting different mechanisms for airway contractility and lung parenchymal inflammation, respectively. To the best of our knowledge, this is the first report to demonstrate an in vivo functional role for the TLR2 pathway in mediating airway inflammatory responses to complex organic dust exposures.
Dusts from large animal confinement facilities, such as modern swine barns, are recognized to be complex mixtures of microbial products from gram-negative and gram-positive bacteria, molds, particulate matter from grain particles, and fecal material. Although endotoxin is the major driver of corn (grain) dust–induced airway inflammation due to its predominance in these environments, and mice genetically hyporesponsive to endotoxin are protected from airway inflammatory outcomes after respective exposures (33, 34), endotoxin is likely not the principal component of animal facility dusts. Several studies disagree about the association of endotoxin and disease outcomes in exposed workers (2–4). Furthermore, others found only a significant decrease in lavage cellularity and neutrophils after single (not repetitive) exposure to swine barn air in endotoxin-hyporesponsive mice (C3H/HeJ), but no difference in AHR and cytokine levels after single and repetitive exposures (35). In our earlier studies, we found no difference in lavage cellularity or cytokine/chemokine production after a single intranasal swine facility DE (12.5%) challenge in endotoxin-nonresponsive mice (C3H/HeJ) (data not shown). Another potential component in the DE is particulate matter as coarse particulate matter (>2.5 μm) has been shown to be a potent stimulus (15). However, it is not likely to be a major contributor to explain our results, because coarse particulate matter was removed in our sterile filtering process. In addition, when dust was heat inactivated (heating DE to 120°C for 24 h), which is a process that inactivates the biologics and leaves the metals and particles intact, the heat-inactivated DE failed to elicit an airway inflammatory response (data not shown).
Due to recent advances in this field, there is an emerging shift to understand the potential role of gram-positive bacteria components in mediating animal farming–induced disease. As there is no way to remove or scrub only PGNs from the DEs, targeting key receptors/pathways that recognize and activate innate immune responses to PGNs can be used. To this end, we first found that TNF-α, IL-6, CXCL1, but not CXCL2, were significantly reduced in isolated, dust-exposed, TLR2-deficient lung macrophages. Other studies reported diminished mediator release after swine facility DE in airway epithelial cells in vitro when TLR2 is blocked (17). We next found that a TLR2-dependent mechanism was involved at both relatively low and high concentrations of the DE, suggesting specific and sensitive activation pathway(s) for these DE-triggered responses. Specifically, cellular influx was significantly reduced in the absence of TLR2, and release of some cytokines/chemokines in the airway was also reduced after a one-time challenge with DE. Furthermore, there was no rebound in inflammatory cells or cytokines/chemokines at later time points (24 and 48 h after exposure) in the absence of TLR2. Thus, these findings support a central role, not a temporal role, of the TLR2 pathway in regulating acute organic dust–induced airway inflammation indices. Of note, our findings were not explained by a “general hyporesponsiveness” in the TLR2-deficient mice, because these TLR2-deficient mice respond appropriately to LPS challenge (see online supplement), which is also consistent with prior reports (13). However, although there was a reduction in some cytokine/chemokine release in TLR2-deficient compared with WT mice, significant differences in all mediators were not globally observed. Thus, acute swine confinement facility organic dust exposure–induced airway inflammation is largely, but not completely, dependent on the TLR2 pathway. Potential alternative receptors that may cooperate with TLR2 to mediate pathology after complex organic dust exposure include extracellular TLR1, -4, and -6, and/or intracellular TLR9 and the family of nucleotide-binding domain, leucine rich containing (NLR) protein family, which includes nucleotide oligomerization domains 1 and 2 (36).
