Abstract
Rationale: Sepsis syndrome is characterized by inappropriate amplified systemic inflammatory response and bacteremia that promote multiorgan failure and mortality. Nuclear factor–erythroid 2 p45-related factor 2 (Nrf2) regulates a pleiotropic cytoprotective defense program including antioxidants and protects against several inflammatory disorders by inhibiting oxidative tissue injuries. However, the role of enhanced Nrf2 activity in modulating innate immune responses to microbial infection and pathogenesis of sepsis is unclear.
Objectives: To determine whether Nrf2 in myeloid leukocytes alters inflammatory response and protects against sepsis.
Methods: Mice with deletion of Nrf2 or kelch-like ECH-associated protein (Keap1) in myeloid leukocyte cells and respective floxed controls were subjected to cecal ligation and puncture–induced sepsis and were assessed for survival, organ injury, systemic inflammation, and bacteremia. Using LPS-stimulated peritoneal macrophages, Toll-like receptor (TLR) 4 surface trafficking and downstream signaling events were analyzed.
Measurements and Main Results: Mortality, organ injury, circulating levels of inflammatory mediators, and bacteremia were markedly reduced in LysM-Keap1−/− compared with respective floxed controls (Keap1f/f or Nrf2f/f) and significantly elevated in LysM-Nrf2−/− mice after cecal ligation and puncture. Peritoneal macrophages from septic LysM-Keap1−/− mice showed a greater bacterial phagocytic activity compared with LysM-Nrf2−/− and floxed controls. LPS stimulation resulted in greater reactive oxygen species–induced cell surface transport of TLR4 from trans-Golgi network and subsequent TLR4 downstream signaling (recruitment of MYD88 and TRIF, phosphorylation of IkB and IRF3, and cytokine expression) in macrophages of LysM-Nrf2−/− compared with LysM-Keap1−/− mice and floxed controls.
Conclusions: Our study shows that Nrf2 acts as a critical immunomodulator in leukocytes, controls host inflammatory response to bacterial infection, and protects against sepsis.
Keywords: Nrf2, Keap1, sepsis, antioxidants, inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
Host factors that control innate immune-inflammatory responses during sepsis are less understood. Nuclear factor–erythroid 2 p45-related factor 2 (Nrf2) regulates antioxidant defenses that protect against inflammation by inhibiting oxidative tissue injuries. However, whether enhancing Nrf2 in leukocytes affects innate immune-inflammatory responses to microbial infection and modulates the pathogenesis of sepsis is unclear.
What This Study Adds to the Field
Enhancing Nrf2 signaling in macrophages and neutrophils improves host survival by increasing antibacterial and antiinflammatory defenses during sepsis.
Sepsis is a complex syndrome characterized by infection and systemic inflammatory response (1). It is a leading cause of death in intensive care units with mortality rates that range from 30–70%, and the mortality rate is especially high in patients with nosocomial infections (1–3). Other than supportive care, there are no effective therapies to improve survival in patients with sepsis (1). Recombinant activated protein C is the only approved therapy for the treatment of patients with severe form of sepsis (4). Recent advancement in research in sepsis has indicated that the pathogenesis of sepsis is mediated by dysregulated host inflammatory response that involves amplified innate inflammatory response along with complement activation against infection followed by immune suppression (5). Dysregulated host inflammatory response results in persistent infection and excess cytokines and secondary mediators, such as lipid mediators, reactive oxygen species (ROS), and reactive nitrogen species, which cause tissue damage (1). Pathologic sequelae of sepsis include vascular injury, apoptosis, tissue injury, and ultimately multiorgan failure.
