Abstract
Rationale: The Xpert MTB/RIF is an automated molecular test for Mycobacterium tuberculosis that estimates bacterial burden by measuring the threshold-cycle (Ct) of its M. tuberculosis–specific real-time polymerase chain reaction. Bacterial burden is an important biomarker for disease severity, infection control risk, and response to therapy.
Objectives: Evaluate bacterial load quantitation by Xpert MTB/RIF compared with conventional quantitative methods.
Methods: Xpert MTB/RIF results were compared with smear-microscopy, semiquantiative solid culture, and time-to-detection in liquid culture for 741 patients and 2,008 samples tested in a multisite clinical trial. An internal control real-time polymerase chain reaction was evaluated for its ability to identify inaccurate quantitative Xpert MTB/RIF results.
Measurements and Main Results: Assays with an internal control Ct greater than 34 were likely to be inaccurately quantitated; this represented 15% of M. tuberculosis–positive tests. Excluding these, decreasing M. tuberculosis Ct was associated with increasing smear microscopy grade for smears of concentrated sputum pellets (rs = −0.77) and directly from sputum (rs = −0.71). A Ct cutoff of approximately 27.7 best predicted smear-positive status. The association between M. tuberculosis Ct and time-to-detection in liquid culture (rs = 0.68) and semiquantitative colony counts (rs = −0.56) was weaker than smear. Tests of paired same-patient sputum showed that high-viscosity sputum samples contained ×32 more M. tuberculosis than nonviscous samples. Comparisons between the grade of the acid-fast bacilli smear and Xpert MTB/RIF quantitative data across study sites enabled us to identify a site outlier in microscopy.
Conclusions: Xpert MTB/RIF quantitation offers a new, standardized approach to measuring bacterial burden in the sputum of patients with tuberculosis.
Keywords: tuberculosis, molecular diagnostics, diagnostic techniques and procedures, diagnosis, clinical trial
At a Glance Commentary
Scientific Knowledge on the Subject
The Xpert MTB/RIF assay is a rapid diagnostic test for tuberculosis and rifampin resistance. A number of studies have examined the sensitivity and specificity of the assay in various clinical settings and with various sample types. The test can also provide an estimate of the bacterial load present in the sample, although little data have been published on the accuracy or performance of this capability.
What This Study Adds to the Field
Xpert MTB/RIF assay provides a measure of the number of Mycobacterium tuberculosis bacilli that are present in a defined volume of sputum. This measure correlated well with other estimates of bacterial load, such as sputum acid-fast bacilli smear and culture time-to-positive. The correlation was strongest for assays that had internal control cycle threshold values less than or equal to 34. Despite this generally good correlation, the Xpert MTB/RIF assay detected considerable variation in the Xpert's quantitative estimate among sputum samples within the same acid-fast bacilli grade. This observation could be caused by variability within individual sputum samples, the inherent inaccuracy of smear grading, or unexplained variation in the Xpert MTB/RIF assay. The Xpert MTB/RIF assay could be used to distinguish between samples that were smear-positive versus smear-negative with varying degrees of sensitivity and specificity depending on the chosen cutoff. A cycle-threshold cutoff of approximately 27.7 best predicted smear-positive status.
Measurements of bacterial load have long played an important role in tuberculosis diagnostics. Semiquantitative or quantitative measures of the number of Mycobacterium tuberculosis bacilli present within a clinical sample have been clinically useful for determining disease severity, assessing transmission risk, or monitoring therapy (1–3). Quantitative readouts have also aided in the investigation of potentially false-positive results (4–6).
The Xpert MTB/RIF (Cepheid, Sunnyvale, CA) assay simultaneously detects the presence of M. tuberculosis and its susceptibility to the important first-line drug rifampin in less than 2 hours (7). The assay is contained in a small plastic cartridge, and it is run on the GeneXpert platform (Cepheid), a diagnostic system that accepts a variety of other cartridges intended for other diseases or disease panels. Several recent studies have described the analytic and clinical performance of Xpert MTB/RIF (7–15).
The real-time polymerase chain reaction (PCR) used to detect M. tuberculosis within the Xpert MTB/RIF assay has been shown to be quantitative. Analytic experiments conducted by spiking known quantities of M. tuberculosis into M. tuberculosis–negative sputum have shown that there is a linear relationship between the M. tuberculosis–specific cycle-threshold (M. tuberculosis Ct) that is generated in each positive assay and the log of M. tuberculosis cfu present in the sample (8). The assay also contains an internal control intended to indicate suboptimal sample processing and PCR conditions that can alter the relationship between M. tuberculosis Ct and the number of bacteria present in the tested sample.
Several small studies have compared quantitative Xpert MTB/RIF results with more conventional measures of bacterial load, such as direct or concentrated sputum smears, counts of cfu on solid agar growth media, or measures of time-to-detection (TTD) in liquid culture (11, 13, 14, 16). However, these studies have been performed at single sites with limited numbers of samples, which has precluded detailed and generalizable analyses. No study has compared the Xpert MTB/RIF assay with all four conventional quantitative measures. Finally, no study has included the ability of Xpert MTB/RIF to identify samples with PCR inhibition in their analysis of assay quantitation. A failure to consider the effect of inhibition can result in a serious misinterpretation of the assay's quantitative result, because PCR inhibition might impact M. tuberculosis Ct independently of bacterial load. To address the limitations of previous studies, we compared the quantitative capabilities of Xpert MTB/RIF with each existing method with and without adjustment for PCR inhibition measures. We used data from a large recently published, multisite clinical trial to increase the precision of our analysis, to examine quantitative performance between and across study sites, and to investigate potential applications of quantitative data produced by the Xpert MTB/RIF assay (9).
Methods
Study Population and Methods
We analyzed quantitative measures recorded in a previously reported clinical evaluation of the Xpert MTB/RIF assay (Cepheid) (9). The patient populations, study protocol, and methods have been reported previously in detail (9). In brief, patients were enrolled at five sites: Lima, Peru; Baku, Azerbaijan; Cape Town and Durban, South Africa; and Mumbai, India. For this analysis each site was randomly assigned a single letter alias (A, B, C, D, or E). Each patient provided three sputum samples. Two of these were randomly selected and were processed by the NALC-NaOH method and then resuspended in 1.5 ml of phosphate buffer (17). A total of 500 μl of the resulting pellet was used to inoculate a mycobacterial growth indicator tube (MGIT); 500 μl was tested in the Xpert MTB/RIF; 100 μl was inoculated onto Löwenstein–Jensen media (site E used Middlebrook 7H11); and a Ziehl-Neelsen (ZN)–stained smear was prepared from the remaining volume. The Xpert MTB/RIF aliquot was brought up to 2 ml in the assay's sample reagent before testing. A direct ZN smear was prepared from a portion of the unprocessed third sputum and the remaining sample was tested directly in the Xpert MTB/RIF assay according to the manufacturer's instructions without NALC-NaOH decontamination. Operators graded each sputum sample as “very viscous,” “viscous,” or “not viscous/watery,” although no standardized training on how to classify sample viscosity was provided. We performed a per sample analysis of sputum samples collected from patients with culture-confirmed tuberculosis. Samples with contaminated cultures were excluded from analysis where applicable. This study was approved by the institutional review board at each clinical site as part of the parent clinical study approval (which included an analysis of the Xpert MTB/RIF assay's quantitative functions in the original study protocol). The data analysis reported here was also approved by the University of Medicine and Dentistry of New Jersey institutional review board. Patient data collected from the parent study were anonymized before use in the present work.
