Abstract
Substantial information regarding the role of lung volume reduction surgery (LVRS) in severe emphysema emanates from the National Emphysema Treatment Trial (NETT). The NETT was not a crossover trial and therefore was able to examine the effects of optimal medical management and LVRS on short- and long-term survival, as well as lung function, exercise performance, and quality of life. The NETT generated multiple insights into the preoperative, perioperative, and postoperative management of patients undergoing thoracotomy; described pain control techniques that were safe and effective; and emphasized the need to address nonpulmonary issues to optimize surgical outcomes. After the NETT, newer investigation has focused on bronchoscopic endobronchial interventions and other techniques less invasive than LVRS to achieve lung reduction. In this review, we summarize what we currently know about the role of LVRS in the treatment of severe emphysema as a result of insights gained from the NETT and provide a brief review of the newer techniques of lung volume reduction.
Keywords: emphysema, COPD, lung volume reduction surgery
Much of the information regarding lung volume reduction surgery (LVRS) emanates from the National Emphysema Treatment Trial (NETT). NETT was a multicenter prospective randomized controlled trial that compared optimal medical treatment, including pulmonary rehabilitation, with optimal medical treatment plus LVRS (1). NETT was not a crossover trial and thus was able to examine the effects of optimal medical management and LVRS on short- and long-term survival, as well as lung function, exercise performance, and quality of life.
After NETT, newer investigation has focused on bronchoscopic endobronchial interventions and other techniques less invasive than LVRS to achieve lung reduction (2). Moreover, new data have surfaced regarding the effects of LVRS on ameliorating the manifestations of systemic inflammation (3), the cardiovascular ramifications of hyperinflation (4), and improved radiological techniques that identify optimal LVRS candidates (5).
In this review, we summarize what we currently know about LVRS as a result of NETT in the treatment of severe emphysema and provide a brief review of lung volume reduction techniques.
LVRS: Background
Data regarding LVRS before NETT mainly consisted of uncontrolled, single-center case series characterized by small patient numbers and substantial variability in selection criteria, surgical technique, duration of follow-up, and definitions of complications and outcomes (6–15).
NETT was a randomized, controlled, multicenter, long-term trial that examined the effects of LVRS on the primary end points of survival and maximal exercise performance and the secondary end points of lung function, patient symptoms, and quality of life in contrast to medical therapy (16).
In 2003, NETT first reported the effects of LVRS on survival and maximal exercise capacity in 1,218 patients with emphysema who were randomized to LVRS or medical treatment between January 1998 and July 2002 and monitored for a mean of 2.4 years (17). The effects of LVRS on pulmonary function, oxygen requirement, 6-minute-walk distance(6MWD), quality of life, respiratory symptoms, and health care use were also reported at that time. In 2006, NETT reported updated analyses with a median follow-up of 4.3 years regarding survival and functional measures (18). The updated analyses included 40% more patients with data measured at 2 years postrandomization compared with the initial 2003 report.
Major NETT Outcomes: All Patients
Between January 1998 and July 2002, 3,777 patients were screened for NETT and 1,218 underwent randomization: 608 to LVRS and 610 to medical treatment. Enrollment criteria are provided in Table 1. Baseline characteristics were similar between the groups. Of 608 patients assigned to LVRS, 580 (95.4%) underwent LVRS (406 [70%] by median sternotomy, 174 [30%] by video-assisted thoracoscopic surgery), 21 (3.5%) declined LVRS, and 7 (1.2%) were considered unsuitable by the surgeon for LVRS after randomization.
TABLE 1.
ENROLLMENT CRITERIA
| Inclusion Criteria |
| • History and physical examination consistent with emphysema |
| • CT scan evidence of bilateral emphysema |
| • Prerehabilitation postbronchodilator TLC ≥ 100% predicted |
| • Prerehabilitation postbronchodilator RV ≥ 150% predicted |
| • Prerehabilitation FEV1 (maximum of pre- and postbronchodilator values) ≤ 45% of predicted and, if age ≥ 70 yr prerehabilitation, FEV1 (maximum of pre- and postbronchodilator values) ≥ 15% of predicted |
| • Prerehabilitation room air, resting PaCO2 ≤ 60 mm Hg (≤55 mm Hg in Denver) |
| • Prerehabilitation room air, resting PaO2 ≥ 45 mm Hg (≥30 mm Hg in Denver) |
| • Prerehabilitation plasma cotinine ≤ 13.7 ng/ml (if not using nicotine products)or prerehabilitation arterial carboxyhemoglobin ≤ 2.5% (if using nicotine products) |
| • Body mass index ≤ 31.1 (males) or ≤ 32.3 (females) as of randomization |
| • Nonsmoker (tobacco products) for 4 mo before initial interview |
| • Approval for surgery by cardiologist if any of the following: unstable angina, left ventricular ejection fraction cannot be estimated from the echocardiogram, left ventricular ejection fraction < 45%, dobutamine-radionuclide cardiac scan indicates coronary artery disease or ventricular dysfunction, >5 premature ventricular beats/min (rest), cardiac rhythm other than sinus or premature atrial contractions noted during resting EKG, S3 gallop on physical examination |
| • Completion of all prerehabilitation assessments |
| • Judgment by study physician that patient is likely to be approved for surgery on completion of the rehabilitation program |
| • Completion of NETT rehabilitation program |
| • Completion of all postrehabilitation and all randomization assessments |
| Exclusion Criteria |
| • CT scan evidence of diffuse emphysema judged unsuitable for LVRS |
| • Previous LVRS (laser or excision) |
| • Pleural or interstitial disease that precludes surgery |
| • Giant bulla (≥one-third of the volume of the lung) |
| • Clinically significant bronchiectasis |
| • Pulmonary nodule requiring surgery |
| • Previous sternotomy or lobectomy |
| • Myocardial infarction within 6 mo of interview and ejection fraction < 45% |
| • CHF within 6 mo of interview and ejection fraction < 45% |
| • Uncontrolled hypertension (systolic > 200 mm Hg or diastolic > 110 mm Hg) |
| • Pulmonary hypertension: mean Ppa on right heart catheterization ≥ 35 mm Hg (≥38 mm Hg in Denver) or peak systolic Ppa on right heart catheterization ≥ 45 mm Hg (≥50 mm Hg in Denver); right heart catheterization is required to rule out pulmonary hypertension if peak systolic Ppa on echocardiogram > 45 mm Hg |
| • Unplanned, unexplained weight loss > 10% usual weight in 90 d before interview or unplanned, explained weight loss > 10% usual weight in 90 d before interview |
| • History of recurrent infections with daily sputum production judged clinically significant |
| • Daily use of >20 mg of prednisone or its equivalent |
| • History of exercise-related syncope |
| • Resting bradycardia (<50 beats/min), frequent multifocal PVCs, or complex ventricular arrhythmia or sustained SVT |
| • Cardiac dysrhythmia that poses a risk to the patient during exercise testing or training |
| • Oxygen requirement during resting or oxygen titration exceeding 6 L/min to keep saturation ≥90% |
| • Evidence of systemic disease or neoplasia that is expected to compromise survival |
| • Any disease or condition that may interfere with completion of tests, therapy, or follow-up |
| • 6MWD ≤ 140 m postrehabilitation |
| • Inability to complete successfully any of the screening or baseline data collection procedures |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CHF = congestive heart failure; CT = computed tomography; EKG = electrocardiogram; LVRS = lung volume reduction surgery; NETT = National Emphysema Treatment Trial; Ppa = pulmonary arterial pressure; PVCs = premature ventricular contractions; RV = residual volume; SVT = supraventricular tachycardia; TLC = total lung capacity.
Modified from Reference 17.
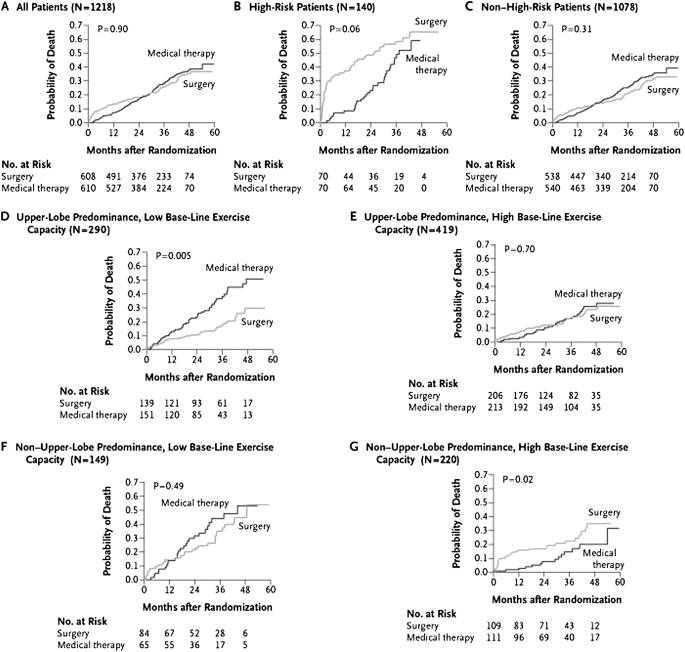
The 90-day mortality rate was 7.9% (95% confidence interval, 5.9–10.3%) in the LVRS group compared with 1.3% in the medical group (P < 0.001). During a mean postrandomization follow-up of 29.2 months, 160 patients assigned to medical treatment died compared with 157 receiving LVRS. Overall mortality was similar in both groups, although a higher initial mortality rate was identified in the LVRS group as expected in the immediate postoperative period (Figure 1A).
