Abstract
Rationale: Opioids are commonly used to relieve dyspnea, but clinical data are mixed and practice varies widely.
Objectives: Evaluate the effect of morphine on dyspnea and ventilatory drive under well-controlled laboratory conditions.
Methods: Six healthy volunteers received morphine (0.07 mg/kg) and placebo intravenously on separate days (randomized, blinded). We measured two responses to a CO2 stimulus: (1) perceptual response (breathing discomfort; described by subjects as “air hunger”) induced by increasing partial pressure of end-tidal carbon dioxide (PetCO2) during restricted ventilation, measured with a visual analog scale (range, “neutral” to “intolerable”); and (2) ventilatory response, measured in separate trials during unrestricted breathing.
Measurements and Main Results: We determined the PetCO2 that produced a 60% breathing discomfort rating in each subject before morphine (median, 8.5 mm Hg above resting PetCO2). At the same PetCO2 after morphine administration, median breathing discomfort was reduced by 65% of its pretreatment value; P < 0.001. Ventilation fell 28% at the same PetCO2; P < 0.01. The effect of morphine on breathing discomfort was not significantly correlated with the effect on ventilatory response. Placebo had no effect.
Conclusions: (1) A moderate morphine dose produced substantial relief of laboratory dyspnea, with a smaller reduction of ventilation. (2) In contrast to an earlier laboratory model of breathing effort, this laboratory model of air hunger established a highly significant treatment effect consistent in magnitude with clinical studies of opioids. Laboratory studies require fewer subjects and enable physiological measurements that are difficult to make in a clinical setting. Within-subject comparison of the response to carefully controlled laboratory stimuli can be an efficient means to optimize treatments before clinical trials.
Keywords: opioids, breathlessness, signs and symptoms, respiratory, pain
At a Glance Commentary
Scientific Knowledge on the Subject
Clinical studies show that opioids reduce refractory dyspnea (breathing discomfort), but individual studies vary from no effect to strong effect, perhaps because of variability of clinical conditions. There was a single previous attempt to study the effect of opioids on dyspnea under controlled laboratory conditions. In that study, opioids did not reduce dyspnea produced by a laboratory challenge that evoked a sense of increased breathing work and effort. Thus, laboratory results did not match clinical outcomes.
What This Study Adds to the Field
The present study showed that opioids profoundly reduced dyspnea during a laboratory challenge that evoked a sense of air hunger. Treatment effect was quantitatively similar to clinical studies. This study strengthens confidence in the laboratory model of air hunger dyspnea as an adjunct to clinical trials of palliative treatments, focuses attention on air hunger as the primary target of therapy, and reaffirms the effectiveness of opiates for dyspnea palliation.
Dyspnea, the perception of breathing discomfort, is a powerfully aversive sensation (1, 2). Dyspnea is one of the most troubling symptoms in palliative care (3, 4), is common in mechanically ventilated patients, and is probably connected to poor psychological outcomes (5, 6). The need for effective dyspnea palliation is acute.
Reviews conclude that no drugs other than opioids are effective for this purpose (7, 8). However, dyspnea palliation is not a Food and Drug Administration–listed indication for opioids, and their use is limited by concerns about respiratory depression and other side effects (e.g., Reference 9). Evidence supporting the use of systemic opiates for dyspnea palliation includes a meta-analysis of 9 clinical studies, each having 7 to 18 patients (10). The authors concluded that opiates reduce dyspnea, but several individual studies reported little or no benefit. A single adequately powered study of slow-release opioid showed a statistically significant but modest effect (11).
The clinical setting provides relatively noisy data because both the underlying pathophysiology and treatment constantly vary. The ability to employ a laboratory model in the testing of palliative treatments for dyspnea could allow more certain progress: dose escalation studies, drug comparison studies, and placebo controls can be done within individuals, allowing paired comparison; repeated laboratory tests are not subject to variations in pathophysiological state; physiological measurements that are difficult in the clinical setting can be obtained easily in the laboratory. A published laboratory model of respiratory work did not show a treatment effect of opiates, in contrast to published clinical studies (12). We sought to determine whether a laboratory model of air hunger is more appropriate for predicting clinical effect.
The present study addresses several questions: Does morphine relieve air hunger? Can laboratory-induced dyspnea in healthy humans provide a useful model to assess potential clinical dyspnea treatments in a controlled environment? Is dyspnea relief by morphine a simple outcome of brainstem respiratory depression, or might opiate effects on perceptual processing contribute to relief? We used a well-characterized laboratory model to induce dyspnea in healthy subjects to test the effect of intravenous morphine and placebo. A combination of mild hypercapnia with restricted minute ventilation has been shown to evoke reports of “air hunger,” “unsatisfied inspiration,” and, to some extent, “respiratory work” (e.g., References 13–17). Although this not a perfect model of clinical dyspnea, it evokes both the immediate unpleasantness and the emotional responses reported by patients to a greater degree than the other widely used model, external resistance (18).
Some of the results of these studies have been previously reported in abstract form (19).
Methods
The Committee on Clinical Investigations (Institutional Review Board, IRB) at Beth Israel Deaconess Medical Center (Boston, MA) approved this protocol, and written consent was obtained from all subjects. Exclusion criteria included pregnancy, history of hypersensitivity to any opiate, opiate use in the past month, unstable cardiac or vascular disease, stroke, seizure disorder, severe migraine headaches, hepatic or renal disease, pheochromocytoma, peripheral neuropathies, known brain metastasis, drug addiction, major psychiatric disorder, and panic disorder. In addition, we excluded subjects not fluent in English. Fifteen subjects were enrolled (i.e., signed consent forms), and full data sets were obtained and analyzed for 6 subjects, as explained below.