Sample Quantitation
The Xpert MTB/RIF assay is a hemi-nested PCR assay that targets the M. tuberculosis rpoB gene (7). The GeneXpert instrument, which automatically performs the assay, assigns each sample a semiquantitative grade on the basis of the Ct of the first positive probe that detects M. tuberculosis. Semiquantitative cutoffs had been previously assigned to roughly quarter the number of tests falling into each group using results from initial clinical pilot studies as follows: “high,” Ct less than or equal to 16; “medium,” Ct greater than 16 and less than or equal to 22; “low,” Ct greater than 22 and less than or equal to 28; and “very low,” Ct greater than 28 and less than or equal to 38. Sputum smears and culture on Löwenstein–Jensen agar slants (LJ) were graded according to the International Union Against Tuberculosis and Lung Disease (IUATLD) scale (18) provided in Table 1 along with the conversion to the alternate American Thoracic Society scale (19). The TTD for positive MGIT cultures was determined as the time elapsed in days between the inoculation of a given culture and the day in which it was identified as a positive result by the BACTEC 960 (BD Diagnostics, Sparks, MD) instrument, but excluding any time necessary to confirm the positive result as M. tuberculosis. In the event that a culture was contaminated, sites reinoculated secondary cultures from the initial sputum pellet, which was stored at −80°C. TTD data reporting for these reinoculated cultures were not handled uniformly at all sites because this was not an end point of the parent study. Sites A and B reported the time from repeat inoculation to positivity. Sites C and D reported only the initial inoculation date of the primary culture and the date of positivity for the reinoculated culture. TTD data were not collected at site E. Incomplete data on sample reinoculation precluded the possibility of excluding all reinoculated cultures.
TABLE 1.
IUATLD QUANTITATIVE GRADING CONVENTIONS
| ZN-Smear Microscopy | ||
| Grade |
||
| IUATLD | ATS | AFB* |
| Negative | Negative† | 0 AFB/100 fields |
| Scanty | 1+ | 1–9 AFB/100 fields |
| 1+ | 2+ | 1–9 AFB/10 fields |
| 2+ | 3+ | 1–10 AFB/field |
| 3+ | 4+ | >10 AFB/field |
| LJ–Agar Growth Medium | ||
| IUATLD | ||
| Grade | CFU | |
| <20 | 1–19 | |
| 1+ | 20–99 | |
| 2+ | 200–199 | |
| 3+ | >200 | |
Definition of abbreviations: AFB = acid-fast bacilli; ATS = American Thoracic Society; IUATLD = International Union Against Tuberculosis and Lung Disease; LJ = Löwenstein–Jensen agar slants; ZN = Ziehl-Neelson stain.
Field of view (×1,000 oil immersion, bright-field).
The ATS scale requires 300 fields to be observed before reporting a negative, includes an additional category of “doubtful” for one to two bacilli observed per 300 fields, and defines 3+ and 4+ as one to nine and greater than nine AFB per field, respectively.
Calculation of Ideal M. tuberculosis Ct for Each Smear Grade
Our study includes a comparison between actual M. tuberculosis Ct, acid-fast bacilli (AFB) smear grade, and an “ideal” M. tuberculosis Ct that would be expected for each AFB smear grade under ideal conditions (no PCR inhibitors, linear Xpert MTB/RIF assay quantitative dynamic range, and accurate AFB smear grade assignments). This calculation presumed that the limit of detection for smear microscopy is 5,000 cfu/ml (20, 21), and that each increasing grade reflected a 10-fold increase in the number of bacilli present in the sputum sample. Estimates of cfu per milliliter of sputum were converted to M. tuberculosis Ct based on an experimentally determined relationship between known numbers of tuberculosis cfu spiked into sputum and M. tuberculosis Ct (8), wherein M. tuberculosis Ct = −2.987 * Log(cfu/ml) + 39.2.
Detecting PCR Inhibition
The Xpert MTB/RIF includes an internal control PCR. Bacillus globigii spores are resuspended during the automated sample processing steps, processed along with the sputum sample, and the B. globigii DNA is detected in a hemi-nested real-time PCR reaction multiplexed with the M. tuberculosis assay. If the internal control assay fails to produce a positive result the instrument reports the assay as invalid in the event that the M. tuberculosis probes are also negative. We have previously shown that the Ct of the internal control assay is not affected by the amount of M. tuberculosis present in the sample (8).
Statistical Analysis
The strength of correlation between M. tuberculosis Ct and AFB smear or solid culture grade was determined by calculating the Spearman rank correlation coefficient (rs) for all valid samples and separately for each individual site. Similarly, Spearman rank coefficients were estimated to assess the correlation between M. tuberculosis Ct and MGIT TTD again overall, and by site. Correlations were determined before and after omitting tests with an internal control Ct greater than 34. SAS version 9.2 (SAS, Cary, NC) was used for these calculations. To generate a receiver operating curve (ROC) we defined all samples with a smear grade of 1+ or above as smear positive, and those with grades of scanty or negative as smear negative. We then iteratively calculated the sensitivity and specificity of a M. tuberculosis Ct threshold for defining smear positivity for the range of possible M. tuberculosis Ct values, wherein all tests with a Ct below a threshold were defined as smear-positive, and those above as smear-negative.
Results
Patients and Samples
We examined the correlation between M. tuberculosis Ct and conventional quantitative results in the 2,223 sputum samples from patients with culture-confirmed tuberculosis that had been included in the core analysis of the parent study (Figure 1). We excluded 215 of these samples that were Xpert MTB/RIF negative. Of the remaining 2,008 samples, 1,333 had been processed with NALC-NaOH and had ZN microscopy, liquid culture, solid culture, and Xpert results; and 675 samples had been tested directly with direct ZN microscopy and Xpert. In 53 cases, the initial Xpert MTB/RIF test failed, whereas a repeat test was positive for tuberculosis. These repeat tests were included in the study.