Figure 1.
Probability of death as a function of the number of months after randomization (Kaplan-Meier estimates). High-risk patients were defined as having an FEV1 not greater than 20% predicted and either homogeneous emphysema or DLCO (diffusing capacity for carbon monoxide) not exceeding 20% predicted. Low baseline exercise capacity was defined as a maximal workload at or below the sex-specific 40th percentile (25 W for women and 40 W for men); high exercise capacity was defined as a workload above this threshold. P values were derived by Fisher's exact test for e comparison between groups over a mean follow-up period of 29.2 months. (Reprinted by permission from Reference 17.)
A 10-W change in exercise performance and an eight-point change in St. George's Respiratory Questionnaire (SGRQ) were proposed by the NETT steering committee a priori to signify thresholds for meaningful important clinical changes in a surgical procedure that had associated morbidity and mortality. Exercise capacity improved by more than 10 W in 28, 22, and 15% after LVRS after 6, 12, and 24 months, respectively, compared with 4, 5, and 3% of the medically treated patients (P < 0.001 at each time point; Table 2). patients with LVRS were more likely to have had improvements in 6MWD, FEV1, the severity of dyspnea, and in general as well as disease-specific quality of life assessments than medically treated patients (Table 2).
TABLE 2.
IMPROVEMENT IN EXERCISE CAPACITY AND HEALTH-RELATED QUALITY OF LIFE AT 24 MONTHS
| Improvement in Exercise Capacity |
Improvement in Health-related Quality of Life |
|||||||
| Surgery Group | Medical-Therapy Group | Surgery Group | Medical-Therapy Group | |||||
| Patients | No./Total No. (%) | No./Total No. (%) | Odds Ratio | P Value | No./Total No. (%) | No./Total No. (%) | Odds Ratio | P Value |
| All patients | 54/371 (15) | 10/378 (3) | 6.27 | <0.001 | 121/371 (33) | 34/378 (9) | 4.90 | <0.001 |
| High-risk* | 4/58 (7) | 1/48 (2) | 3.48 | 0.37 | 6/58 (10) | 0/48 | — | 0.03 |
| Other | 50/313 (16) | 9/330 (3) | 6.78 | <0.001 | 115/313 (37) | 34/330 (10) | 5.06 | <0.000 |
| Subgroups† | ||||||||
| Predominantly upper lobe emphysema | ||||||||
| Low exercise capacity | 25/84 (30) | 0/92 | — | <0.001 | 40/84 (48) | 9/92 (10) | 8.38 | <0.001 |
| High exercise capacity | 17/115 (15) | 4/138 (3) | 5.81 | 0.001 | 47/115 (41) | 15/138 (11) | 5.67 | <0.001 |
| Predominantly non−upper lobe emphysema | ||||||||
| Low exercise capacity | 6/49 (12) | 3/41 (7) | 1.77 | 0.50 | 18/49 (37) | 3/41 (7) | 7.35 | 0.001 |
| High exercise capacity | 2/65 (3) | 2/59 (3) | 0.90 | 1.00 | 10/65 (15) | 7/59 (12) | 1.35 | 0.61 |
Reprinted by permission from Reference 17.
Improvement in exercise capacity in patients, monitored for 24 months after randomization, was defined as an increase in the maximal workload of more than 10 W from the patient's postrehabilitation baseline value. Improvement in the health-related quality of life in patients, monitored for 24 months after randomization, was defined as a decrease in the score on the St. George's Respiratory Questionnaire of more than 8 points (on a 100-point scale) from the patient's postrehabilitation baseline score. For both analyses, patients who died or who missed the 24-month assessment were considered not to have improvement. Odds ratios are for improvement in the surgery group as compared with the medical therapy group. P values were calculated by Fisher's exact test. A low baseline exercise capacity was defined as a postrehabilitation on baseline maximal workload at or below the sex-specific 40th percentile (25 W for women and 40 W for men); a high exercise capacity was defined as a workload above this threshold.
High-risk patients were defined as those with an FEV1 that was 20% or less of the predicted value and either homogeneous emphysema on computed tomography or a carbon monoxide diffusing capacity that was 20% or less than the predicted value.
High-risk patients were excluded from the subgroup analyses. For improvement in exercise capacity, P for interaction = 0.005; for improvement in health-related quality of life, P for interaction = 0.03. These P values were derived from binary logistic-regression models with terms for treatment, subgroup, and the interaction between the two, with the use of an exact-score test with 3 degrees of freedom. Other factors that were considered as potential variables for the definition of subgroups included the baseline FEV1, carbon monoxide diffusing capacity, partial pressure of arterial carbon dioxide, residual volume, ratio of residual volume to total lung capacity, ratio of expired ventilation in 1 minute to carbon dioxide excretion in 1 minute, distribution of emphysema (heterogeneous vs. homogeneous), perfusion ratio, Health-related Quality of Life score, Quality of Well-Being score, age, race or ethnic group, and sex.
Identifying aN LVRS Subgroup at High Risk of Death
During the planning of NETT, a 30-day surgical mortality greater than 8% in either treatment group was prespecified as a stopping end point. A subgroup defined by FEV1 less than or equal to 20% predicted and either a diffusing capacity for carbon monoxide (DLCO) less than or equal to 20% predicted or homogeneous emphysema met the prespecified stopping criteria in May 2001, because of excessive mortality after LVRS (19). Thirty-day mortality in patients who received LVRS identified by the previously described criteria was 16% (P < 0.001), and those who survived had little chance of clinically meaningful improvements in lung function, exercise tolerance, or quality of life. Because of the high mortality and limited improvements, patients with high-risk characteristics are not candidates for LVRS.
Outcomes in Non–High-Risk Patients
In the remaining 1,078 NETT patients who were not high risk, the 30-day mortality was 2.2% with LVRS and 0.2% with medical treatment (P < 0.001). The 90-day mortality rate was 5.2% with LVRS and 1.5% with medical treatment (P = 0.001; Table 3). Improvements in 6MWD, maximal exercise capacity, FEV1 % predicted, and quality of life (disease specific and general) were more likely to occur after LVRS compared with medical treatment (P < 0.001 for each comparison).
TABLE 3.
MORTALITY AMONG ALL PATIENTS AND IN SUBGROUPS
| 90-Day Mortality |
Total Mortality |
||||||||
| Surgery Group | Medical-Therapy Group | Surgery Group |
Medical-Therapy Group |
||||||
| Patients | No. of Deaths/Total No. (% [95% CI]) | No. of Deaths/Total No. (% [95% CI]) | P Value | No. of Deaths/Total No. | No. of Deaths/Person-Year | No. of Deaths/Total No. | No. of Deaths/Person-Year | Risk Ratio | P Value |
| All patients | 48/608 (7.9 [5.9−10.3]) | 8/610 (1.3 [0.6−2.6]) | <0.001 | 157/608 | 0.11 | 160/610 | 0.11 | 1.01 | 0.90 |
| High-risk* | 20/70 (28.6 [18.4−40.6]) | 0/70 (0 [0−5.1]) | <0.001 | 42/70 | 0.33 | 30/70 | 0.18 | 1.82 | 0.06 |
| Other | 28/538 (5.2 [3.5−7.4]) | 8/540 (1.5 [0.6−2.9]) | 0.001 | 115/538 | 0.09 | 130/540 | 0.10 | 0.89 | 0.31 |
| Subgroups† | |||||||||
| Patients with predominantly upper lobe emphysema | |||||||||
| Low exercise capacity | 4/139 (2.9 [0.8−7.2]) | 5/151 (3.3 [1.1−7.6]) | 1.00 | 26/139 | 0.07 | 51/151 | 0.15 | 0.47 | 0.005 |
| High exercise capacity | 6/206 (2.9 [1.1−6.2]) | 2/213 (0.9 [0.1−3.4]) | 0.17 | 34/206 | 0.07 | 39/213 | 0.07 | 0.98 | 0.70 |
| Patients with predominantly non–upper lobe emphysema | |||||||||
| Low exercise capacity | 7/84 (8.3 [3.4−16.4]) | 0/65 (0 [0−5.5]) | 0.02 | 28/84 | 0.15 | 26/65 | 0.18 | 0.81 | 0.49 |
| High exercise capacity | 11/109 (10.1 [5.1−17.3]) | 1/111 (0.9 [0.02−4.9]) | 0.003 | 27/109 | 0.10 | 14/111 | 0.05 | 2.06 | 0.02 |
Definition of abbreviation: CI = confidence interval.