Test Stimuli
We employed two stimuli, both with graded hypercapnia: (1) laboratory dyspnea challenge, in which minute ventilation was limited; and (2) hypercapnic ventilatory response (HCVR), in which breathing was unimpeded. End-tidal PCO2 (PetCO2) was manipulated by altering inspired PCO2 and holding each PetCO2 level for 3 minutes to permit steady state response in both tests (20, 21).
Measurement of Dyspnea
Dyspnea challenge.
The effects of morphine and placebo on dyspnea were assessed from the dyspnea challenge tests, in which a system previously described was used to limit minute ventilation during hypercapnia to 0.13 L/minute/kg (13, 16, 18). (The breathing system is shown in Figure E1 in the online supplement). We controlled inspired PCO2 to produce four to six stepwise PetCO2 increases and decreases of 2 to 8 mm Hg in unpredictable order; each step was held for approximately 3 minutes.
Breathing discomfort scale.
Subjects were instructed to rate “Breathing Discomfort…how unpleasant or bad your breathing feels” using a Breathing Discomfort Visual Analog Scale (BDVAS) labeled neutral/unpleasant/annoying/distressing/unbearable. They were encouraged to change their rating whenever discomfort changed. The scale was implemented electronically, with a linear LED visual readout that the subject adjusted by turning a knob. An analog signal was provided for continuous recording. BDVAS rating was the primary outcome measure for dyspnea challenge tests. Instructions to the subject are given in the online supplement.
Multidimensional dyspnea profile.
To enhance our understanding of the action of morphine, subjects were also questioned immediately after each dyspnea challenge trial regarding respiratory sensations, using the Multidimensional Dyspnea Profile (MDP) (17, 18, 22, 23). Dyspnea is a multidimensional perception (24), which can be viewed as having an immediate sensory component comprising the intensity and duration of several sensory qualities (SQs), an immediate affective discomfort or unpleasantness (affective stage A1), and an evaluative emotional component (affective stage A2) (see Reference 25). This instrument presents a single scale for immediate unpleasantness of respiratory sensation, five scales for the intensity of several qualities of respiratory sensation, and five scales for the negative emotional response to respiratory discomfort; individual items are listed in Table E2. Subjects were asked to focus on the last 30 seconds of the trial when completing the MDP. Data from the MDP were used as secondary outcome measures, not as primary tests of the effect of morphine on dyspnea.
Measurement of Respiratory Drive (HCVR)
The effects of morphine and placebo on respiratory drive were assessed from the steady state HCVR test, in which subjects were free to breathe spontaneously (15). Four to six stepwise PetCO2 elevations were applied by raising inspired PCO2 in steps of 2 to 4 mm Hg to achieve PetCO2 levels equivalent to those used in the prior dyspnea challenge test. Minute ventilation derived from a calibrated pneumotachometer signal was the primary outcome measure from HCVR tests.
Blinding and Randomization
The physician providing medical coverage randomized the order of presentation of morphine and saline injections. The first two subjects were informed that on a given day they would receive either drug or saline placebo. Because an early subject reported detecting drug side effects, we increased subterfuge to reduce the chance likelihood that subjects might discern treatment condition from side effects. We informed subjects 3–15 that they would get either morphine or a benzodiazepine tranquilizer (but the “benzodiazepine” was actually saline). Experimenters were blinded to whether the drug was morphine or saline, with the exception of the attending physician, who had no involvement in stimulus administration or dyspnea measurement.
An additional blinding measure was added to prevent subjects from discerning which treatment reduced dyspnea (especially important in the case of the investigator-subject): PetCO2 was elevated if necessary to achieve the same maximal level of BDVAS during the dyspnea challenge after treatment as had been observed during predrug baseline. Thus, if treatment was effective, the subject experienced higher PetCO2 after treatment during both the dyspnea challenge and the following HCVR.
Protocol
On arrival for a laboratory session, the subject's resting PetCO2 was measured with an unobtrusive nasal sampling catheter during quiet undisturbed reading of interesting but nonemotive material.
Each subject underwent one or two sessions of familiarization with both dyspnea challenge and HCVR, which included a stimulus strength that produced intolerable discomfort during the dyspnea challenge. These sessions stabilize the relationship between stimulus and rating. At this stage we identified and excluded one subject whose ratings were correlated poorly with PetCO2 as per the a priori criterion (r2 < 0.5; see Reference 15).
Test days: After measuring resting PetCO2, we placed an intravenous catheter. We then performed one or two predrug baseline assessments of the dyspnea challenge response and a steady state HCVR. Each dyspnea challenge assessment comprised three to five steps of end-tidal PetCO2 delivered in an assessment trial lasting 10 to 20 minutes. Our goal was to achieve BDVAS ratings ranging from zero to approximately 75% of full scale (%FS) before and after treatment. To reduce the influence of subject expectation, each trial was started at a different PetCO2 than the prior trial, and each trial included both increasing and decreasing PetCO2 steps in varied order. Examples of time traces are shown in Figure E2.
We then infused morphine sulfate (0.07 mg/kg) or saline placebo over the course of 5 minutes (separate days, order randomized). This dose is in the range of the suggested starting dose when morphine is given for acute pain. Resting ventilation was measured for at least 5 minutes after the end of drug infusion; we then commenced alternating trials of dyspnea challenge and HCVR. All subjects completed at least two trials of each type after drug infusion. Two subjects (1 and 13) underwent a second experiment at a higher dose (180 and 145% of the standard dose, respectively) at least 10 days after the initial experiment.
Subjects were also questioned about side effects that might be expected with the drug.