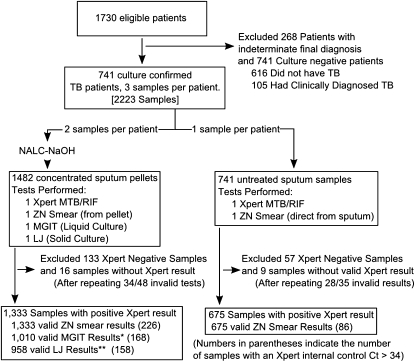
Figure 1.
Patient samples used in this study. A flow diagram for sample inclusion and exclusion is shown. LJ = culture on Löwenstein–Jensen agar slants; MGIT = mycobacterial growth indicator tubes; NACL-NaOH = treatment with NALC-NaOH as in (17); TB = tuberculosis; ZN = Ziehl-Neelsen staining. *MGIT results excluded 80 samples from Site E for which no time-to-detection data were collected, 234 samples collected from patients being treated for Mycobacterium tuberculosis at the time of sample collection, and 9 samples for which the MGIT culture was contaminated. **LJ results excluded 80 samples from Site E that were cultured on a different solid media (Middlebrook 7H11) than at the rest of the sites, 234 samples collected from patients on M. tuberculosis therapy at time of collection, and 61 contaminated cultures.
Mycobacterium tuberculosis Ct and Internal Control Ct
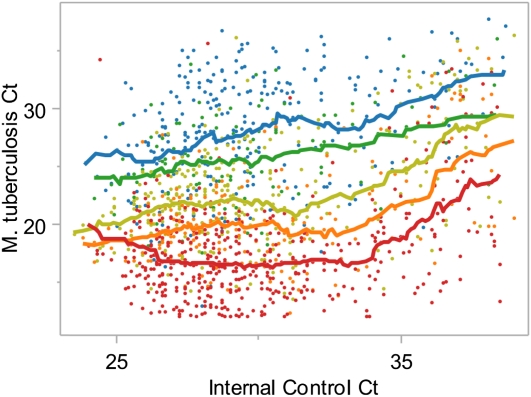
We wondered whether the internal control PCR reaction, which is embedded within the Xpert MTB/RIF assay, could be used to identify tests that were at risk for inaccurate quantification because of PCR inhibitors or other test errors. We examined the relationship between the average M. tuberculosis PCR Ct and AFB smear grade as a function of the internal control PCR Ct (Figure 2). We found that the moving average of M. tuberculosis Ct was constant within each smear grade when tests had internal control Cts between 24 and 34. Within any given smear grade M. tuberculosis Cts began to increase when internal control Cts were greater than 34. For example, the mean M. tuberculosis Ct for 3+ smear samples with an internal control Ct between 30 and 31 was 16.3, whereas the mean M. tuberculosis Ct for 3+ smear samples with an internal control Ct between 36 and 37 was 21.2. The positive correlation between M. tuberculosis Ct and internal control Cts greater than 34 was strongest in smear-positive samples and weakest in smear-negative samples. Internal control Cts greater than 34 were observed in 15.5% of the 2,008 M. tuberculosis–positive Xpert tests comprising 17% of tests from pellets and 12.7% of tests from sputa.
Figure 2.
Relationship between the internal control cycle-threshold (Ct) and accuracy of quantitation. The Mycobacterium tuberculosis Cts for all concentrated sputum samples that produced a positive internal control result are shown plotted against the internal control Ct. Each dot represents a single Xpert test. Colors indicate acid-fast bacilli smear grade of a given sample: red, 3+; orange, 2+; yellow, 1+; green, scanty; and blue, smear-negative. Matched line colors show a moving average of M. tuberculosis Ct within the corresponding smear grade looking back 15 tests and ahead five tests.
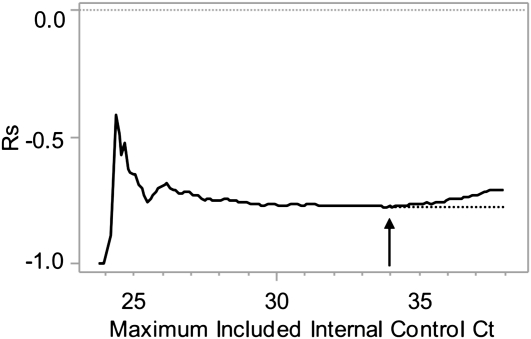
The internal control is designed to identify PCR inhibition; therefore, we hypothesized that the correlation between M. tuberculosis Ct and conventional quantitative methods would diminish as internal control Ct increased. We iteratively calculated rs between M. tuberculosis Ct and the grade of the AFB smear starting with the tests with the lowest internal control Ct and adding the next lowest tests in each subsequent calculation for all tests performed from pellets. After an initial instability caused by low sample size, Spearman correlation values stabilized at approximately −0.76 when including tests with an internal control Ct of 29 up to 34, and then declined as samples with internal control Ct greater than 34 were included (Figure 3). These results strongly suggest that Xpert MTB/RIF tests that produce internal control Cts greater than 34 provide less accurate quantitation than tests with internal control Cts less than or equal to 34.
Figure 3.
Influence of internal control cycle-threshold (Ct) on calculated Spearman rank. The calculated Spearman rank correlation (rs) between Mycobacterium tuberculosis Ct and acid-fast bacilli smear grade for all concentrated sputum samples is shown for each maximum allowed internal control Ct. The arrow indicates the point of maximum correlation. The dotted line indicates the trend in Spearman rank correlation that would have been expected in the absence of polymerase chain reaction inhibition.