Reprinted by permission from Reference 17.
Mortality was measured from the date of randomization in both treatment groups. Total mortality rates are based on a mean follow-up of 29.2 months. P values were calculated by Fisher's exact test. Risk ratios are for the risk in the surgery group as compared with the risk in the medical-therapy group. A low baseline exercise capacity was defined as a postrehabilitation baseline maximal workload at or below the sex-specific 40th percentile (25 W for women and 40 W for men); a high-exercise capacity was defined as a workload above this threshold.
High-risk patients were defined as those with an FEV1 that was 20% or less of the predicted value and either homogeneous emphysema on computed tomography or a carbon monoxide diffusing capacity that was 20% or less of the predicted value.
High-risk patients were excluded from the subgroup analyses. For total mortality, P for interaction = 0.004; this P value was derived from binary logistic-regression models with terms of treatment, subgroup, and the interaction between the two, with the use of an exact-score test with 3 degrees of freedom. Other factors that were considered as potential variables for the definition of subgroups included the baseline FEV1, carbon monoxide diffusing capacity, partial pressure of arterial carbon dioxide, residual volume to total lung capacity, ratio of expired ventilation in 1 minute to carbon dioxide excretion in 1 minute, distribution of emphysema (heterogeneous vs. homogeneous), perfusion ratio, Health-related Quality of Life score, Quality of Well-Being score, age, race or ethnic group, and sex.
Predicting LVRS Outcomes in Non–High-Risk NETT Patients
The craniocaudal distribution of emphysema on chest computed tomography (CT) (presence or absence of upper lobe–predominant emphysema; P for interaction = 0.02) and postrehabilitation exercise test maximal wattage (low or high exercise; P for interaction = 0.01) were the only baseline factors that were found to discriminate mortality differences between the two treatment groups.
On the basis of combinations of high and low exercise maximal wattage with upper lobe– or non–upper lobe–predominant emphysema identified by chest CT, patients were divided into four subgroups. In 290 patients with upper lobe–predominant emphysema and low exercise capacity, LVRS had a lower risk of death than medical therapy (P = 0.005; Figure 1D and Table 3). The LVRS group was more likely to achieve a greater than 10-W improvement in maximal exercise wattage at 24 months (30 vs. 0%; P < 0.001; Table 2) and a greater than eight-point improvement in SGRQ score at 24 months (48 vs. 10%, P < 0.001; Table 2).
In 419 patients with upper lobe–predominant emphysema and high exercise wattage, LVRS had no effect on survival (P = 0.70). After LVRS, however, patients were more likely to have a greater than 10-W improvement in maximal exercise wattage at 24 months (15 vs. 3%, P = 0.001; Table 2) and a more than eight-point improvement in SGRQ score (41 vs. 11%, P < 0.001; Table 2) compared with medical therapy.
In 149 patients with non–upper lobe–predominant disease and low exercise capacity, LVRS had no effect on the risk of death (P = 0.49) or maximal exercise capacity at 24 months (12 vs. 7%; P = 0.50). However, patients with LVRS were more likely to improve their SGRQ score at 24 months (37 vs. 7%; P = 0.001; Table 2).
In 220 patients with non–upper lobe–predominant emphysema and high exercise at baseline, LVRS increased the risk for death (P = 0.02) and had no beneficial impact on maximal exercise capacity at 24 months (3% both groups; P = 1.0) or SGRQ score (15 vs. 12%, P = 0.61; Table 2).
At a mean 29.2 months, LVRS provided no survival benefit over medical treatment, even with excluding the high risk for death LVRS subgroup. However, LVRS produced significant and sustained improvements in exercise capacity and 6MWD, reduction in dyspnea, and improvements in disease-specific and general quality of life measurements. The NETT Steering Committee noted that the mean follow-up was only 2.4 years at the time of this analysis and proposed a longer follow-up assessment to establish the effect of LVRS on long-term survival and quality of life.
Long-term Follow-up in NETT: Effect of LVRS versus Medical Therapy on Survival and Maximal Exercise
NETT patients continued to have regularly scheduled follow-up tests, telephone interviews, and clinic visits, and completed quality of life questionnaires after the initial major outcomes report. Long-term survival was updated by clinical center reports and reviews of the Social Security Master Death file.
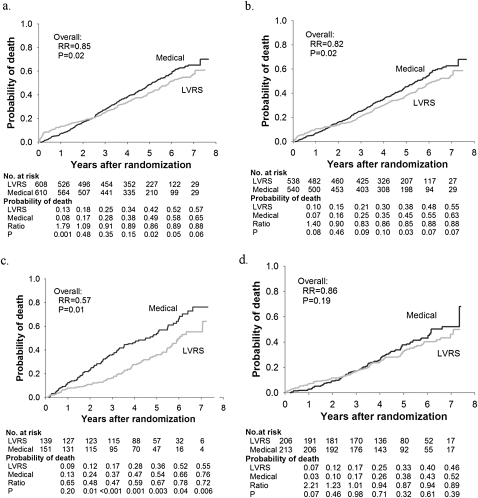
Figure 2A shows probability of death as a function of years after LVRS or medical treatment in all 1,218 NETT patients after the prolonged follow-up assessment (median follow-up, 4.3 yr) (18). The total mortality rate was 0.11 deaths per person-year with LVRS and 0.13 with medical treatment (P = 0.02). Survival improved after LVRS compared with medical treatment despite the expected higher postoperative immediate mortality after LVRS.
Figure 2.
Kaplan-Meier estimates of the cumulative probability of death as a function of years postrandomization to lung volume reduction surgery (LVRS) (gray line) or medical therapy (black line) for (a) all patients and (b–d) non–high-risk and upper lobe–predominant subgroups of patients. The P value is from the Fisher's exact test for difference in the proportions of patients who died during the 4.3 years (median) of follow-up. Shown below each graph are the numbers of patients at risk, the Kaplan-Meier probabilities, the ratio of the probabilities (LVRS:Medical), and P value for the difference in these probabilities. This is an intention-to-treat analysis. (a) All patients (n = 1,218). (b) Non–high-risk patients (n = 1078). (c) Upper lobe–predominant and low baseline exercise capacity (n = 290). (d) Upper lobe–predominant and high exercise capacity (n = 419). RR = relative risk. Reprinted by permission from Reference 18.
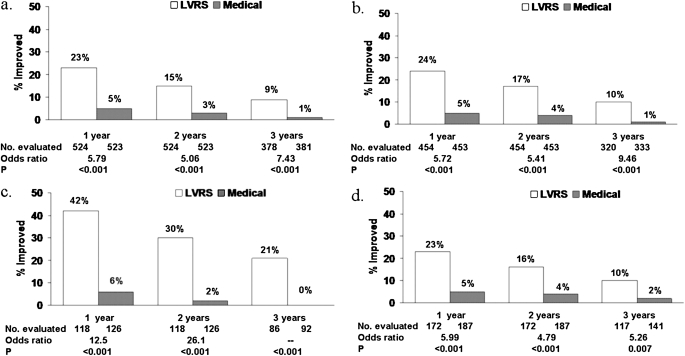
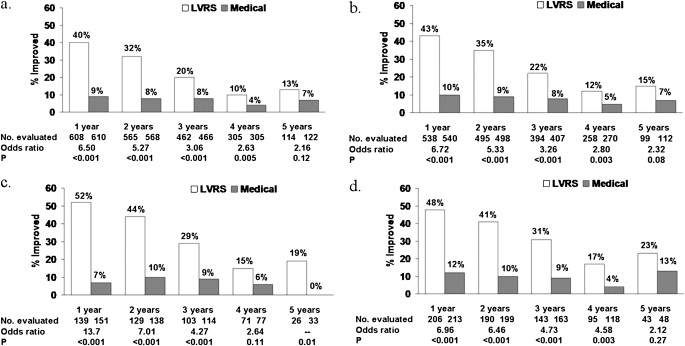
Exercise capacity improved by more than 10 W in 23, 15, and 9% after LVRS compared with 5, 3, and 1% after medical therapy at 1, 2, and 3 years of follow-up (P < 0.001 at each time point) (Figure 3). After LVRS, SGRQ score decreased by more than 8 units in 40, 32, 20, 10, and 13% compared with 9, 8, 8, 4, and 7% after medical treatment at 1–5 years of follow-up (P < 0.001, Year 1–3; P = 0.005, Year 4; P = 0.12, Year 5) (Figure 4).
Figure 3.
Improvement in exercise capacity (increase in maximal work > 10 W above the patient's postrehabilitation baseline) at 1, 2, and 3 years postrandomization to lung volume reduction surgery (LVRS) (open columns) or medical therapy (solid columns) for (a) all patients and (b–d) non–high-risk and upper lobe–predominant patient subgroups. Shown below each graph are the numbers of patients evaluated, the odds ratio for improvement (LVRS:Medical), and the Fisher's exact P value for difference in proportion improved. Patients who died or who did not complete the assessment were considered not improved. This is an intention-to-treat analysis. (a) All patients (n = 1,218). (b) Non–high-risk patients (n = 1,078). (c) Upper lobe–predominant and low baseline exercise capacity (n = 290). (d) Upper lobe–predominant and high exercise capacity (n = 419). Reprinted by permission from Reference 18.