Analysis
Analyses were based on a “steady state” epoch of data beginning at least 100 seconds after a step change in PetCO2 and lasting at least 30 seconds (20, 21). Because the analgesic effect of intravenous morphine lasts several hours, and because initial analysis showed no difference between the first two dyspnea challenge trials postdrug, data from these trials were combined for analysis (the first trial commenced 9–15 min after the end of drug infusion, and the second commenced 49–73 min after drug infusion).
By a priori design, we used a “response feature” analysis to provide a single number for treatment effect in each subject (see Figure E3). This approach uses the data collected in repeated measures over a range of stimulus values, thus minimizing the effect of “noise” in the psychophysical ratings. It does not require exact matching of stimuli before and after treatment, eliminating noise in the independent variable. The single response feature enabled statistical testing using a two-tail paired t test. On the basis of linear regression we interpolated the PetCO2 at which the BDVAS rating was 60% full scale (%FS) before treatment. This level is typical of patients entered in clinical studies using morphine for palliation of dyspnea, and allows sufficient scope to enable measurement of a large treatment effect. Treatment response was defined as the fall in BDVAS rating at the same PetCO2 after treatment with morphine, and was compared with mean placebo effect. We examined the change in HCVR by a similar approach, measuring change in ventilation at the same PetCO2 as used for each subject in the analysis of BDVAS.
To estimate the effect of morphine throughout a range of dyspnea, we also calculated mean regression lines for both BDVAS and HCVR, using the mean slope and mean x intercept, with each subject weighted equally; we did not use these for statistical tests.
Results
Subjects
We studied six opiate-naive healthy volunteers (see Table 1). We enrolled 15 subjects, but 7 subjects were dropped between enrollment and drug testing for the following reasons: 4 were excluded based on a priori medical criteria (medical histories were taken only after the consent form was signed); 1 failed to show an adequate correlation between the CO2 stimulus and BDVAS; 2 were dropped because contact was lost.
TABLE 1.
SUBJECT CHARACTERISTICS
| Subject (Sex) | Height (cm) | Weight (kg) | Age (yr) | PetCO2 at Rest (mm Hg) | HCVR Slope (L/min/mm Hg) | HCVR Intercept (mm Hg) | BDVAS Slope (%FS, mm Hg) | BDVAS Intercept (mm Hg) | Experience Relevant to Respiratory Testing |
| 1 (M) | 178 | 78 | 57 | 40 | 1.9 | 35.9 | 9.4 | 42.8 | Ph.D. in physiology, participating investigator |
| 3 (M) | 163 | 68 | 34 | 41 | 0.9 | 17 | 6.7 | 41.1 | College, no biology. Mouthpiece experience, breathing exercises in martial arts |
| 6 (M) | 188 | 80 | 21 | 42 | 2.0 | 38.2 | 6.9 | 41.3 | Some college, no biology, no controlled breathing experience |
| 11 (M) | 168 | 70 | 26 | 41 | 1.4 | 34.8 | 5.9 | 38.1 | College, biology major. No controlled breathing experience |
| 13 (M) | 183 | 77 | 20 | 45 | 1.4 | 40.6 | 5.3 | 50.3 | High school. Some scuba experience |
| 14 (F) | 183 | 64 | 23 | 39 | 1.2 | 33.7 | 10.7 | 38.7 | College (premed), emergency room aide |
| Mean | 177.2 | 72.8 | 30.2 | 41.3 | 1.5 | 33.4 | 7.5 | 42.1 | |
| Median | 180.5 | 73.5 | 24.5 | 41 | 1.4 | 35.3 | 6.8 | 41.2 | |
| SD | 9.7 | 6.4 | 14.1 | 2.1 | 0.4 | 8.4 | 2.1 | 4.4 | |
| Coefficient of variation | 5% | 9% | 47% | 5% | 29% | 25% | 28% | 10% |
Definition of abbreviations: BDVAS = Breathing Discomfort Visual Analog Scale; F = female; FS = full scale; HCVR = hypercapnic ventilatory response; M = male; PetCO2 = partial pressure of end-tidal carbon dioxide.
Note: Subject numbers were assigned in recruitment order; missing numbers reflect dropout as explained in text.
In addition, one subject failed to complete the morphine study due to strong nausea, and one subject was dropped during data analysis because we had not been able to induce the prescribed baseline dyspnea within the IRB-agreed limit for PetCO2.
Effect of Morphine on Dyspnea
Breathing Discomfort Visual Analog Scale.
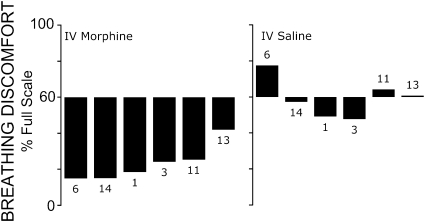
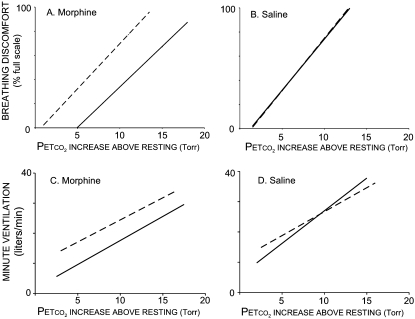
After morphine, breathing discomfort fell 39%FS (median) at the same PetCO2 that had induced a pretreatment BDVAS of 60%FS (P < 0.001); see Figure 1. This is a relative decrease of 65% of the prevailing level of pretreatment dyspnea (see Alternate Statistical Test in the online supplement). Morphine produced a parallel rightward shift in breathing discomfort versus PetCO2, such that breathing discomfort at any given PetCO2 was substantially less, as shown in Figure 2 (top). Placebo produced no effect (median change, 0%FS; P = 0.31).