Xpert Quantitation Versus Smear Microscopy
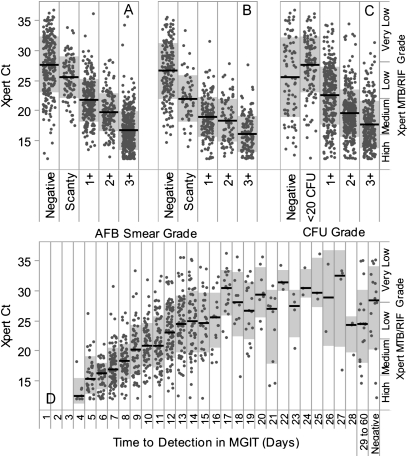
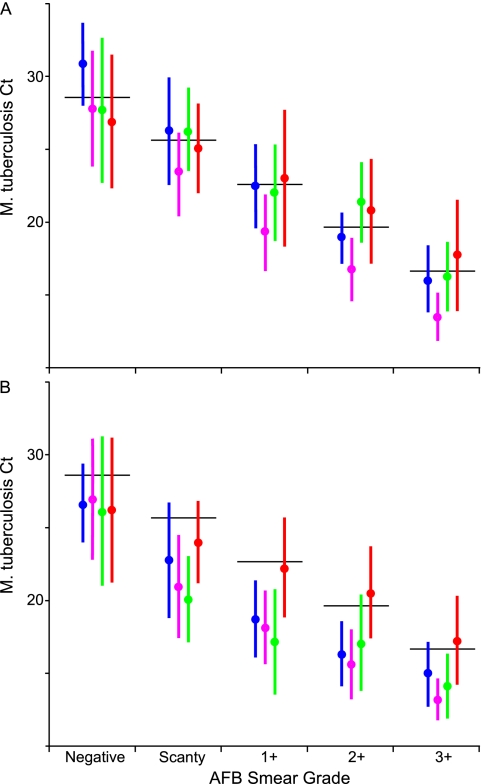
Smear microscopy is the only widely implemented method for quantifying bacterial burden at the time of initial diagnosis. We examined the relationship between M. tuberculosis Ct and the grade of the AFB smear for samples prepared from either processed sputum pellets or directly from the sputa. The rs was calculated for all samples and for each individual study site, with and without omitting assays with internal control Cts greater than 34 (Table 2). We found that there was an inverse relationship between increasing AFB smear grade and M. tuberculosis Ct for both tests performed on sputum pellets (rs = −0.77) and for tests performed directly on unprocessed sputum (rs = −0.71). Despite these strong correlations, even samples with internal control Cts less than or equal to 34 showed a wide range of Ct values within each smear grade. For example 3+ smears from pellet had a mean M. tuberculosis Ct of 16.7 and a standard deviation of 3.2. This corresponds to a mean of 3.1 · 107 cfu/ml and a ×137 difference in concentration from +1 to −1 standard deviation (2.7 · 106 to 3.7 · 108 cfu/ml). A substantial overlap of Ct values between smear grades was noted for both concentrated smears (Figure 4A) and direct smears (Figure 4B).
TABLE 2.
PER SITE CORRELATION BETWEEN MYCOBACTERIUM TUBERCULOSIS CYCLE-THRESHOLD AND CONVENTIONAL DIAGNOSTIC TECHNIQUES
| rs Internal Control Ct <34 | N Internal Control Ct Ct >34 | % Internal Control Ct Ct >34 | AFB Smear Grade |
||||||||
| Method | Site | rs* | N | 3+ | 2+ | 1+ | Scanty | Negative | |||
| AFB smear (pellet) | A | −0.77 | −0.83 | 397 | 69 | 17.4 | 154 | 59 | 126 | 50 | 8 |
| B | −0.83 | −0.86 | 247 | 30 | 12.1 | 31 | 30 | 55 | 25 | 106 | |
| C | −0.72 | −0.77 | 260 | 31 | 11.9 | 60 | 35 | 56 | 23 | 86 | |
| D | −0.59 | −0.68 | 349 | 86 | 24.6 | 185 | 49 | 43 | 10 | 62 | |
| E | −0.55 | −0.57 | 80 | 10 | 12.5 | 27 | 12 | 16 | 2 | 23 | |
| Total | −0.69 | −0.77 | 1,333 | 226 | 17 | 457 | 185 | 296 | 110 | 285 | |
| AFB smear (sputum) | A | −0.71 | −0.76 | 201 | 21 | 10.4 | 48 | 20 | 79 | 36 | 18 |
| B | −0.81 | −0.83 | 123 | 12 | 9.8 | 10 | 12 | 24 | 14 | 63 | |
| C | −0.69 | −0.73 | 136 | 19 | 14 | 5 | 20 | 31 | 13 | 67 | |
| D | −0.55 | −0.70 | 179 | 30 | 16.8 | 82 | 34 | 25 | 3 | 35 | |
| E | −0.65 | −0.62 | 36 | 4 | 11.1 | 8 | 11 | 7 | 2 | 8 | |
| Total | −0.63 | −0.71 | 675 | 86 | 12.7 | 153 | 97 | 166 | 68 | 191 | |
| Solid Culture CFU Grade | |||||||||||
| 3+ | 2+ | 1+ | <20 CFU | Negative | |||||||
| LJ solid culture† | A | −0.41 | −0.46 | 397 | 69 | 17.4 | 71 | 126 | 173 | 21 | 6 |
| B | −0.71 | −0.73 | 187 | 24 | 12.8 | 50 | 40 | 44 | 47 | 6 | |
| C | −0.56 | −0.60 | 220 | 29 | 13.2 | 97 | 40 | 43 | 23 | 17 | |
| D | −0.54 | −0.50 | 154 | 36 | 23.4 | 4 | 49 | 39 | 33 | 29 | |
| Total | −0.53 | −0.56 | 958 | 158 | 16.5 | 222 | 255 | 299 | 124 | 58 | |
| MGIT Time to Detection (days) | |||||||||||
| 1–28 | >28 | Negative | |||||||||
| MGIT liquid culture†‡ | A | 0.56 | 0.66 | 397 | 71 | 17.9 | 387 | 4 | 6 | ||
| B | 0.77 | 0.77 | 218 | 25 | 11.5 | 214 | 0 | 4 | |||
| C | 0.73 | 0.78 | 235 | 30 | 12.8 | 214 | 15 | 6 | |||
| D | 0.49 | 0.66 | 160 | 42 | 26.3 | 155 | 3 | 2 | |||
| Total | 0.67 | 0.68 | 1,010 | 168 | 16.6 | 970 | 22 | 18 | |||
Definition of abbreviations: AFB = acid-fast bacilli; Ct = cycle-threshold; LJ = Löwenstein–Jensen agar slants; MGIT = mycobacterial growth indicator tube.
Spearman rank correlation coefficient.
Site E used alternative solid media (Middlebrook 7H11), and liquid culture time-to-detection was not recorded at this site.
Excluding MGIT cultures with TTD greater than 28 days.
Figure 4.
(A–D) Xpert quantitation compared with conventional quantitative measures. The distribution of Mycobacterium tuberculosis cycle-threshold (Cts) within each smear grade or quantitative culture category is shown. Only tests with an internal control Ct less than 34 are included. Each dot indicates a single tested sputum sample; dots are randomly distributed horizontally within each category to avoid overlap. Black lines indicate the median Xpert M. tuberculosis Ct and gray boxes indicate average ± one standard deviation. Xpert M. tuberculosis Ct is compared with (A) smear grade of concentrated samples, (B) smear grade of direct sputum smears, (C) semiquantitative solid culture cfu grade on culture on Löwenstein–Jensen agar slants, and (D) time to detection in days in a BACTEC mycobacterial growth indicator tubes (MGIT) 960. The semiquantitative scale produced by Xpert software is indicated on the right margin. For (D) days 29–60 have been combined in a single column and “negative” indicates all tests with no growth after 60 days. AFB = acid-fast bacilli.