Figure 4.
Improvement in health-related quality of life (decrease in St. George's Respiratory Questionnaire total score of >8 units below the patient's postrehabilitation baseline) at 1, 2, 3, 4, and 5 years after randomization to LVRS (open columns) or medical therapy (solid columns) for (a) all patients and (b–d) non–high-risk and upper lobe–predominant subgroups of patients. Shown below each graph are the numbers of patients evaluated, the odds ratio for improvement (LVRS:Medical), and the Fisher's exact P value for difference in proportion improved. Patients who died or who did not complete the assessment were considered not improved. This is an intention-to-treat analysis. (a) All patients (n = 1,218). (b) Non–high-risk patients (n = 1,078). (c) Upper lobe–predominant and low baseline exercise capacity (n = 290). (d) Upper lobe–predominant and high exercise capacity (n = 419). Reprinted by permission from Reference 18.
Effect of NETT Subgroup Classification on Long-term Survival and Functional Outcome After LVRS
The updated long-term analyses reaffirmed the differential risks and benefits of LVRS by subclassifying patients on the basis of chest CT emphysema pattern and maximal wattage attained during lower extremity ergometry testing postpulmonary rehabilitation.
In 290 patients with upper lobe–predominant emphysema and low exercise capacity, LVRS afforded a substantial survival advantage (P = 0.01; Figure 2c), and improved exercise capacity and quality of life (Figures 3c and 4c, respectively) compared with medical therapy.
Perfusion Scintigraphy and Patient Selection for LVRS
A post hoc analysis was performed to determine whether lung perfusion could predict response to LVRS in 1,045 NETT patients who had complete scintigraphy results at baseline (20). Low upper lobe perfusion was defined as less than 20% of total perfusion directed to the upper third of the lungs as measured on perfusion scintigraphy. In 284 patients with upper lobe–predominant emphysema and low exercise capacity, the 202 patients with low upper zone perfusion had lower mortality with LVRS versus medical therapy (P = 0.008) as opposed to the 82 patients with high perfusion in whom mortality was unchanged. In 404 patients with upper lobe–predominant emphysema and high exercise, 278 patients with low upper lobe zone perfusion had lower mortality with LVRS (P = 0.02) compared with 126 patients with high perfusion (P = 1.00). In the remaining patients with non–upper lobe–predominant emphysema, measurement of upper zone perfusion did not provide any prognostic information. These data indicate that low upper zone perfusion measured by perfusion scintigraphy indicates a survival advantage with LVRS in patients with upper lobe–predominant emphysema.
Lung function and prediction of LVRS outcome
A subset of NETT patients who underwent LVRS had measurements of static lung recoil at total lung capacity (SRTLC) and inspiratory resistance (RI) (5). Relationships between high-resolution chest computed tomography (HRCT) measures of emphysema and airway disease, SRTLC, RI, the ratio of residual volume to total lung capacity (RV/TLC), and the 6-month postoperative changes in FEV1 and maximal exercise workload were assessed to determine their ability to predict outcome. SRTLC, RI, and CT measures of airway disease did not predict improvements in either FEV1 or maximal workload. RV/TLC and CT measures of emphysema (upper-to-lower lobe ratios of percent emphysema) were only weakly predictive of postoperative changes in FEV1 and maximal exercise capacity.
Operative Mortality and Cardiopulmonary Morbidity after LVRS
A secondary goal of NETT was to identify predictors of LVRS mortality and morbidity (21). Data from 511 non–high-risk patients who underwent LVRS were analyzed. Of these patients, 5.5% died within 90 days of LVRS. The existence of non–upper lobe–predominant emphysema was the sole predictor of operative mortality (P = 0.009). During the intraoperative period, 91% of patients had no complications, 2.2% had transient hypoxemia, and 1.2% developed an arrhythmia.
Of patients with LVRS, 58.7% had at least one postoperative complication within 30 days postsurgery. Cardiac arrhythmia was the most common complication and occurred in 23.5%. Pneumonia developed in 18.2%, 21.8% required at least one reintubation, 11.7% were readmitted to the intensive care unit, 8.2% underwent tracheotomy, and 5.1% of patients failed to wean successfully from mechanical ventilation within 3 days of LVRS.
Major pulmonary and cardiovascular morbidity (assessed in the 30 d after LVRS) occurred in 29.8 and 20% of patients, respectively. Pulmonary morbidity was greater in older patients (P = 0.02), and those with lower FEV1 (P = 0.05) or DLCO (0.97; P = 0.01). Cardiovascular morbidity was higher with age (1.07; P = 0.004), use of oral steroids (P = 0.04), or in the presence of non–upper lobe–predominant emphysema (P < 0.001).
LVRS and Air Leaks
After LVRS, 90% of patients with LVRS had air leaks at some point within 30 days of thoracotomy (22). Median air leak duration was 7 days, but 12% had air leaks for 30 days or more postoperatively. Air leak duration was longer in white patients (P < 0.0001), patients with lower FEV1 (P = 0.0003) or diffusion capacity (P = 0.06), those using inhaled steroids (P = 0.004), in the presence of upper lobe–predominant emphysema (P = 0.04), and if pleural adhesions were present (P = 0.007).
Surgical technique (median sternotomy [MS] vs. video-assisted thoracoscopic surgery [VATS]) and the use of buttressing materials and stapler brand had no effect on air leak occurrence or their duration (P ≥ 0.1). Postoperative complications were greater in patients with air leaks (57 vs. 30%; P = 0.0004) and their postoperative stay was prolonged (11.8 ± 6.5 d vs. 7.6 ± 4.4 d; P = 0.0005).
Surgical Approach and its Effects on LVRS Outcomes in NETT
NETT also investigated whether the LVRS surgical approach (e.g., MS vs. VATS) affected patient mortality, morbidity, and functional outcomes (11).
Ninety-day mortality was similar between the two techniques (5.9% for MS and 4.6% for VATS; P = 0.67). All-cause mortality was 0.08 deaths per person-year for MS and 0.10 deaths per person-year for VATS (VATS:MS relative risk, 1.18; P = 0.42).
MS and VATS were similar in terms of mean intraoperative blood loss (P = 0.55) or transfusion needs (P = 0.99). Mean operating time was 21.7 minutes shorter for MS than VATS (P < 0.001), hypoxemia was rarer with MS than VATS (0.8 vs. 5.3%; P = 0.004), and intraoperative complications were fewer with MS compared with VATS (93 vs. 86.2% no intraoperative complications; P = 0.02).
Median hospital length of stay after LVRS was longer for MS than VATS patients (10 vs. 9 d; P = 0.01). At 30 days after LVRS, 70.5% of MS patients were living independently compared with 80.9% of VATS patients (P = 0.02). Functional outcomes were similar between the MS and VATS groups at 12 and 24 months of follow-up. LVRS-related costs and associated hospitalization were less for VATS compared with MS (P = 0.03), as were total costs (medical and nonmedical) during the subsequent 6-month period after LVRS (P = 0.005).
Effects of α1-Antitrypsin Deficiency and LVRS in NETT
Of 1,218 patients randomized into NETT, 16 (1.3%) had severe α1-antitrypsin (AAT) deficiency (serum level < 80 mg/dl) (23) and 10 patients underwent LVRS. Two-year mortality was higher with LVRS compared with medical therapy (20 vs. 0%) in the AAT-deficient NETT patients. AAT-deficient subjects had lower and shorter durations of increases in FEV1 and exercise capacity compared with patients with LVRS without AAT deficiency.
Effects of LVRS on breathing pattern during exercise
The effects of LVRS on breathing pattern, gas exchange and dyspnea during maximal exercise was assessed in the NETT exercise substudy (24). In 238 patients, minute ventilation (e), tidal volume (Vt), carbon dioxide production (co2), dyspnea rating, and workload were recorded during exercise testing at baseline, postrehabilitation, and then 6, 12, and 24 months postrandomization. At 6 months, patients with LVRS had higher maximal e (32.8 vs. 29.6 L/min; P = 0.001), co2 (0.923 vs. 0.820 L/min; P = 0.0003), VT (1.18 vs. 1.07 L; P = 0.001), heart rate (124 vs. 121 beats/min; P = 0.02), and workload (49.3 vs. 45.1 W; P = 0.04), but less breathlessness (4.4 vs. 5.2 on the Borg dyspnea scale; P = 0.0001) and exercise ventilatory limitation (49.5 vs. 71.9%; P = 0.001) than medical control subjects. After LVRS, patients breathed slower and deeper during exercise at 6 months (P = 0.01) and 12 months (P = 0.006) with reduced dead space at 6 months (P = 0.007) and 24 months (P = 0.006). Patients with upper lobe–predominant emphysema showed a downward shift in the Pco2 versus co2 relationship during restful breathing and throughout exercise (P = 0.001). These data show that after LVRS, patients breathe slower and deeper during exercise and have improved CO2 elimination and less dyspnea and dead space ventilation.