Figure 1.
Change in “response feature”: bar labels refer to individual subject codes, as shown in Table 1. Bars depict change in breathing discomfort at the partial pressure of end-tidal carbon dioxide (PetCO2) that elicited a 60% rating on the Breathing Discomfort Visual Analog Scale (BDVAS) under baseline conditions on that day; thus, the maximal possible decrease would be 60%. IV = intravenous.
Figure 2.
Regression lines showing average perceptual and ventilatory responses. Top: Breathing discomfort versus partial pressure of end-tidal carbon dioxide (PetCO2) while minute ventilation was held constant at 0.13 L · minute−1 · kg−1. Bottom: Minute ventilation versus PetCO2 during unrestricted breathing. Dashed lines depict same-day baseline values before drug or placebo (because placebo had no effect, pre- and postinjection Breathing Discomfort Visual Analog Scale [BDVAS] regressions are superimposed). Average regression was obtained by averaging the slopes and intercepts for individual subjects’ regressions.
The higher dose given to subjects 1 and 13 on a second occasion produced no discernable improvement in dyspnea compared with the initial dose given to these subjects.
Multidimensional Dyspnea Profile.
The affective response to dyspnea (and pain) can be viewed as having an immediate unpleasantness component (A1) and an evaluative emotional component (A2) (25). The instruction given for BDVAS rating corresponds closely to immediate unpleasantness; thus, to verify the performance of the MDP, we tested the correlation of immediate unpleasantness rated just after the trial to the online BDVAS rating during the focus period, using all 50 MDP responses collected from all 6 subjects on morphine or placebo administration days (18 predrug trials, 14 postsaline trials, and 18 postmorphine trials). The mean online BDVAS for these periods was 60%FS (SD, 26%FS). VAS and immediate unpleasantness were highly correlated (r2 = 0.84, P < 0.0001).
The descriptor group “air hunger, not getting enough air, smothering” was selected by subjects as the best description of the dyspnea challenge. In the 50 MDP responses after dyspnea challenge, the overall average BDVAS rating was 59%FS. The average rating for air hunger was 61%FS, followed closely by the essentially synonymous descriptor groups “want more air, not breathing enough, breaths don't satisfy,” both at 60%FS. The second most highly rated descriptor group was “breathing requires mental effort or concentration,” averaging 43%FS. The descriptors “chest and lungs feel tight or constricted” and “breathing requires muscle work or effort” were rated 32%FS and 31%FS, respectively. There was no difference in the rank order of these descriptors before versus after morphine.
Our measurement of negative emotional response comprised rating scales for “depressed,” “anxious,” “frustrated,” “angry,” and “afraid.” The most prominent emotional response in the control state was anxiety, with a mean rating of 21%FS, followed by frustration (mean, 15%FS); the other emotions averaged 3%FS or less. To determine whether morphine had a differential effect on the affective dimension of dyspnea (as proposed for pain), we selected one MDP response pair that was matched to have the same immediate unpleasantness pre- versus posttreatment in each of the six subjects. (To obtain equal unpleasantness PetCO2 was on average 5 mm Hg higher after morphine.) Anxiety ratings fell disproportionately after morphine: anxiety fell significantly from 0.63 to 0.22 (P = 0.025). Placebo had no effect on the ratio of anxiety to unpleasantness.
Effect of Morphine on Ventilation
The mean regression for HCVR showed a parallel rightward shift of 5 mm Hg after morphine; that is, it required greater PetCO2 to produce the same ventilation (see Figure 2, bottom). The median fall in minute ventilation at the PetCO2 identified for response feature analysis was 29% (P < 0.05). The median increase in PetCO2 needed to restore ventilation to the premorphine level was 3.0 mm Hg (range, 2–7 mm Hg). This is lower than the 5.2–mm Hg increase needed to restore the same BDVAS (P = 0.07). The fall in HCVR was not correlated with the reduction of dyspnea across subjects (r2 = 0.18).
Resting ventilation measured 5 minutes after the end of morphine infusion was 106 ml/kg/minute, not different from the resting ventilation after placebo (110 ml/kg/min). Respiratory rate was also similar (16.5/min after morphine, 17.7/min after placebo). There was no significant change in resting PetCO2 (mean PetCO2 was 1 mm Hg higher after morphine).
Adverse Effects
Side effects of morphine were minimal in all but one subject. Two subjects reported mild nausea; a third, subject 7, experienced strong nausea that required discontinuance of the experiment, and that later evolved to vomiting (subject 7 was not included in analysis). Six subjects reported mild fatigue, three subjects reported lightheadedness or difficulty concentrating (two of these reported similar effects after placebo), one subject reported itching and dizziness, and one subject reported tingling and dry mouth. No subject experienced urinary retention. We did not assess constipation, a clinically important adverse effect of morphine, because the short duration of the study would not have produced meaningful results.
Discussion
Main Finding
Morphine produced a substantial and consistent reduction of laboratory-induced air hunger, while placebo had no effect. We saw a disproportionately large reduction of dyspnea-related anxiety, and a more modest reduction of ventilation. Consistent with this finding, remifentanil has been shown to significantly reduce the discomfort of a short breath hold in concert with reduced activation of cerebral areas previously associated with air hunger (26). The overall evidence shows opioids are efficacious for dyspnea in the clinical setting. The largest treatment effects seen in published clinical studies are comparable to the effect seen in this study, but some studies have shown no change in the mean level of dyspnea (10, 11, 27). The present study reinforces positive clinical findings, and suggests that a substantial treatment effect is possible. Tests of the dose–response relationship and other treatment variables are difficult to carry out against the background of constantly varying clinical condition. Basic laboratory work can narrow the range of possibilities for clinical testing, as well as provide a model useful for testing the neurophysiological mechanisms that underlie treatments.