Xpert Semiquantitative Reporting
The Xpert MTB/RIF assay assigns a semiquantitative category to each test positive for M. tuberculosis. We examined the distribution of smear grades for all pelleted samples within each semiquantitative category (Table 3). Tests with internal control Ct greater than 34 were excluded. We found that 94.9% of “high” samples were graded 2+ or above; 93.7% of “medium” samples were 1+ and above, 81.9% of “low” samples were 1+ and below, and 86% of “very low” samples were scanty and below. Thus, although the Xpert MTB/RIF assay's current semiquantitative categories seem to be generally predictive of bacterial load as assessed by AFB smears, the Xpert MTB/RIF assay quantitative categories do not consistently segregate samples into the same categories as sputum microscopy.
TABLE 3.
COMPARISON OF XPERT SEMIQUANTITATIVE RANGES AND AFB SMEAR RESULTS FOR PELLETED SAMPLES
| AFB Smear Grade* |
|||||||
| Xpert result (TB Ct range) | Negative | Scanty | 1+ | 2+ | 3+ | Total | |
| Very low (28 < Ct ≤ 38) | % | 65.4 | 20.6 | 8.4 | 2.8 | 2.8 | 100 |
| n | 70 | 22 | 9 | 3 | 3 | 107 | |
| Low (22 < Ct ≤ 28) | % | 25.5 | 19.3 | 37 | 12.8 | 5.4 | 100 |
| n | 62 | 47 | 90 | 31 | 13 | 243 | |
| Medium (16 < Ct ≤ 22) | % | 4.4 | 1.8 | 21.9 | 22.7 | 49.1 | 100 |
| n | 17 | 7 | 84 | 87 | 188 | 383 | |
| High (Ct ≤ 16) | % | 1.9 | 0 | 3.2 | 4.4 | 90.5 | 100 |
| n | 3 | 0 | 5 | 7 | 143 | 158 | |
Definition of abbreviations: AFB = acid-fast bacilli; Ct = cycle-threshold; TB = tuberculosis.
Excludes tests with internal control Ct greater than 34 and tests from site B, which was found to be an outlier in comparisons of average Mycobacterium tuberculosis Cts per smear grade (Figure 6).
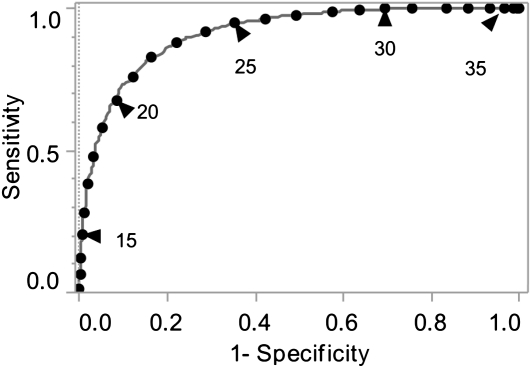
Smear-negative patients are generally thought to be less infectious than smear-positive patients (22, 23). A ROC curve of all samples with internal control Cts less than or equal to 34 was generated to examine the sensitivity versus specificity of various M. tuberculosis Ct cutoffs for predicting smear-positive (AFB grade of +1 and greater) status (Figure 5). A Ct of 25 was 95% sensitive and 65% specific for smear-positive status, and a Ct of 27.7 was 98% sensitive and 48% specific for smear-positive status.
Figure 5.
Cycle-threshold (Ct) cutoff for smear-positive samples. The receiver operating characteristic (ROC) of Mycobacterium tuberculosis Ct as a test for smear-positive sputum is shown. Only tests with an internal control Ct less than 34 were included. Smear positivity was defined as all concentrated sputum samples that were grade 1+ or greater. The gray line indicates the ROC curve. The black circles indicate 1-Ct increments on the ROC curve, and black circles with a black triangle indicate each 5th Ct as labeled.
Culture Techniques
Culture-based diagnostics are significantly more sensitive than smear microscopy, but they require an incubation interval that precludes their use for initial patient management. Culture-based quantitation remains important for public health reporting and scientific applications. We assessed the association between M. tuberculosis Ct and the quantitative estimates of bacterial burden provided by the culture-based techniques used in this study. The parent study used a semiquantitative reporting system instead of actual cfu counts, consistent with IUTALD recommended practice. This system precluded a precise analysis of the cfu to Ct correlation. Nonetheless, the results showed a weak (rs = −0.56) inverse correlation between increasing solid culture grade and M. tuberculosis Ct across all sites (Figure 4C) when excluding tests with internal control Ct greater than 34. There was significant overlap in M. tuberculosis Ct distribution between culture-negative samples and those in the less than 20 cfu grade that likely reflects the relatively low sensitivity of solid culture (9). We also assessed the correlation between M. tuberculosis Ct and TTD in a BACTEC MGIT 960 automated liquid culture system (Figure 4D) and found a positive correlation between increasing M. tuberculosis Ct and TTD less than or equal to 28 days (rs = 0.68) after excluding tests with internal control Ct greater than 34 (Table 2). There was no significant association between TTD and Ct for samples with a TTD greater than 28 days. There was substantial variation in the M. tuberculosis Ct for samples with identical TTD results, with an average standard deviation of ± 4 Cts per day, or a roughly ×500 difference in concentration within ± one standard deviation of the mean for all samples positive on an average day. Many factors other than number of cfu in the initial sputum sample influence TTD, including variation in viability after decontamination and strain fitness, potentially contributing to the observed variability.
Site-to-Site Smear Microscopy Comparisons
Both the sensitivity of smear microscopy and the accuracy of the resultant quantitative grades can vary with training, workload, and equipment. We compared the average M. tuberculosis Ct for all samples assigned a particular smear grade at each site with each other, and with an “ideal” average Ct that was expected for each smear grade at all sites with at least 100 results. Tests performed on pellets (Figure 6A) or directly from sputum (Figure 6B) were studied separately. We found that smear grading based on smears prepared from pellets was relatively consistent across sites. The average M. tuberculosis Cts for each smear grade was also very close to the average “ideal” M. tuberculosis Cts predicted for each, although standard deviation in M. tuberculosis Ct was high with an average standard deviation of 3.3 Cts per grade. Smears prepared from pellet at site B were associated with consistently earlier M. tuberculosis Cts when compared with the other sites. Interestingly, smears prepared directly from sputum deviated consistently from “ideal” Cts, except at site D. This is likely because of the difficulty in accurately quantitating AFB numbers in smears that have not been digested and concentrated. Our results suggest that the Xpert assay can be used to test and monitor laboratories that perform sputum AFB tests for accuracy and consistency.