Effects of LVRS on pulmonary hemodynamics
The cardiac substudy of NETT assessed the effects of LVRS on resting pulmonary hemodynamics. Fifty-five of 110 patients underwent baseline right heart catheterization postrehabilitation and then 6 months after randomization to medical therapy or LVRS (25). Baseline demographics and lung function were similar between groups and moderate pulmonary hypertension was present at baseline (mean Ppa, 24.8 ± 4.99 mm Hg). Changes from baseline pressures to 6 months after medical or LVRS treatment were similar except for a decrease in pulmonary capillary wedge pressure at end-expiration with LVRS (–1.8 vs. 3.5 mm Hg; P = 0.04) These data confirm that LVRS does not raise pulmonary artery pressures and reduces intracardiac pressures by a decrease in intrathoracic pressures.
Effects of LVRS on oxygenation
The effects of LVRS on oxygenation was evaluated in 1,078 NETT subjects, using arterial blood gases, the need for supplemental oxygen during treadmill testing, and the self-reported use of oxygen during rest, exertion, and sleep. Fewer patients with LVRS required oxygen at 6 months (33 vs. 49%; P < 0.001), 12 months (50 vs. 36%; P < 0.001), and 24 months (52 vs. 42%; P = 0.02) compared with medical control subjects. In addition, self-reported oxygen use during rest, exercise, and sleep was lower with LVRS compared with medical therapy at 6, 12, and 24 months. Multivariate analysis of preoperative characteristics showed that baseline oxygenation predicted best the need for postoperative supplemental oxygen (26).
Cost-effectiveness of LVRS
NETT conducted a parallel prospective cost-effectiveness study of LVRS that analyzed the estimated value of medical goods and services, transportation to and from health care facilities, time spent by the patient in receiving treatment, and time spent by family and friends in caring for the patients (27).
Cost-effectiveness was calculated as the ratio of the difference in costs between the LVRS and medical treated groups divided by the difference in the quality-adjusted life years gained between the two groups. The cost-effectiveness ratio was then computed for the 3-year trial period and projections were computed for 5 and 10 years postrandomization.
The mean total cost per patient was higher in the LVRS than the medical group in the first 12 months after surgery ($71,515 vs. $23,371; P < 0.001), mainly because of the operative and postoperative hospitalization costs within the first 6 months ($62,753 vs. $12,932; P < 0.001). However, the mean total cost of care was lower in LVRS compared with medical patients in the second year posttreatment ($13,222 vs. $21,319; P < 0.001). In the third year, costs in the LVRS group remained lower but the difference was no longer statistically significant ($14,215 vs. $17,870; P = 0.08). Mean total medical costs per patient during months 7–36 was almost $10,000 lower in the LVRS compared with the medical group ($36,199 vs. $49,628; P < 0.001), due mainly to fewer hospitalization days with LVRS.
The mean total costs per person ($98,952 vs. $62,560; P < 0.001) and per-person medical costs ($80,818 vs. $43,689; P < 0.001) at 3 years were higher with LVRS compared with medical care. The nonmedical costs were not different between the two groups.
At 3 years of follow-up, the mean number of quality-adjusted life years gained was greater in the LVRS compared with the medical group (1.46 vs. 1.27; P < 0.001) and the mean number of quality-adjusted life years gained was also significantly greater at 1 and 2 years of follow-up.
The cost-effectiveness ratio for LVRS versus medical treatment at 3 years was $190,000 per quality-adjusted life year gained and was projected to be $53,000 per quality-adjusted life year gained at 10 years. The cost-effectiveness of LVRS compared with medical treatment in the subgroup with upper lobe–predominant disease by chest CT and low exercise was $98,000 per quality-adjusted life year gained at 3 years and projected to be $21,000 at 10 years.
The cost-effectiveness analysis was updated using data obtained on patients during the extension of NETT follow-up performed in 2003 (28). Updated data showed that the cost-effectiveness ratio fell for the LVRS group compared with the medical group from $190,000 to $140,000 per patient. These data show that medical care for the patient with severe emphysema is costly, and that the cost-effectiveness of LVRS depends on patient characteristics determined by chest CT and performance on maximal exercise testing.
LVRS: An Infrequently Performed Therapy
Despite extensive study demonstrating the many benefits that may result from LVRS, relatively few patients receive the procedure. In 2004, only 254 Medicare beneficiaries underwent LVRS at 42 approved centers, and in 2005 and 2006 only 120 and 105 Medicare beneficiaries underwent LVRS, respectively.
Specific reasons for the underperformance of LVRS are not known, but NETT investigators have proposed the following explanations: (1) restricting LVRS performance to NETT, lung transplant, or JCAHO (Joint Commission on Accreditation of Healthcare Organizations)-approved centers limits patient access; (2) LVRS assessment is perceived as overly complicated; (3) outpatient pulmonary rehabilitation programs have limited availability; (4) many physicians still remain unaware of the benefits of LVRS and what constitutes an appropriate patient candidate; (5) the publication (19) that described the patient group at high risk for death with LVRS (e.g., FEV1 ≤ 20% predicted and either nonheterogeneous emphysema on chest CT or diffusion capacity ≤ 20% predicted) has been erroneously misinterpreted as reporting that all patients are at high risk for death with LVRS and stigmatized LVRS as unduly risky for all patients with emphysema; and (6) LVRS is perceived by many in the medical community as too costly (29). In addition, the beneficial outcomes that occur after LVRS are not uniform and even in the group of high benefit (upper lobe–predominant disease and low exercise performance), the variability of changes in physiological and functional parameters after LVRS are great and may be partially explained by the presence of unrecognized small airways disease that escapes detection by current preoperative testing (30, 31).
As a result, nonsurgical approaches to lung volume reduction (LVR) have been pursued to achieve the benefits of LVRS, but with less patient morbidity and mortality (2, 32–39).
Lung Volume Reduction: Investigational Approaches
At present, there is no U.S. Food and Drug Administration (FDA)–approved method of LVR; all approaches remain investigative at the present time. Investigative LVR techniques can be parsed into the following categories: (1) one-way endobronchial valves implanted into the airway, (2) self-activating coils placed into the airway, (3) targeted destruction and remodeling of emphysematous tissue, (4) bypass tract airway stenting, and (5) transpleural ventilation. The first four techniques are performed via a bronchoscope, and the last is performed via a minithoracotomy (Table 4).
TABLE 4.
COMPARISON OF INVESTIGATIONAL LUNG VOLUME REDUCTION TECHNIQUES
| Technique | Mechanism | PRCT Data | Procedure Type | Procedure Reversible? | Affected by Collateral Ventilation |
| One-way endobronchial valves (EBVs) implanted in airway | Promotes regional atelectasis by blocking inspiration but allowing expiration | After EBV, mean FEV1 increased 6.8% (60 ml) and mean 6MWD increased 2.55 (9.3 m) compared with control | Bronchoscopy | Yes | Yes |
| Self-activating coils placed in airway | Atelectasis induced by assuming preformed coiled shape: bends airway and collapses surrounding lung tissue | No | Bronchoscopy | Yes | No |
| Targeted destruction of emphysematous tissue | BioLVR: Regional instillation of biological agents that collapse and remodel emphysematous regionsBTVA heats and destroys targeted emphysematous tissue | No | Bronchoscopy | No | No |
| Bypass tract airway stenting | Endobronchial stents placed into emphysematous tissue to enhance deflation | Pending | Bronchoscopy | Yes | No |
| Transpleural ventilation | Modified tubes placed externally into emphysematous tissue to enhance external deflation | No | Minithoracotomy | Yes | No |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BTVA = bronchoscopic thermal vapor ablation; BioLVR = Biological lung volume reduction; PRCT = prospective randomized controlled trial.
Endobronchial One-Way Valves
One–way endobronchial valves (placed at a segmental or lobar level) regionally block inspiration but permit expiration, and conceptually work by promoting lobar atelectasis. The two available and most studied endobronchial one-way valve systems have similar characteristics. The valves are available in multiple diameters designed to occlude airways ranging from approximately 4 to 8.5 mm in diameter. Both valves are composed of materials that have inherent plasticity to allow some degree of scalability with the airway lumen throughout the phases of respiration. Expiratory air and secretions escape around the outer edges of the flexible Spiration valve (Spiration Incorporated, Redmond, WA) and through the one-way valve lumen of the Zephyr valve (Emphasys Medical, Redwood City, CA). The Spiration intrabronchial valve system has an “umbrella design” in which an occlusive cover is stretched over a titanium wire frame. The Emphasys endobronchial valve (EBV) is a cylindrical device with a “duck bill” one-way valve seated in a nitinol wire cage. Both valves can be easily removed via a bronchoscope if the clinical need arises.