In contrast to the inflammatory cellular influx and cytokine/chemokine production findings, there appears to be an opposite, time-dependent role for the TLR2 pathway in mediating DE-induced NO production. NO is important in airway inflammatory responses and host defense against infection (30), and, in humans, there are subtle, but statistically significant, increases in exhaled NO in normal subjects and workers exposed to swine confinement facility dusts (26, 27). We found an increase in NO after an acute challenge with DE, which was significant at 24 and 48 hours, but not 5 hours after exposure as compared with saline control. Although there was a significant decrease of NO at 5 hours after exposure in the TLR2-deficient mice as compared with WT mice, this effect was subtle. More importantly, the TLR2-deficient mice demonstrated significant increases in DE-induced NO production as compared with WT animals at 48 hours after exposure. Although the explanation for this finding is not clear, it might suggest the lack of a negative regulatory signal normally delivered by TLR2 at later time points. Alternatively, others have described defects in impaired clearance of microorganisms in states of TLR2 deficiency (21), and it is possible that this later increase in NO might be a compensatory effect to clear toxins from the airway.
There was also an overall protection against repetitive organic dust–induced airway inflammation in the TLR2-deficient mouse. Specifically, total BAL cellular and neutrophil influx and TNF-α, IL-6, and CXCL2, but not CXCL1, were reduced in TLR2-deficient mice after repetitive challenge. Importantly, lung parenchymal changes differed and were marked by a reduction in bronchiolar compartment inflammation indices, but not alveolar compartment inflammation indices and mononuclear cellular aggregates. This finding, that the alveolar compartment inflammation was not altered, is important. We speculate that the TLR2 pathway is important for controlling inhalation challenges to toxins/microbial cell wall component(s) at or before the terminal bronchioles. Another potential explanation is that deficiencies in the TLR2 pathway can be associated with impaired pulmonary clearance. In agreement with this hypothesis, several other investigators have demonstrated that a deficiency in TLR2 is associated with a higher bacterial burden in the lower respiratory tract after infection due to impaired pulmonary clearance (21, 37, 38). Thus, our data suggest that there are also deleterious effects in the lower respiratory tract after repetitive, complex organic DE inhalation challenges in states of TLR2 deficiency.
In contrast to the striking TLR2-dependent changes that we observed in DE-triggered airway inflammation, we did not observe changes in airway AHR after a one-time challenge with DE in TLR2-deficient mice. As stated above, others have also demonstrated that the TLR4 pathway does not mediate swine barn air–induced AHR (35). Thus, the mechanism(s) responsible for mediating complex organic dust–induced AHR remain unresolved, but these mechanism(s) appear to be TLR2 and TLR4 independent. Future studies should investigate targeting signaling proteins downstream of TLRs, such as myeloid differentiation factor (MyD) 88, MyD88 adapter–like/Toll IL-1 receptor–associated protein, TLR-associated activator of IFN, and TLR-associated molecule.
Although the TLR2 pathway was chosen here because recent analysis found a potential strong role for gram-positive cell wall products in mediating large animal farming dust(s)–induced inflammatory outcomes (6, 9, 10), we recognize that TLR2 can bind to lipoarabinomannan from mycobacteria, and zymosan from fungi, and other lipoproteins from gram-negative bacteria (39). It is possible that these other microbial products, which may be present in the dust, are mediating inflammatory outcomes after exposure challenges, and that the binding of these agents are reduced in the absence of TLR2. However, in support of the role of PGN, we found that, when we intranasally challenged mice with a high concentration of a TLR2-agonist, PGN (100 μg, a concentration approximating half the protein concentration in the DE [see online supplement]), the airway inflammatory results were similar to those observed with the DE. Namely, there was a robust increase in airway inflammatory cell influx, cytokine/chemokine production, and AHR after a single challenge, and, after repetitive exposures, there was a chronic adaptation response marked by reduction in cytokine/chemokine production and AHR, but increased lung parenchymal cellular inflammation.
In summary, this study demonstrates several new aspects related to organic dust–induced airway inflammation. Namely, TLR2 appears to be a positive regulator of organic dust–induced proinflammatory cytokine/chemokine production in lung macrophages, and that organic dust–induced airway inflammation in vivo is dependent, in part, on the TLR2 pathway. Together, these studies highlight a significant role for TLR2 agonists present in the organic dust, which underscores the importance of future environmental sampling strategies that could ultimately lead to reduction of these critical exposure agents in agricultural environments. Finally, modulating the TLR2 pathway might be an important adjunctive therapy when investigating potential prevention and therapeutic interventions in humans.