Figure 6.
Mycobacterium tuberculosis cycle-threshold (Ct) compared with expected “ideal” Ct for each smear grade. The average M. tuberculosis Ct for each smear grade at each test site is shown compared with the “expected” ideal average Ct. Tests with an internal control Ct greater than 34 were excluded. (A) Tests performed from sputum pellets. (B) Tests performed directly from sputum. Filled circles indicate the average M. tuberculosis Ct per grade and vertical bars indicate ± one standard deviation. Sites are ordered alphabetically: A (blue); B (pink); C (green); and D (red). Site E was excluded because of low sample size. The calculated “ideal” Cts for each grade are indicated by a solid black horizontal line and indicate a difference in Ct equivalent to a ×10 change in cfu per milliliter in adjacent categories. AFB = acid-fast bacilli.
Bacterial Load and Sputum Type
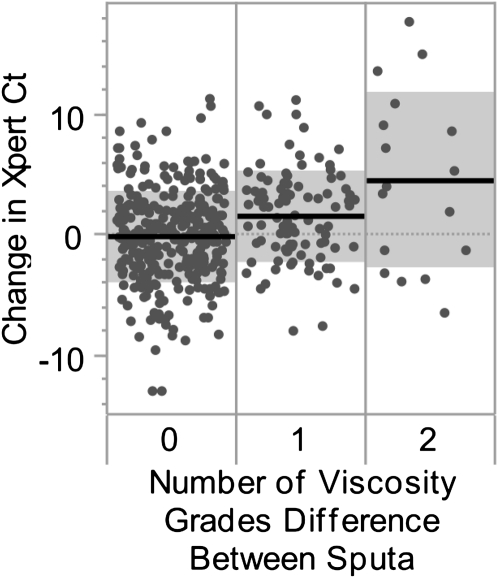
Specimen quality has been implicated as an important component in TB diagnosis. We hypothesized that sputum viscosity grades assigned to each sputum might function as a surrogate marker of specimen quality. Specimens were graded as “very viscous” (VV), “viscous” (V), or “not viscous/watery” (NV/W). We determined the difference in M. tuberculosis Ct for each pair of NALC-NaOH–treated sputum samples submitted by a single patient (∆MtbCt), for all patients with two positive Xperts both having internal control Cts less than or equal to 34, by subtracting the M. tuberculosis Ct of the higher-viscosity sample from that of the lower-viscosity sample. If both samples were assigned the same viscosity grade we subtracted the Ct from one of the sputum samples selected at random from the other. The average ∆MtbCt for all sample pairs with identical viscosity estimates (both VV, V, or NV/W) was −0.11 (n = 319; 95% confidence interval [CI], −0.5 to 0.3). For paired samples with a one grade level difference between estimates (VV → V or V → NV/W) the higher viscosity sample was an average of 1.6 Cts (n = 95; 95% CI, 0.8–2.4) earlier than the low viscosity sample, and for a two grade level difference between estimates (VV → NV/W) the higher viscosity sample was 4.52 Cts (n = 17; 95% CI, 0.8–8.3) earlier (Figure 7). It is possible to extrapolate a difference in sputum cfu per milliliter from a known difference in assay Ct using the relationship between Ct and cfu described in Methods. A one grade difference corresponds to an average of ×3.4 more cfu per milliliter in the more viscous sample, whereas a two grade difference corresponds to an average of ×32.6 more cfu per milliliter between the least viscous and most viscous. These results suggest that higher viscosity samples will be more likely to contain large numbers of bacteria.
Figure 7.
Cycle-threshold (Ct) differences between paired samples with same or different viscosities. The Mycobacterium tuberculosis Ct between concentrated sputum samples collected from a single patient is shown. Only tests with an internal control Ct less than 34 were included. Specimens were graded as “very viscous” (VV), “viscous” (V), or “not viscous/watery” (NV/W). Specimen pairs assigned the same viscosity category (both VV, both V, or both NV/W) are indicated in the left column. Specimen pairs with a one-grade difference (VV and V, or V and NV/W) are in the middle column. Specimen pairs with a two-grade difference (VV and NV/W) are in the right column. Each dot indicates a pair of specimens and dots are randomly distributed horizontally to reduce overlap. The mean change in M. tuberculosis Ct in each group is indicated by a solid black line. Gray boxes indicate ± one standard deviation.
Discussion
Smear microscopy is the primary means of diagnosing tuberculosis in high-burden countries. The distinction between smear-negative and smear-positive cases can be particularly important. Smear-positive cases pose an increased clinical, infection control, and public health risk because of their higher disease burden and infectiousness. Smear-negative cases mark patients who might not have been identified and treated if sputum microscopy was the sole diagnostic modality. Laboratory cross-contamination and pseudo outbreaks of tuberculosis most commonly occur in smear-negative cases. Here, we showed that the Xpert MTB/RIFs M. tuberculosis Ct results correlate well with smear microscopy grades. The correlation can be further improved if tests with Cts delayed by PCR inhibition are removed.
The AFB smear has an effective dynamic range of 104 cfu/ml to approximately 108 cfu/ml. The lower bound of this range is set by a microscopy's operational limit of detection and the upper bound is set by the maximum bacterial load observed in clinical specimens. This four-log range is divided by a classification scheme with four grades each corresponding to a 10-fold change from the grade on either side. The semiquantitative grades provided by the Xpert MTB/RIF assay could possibly substitute for the AFB smear grade. However, the assay has a dynamic range of 102 to at least 107 cfu/ml; yet, the assay assigns positive results to one of only four semiquantitative categories. Furthermore, these categories are not based on log measures, but were instead designed to roughly quarter the samples tested. It will take an adjustment for health care workers to adapt their practices to the Xpert MTB/RIF grading system. An alternative is for clinicians to use the M. tuberculosis Ct value of each assay (perhaps adjusted for internal control Ct if >34) to measure bacterial load rather than relying on the semiquantitative grades. Our study suggests it may be possible to detect the equivalent of smear-positive patients based on the M. tuberculosis Ct reported by the assay. The association between a particular M. tuberculosis Ct cutoff and patient or public health outcomes needs to be rigorously examined in prospective studies.