Phase III Endobronchial One-way Valve Trials
VENT (Endobronchial Valve for Emphysema Palliation Trial) was the first prospective randomized, multicenter, controlled trial to evaluate bronchoscopic LVR (Zephyr endobronchial valve; Emphasys Medical) compared with medical care in severe heterogeneous emphysema (2). VENT randomized 321 patients (age, 40–75 yr) to endobronchial valve (EBV) placement (n = 220) or to medical management (n = 101) defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2001 guidelines. Primary efficacy end points were percent changes in FEV1 and 6-MWD at 6 months compared with baseline. Secondary end points included mean changes in quality of life (SGRQ), incremental cycle exercise capacity, dyspnea measured by a modified Medical Research Council (mMRC) score, the extent of targeted lobe volume reduction measured by quantitative HRCT, and daily oxygen use. The difference in the major complication composite (MCC) rate at 180 days postrandomization between groups was the primary safety end point. The MCC included death, massive hemoptysis, empyema, pneumonia distal to valves, and ventilator dependency equal to or greater than 24 hours. HRCT was performed at baseline and 180 days postrandomization and analyzed for lobar emphysema severity, fissure integrity, and targeting lobes for EBV placement. The lobe with the highest percentage of emphysema and greatest degree of heterogeneity (difference in percent emphysema between ipsilateral lung lobes) was preferentially selected for EBV placement.
At 6 months postrandomization, FEV1 increased 4.3% (mean change, 34.5 ml) in the EBV group but decreased 2.5% (mean change, –25.4 ml) in the control group; therefore FEV1 was a mean 6.8% greater (60 ml) after EBV compared with control (P = 0.005). The 6-MWD increased 2.5% (median change 9.3 m) in the EBV group and decreased 3.2% (median change –10.7 m) in control subjects at 6 months postrandomization; thus there was a mean increase in 6-MWD distance of 5.8% (median change, 19.1 m) after EBV compared with control (P = 0.04). Secondary outcomes also showed modest improvements after EBV placement at 6 months. Comparing changes for EBV to control, in mean differences at 6 months relative to baseline, SGRQ was –3.4 (P = 0.04), mMRC was –0.3 (P = 0.04), cycle ergometry peak workload was 3.8 W greater (P = 0.05), and supplemental oxygen use was less (P = 0.005), data that all favored EBV over control. Control subjects had an MCC rate of 1.2% at 6 months compared with a rate of 6.1% in EBV patients. Included in the MCC was a 2.8% mortality rate associated with EBV compared with no deaths in the control subjects (P = 0.19). Pneumonia developed distal to the EBV valve in 4.2% of treated patients; all pneumonias resolved with antibiotic therapy alone. Hemoptysis occurred in 5.6% of EBV patients over the 6 months postplacement but in no control subjects (P = 0.02). Acute chronic obstructive pulmonary disease (COPD) exacerbations requiring hospitalization occurred more commonly after EBV (7.9%) than control (1.2%; P < 0.03). In the 12 months after EBV placement, 31 patients had EBVs removed for the following reasons: retrieval of migrated valve (n = 8), patient's request (n = 7), pneumonia distal to the valve (n = 3), and recurring COPD exacerbations or episodes of hemoptysis.
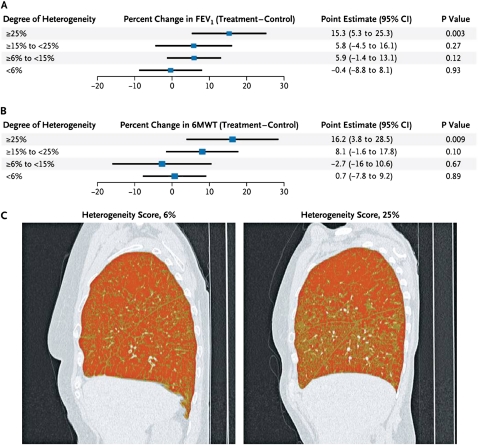
Heterogeneity (i.e., the difference in the percentage of emphysema between lobes in the treated lung) and fissure integrity were predictive of improvements in FEV1 and 6-MWD distance (Figure 5). The percentage of emphysema heterogeneity and presence of intact fissures were identified a priori as possible discriminators for patient responses to EBV, but the magnitudes of these variables that indicated clinical improvement were identified by retrospective analyses. At a median cutoff of 15%, the high-heterogeneity subgroup had relatively greater improvements in FEV1 and 6-MWD at 6 months after EBV compared with control. EBV subjects with intact fissures had incremental improvements in FEV1 of 16.2% (P < 0.001) at 6 months and 17.9% at 12 months (P < 0.001) in comparison with those with incomplete fissures, who had insignificant changes of 2 and 2.8% at 6 and 12 months, respectively.
Figure 5.
Effect of heterogeneity on endobronchial valve (EBV) response at 6 months. Shown is the effect of heterogeneity on change in (A) FEV1 and (B) 6-minute walk distance (6MWD) at 6 months after EBV implantation. Percent heterogeneity was the difference in quantitative emphysema score (the proportion of pixels less than −910 Hounsfield units) between EBV-treated and ipsilateral nontreated lobes. In (C), sagittal high-resolution chest computed tomography views with density mask views show low (6%, left) and high heterogeneity (25%, right). Darker areas represent pixels less than −910 Hounsfield units, consistent with emphysema. Reprinted by permission from Reference 2.
Emphasys Medical Inc. was denied FDA approval in December 2008. The FDA review commented that the mean changes in FEV1 and 6-MWD were not clinically meaningful and that additional data regarding long-term device safety were needed. Emphasys dissolved as a company and the Zephyr valve was purchased by Pulmonx Inc. (Redwood City, CA). Plans for future investigation using the Zephyr valve are currently under development. A European study using patient characteristics identified by VENT to favor EBV placement (all eight subjects had high heterogeneity and intact fissures) has preliminarily reported improvements in lung function (compared with baseline, EBV treatment resulted in the following changes: VC, +16.6%; FEV1, +12.4%; and RV, –52%; for all values, P < 0.05) (40).
Spiration, Inc. has just completed enrolling patients in a prospective, randomized, controlled, multicenter clinical trial to evaluate the safety and effectiveness of the IBV valve system for the treatment of severe emphysema. Results are pending at this time.
Biological Remodeling of Emphysematous Tissue to Create Lung Volume Reduction
Biological lung volume reduction uses a biodegradable sclerosant gel (BioLVR; Aeris Therapeutics Inc., Woburn, MA) to polymerize the small airways and alveolar airspaces (36, 37). Focal reductions in lung volume occur as collapse, remodeling, and scarring of targeted lung regions occur over several weeks. In an open-label, multicenter phase II dose-ranging study, BioLVR hydrogel was administered to eight subsegmental sites (four pulmonary subsegments in each upper lobe) via the following dosing schemes: (1) low dose (n = 28) with 10 ml/site (LD); and (2) high-dose treatments (n = 22) with 20 ml/site (HD) (41). Safety was determined by the incidence of serious complications that developed postinstillation. Efficacy was assessed by changes at 12 and 24 weeks posttreatment compared with baseline in lung function, dyspnea score, 6-MWD, and quality of life. A reduction in RV/TLC at 12 weeks (primary efficacy outcome) was reported with both LD (–6.4 ± 9.3%; P = 0.002) and HD (–5.5 ± 9.4%; P = 0.028) treatments. Lung function improvements were greater with HD (6 mo: FEV1, +15.6% [P = 0.002]; FVC, +9.1% [P = 0.034]) than LD (6 mo: FEV1, +6.7% [P = 0.021]; FVC, +5.1% [P = 0.139]) treatments. Both LD- and HD-treated groups demonstrated improved symptom and health-related quality of life (HRQOL) scores. The overall improvements were larger, and responses more durable with 20 ml/site than 10 ml/site dosing. In a separate study, BioLVR was also administered to 25 patients with homogeneous emphysema in an open-label, phase II study; 8 subjects received LD treatment with 10 ml/site at 8 subsegments and 17 received HD treatment with 20 ml/site at 8 subsegments (42). Compared with baseline, changes at 6 months in FEV1, FVC, RV/TLC, dyspnea scores, and SGRQ were better with HD than LD treatment, but attained statistical significance only after HD doses in FEV1 (±13.8 ± 20.2; P = 0.007), dyspnea (–0.8 ± 0.7 mMRC score; P = 0.001), and SGRQ total score (–12.2 ± 12.3; P = 0.0001). Aeris Therapeutics halted plans for a multicenter, phase III, prospective, controlled trial of BioLVR to redirect efforts toward development of a new polymeric sealant coined AeriSeal (43). AeriSeal is currently undergoing active investigation in Europe. Preliminary data presented in 15 patients with upper lobe–predominant emphysema treated at 2–4 subsegments showed a reduction in CT-measured lung volume in treated lobes and an increase in ipsilateral volume in nontreated lobes. Reductions in lobar volume correlated with a reduction in gas trapping (RV/TLC, r = 0.59, P = 0.02) and increases in FEV1 (r = –0.65, P = 0.009) and 6-MWD (r = 0.48, P = 0.07). Future prospective randomized controlled phase III trials are planned.