Toll-like receptors (TLRs) in phagocytes (macrophages and neutrophils) mediate immune recognition of pathogens by detecting conserved pathogen-associated molecular patterns (5). They play a crucial role in modulating innate immune-inflammatory response and outcome of sepsis. Disruption or antibody neutralization of specific TLRs including TLR2, TLR3, TLR4, and TLR9 has been shown to improve survival in a polymicrobial mice model of sepsis (6–8). Further, a recent study has reported cross-talk between TLR4 and TLR2 signaling pathways in augmenting proinflammatory response and mortality in sepsis (9). Overall, there is an imbalance of regulatory inflammatory networks resulting in inappropriate host immune response in sepsis (5). However, the role of host factors that modulate inflammatory signaling pathways and determine severity of sepsis or decrease mortality is less clear.
Nuclear factor–erythroid 2 p45-related factor 2 (Nrf2), a bZIP transcription factor, regulates cellular redox homeostasis by mounting a coordinated induction of antioxidants and other associated defenses (glutathione biosynthesis, NADP reduced regeneration, and heme oxygenase-1) (10–12). In response to inflammatory or oxidative stress, Nrf2 dissociates from its cytosolic inhibitor, kelch-like ECH-associated protein (Keap1), translocates to the nucleus, binds to a cis-element referred to as “antioxidant response element,” and transcribes its target genes (10). Global deletion of Nrf2 in mice impairs induction of cellular antioxidant defenses, lowers redox potential, and enhances susceptibility and severity to several inflammatory disorders including asthma, fibrosis, emphysema, and colitis (10, 11, 13). Clinical studies support inverse correlation between Nrf2 activity and severity of inflammatory disorders, such as chronic obstructive pulmonary disease and Friedreich ataxia (14, 15). Previously, we reported that global disruption of Nrf2 enhances LPS- and cecal ligation and puncture (CLP)–induced mortality (16). However, it remained unclear whether increasing Nrf2 activity alters innate-inflammatory responses and protects against sepsis pathogenesis. Here, we report that enhancement of Nrf2 activity in effector cells of innate immunity, macrophages and neutrophils, by myeloid cell–specific deletion of Keap1 markedly improves survival, whereas the deletion of Nrf2 augments mortality because of dysregulated inflammatory response in an experimental model of sepsis.
Methods
Generation of Lineage-specific Deletion of Keap1 and Nrf2 Genes in the Myeloid Cells of Mice
We generated mice for conditional deletion of Keap1 and Nrf2 in myeloid cells. A targeting vector for Keap1 was constructed by inserting LoxP sites flanking Exons 2 and 3. A neomycin cassette flanked by flippase recognition target (FRT) sites was used for the selection of positive clones. The vector was linearized by Not1 and transfected by electroporation into C57BL/6J embryonic stem cells. After selection of clones with neomycin, surviving clones were expanded for reverse transcription polymerase chain reaction (PCR) analysis to identify ES clones with homologous recombination at Keap1 locus. Two recombinant clones were identified and injected into recipient female mice. Chimeras with the targeted allele were backcrossed with C57BL/6 mice to generate Keap1flox/WT Neo+ mice. Keap1flox/WT Neo+ mice were subsequently crossed with mice carrying the flippase (FLP) recombinase (FLP+ mice on the C57BL/6 background), which targets the FRT sequence flanking the neomycin cassette to generate Keap1flox/wt-Neo+-FLP+ mice. The presence of the FLP transgene was segregated by backcrossing with C57/BL6J mice to generate the Keap1flox/wt FLP− mice. The confirmation of the floxed allele without the FLP transgene was done by PCR analysis. Keap1flox/wt FLP− mice were further inbred to generate Keap1flox/flox mice, which are aphenotypic. Myeloid cell–specific Keap1-deficient (LysM-Keap1−/−) mice were generated by breeding Keap1flox/flox mice with LysM-Cre+ mice (Jackson Laboratories, Bar Harbor, ME), which express Cre recombinase only in the myeloid cells including macrophages and granulocytes (17). Initial experiments have revealed no reduction in survival or fertility of conditionally targeted mice. The deletion of Exons 2 and 3 of the Keap1 gene resulted in the loss of most of the intervening region domain and four of six of the Kelch domains in the truncated Keap1 protein. The predicted size of the truncated Keap1 protein is 25 kD.