Supplementary Material
Acknowledgments
The authors thank Chris Bauer, Jacqueline Pavlik, and Jane DeVasure for assisting in the experiments. The authors also thank Dr. Shizuo Akira for generously providing Toll-like receptor 2 knockout mice for experimental studies, Thomas Jerrells, Ph.D., for assistance with digital microscopy images prepared for the manuscript, and Lisa Chudomelka for manuscript preparation assistance.
Footnotes
This work was supported by National Institute of Environmental Health Sciences grants K08 ES015522-01 and ES015522-03S1[ARRA], and R01 ES019325 (J.A.P.), National Institute of Occupational Safety Health grant R01 OH008539-01 (D.J.R.), National Institute on Alcohol Abuse and Alcoholism grant R01 AA017993 (T.A.W.), and Kuhl Testamentary Fund University of Nebraska Medical Center Intramural grant (G.G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0427OC on January 28, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest 2009;136:716–725 [DOI] [PubMed] [Google Scholar]
- 2.Rask-Andersen A, Malmberg P, Lundholm M. Endotoxin levels in farming: absence of symptoms despite high exposure levels. Br J Ind Med 1989;46:412–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormier Y, Israel-Assayag E, Racine G, Duchaine C. Farming practices and the respiratory health risks of swine confinement buildings. Eur Respir J 2000;15:560–565 [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang PF, van der Gulden JW, Folgering H, Heederik D, Tielen MJ, van Schayck CP. Longitudinal changes in bronchial responsiveness associated with swine confinement dust exposure. Chest 2000;117:1488–1495 [DOI] [PubMed] [Google Scholar]
- 5.Muller-Suur C, Larsson K, Grunewald J. Organic dust–induced interleukin-12 production activates T- and natural killer cells. Eur Respir J 2002;20:686–690 [DOI] [PubMed] [Google Scholar]
- 6.Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, Larsson L, Allen-Gipson D, Von Essen SG, Romberger DJ. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol 2008;122:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, Romberger DJ. Repeat organic dust exposure–induced monocyte inflammation is associated with protein kinase C activity. J Allergy Clin Immunol 2007;120:366–373 [DOI] [PubMed] [Google Scholar]
- 8.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 2002;93:289–296 [DOI] [PubMed] [Google Scholar]
- 9.Nehme B, Letourneau V, Forster RJ, Veillette M, Duchaine C. Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ Microbiol 2008;10:665–675 [DOI] [PubMed] [Google Scholar]
- 10.Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A 2010;73:684–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole JA, Thiele GM, Alexis NE, Burrell AM, Parks C, Romberger DJ. Organic dust exposure alters monocyte-derived dendritic cell differentiation and maturation. Am J Physiol Lung Cell Mol Physiol 2009;297:L767–L776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801 [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999;11:443–451 [DOI] [PubMed] [Google Scholar]
- 14.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 2009;296:L1085–L1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol 2006;117:1396–1403 [DOI] [PubMed] [Google Scholar]
- 16.Bailey KL, Poole JA, Mathisen TL, Wyatt TA, Von Essen SG, Romberger DJ. Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6–dependent manner in the airway epithelium. Am J Physiol Lung Cell Mol Physiol 2008;294:L1049–L1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Scheele I, Larsson K, Palmberg L. Budesonide enhances Toll-like receptor 2 expression in activated bronchial epithelial cells. Inhal Toxicol 2010;22:493–499 [DOI] [PubMed] [Google Scholar]
- 18.Vargas-Inchaustegui DA, Tai W, Xin L, Hogg AE, Corry DB, Soong L. Distinct roles for MyD88 and Toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect Immun 2009;77:2948–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez N, Wantia N, Fend F, Durr S, Wagner H, Miethke T. Differential involvement of TLR2 and TLR4 in host survival during pulmonary infection with Chlamydia pneumoniae. Eur J Immunol 2006;36:1145–1155 [DOI] [PubMed] [Google Scholar]