Airway Bypass Tract
Airway bypass transbronchial fenestration is a bronchoscopic technique using a needle-tipped catheter designed to create extraanatomical bronchial fenestrations that remain patent using drug-eluting stents (Broncus Technologies, Mountain View, CA). Broncus Technologies announced the results of a phase III double-blinded, randomized, sham-controlled trial of the airway bypass procedure using Exhale drug-eluting stents (EASE) (44). EASE was designed to demonstrate the safety and efficacy of the procedure, but it failed to meet its two coprimary end points of improving FVC and mMRC dyspnea score when compared with sham controls. Dyspnea, as measured by the mMRC, however, did show significant improvement after airway bypass. Post hoc analysis revealed that patients who met both coprimary end points showed a reduction in RV equal to or greater than 500 ml posttreatment at 1 month. The reduction in RV correlated with significant improvements in lung function and patient symptoms. At this date peer-reviewed publication of the results is pending.
Other LVR Techniques
Several new techniques have been described in uncontrolled small studies that show potential in achieving lung volume reduction. Although these techniques vary significantly in their approach, all share the common feature of addressing the issue of collateral ventilation. The essentials of the most promising techniques are briefly discussed below.
In a pilot study, lung volume reduction coils (PneumRx) were bronchoscopically placed into the most diseased regions in 11 patients with severe emphysema (8 homogeneous, 3 heterogeneous) to achieve lung tissue compression (45). Safety was the primary end point; efficacy outcomes were secondary end points. The 11 patients underwent 21 treatments with a total placement of 101 lung volume reduction coils. Thirty-three adverse events were reported: none were severe, 36% were mild ,and 64% were moderate. Adverse events possibly attributed to the procedure or device included dyspnea (10 events), cough (5 events), COPD exacerbations (3 events), and chest pain (1 event). Group mean values for FEV1, RV, TLC, SGRQ, and 6-MWD at 1 and 3 months improved after the first procedure. The greatest relative changes were observed in 6-MWD, SGRQ, and mMRC in the patients with heterogeneous emphysema. Further testing of the device is ongoing.
In 11 patients with severe heterogeneous emphysema, bronchoscopic administration of thermal energy (bronchoscopic thermal vapor ablation [BTVA]; Uptake Medical Corp., Seattle, WA) to a targeted area of emphysema in the upper lobe was applied (46). BTVA induces an inflammatory response to the airway and parenchyma and thereby initiates LVR. BTVA uses a vapor generator and metal balloon vapor catheter, with target dosing at 3–7.5 cal/g according to a prior CT-based tissue–air algorithm. Eleven patients (FEV1, 0.77 ± 0.17 L [32% predicted]; RV, 4.1 ± 0.9 L [219% predicted]) underwent 9 right and 2 left upper lobe unilateral treatments (approximately 3 applications per lobe) over 22 ± 5 minutes. All had immediate mild radiographic opacification of the lung in the target area. Serious adverse events (n = 5) were bacterial pneumonia, anxiety and atrial tachycardia (n = 3), and acute COPD exacerbation (n = 2). Minor adverse events included minor hemoptysis (n = 6) and inflammatory pneumonitis (n = 2). All had less dyspnea at 3–16 weeks postprocedure. Seven patients completed a 1-month follow-up with a mean 9 ± 8% increase in FEV1 and 7.4 ± 9% decrease in RV; however, at 6 months there were no significant differences compared with baseline FVC, FEV1, 6MWD, and RV. In contrast, MRC dyspnea scores (baseline, 2.6; 6 mo, 2.1) and SGRQ (baseline, 64.4; 6 mo, 49.1) were both improved 6 months after BTVA. BTVA continues to undergo further investigation at this time.
External placement of modified chest tubes in the most emphysematous regions of three patients reportedly increased FEV1 and 6MWD and reduced RV, TLC, mMRC, and SGRQ values (47). These data support the concept that placing artificial spiracles into diseased emphysematous lung could enhance gas emptying and thereby ameliorate the consequences of hyperinflation.
Summary
Investigation of LVRS over the last two decades has rapidly added to our knowledge of the pathogenesis, evaluation, and array of medical, surgical, and interventional treatments for severe emphysema. NETT has provided substantial evidence that treating hyperinflation in emphysema can improve survival and thereby represents a disease-modifying form of therapy. LVRS offers a select group of patients with emphysema the opportunity for clinically meaningful improvements in exercise tolerance, lung function, and quality of life; and in those with upper lobe–predominant disease and low exercise, survival. Specifically, LVRS in addition to supplemental oxygen and smoking cessation is one of only a few therapies that can improve survival in selected patients with severe emphysema. At present, LVRS remains an underused therapy and its place in the treatment of severe emphysema as a potentially disease-modifying therapy is not yet appropriately recognized. Various novel, less invasive bronchoscopic techniques are currently undergoing study and show potential to effectively treat a broader group of patients with advanced emphysema who suffer from severe hyperinflation. Future prospective, controlled studies are needed to determine the optimal role of LVRS versus bronchoscopic LVR in the treatment of severe emphysema (48).
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201103-0455CI on June 30, 2011
Author Disclosure: G.J.C. has acted as a consultant for Uptake Medical, PortAero, and Pulmonx; he has received travel support from Emphasys Medical; his institution has received grants from Aeris Therapeutics, and Emphasys Medical. F.C. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.L.S. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.J.M. has served as a board member for GlaxoSmithKline (GSK), MedImmune/AstraZeneca (AZ), Merck, Pearl, Novartis, Mpex, Ikaria, and UBC; he has acted as a consultant for Forest/Almirall, Boehringer Ingelheim (BI), Nycomed/Forest, Roche, Bayer, Schering, HLS, Talecris, Comgenix, F&B Communications, BoomComm, Actelion, Elan, Genzyme, Quark, Merck, Pfizer, and Sanofi-Aventis; he has received grants from Gilead, Johnson & Johnson/Centocor, and Actelion; he has received lecture fees from GSK, NACE, MedEd, Potomac, Pfizer, BI, Schering, Vox Medic, the American Lung Association, WebMD, ePocrates, AZ, the France Foundation, and Altana/Nycomed; he has received royalties from Associates in Medical Marketing, and Castle Connolly; he has received payment for the development of educational presentations from the France Foundation, HIT Global, and UpToDate; his institution has received grants from BI.