To delete the Nrf2 gene in cells from the myeloid lineage, a targeting vector was generated by inserting LoxP sites flanking the DNA-binding domain (Exon 5) of the Nrf2 gene and a neomycin cassette flanked by FRT sites for the selection of positive clones. The linearized vector was transfected into C57BL/6J embryonic stem cells. After selection with neomycin, surviving clones were expanded for PCR analysis to identify ES clones with homologous recombination at Nrf2 locus. Two recombinant clones were identified and used for injection into recipient female mice. Chimeras with the targeted allele were backcrossed with C57BL/6 mice to generate the Nrf2flox/flox and Neo+ mice. The crossing strategy to generate LysM-Nrf2−/− was the same as described previously for LysM-Keap1−/− mice.
Microarray Analysis
The mRNA isolated from bone marrow–derived macrophages (BMDM) was applied to Murine Genome MOE 430 2.0 GeneChip arrays (Affymetrix, Santa Clara, CA) according to procedures described previously (16). Intensity of hybridization for each probe pair was computed by Command Console software (Affymetrix). Genes differentially expressed were defined as significant with P less than or equal to 0.01 with a fold change greater than 1.5-fold.
CLP Model
Sepsis was induced by CLP using methods described previously (16, 18). The distal 50% of exposed cecum was ligated with 3–0 silk suture and punctured with one pass of a 21-gauge needle. For serum cytokine analysis, a less severe CLP model (one pass of a 22-gauge needle) was used. Postoperatively, the animals were resuscitated with subcutaneous injection of 1 ml sterile 0.9% NaCl.
Microarray Immunoassay
The microarray immunoassay was performed using standard spotting equipment and fluorescence slide readers as described previously (18).
Peritoneal Macrophages
Thioglycollate-elicited peritoneal macrophages were isolated from mice using methods described previously (16, 19).
Phagocytosis Assay
Peritoneal macrophages were isolated from healthy or CLP- treated mice and cocultured with fluorescent-labeled (fluorescein isothiocyanate) Pseudomonas aeruginosa (ATCC) in phosphate-buffered saline at 37°C for 1 hour. Bacterial phagocytosis was analyzed by flow cytometry as described previously (20). For analyzing bactericidal activity, macrophages were incubated with P. aeruginosa for 4 hours and bacterial growth was assessed in cell-free culture medium as described previously (20).
Quantitative Real-time PCR
Quantitative real-time PCR analyses were performed by using probe sets commercially available from Applied Biosystems (Foster City, CA) as described previously (19). Assays were performed by using the ABI 7000 Taqman system (Applied Biosystems).
Flow Cytometry Analysis of TLR4 Surface Expression
Cell surface expression of TLR4 was detected by flow cytometry with fluorescein isothiocyanate–conjugated anti-TLR4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) as described elsewhere (18).
Measurement of ROS
Intracellular levels of ROS were determined using the redox-sensitive probes DCFH-DA in conjunction with flow cytometry as described previously (18).
Immunoblot Analysis
Antibodies (anti-TLR4, anti-MYD88, anti-IkB, and antiphosphorylated IkB) were obtained from Santa Cruz Biotechnology. Anti-IRF3 and antiphosphorylated IRF3 were obtained from Cell Signaling Technology (Danvers, MA). Anti-high mobility group 1 (HMGB1) and anti-toll-interleukin-1 receptor domain-containing adapter protein inducing interferon beta (TRIF) were procured from Abcam (Cambridge, MA). Immunoprecipitation and immunoblot analysis was performed as described previously (15).
Statistical Analysis
Survival studies were analyzed by using the log-rank test. All other data were analyzed with the unpaired Student t test or U test. Statistical significance was accepted at P less than 0.05.