Relationship of Dyspnea Relief to Ventilatory Drive
There are two reasons to assess the relationship of dyspnea relief to ventilatory drive: (1) concern about ventilatory depression in patients; and (2) a leading mechanistic hypothesis that suppression of brainstem respiratory drive is the mechanism through which opioids reduce dyspnea; air hunger is thought to arise from awareness of brainstem drive (28).
Most clinical studies have reported respiratory depression to be absent or minimal (e.g., Reference 29), but the measures used—respiratory rate, pulse oximetry, or reported clinical incident—are relatively insensitive. Our data support the idea that, at least in waking subjects, morphine sufficient to relieve dyspnea has little effect on resting ventilation. The parallel shift of the HCVR to higher PCO2 would have predicted a small rise in PetCO2 (30, 31); however, resting breathing is also influenced by the “wakefulness drive to breathe,” which produces a plateau in the HCVR below resting PCO2. This may explain the absence of measurable ventilatory depression in our study, and in many clinical studies. The shift in HCVR has more relevance to breathing during sleep; our finding suggests that care should be taken in patients with a history of sleep apnea (32).
Opiates act to suppress pain at nearly every level from initial spinal synapse to cortex. Given the similarity in cortical structures activated by pain and air hunger (33, 34), it is reasonable to ask whether opiates act on central pathways subserving the perception of air hunger. Our surrogate measure for brainstem drive is provided by the HCVR test. The mean rightward shifts of the HCVR and BDVAS lines were not significantly different, but the individual HCVR shift did not predict that individual's BDVAS shift. Although the latter may suggest that morphine is also acting at cortical synapses to alter dyspnea perception, we cannot rule out decreased ventilatory drive as the sole mechanism for dyspnea relief.
Critique of This Study
Analysis approach.
The “response feature” analysis approach we employed provides an outcome measure that is useful in thinking about clinical effect. This analysis does depend on the assumption of linearity in the stimulus–response characteristic, but this has been repeatedly demonstrated for the air hunger–PetCO2 relationship during limited ventilation (e.g., References 15 and 16). The response feature described here is a robust approach. The median correlation coefficient between PetCO2 stimulus and BDVAS response for all three conditions (pretreatment, postplacebo, and postmorphine) in this selected group of subjects was high (r = 0.87). However, in 6 of 28 trials subjects had a single “bad trial” (i.e., r2 < 0.49, i.e., less than half the variation in BDVAS was explained by PetCO2). We included these trials in the analysis, and the results nonetheless showed a consistent treatment response and high statistical significance with only six subjects.
Method of adjusting for placebo effect.
Measurement of subjective sensations is inherently noisy, especially when the subject is rating an unfamiliar internal sensation. The result of our placebo intervention indicates the level of noise one can expect in such circumstances—the standard deviation of placebo effect was 10.9%FS. Because subtracting the individual placebo effect from the individual treatment effect would simply increase measurement noise, we compared morphine effect with mean placebo effect. To provide assurance that this approach was valid, we also performed the analysis in a more usual manner (see the online supplement: Results, Alternate Statistical Test).
Subject selection and dropout.
Subjects were self-selected, as they responded to posted advertisements; this may have biased the sample toward those who have less fear of respiratory discomfort. It is difficult to see how it would bias the sample toward those who have an atypical response to morphine. We dropped two subjects from analysis after data were collected; however, the available data on both these subjects indicated a strong treatment effect of morphine. See the online supplement (Results, Dropped Subjects).
Multiple Dimensions of Dyspnea
Our main outcome measure was a unidimensional VAS rating of “breathing discomfort,” which has been widely validated for dyspnea. This electronically implemented VAS was chosen because it allows moment-to-moment ratings, and thus permits measurement of several steady state data points at different stimulus levels within the same trial (i.e., without interrupting the subject's task for questioning). As mentioned, the correlation between VAS and stimulus was high in the individuals tested. We also employed the Multidimensional Dyspnea Profile, which required about 2 minutes to complete at the end of each trial. This questionnaire was focused on the final 30 seconds of each trial. The extremely high correlation between BDVAS during the last 30 seconds and MDP unpleasantness rating substantiates the validity of the MDP immediate unpleasantness scale.
Morphine did not alter the ratio between ratings of unpleasantness and ratings of air hunger intensity. Although some reports suggest that opioids reduce the immediate unpleasantness of pain relative to the sensory intensity of pain (35), other reports suggest that pain unpleasantness is not differentially lowered (36, 37). Our results are comparable to these latter studies.
We did observe a strong differential effect of morphine on the dyspnea-related anxiety reported by five subjects. At a matched level of unpleasantness, anxiety fell in all five subjects. Placebo had no effect on anxiety. This differential effect on anxiety suggests that morphine acts on synapses above the brainstem involved in perceptual processing, as it does with pain.
Relationship to Clinical Studies
Relevance of the limited ventilation dyspnea challenge to clinical situations.