References
- 1.Weinmann GG, Chiang YP, Sheingold S. The National Emphysema Treatment Trial (NETT): a study in agency collaboration. Proc Am Thorac Soc 2008;5:381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J, McLennan G, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–1244 [DOI] [PubMed] [Google Scholar]
- 3.Mineo D, Ambrogi V, Cufari ME, Gambardella S, Pignotti L, Pompeo E, Mineo TC. Variations of inflammatory mediators and α1-antitrypsin levels after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med 2010;181:806–814 [DOI] [PubMed] [Google Scholar]
- 4.Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, Magnussen H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest 2010;138:32–38 [DOI] [PubMed] [Google Scholar]
- 5.Washko GR, Martinez FJ, Hoffman EA, Loring SH, Estepar RS, Diaz AA, Sciurba FC, Silverman EK, Han MK, Decamp M, et al. Physiological and computed tomographic predictors of outcome from lung volume reduction surgery. Am J Respir Crit Care Med 2010;181:494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney PA, Sundaresan RS, Roper CL. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109:106–116; discussion 116–119 [DOI] [PubMed] [Google Scholar]
- 7.Bingisser R, Zollinger A, Hauser M, Bloch KE, Russi EW, Weder W. Bilateral volume reduction surgery for diffuse pulmonary emphysema by video-assisted thoracoscopy. J Thorac Cardiovasc Surg 1996;112:875–882 [DOI] [PubMed] [Google Scholar]
- 8.Cooper JD, Patterson GA, Sundaresan RS, Trulock EP, Yusen RD, Pohl MS, Lefrak SS. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996;112:1319–1329; discussion 1329–1330 [DOI] [PubMed] [Google Scholar]
- 9.Argenziano M, Moazami N, Thomashow B, Jellen PA, Gorenstein LA, Rose EA, Weinberg AD, Steinglass KM, Ginsburg ME. Extended indications for lung volume reduction surgery in advanced emphysema. Ann Thorac Surg 1996;62:1588–1597 [DOI] [PubMed] [Google Scholar]
- 10.Daniel TM, Chan BB, Bhaskar V, Parekh JS, Walters PE, Reeder J, Truwit JD. Lung volume reduction surgery: case selection, operative technique, and clinical results. Ann Surg 1996;223:526–531; discussion 532–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenna RJ, Jr, Benditt JO, DeCamp M, Deschamps C, Kaiser L, Lee SM, Mohsenifar Z, Piantadosi S, Ramsey S, Reilly J. Safety and efficacy of median sternotomy versus video-assisted thoracic surgery for lung volume reduction surgery. J Thorac Cardiovasc Surg 2004;127:1350–1360 [DOI] [PubMed] [Google Scholar]
- 12.McKenna RJ, Jr, Brenner M, Fischel RJ, Gelb AF. Should lung volume reduction for emphysema be unilateral or bilateral? J Thorac Cardiovasc Surg 1996;112:1331–1338; discussion 1338–1339 [DOI] [PubMed] [Google Scholar]
- 13.Miller JI, Jr, Lee RB, Mansour KA. Lung volume reduction surgery: lessons learned. Ann Thorac Surg 1996;61:1464–1468; discussion 1468–1469 [DOI] [PubMed] [Google Scholar]
- 14.Wisser W, Tschernko E, Senbaklavaci O, Kontrus M, Wanke T, Wolner E, Klepetko W. Functional improvement after volume reduction: sternotomy versus videoendoscopic approach. Ann Thorac Surg 1997;63:822–827; discussion 827–828 [DOI] [PubMed] [Google Scholar]
- 15.Health Care Financing Administration Lung volume reductionsurgery and Medicare coverage policy: implications of recently published evidence [report to Congress]. Washington DC: Department of Health and Human Services; 1998 [Google Scholar]
- 16.NETT Research Group Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999;116:1750. [DOI] [PubMed] [Google Scholar]
- 17.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE; National Emphysema Treatment Trial Research Group A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073 [DOI] [PubMed] [Google Scholar]
- 18.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, Deschamps CC, Martinez FJ, Sciurba FC, Tonascia J. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial research group. Ann Thorac Surg 2006;82:431–443 [DOI] [PubMed] [Google Scholar]
- 19.National Emphysema Treatment Trial Research Group Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083 [DOI] [PubMed] [Google Scholar]
- 20.Chandra D, Lipson DA, Hoffman EA, Hansen-Flaschen J, Sciurba FC, Decamp MM, Reilly JJ, Washko GR; National Emphysema Treatment Trial Research Group Perfusion scintigraphy and patient selection for lung volume reduction surgery. Am J Respir Crit Care Med 2010;182:937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naunheim KS, Wood DE, Krasna MJ, DeCamp MM, Jr, Ginsburg ME, McKenna RJ, Jr, Criner GJ, Hoffman EA, Sternberg AL, Deschamps C, et al. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg 2006;131:43–53 [DOI] [PubMed] [Google Scholar]
- 22.DeCamp MM, Blackstone EH, Naunheim KS, Krasna MJ, Wood DE, Meli YM, McKenna RJ Jr; NETT Research Group Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197–206; discussion 206–207 [DOI] [PubMed] [Google Scholar]
- 23.Stoller JK, Gildea TR, Ries AL, Meli YM, Karafa MT; National Emphysema Treatment Trial Research Group Lung volume reduction surgery in patients with emphysema and α1-antitrypsin deficiency. Ann Thorac Surg 2007;83:241–251 [DOI] [PubMed] [Google Scholar]
- 24.Criner GJ, Belt P, Sternberg AL, Mosenifar Z, Make BJ, Utz JP, Sciurba F; National Emphysema Treatment Trial Research Group Effects of lung volume reduction surgery on gas exchange and breathing pattern during maximum exercise. Chest 2009;135:1268–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Criner GJ, Scharf SM, Falk JA, Gaughan JP, Sternberg AL, Patel NB, Fessler HE, Minai OA, Fishman AP; National Emphysema Treatment Trial Research Group Effect of lung volume reduction surgery on resting pulmonary hemodynamics in severe emphysema. Am J Respir Crit Care Med 2007;176:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder ML, Goss CH, Neradilek B, Polissar NL, Mosenifar Z, Wise RA, Fishman AP, Benditt JO; National Emphysema Treatment Trial Research Group Changes in arterial oxygenation and self-reported oxygen use after lung volume reduction surgery. Am J Respir Crit Care Med 2008;178:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey SD, Berry K, Etzioni R, Kaplan RM, Sullivan SD, Wood DE; National Emphysema Treatment Trial Research Group Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med 2003;348:2092–2102 [DOI] [PubMed] [Google Scholar]
- 28.Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost-effectiveness of lung volume reduction surgery. Chest 2007;131:823–832 [DOI] [PubMed] [Google Scholar]
- 29.Criner GJ, Sternberg AL; National Emphysema Treatment Trial Research Group A clinician's guide to the use of lung volume reduction surgery. Proc Am Thorac Soc 2008;5:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim V, Criner GJ, Abdallah HY, Gaughan JP, Furukawa S, Solomides CC. Small airway morphometry and improvement in pulmonary function after lung volume reduction surgery. Am J Respir Crit Care Med 2005;171:40–47 [DOI] [PubMed] [Google Scholar]
- 31.Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, Martinez FJ, Rogers RM, Make BJ, Criner GJ. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007;176:454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, Polkey MI, Geddes DM. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003;361:931–933 [DOI] [PubMed] [Google Scholar]
- 33.Watanabe Y, Matasuo K, Tamaoki A, Komoto R, Hiraki S. Bronchial occlusion with endobronchial Watanabe spigot. J Bronchol 2003;10:264–267 [Google Scholar]
- 34.Sabanathan S, Richardson J, Pieri-Davies S. Bronchoscopic lung volume reduction. J Cardiovasc Surg (Torino) 2003;44:101–108 [PubMed] [Google Scholar]
- 35.Rendina EA, De Giacomo T, Venuta F, Coloni GF, Meyers BF, Patterson GA, Cooper JD. Feasibility and safety of the airway bypass procedure for patients with emphysema. J Thorac Cardiovasc Surg 2003;125:1294–1299 [DOI] [PubMed] [Google Scholar]
- 36.Ingenito EP, Reilly JJ, Mentzer SJ, Swanson SJ, Vin R, Keuhn H, Berger RL, Hoffman A. Bronchoscopic volume reduction: a safe and effective alternative to surgical therapy for emphysema. Am J Respir Crit Care Med 2001;164:295–301 [DOI] [PubMed] [Google Scholar]
- 37.Ingenito EP, Berger RL, Henderson AC, Reilly JJ, Tsai L, Hoffman A. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med 2003;167:771–778 [DOI] [PubMed] [Google Scholar]
- 38.Snell GI, Holsworth L, Borrill ZL, Thomson KR, Kalff V, Smith JA, Williams TJ. The potential for bronchoscopic lung volume reduction using bronchial prostheses: a pilot study. Chest 2003;124:1073–1080 [DOI] [PubMed] [Google Scholar]
- 39.Sterman DH, Mehta AC, Wood DE, Mathur PN, McKenna RJ, Jr, Ost DE, Truwit JD, Diaz P, Wahidi MM, Cerfolio R. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration 2010;79:222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Biggelaar R, Kerstjens H, Slebos D. Bronchoscopic lung volume reduction with endobronchial valves in selected heterogeneous emphysema: first experience in The Netherlands [abstract]. Eur Respir J 2010:747S [Google Scholar]
- 41.Criner GJ, Pinto-Plata V, Strange C, Dransfield M, Gotfried M, Leeds W, McLennan G, Refaely Y, Tewari S, Krasna M. Biologic lung volume reduction in advanced upper lobe emphysema: phase 2 results. Am J Respir Crit Care Med 2009;179:791–798 [DOI] [PubMed] [Google Scholar]
- 42.Refaely Y, Dransfield M, Kramer MR, Gotfried M, Leeds W, McLennan G, Tewari S, Krasna M, Criner GJ. Biologic lung volume reduction therapy for advanced homogeneous emphysema. Eur Respir J 2010;36:20–27 [DOI] [PubMed] [Google Scholar]
- 43.Magnussen H, Kirsten A, Eberhardt R, Schmidt B, Behr J, Stanzel F, Bonnet R, Herth FJ. CT assessment of regional lung volume changes following endobronchial volume reduction therapy in emphsyema patients using a synthetic adhesive hydrogel-foam [abstract]. Am J Respir Crit Care Med 2010;181:A5533 [Google Scholar]
- 44.Broncus Technologies Inc Broncus reports early EASE trial results for airway bypass with EXHALE drug-eluting stents. [Accessed May 11, 2011]. Available from: http://markets.hpcwire.com/taborcomm.hpcwire/?guid=1081574
- 45.Herth FJ, Eberhard R, Gompelmann D, Slebos DJ, Ernst A. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis 2010;4:225–231 [DOI] [PubMed] [Google Scholar]
- 46.Snell GI, Hopkins P, Westall G, Holsworth L, Carle A, Williams TJ. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 2009;88:1993–1998 [DOI] [PubMed] [Google Scholar]
- 47.Saad R, Neto V, Botter M, Stirbulov R, Rivaben J, Goncalves R. Therapeutic application of collateral ventilation with pulmonary drainage in the treatment of diffuse emphysema: report of the first three cases. J Bras Pneumol 2009;1:14–19 [DOI] [PubMed] [Google Scholar]
- 48.Criner GJ. Endoscopic interventions in severe emphysema. Am J Respir Crit Care Med 2009;180:684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.