Despite the strong correlation between M. tuberculosis Ct and sputum smear grade, our results also revealed a relatively large variation of M. tuberculosis Ct within each smear grade and a relatively large overlap of M. tuberculosis Ct between adjoining smear grades. Importantly, a substantial number of smear-negative samples had M. tuberculosis Cts below the mean M. tuberculosis Ct for smear-positive sputum samples. Our ROC analysis demonstrates the consequence of these results, because it shows the difficulty of selecting a single Ct value that is both a sensitive and specific predictor of smear-positive sputum status. Smear classification is operator dependant and has highly variable operational sensitivity (24). It is difficult to assess what proportion of the variation is caused by variation in the reproducibility of the M. tuberculosis Ct and what is attributable to variation in smear grading procedures. We suggest that the Xpert may provide quantitative results that are more consistent across and between sites than smear microscopy, especially in settings where smear sensitivity is low because of inadequacies in personnel or equipment. The consistency of the Xpert assay may be particularly important when dividing patients who are deemed likely to be infectious versus patients who are unlikely to be infectious because of low bacterial sputum burdens. Our results suggest that a significant number of samples that are classified as smear-negative do in fact contain relatively high numbers of M. tuberculosis bacteria in their sputum. At least one large study has suggested that sputum smear-negative patients are a substantial source of tuberculosis transmission (25). It is possible that the Xpert quantitation can be used to identify the subset of potentially infectious smear-negative patients. Taken together, these considerations suggest that the Ct cutoff value most relevant for predicting increased infectivity should likely include almost all smear-positive samples and also include smear-negative samples that have low Ct values. This is because a low Ct value is likely to identify samples with increased numbers of M. tuberculosis bacilli, even if that sample is smear-negative. For example, a Ct of 27.7, which is 98% sensitive and 48% specific for smear-positive status, may detect patients with increased infectivity more reliably than sputum AFB smear, as long as only samples with internal control Cts of less than or equal to 34 are included.
We observed a weak correlation between M. tuberculosis Ct and LJ-based semiquantitative culture grades. The reporting of cfu grades rather than actual cfu counts precluded a more detailed analysis of the LJ culture results. The correlation between Ct and MGIT TTD was relatively stronger although complicated by nonstandardized reporting of contaminated samples and the fact that TTD was reported in days rather than hours. Variability in the NALC-NaOH decontamination procedure can introduce significant variation in the percentage of viable cells recovered from a processed sputum sample (26). The combination of variability in postdecontamination viability and strain-specific differences in growth rate all likely contributed to the variation in M. tuberculosis Cts observed for samples positive by MGIT on the same day.
We believe that the strong correlations that have been observed between cfu per milliliter and TTD in previous reports (27, 28) reflect the fact that both tests measure the same fraction of metabolically culturable cells that remain after sputum decontamination. In contrast, Xpert quantitation provides a measure of the M. tuberculosis DNA that is independent of bacterial metabolic status or the sample processing protocol used. Our results may indicate that in routine practice TTD is not a particularly robust indicator of initial bacterial load and that the estimate provided by the Xpert MTB/RIF may be preferred. Caution must be used in extrapolating our results to patients undergoing tuberculosis treatment. Prior studies have shown that M. tuberculosis DNA persists for long periods in effectively treated patients (29). We have no reason to believe that studies of treated patients with the Xpert would produce substantially different findings.
The parent study was not designed to assess sample quality in particular detail, and sample classification was inherently subjective and nonstandardized. Even with these shortcomings the substantial increase in bacterial load observed in VV versus NV sputum samples suggests that simple visual inspection may be a potential method of assessing sample quality. Our results support a growing body of evidence that suggests that emphasis on collecting and testing higher-quality samples is a relatively simple low-cost way to improve operational sensitivity (30–34). Studies that use M. tuberculosis Cts to compare bacterial load within or between patients should ensure samples of comparable quality are being assessed.
Whereas conventional techniques have been evaluated over a period of years and their failure modes are relatively well understood, the Xpert MTB/RIF assay is among the first commercially available nucleic acid amplification test tuberculosis tests with a quantitative function. The internal control component was included in the Xpert assay in part to identify situations in which the assay performed suboptimally, and the association between a late internal control Ct and inaccurately quantified samples supports the notion that it is functioning as intended. Importantly, the ability to detect inaccurately quantified samples based on the internal control Cts greater than 34 means that these samples can be flagged and alternative assessments pursued. One attractive option is to incorporate a correction factor based on internal control Cts greater than 34.
The strong correlation between decreasing M. tuberculosis Ct and increasing estimates of bacterial load provided by smear microscopy–based techniques suggests that the Xpert's quantitative capability is robust enough for adoption in settings where microscopy-based techniques are now used. Certainly, the distinction between smear-negative and smear-positive M. tuberculosis will continue to be useful given the widespread use of smear microscopy, but diagnostic classification schemes in public health reporting need to evolve to incorporate new information from this and other new diagnostics.
Footnotes
Supported by the Foundation for Innovative New Diagnostics and the National Institutes of Health (AI080653).
Author Contributions: R.B., D.A., and A.L.D. contributed to the data analysis presented in this paper. C.C.B., M.D.P., P.N., and D.A. contributed to conception and design of the parent study, and along with M.J. and D.H.P. contributed analysis and interpretation of that data. V.V., R.T., V.M., M.N., D.H., S.R.-G., F.L., C.Z., and C.R. provided substantial contributions to acquisition of data. All authors contributed to drafting or revision of the article. R.B. and D.A. take responsibility for the integrity of the work as a whole.
Originally Published in Press as DOI: 10.1164/rccm.201103-0536OC on August 11, 2011
Author Disclosure: R.B.’s institution has received grants, loan of equipment to perform experiments, and license payments on royalties from Cepheid. P.N.’s institution has received grants from the Foundation for Innovative New Diagnostics (FIND) and the Bill and Melinda Gates Foundation. A.L.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.N.’s institution received grants from FIND. M.J. and D.H.P. are employed by and hold stock in Cepheid. D.H. is employed by the Research Center, Borstel, Germany. S.R.-G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.L.’s institution has received grants from FIND. C.Z.’s institution has received grants from FIND; he is employed by the Universidad Peruana Cayetano Heredia. C.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.C.B.’s institution has received grants from FIND and the Bill and Melinda Gates Foundation. M.D.P.’s institution has received grants from FIND and the Bill and Melinda Gates Foundation. D.A.’s institution has received grants and loan of equipment to perform experiments from Cepheid; he has received consultancy fees from Cepheid, although not in the last 4 years; he and his institution have received license payments on royalties from Cepheid.