We chose the dyspnea challenge model used in this study because we believe it has several characteristics in common with many cases of clinical dyspnea: (1) many dyspneic patients are unable to ventilate their lungs in proportion to the existing level of medullary respiratory center drive. This failure to meet demand can arise from diseased lungs or weakened respiratory muscles. The most probable neurophysiological mechanism is that air hunger arises from medullary drive that is disproportionate to feedback from mechanoreceptors such as the pulmonary stretch receptors (38); (2) descriptors related to air hunger are prominent in the language used by patients to describe their dyspnea. Smith and colleagues (39) reported that terms related to air hunger explained the great majority of the variance in dyspnea reported by patients (their components 1 and 2). O'Donnell and coworkers reported that the prominent sensation in patients with chronic obstructive pulmonary disease (COPD) when dyspnea limits exercise is “unsatisfied inspiration,” a descriptor group that includes mainly terms related to air hunger (40, 41); and (3) air hunger is the most unpleasant quality of dyspnea, and as such gives rise to the strongest emotional response (18). This would seem to make it a logical target of efforts to palliate dyspnea.
There are several ways to produce elevated medullary drive in modeling dyspnea. Hypercapnia is convenient and safe, and produces the same sensation as hypoxia (16). If ventilation is not restricted, high levels of PaCO2 are needed to produce strong air hunger (15), presumably because stretch receptor feedback from larger tidal volume minimizes air hunger (14). At these high levels of PaCO2 side effects such as headache and autonomic sequelae often limit the exposure. In addition, the resultant high minute ventilations are not typical of most patients with cardiopulmonary disease. Restriction of ventilation during hypercapnia, using present methods or using inspiratory resistance (e.g., Reference 42), avoids the problems of high PCO2, and better approximates clinical situations. Although no model is a perfect representation of disease, present results show that the current model can simulate clinical outcomes (see the following).
Comparison of model results with existing clinical studies.
The intravenous dose we used is in the lower range of starting intravenous doses recommended by the American Thoracic Society panel on palliative care (43). Because the bioavailability of oral doses is variable among individuals, our results can best be compared with clinical studies using parenteral administration. We therefore searched for all prospective studies using parenteral opiates and scalar measures of dyspnea that can be compared with our outcome. We identified five studies; it happened that all used subcutaneous administration of morphine or diamorphine for relief of dyspnea at rest (four in cancer, one in pulmonary fibrosis), and all used some form of visual analog scale to assess dyspnea (44–48). Doses were similar to the dose we used (except in some cases investigators adjusted individual dose to compensate for prior opiate use by that individual). The mean initial level of dyspnea in these five clinical studies was 59.8%FS (range, 30–83%FS; means weighted by number of patients studied), and the mean fall in dyspnea after morphine or diamorphine was 50.5% of the initial value (range, 40–66%). These results are similar to the 60%FS initial value and the 65.0% mean fall from initial value obtained in the present study. (There were no studies of intramuscular opioids for dyspnea, and the single study of intravenous infusion was limited to a binary measure of dyspnea relief vs. no relief—seven of eight patients experienced relief [49].)
A systematic review identified three clinical studies that failed to find a positive effect of opiates on dyspnea—all of these negative studies employed oral dosing to treat dyspnea in ambulatory patients with COPD (27, 50, 51). However, other studies in similar patients with COPD found a positive effect of oral dihydrocodeine (52–54). Oral morphine and hydromorphone have shown a significant and sizable positive effect in patients with cancer (11, 29, 55, 56), amyotrophic lateral sclerosis (57), and congestive heart failure (58). Oral administration is convenient for clinical use, but doses require titration to account for wide individual variability in bioavailability of enteral drug.
Comparison of the present limited ventilation dyspnea challenge with a respiratory work dyspnea challenge.
Supinski and colleagues assessed the effect of 90 mg of oral codeine on dyspnea, using a laboratory model that produced high ratings of respiratory effort: voluntary hyperventilation against a large inspiratory threshold load (12). This challenge is unlikely to have produced much air hunger because ventilation was high (14 L/min), tidal volume was high (1 L), and no CO2 was added to the inspired air. This breathing pattern is expected to produce hypocapnia (low medullary drive) and elevated traffic from pulmonary stretch receptors, both minimizing air hunger. Codeine produced no change in respiratory discomfort in this loaded breathing model (although it did produce effective analgesia in all subjects). Results from this mechanical loading model differ sharply from most clinical results, including several studies that used similar drugs in similar or smaller doses (52–54, 58). We suggest that the reason for this difference is that mechanical loading with voluntary hyperventilation does not replicate some essential features of clinical dyspnea: it does not involve medullary respiratory drive in excess of achieved ventilation; rather, ventilation is voluntarily driven by the cortex in excess of medullary demand. A similar respiratory work stimulus has been shown to be less unpleasant than the limited ventilation challenge and it evoked less emotional response (18).
Conclusions
We found that morphine is effective in relieving dyspnea in a laboratory model of dyspnea that predominantly evokes air hunger sensation. Our study is in good quantitative agreement with the clinical studies that used parenteral doses of morphine. A previously published study of opioid in a laboratory model of dyspnea that predominantly evokes work/effort sensation failed to agree with clinical findings. These findings provide some mutual reassurance that the laboratory approach is relevant, and that the clinical results are valid. We found no measurable depression of resting ventilation, consistent with clinical reports. Although the results of laboratory studies must always be verified in particular patient populations, laboratory models such as this can enable efficient performance of studies that could be technically difficult (e.g., functional brain imaging) or require large sample sizes if done in patients (e.g., comparison of different doses or different treatments). Laboratory studies can also allow us to measure variables such as minute ventilation that would be difficult in a clinical study of patients with intractable dyspnea.
Supplementary Material
Acknowledgments
The authors thank Daniel Elkin and Tegan Guilfoyle for superb assistance with the performance of experiments and analysis of data. The authors also thank Griffith University for supporting a sabbatical for Dr. Adams that made the performance of these experiments possible.