- 20.Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, Kudoh S, Standiford TJ, Yamaguchi K. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol 2007;56:305–312 [DOI] [PubMed] [Google Scholar]
- 21.Hajishengallis G, Wang M, Bagby GJ, Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J Immunol 2008;181:4141–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dessing MC, van der Sluijs KF, Florquin S, Akira S, van der Poll T. Toll-like receptor 2 does not contribute to host response during postinfluenza pneumococcal pneumonia. Am J Respir Cell Mol Biol 2007;36:609–614 [DOI] [PubMed] [Google Scholar]
- 23.Knapp S, von Aulock S, Leendertse M, Haslinger I, Draing C, Golenbock DT, van der Poll T. Lipoteichoic acid–induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol 2008;180:3478–3484 [DOI] [PubMed] [Google Scholar]
- 24.Wyatt TA, Sisson JH, Von Essen SG, Poole JA, Romberger DJ. Exposure to hog barn dust alters airway epithelial ciliary beating. Eur Respir J 2008;31:1249–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldenburg PJ, Wyatt TA, Factor PH, Sisson JH. Alcohol feeding blocks methacholine-induced airway responsiveness in mice. Am J Physiol Lung Cell Mol Physiol 2009;296:L109–L114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J 1997;10:381–387 [DOI] [PubMed] [Google Scholar]
- 27.Von Essen SG, Scheppers LA, Robbins RA, Donham KJ. Respiratory tract inflammation in swine confinement workers studied using induced sputum and exhaled nitric oxide. J Toxicol Clin Toxicol 1998;36:557–655 [DOI] [PubMed] [Google Scholar]
- 28.Deetz DC, Jagielo PJ, Quinn TJ, Thorne PS, Bleuer SA, Schwartz DA. The kinetics of grain dust–induced inflammation of the lower respiratory tract. Am J Respir Crit Care Med 1997;155:254–259 [DOI] [PubMed] [Google Scholar]
- 29.Sundblad BM, von Scheele I, Palmberg L, Olsson M, Larsson K. Repeated exposure to organic material alters inflammatory and physiological airway responses. Eur Respir J 2009;34:80–88 [DOI] [PubMed] [Google Scholar]
- 30.Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:503–510 [DOI] [PubMed] [Google Scholar]
- 31.Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir Res 2005;6:50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmberg L, Larssson BM, Malmberg P, Larsson K. Airway responses of healthy farmers and nonfarmers to exposure in a swine confinement building. Scand J Work Environ Health 2002;28:256–263 [DOI] [PubMed] [Google Scholar]
- 33.Jagielo PJ, Thorne PS, Watt JL, Frees KL, Quinn TJ, Schwartz DA. Grain dust and endotoxin inhalation challenges produce similar inflammatory responses in normal subjects. Chest 1996;110:263–270 [DOI] [PubMed] [Google Scholar]
- 34.George CL, Jin H, Wohlford-Lenane CL, O'Neill ME, Phipps JC, O'Shaughnessy P, Kline JN, Thorne PS, Schwartz DA. Endotoxin responsiveness and subchronic grain dust–induced airway disease. Am J Physiol Lung Cell Mol Physiol 2001;280:L203–L213 [DOI] [PubMed] [Google Scholar]
- 35.Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B. Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res 2008;3:19–35 [DOI] [PubMed] [Google Scholar]
- 36.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 2006;6:9–20 [DOI] [PubMed] [Google Scholar]
- 37.Love W, Dobbs N, Tabor L, Simecka JW. Toll-like receptor 2 (TLR2) plays a major role in innate resistance in the lung against murine mycoplasma. PLoS ONE 2010;5:e10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abplanalp AL, Morris IR, Parida BK, Teale JM, Berton MT. TLR-dependent control of Francisella tularensis infection and host inflammatory responses. PLoS ONE 2009;4:e7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373–384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.