References
- 1.Palaci M, Dietze R, Hadad DJ, Ribeiro FK, Peres RL, Vinhas SA, Maciel EL, do Valle Dettoni V, Horter L, Boom WH, et al. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J Clin Microbiol 2007;45:4064–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrin FM, Woodward N, Phillips PP, McHugh TD, Nunn AJ, Lipman MC, Gillespie SH. Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int J Tuberc Lung Dis 2010;14:1596–1602 [PubMed] [Google Scholar]
- 3.Small PM, Pai M. Tuberculosis diagnosis—time for a game change. N Engl J Med 2010;363:1070–1071 [DOI] [PubMed] [Google Scholar]
- 4.Multiple misdiagnoses of tuberculosis resulting from laboratory error—Wisconsin, 1996. MMWR Morb Mortal Wkly Rep 1997;46:797–801 [PubMed] [Google Scholar]
- 5.de CRM, Soini H, Roscanni GC, Jaques M, Villares MC, Musser JM. Extensive cross-contamination of specimens with Mycobacterium tuberculosis in a reference laboratory. J Clin Microbiol 1999;37:916–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruddy M, McHugh TD, Dale JW, Banerjee D, Maguire H, Wilson P, Drobniewski F, Butcher P, Gillespie SH. Estimation of the rate of unrecognized cross-contamination with Mycobacterium tuberculosis in London microbiology laboratories. J Clin Microbiol 2002;40:4100–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 2010;48:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, Chakravorty S, Jones M, Alland D. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol 2010;48:2495–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillemann D, Ruesch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extrapulmonary tuberculosis by automated GeneXpert MTB/RIF system. J Clin Microbiol 2011;49:1202–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marlowe EM, Novak Weekley SM, Cumpio J, Sharp SE, Momeny MA, Babst A, Carlson JS, Kawamura M, Pandori M. Evaluation of the cepheid Xpert MTB/RIF assay for the direct detection of Mycobacterium tuberculosis complex from respiratory specimens. J Clin Microbiol 2011;49:1621–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moure R, Munoz L, Torres M, Santin M, Martin R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples using an integrated real time PCR method. J Clin Microbiol 2010;49:1137–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, Dawson R, Whitelaw A, Hoelscher M, Sharma S, et al. Evaluation of the Xpert(r) MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 2011;184:132–140 [DOI] [PubMed] [Google Scholar]
- 14.Friedrich SO, Venter A, Kayigire XA, Dawson R, Donald PR, Diacon AH. Xpert MTB/RIF and genotype mtbdrplus for patient selection for a tuberculosis clinical trial (jcm00138–11). J Clin Microbiol 2011;49:2827–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armand S, Vanhuls P, Delcroix G, Courcol R, Lemaitre N. Comparison of the Xpert MTB/RIF test with an is6110-taqman real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol 2011;49:1772–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rachow A, Zumla A, Heinrich N, Rojas-Ponce G, Mtafya B, Reither K, Ntinginya EN, O'Grady J, Huggett J, Dheda K, et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by cepheid Xpert MTB/RIF assay—a clinical validation study. PLoS ONE 2011;6:e20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent PT, Kubica GP. Control CfD. Public health mycobacteriology: a guide for the Level III laboratory. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Atlanta; 1985 [Google Scholar]
- 18.Enarson D. Management of tuberculosis: a guide for low income countries, 5th ed Paris: International Union Against Tuberculosis and Lung Disease; 2000 [PubMed] [Google Scholar]
- 19.Diagnostic standards and classification of tuberculosis in adults and children This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000;161:1376–1395 [DOI] [PubMed] [Google Scholar]
- 20.Cheesbrough M. District Laboratory Practice in Tropical Countries, 2nd ed Cambridge, UK: Cambridge University Press; 2006 [Google Scholar]
- 21.Woolley JS. When is a sputum negative? Chest 1936;2:20–21 [Google Scholar]
- 22.Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, Boeree MJ, van Soolingen D. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis 2008;47:1135–1142 [DOI] [PubMed] [Google Scholar]
- 23.Tuberculosis Research Centre Indian Council of Medical R Additional risk of developing TB for household members with a TB case at home at intake: a 15-year study. Int J Tuberc Lung Dis 2007;11:282–288 [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention AFB microscopy: characteristics, strategies and interpretation. Atlanta: Centers for Disease Control and Prevention; 2003 [Google Scholar]
- 25.Behr MA, Warren SA, Salamon H, Hopewell PC, de Leon AP, Daley CL, Small PM. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 1999;353:444–449 [DOI] [PubMed] [Google Scholar]
- 26.Burdz TVN, Wolfe J, Kabani A. Evaluation of sputum decontamination methods for Mycobacterium tuberculosis using viable colony counts and flow cytometry. Diagn Microbiol Infect Dis 2003;47:503–509 [DOI] [PubMed] [Google Scholar]
- 27.Bark CM, Okwera A, Joloba ML, Thiel BA, Nakibali JG, Debanne SM, Boom WH, Eisenach KD, Johnson JL. Time to detection of Mycobacterium tuberculosis as an alternative to quantitative cultures. Tuberculosis (Edinb) 2011;9:257–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pheiffer C, Carroll NM, Beyers N, Donald P, Duncan K, Uys P, van Helden P. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis 2008;12:792–798 [PubMed] [Google Scholar]
- 29.Desjardin LE, Hehman GL. Quantification of M. tuberculosis DNA in sputum during the treatment of pulmonary tuberculosis. Methods Mol Med 2001;48:121–131 [DOI] [PubMed] [Google Scholar]
- 30.Alisjahbana B, vanCrevel R, Danusantoso H, Gartinah T, Soemantri E, Nelwan R, Meer J. Better patient instruction for sputum sampling can improve microscopic tuberculosis diagnosis. Int J Tuberc Lung Dis 2003;9:814–817 [PubMed] [Google Scholar]
- 31.Hirooka T, Higuchi T, Tanaka N, Ogura T. The value of proper sputum collection instruction in detection of acid-fast bacillus. Kekkaku 2004;79:33–37 [PubMed] [Google Scholar]
- 32.Macq J, Solis A, Velazquez H, Dujardin B. Informing the TB suspect for sputum sample collection and communicating laboratory results in Nicaragua: a neglected process in tuberculosis case finding. Salud Publica Mex 2005;47:303–307 [DOI] [PubMed] [Google Scholar]
- 33.Mishal Sameer K, Osman D, Charalambos S, Karam S, Peter G-F. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet 2007;369:1955–1960 [DOI] [PubMed] [Google Scholar]
- 34.Sakundarno M, Nurjazuli N, Jati S, Sariningdyah R, Purwadi S, Alisjahbana B, van der Werf M. Insufficient quality of sputum submitted for tuberculosis diagnosis and associated factors, in Klaten District, Indonesia. BMC Pulm Med 2009;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]