Footnotes
Supported by the Breathlessness Research Charitable Trust; and by NIH HL46690 and NR10006.
Author Contributions: All authors contributed to performance of experiments and interpretation of data. R.B.B., L.A., C.R.O., R.W.L., and R.M.S. designed the study. R.B.B. wrote the manuscript, with critical input from all other authors. All authors read and approved the final version of the manuscript.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201101-0005OC on June 21, 2011
Author Disclosure: R.B.B.’s institution has received grants from the Breathlessness Relief Charitable Trust. L.A. has received grants from the Breathlessness Research Charitable Trust. C.R.O. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.A.G. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.W.L. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M.S. has received royalties from Lippincott Williams & Wilkins, and UpToDate; he has received fees for editorial duties from the American College of Chest Physicians.
References
- 1.O'Driscoll M, Corner J, Bailey C. The experience of breathlessness in lung cancer. Eur J Cancer Care (Engl) 1999;8:37–43 [DOI] [PubMed] [Google Scholar]
- 2.Thomas JR, von Gunten CF. Clinical management of dyspnoea. Lancet Oncol 2002;3:223–228 [DOI] [PubMed] [Google Scholar]
- 3.Ben-Aharon I, Gafter-Gvili A, Paul M, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review. J Clin Oncol 2008;26:2396–2404 [DOI] [PubMed] [Google Scholar]
- 4.Teno JM, Clarridge BR, Casey V, Welch LC, Wetle T, Shield R, Mor V. Family perspectives on end-of-life care at the last place of care. JAMA 2004;291:88–93 [DOI] [PubMed] [Google Scholar]
- 5.Cuthbertson BH, Hull A, Strachan M, Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med 2004;30:450–455 [DOI] [PubMed] [Google Scholar]
- 6.Schelling G. Effects of stress hormones on traumatic memory formation and the development of posttraumatic stress disorder in critically ill patients. Neurobiol Learn Mem 2002;78:596–609 [DOI] [PubMed] [Google Scholar]
- 7.Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nat Clin Pract Oncol 2008;5:90–100 [DOI] [PubMed] [Google Scholar]
- 8.Simon ST, Higginson IJ, Booth S, Harding R, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev [serial on the Internet]. 2010 [accessed August 2011]; 2010;1:CD007354 Available from: http://www.cochrane.org/reviews/clibintro.htm [DOI] [PubMed] [Google Scholar]
- 9.Berrill J, Linnasne S. Morphine for management of refractory dyspnea: opiates should be used with caution. BMJ 2003;327:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax 2002;57:939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003;327:523–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supinski G, Dimarco A, Bark H, Chapman K, Clary S, Altose M. Effect of codeine on the sensations elicited by loaded breathing. Am Rev Respir Dis 1990;141:1516–1521 [DOI] [PubMed] [Google Scholar]
- 13.Moosavi SH, Binks AP, Lansing RW, Topulos GP, Banzett RB, Schwartzstein RM. Effect of inhaled furosemide on air hunger induced in healthy humans. Respir Physiol Neurobiol 2007;156:1–8 [DOI] [PubMed] [Google Scholar]
- 14.Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett RB. Reduced tidal volume increases “air hunger” at fixed PCO2 in ventilated quadriplegics. Respir Physiol 1992;90:19–30 [DOI] [PubMed] [Google Scholar]
- 15.Banzett R, Lansing R, Evans K, Shea S. Stimulus–response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol 1996;103:19–31 [DOI] [PubMed] [Google Scholar]
- 16.Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol 2003;94:141–154 [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell CR, Schwartzstein RM, Lansing RW, Banzett RB. Descriptor ratings and choices during various forms of laboratory dyspnea [abstract]. Am J Respir Crit Care Med 2010;181:A4803 [Google Scholar]
- 18.Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med 2008;177:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilman SA, Adams L, Schwartzstein RM, O'Donnell CR, Lansing RW, Banzett RB. Morphine alleviates laboratory dyspnea and diminishes hypercapnic ventilatory response [abstract]. Am J Respir Crit Care Med 2010;181:A4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banzett RB. Dynamic response characteristics of CO2-induced air hunger. Respir Physiol 1996;105:47–55 [DOI] [PubMed] [Google Scholar]
- 21.Cunningham D, Robbins P, Wolff C. Integration of respiratory responses to changes in alveolar partial pressures of CO2 and O2 and in arterial pH. : Cherniack NS, Widdicombe JG, Handbook of physiology, section 3: The respiratory system. Washington, DC: American Physiological Society; 1986. pp. 475–528 [Google Scholar]
- 22.Meek P, Parshall M, Bittner P, Sklar D, Alcock J. Reliability and validity of the multidimensional dyspnea profile (MDP) in the emergency department and with 4–6 week follow-up [abstract]. Am J Respir Crit Care Med 2010;181:A4800 [Google Scholar]
- 23.Parshall M, Meek P, Bittner P, Sklar D, Alcock J. Test–retest reliability of multidimensional dyspnea profile recall ratings in the ED [abstract]. Am J Respir Crit Care Med 2010;181:A4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Thoracic Society Ad Hoc Committee. Dyspnea: mechanisms, assessment, and management [consensus statement]. Am J Respir Crit Care Med 1999;159:321–340 [DOI] [PubMed] [Google Scholar]
- 25.Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol 2009;167:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattinson KT, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG. Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci 2009;29:8177–8186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eiser N, Denman WT, West C, Luce P. Oral diamorphine: lack of effect on dyspnoea and exercise tolerance in the “pink puffer” syndrome. Eur Respir J 1991;4:926–931 [PubMed] [Google Scholar]
- 28.Banzett RB, Lansing RW, Reid MB, Adams L, Brown R. “Air hunger” arising from increased PCO2 in mechanically ventilated quadriplegics. Respir Physiol 1989;76:53–67 [DOI] [PubMed] [Google Scholar]
- 29.Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naive palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med 2008;11:204–216 [DOI] [PubMed] [Google Scholar]
- 30.Defares JG. Principles of feedback control and their application to the respiratory control system. : Fenn W, Rahn H, Handbook of physiology, section 3: Respiration. Washington, DC: American Physiological Society; 1964. pp. 649–680 [Google Scholar]
- 31.Gross JB. When you breathe in you inspire, when you don't breathe, you…expire: new insights regarding opioid-induced ventilatory depression. Anesthesiology 2003;99:767–770 [DOI] [PubMed] [Google Scholar]
- 32.Yue HJ, Guilleminault C. Opioid medication and sleep-disordered breathing. Med Clin North Am 2010;94:435–446 [DOI] [PubMed] [Google Scholar]
- 33.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 2002;88:1500–1511 [DOI] [PubMed] [Google Scholar]
- 34.von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Buchel C. Dyspnea and pain share emotion-related brain network. Neuroimage 2009;48:200–206 [DOI] [PubMed] [Google Scholar]
- 35.Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain 1985;22:261–269 [DOI] [PubMed] [Google Scholar]
- 36.Gracely RH, Dubner R, McGrath PA. Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science 1979;203:1261–1263 [DOI] [PubMed] [Google Scholar]
- 37.Casey KL, Svensson P, Morrow TJ, Raz J, Jone C, Minoshima S. Selective opiate modulation of nociceptive processing in the human brain. J Neurophysiol 2000;84:525–533 [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC, Gelb AF, Mahler DA, Webb KA. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc 2007;4:145–168 [DOI] [PubMed] [Google Scholar]
- 39.Smith J, Albert P, Bertella E, Lester J, Jack S, Calverley P. Qualitative aspects of breathlessness in health and disease. Thorax 2009;64:713–718 [DOI] [PubMed] [Google Scholar]
- 40.O'Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 1997;155:109–115 [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell DE, Ora J, Webb KA, Laveneziana P, Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol 2009;167:116–132 [DOI] [PubMed] [Google Scholar]
- 42.Nishino T, Ide T, Sudo T, Sato J. Inhaled furosemide greatly alleviates the sensation of experimentally induced dyspnea. Am J Respir Crit Care Med 2000;161:1963–1967 [DOI] [PubMed] [Google Scholar]
- 43.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, Levy M, Mularski RA, Osborne ML, Prendergast TJ, et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med 2008;177:912–927 [DOI] [PubMed] [Google Scholar]
- 44.Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Ann Intern Med 1993;119:906–907 [DOI] [PubMed] [Google Scholar]
- 45.Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage 1990;5:341–344 [DOI] [PubMed] [Google Scholar]
- 46.Bruera E, Sala R, Spruyt O, Palmer JL, Zhang T, Willey J. Nebulized versus subcutaneous morphine for patients with cancer dyspnea: a preliminary study. J Pain Symptom Manage 2005;29:613–618 [DOI] [PubMed] [Google Scholar]
- 47.Allen S, Raut S, Woollard J, Vassallo M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med 2005;19:128–130 [DOI] [PubMed] [Google Scholar]
- 48.Mazzocato C, Buclin T, Rapin CH. The effects of morphine on dyspnea and ventilatory function in elderly patients with advanced cancer: a randomized double-blind controlled trial. Ann Oncol 1999;10:1511–1514 [DOI] [PubMed] [Google Scholar]
- 49.Cohen MH, Anderson AJ, Krasnow SH, Spagnolo SV, Citron ML, Payne M, Fossieck BE., Jr Continuous intravenous infusion of morphine for severe dyspnea. South Med J 1991;84:229–234 [DOI] [PubMed] [Google Scholar]
- 50.Light RW, Stansbury DW, Webster JS. Effect of 30 mg of morphine alone or with promethazine or prochlorperazine on the exercise capacity of patients with COPD. Chest 1996;109:975–981 [DOI] [PubMed] [Google Scholar]
- 51.Poole PJ, Veale AG, Black PN. The effect of sustained-release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1877–1880 [DOI] [PubMed] [Google Scholar]
- 52.Light RW, Muro JR, Sato RI, Stansbury DW, Fischer CE, Brown SE. Effects of oral morphine on breathlessness and exercise tolerance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989;139:126–133 [DOI] [PubMed] [Google Scholar]
- 53.Johnson MA, Woodcock AA, Geddes DM. Dihydrocodeine for breathlessness in “pink puffers.” Br Med J (Clin Res Ed) 1983;286:675–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. N Engl J Med 1981;305:1611–1616 [DOI] [PubMed] [Google Scholar]
- 55.Clemens KE, Klaschik E. Symptomatic therapy of dyspnea with strong opioids and its effect on ventilation in palliative care patients. J Pain Symptom Manage 2007;33:473–481 [DOI] [PubMed] [Google Scholar]
- 56.Clemens KE, Klaschik E. Effect of hydromorphone on ventilation in palliative care patients with dyspnea. Support Care Cancer 2008;16:93–99 [DOI] [PubMed] [Google Scholar]
- 57.Clemens KE, Klaschik E. Morphine in the management of dyspnoea in ALS: a pilot study. Eur J Neurol 2008;15:445–450 [DOI] [PubMed] [Google Scholar]
- 58.Chua TP, Harrington D, Ponikowski P, Webb-Peploe K, Poole-Wilson PA, Coats AJ. Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol 1997;29:147–152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.