Results
Generation and Characterization of Mice with Myeloid Cell–Specific Deletion of Keap1 or Nrf2
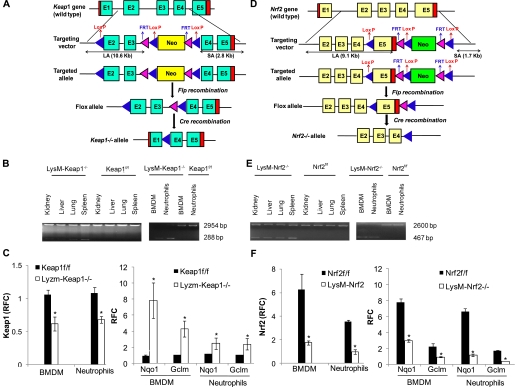
To examine the specific role of Nrf2 in regulation of innate immune responses in sepsis, we generated mice with deletion of Keap1 or Nrf2 specifically in myeloid cells (macrophages and neutrophils) using the cre-loxP recombination approach. We first generated Nrf2flox/flox (Nrf2f/f) and Keap1flox/flox (Keap1f/f) mice by the insertion of two loxP sites flanking Exon 5 and Exons 2 and 3, respectively, (Figures 1A and 1D). Next, we analyzed the expression of Nrf2-regulated transcriptional targets in different organs and confirmed that insertion of loxP sites in Nrf2 or Keap1 gene did not alter the wild-type phenotype of Nrf2f/f and Keap1f/f. The level of the expression of Nrf2-regulated target genes NQO1 and GCLM was similar in BMDM, liver, kidney, and lungs of Nrf2f/f, Keap1f/f, and wild-type mice (data not shown).
Figure 1.
Generation and characterization of conditional knockout mice. (A) Schematic representation of kelch-like ECH-associated protein (Keap1) gene targeting vector for tissue-specific deletion of Exons 2 and 3. LA = long arm; SA = short arm. (B) Specific recombination of the conditional Keap1 allele in the LysM-Keap1−/− mice lungs, liver, kidney, spleen, bone marrow–derived macrophages, and neutrophils. The 288-bp band represents Exons 2 and 3 deleted Keap1 allele and 2,954-bp band represents the floxed or the wild-type allele. No deletion was detected in the macrophages and neutrophils from Keap1f/f mice. (C) mRNA expression by quantitative polymerase chain reaction (PCR) of Keap1, Gclm, and Nqo1 genes in bone marrow–derived macrophages and peritoneal neutrophils from Keap1−/− and Keap1f/f mice. P < 0.05. (D) Schematic representation of nuclear factor–erythroid 2 p45-related factor 2 (Nrf2) gene targeting vector for tissue-specific deletion of Exon 5. LA = long arm; SA = short arm. (E) Specific recombination of the conditional Nrf2 allele in the LysM-Nrf2−/− lungs, liver, kidney, spleen, bone marrow–derived macrophages, and neutrophils. PCR-based genotyping of the DNA revealed a 2,600-bp band of wild-type or the floxed allele and a 467-bp band representing the Exon 5 deleted allele. No deletion was detected in the macrophages and neutrophils from Nrf2f/f mice. (F) mRNA expression by reverse transcriptase PCR of Nrf2, Gclm, and Nqo1 genes in bone marrow–derived macrophages and peritoneal neutrophils from Nrf2−/− and Nrf2f/f mice. *Significant compared with respective flox control mice. P < 0.05. Data were analyzed by Student t test. BMDM = bone marrow-derived macrophages; RFC = relative fold change.
Myeloid cell–specific deletion of Nrf2 or Keap1 was generated by crossing Nrf2f/f and Keap1f/f mice with mice bearing Cre recombinase under the control of lysozyme M promoter (LysM-Nrf2−/− and LysM-Keap1−/−). Lysozyme M has been shown to be exclusively expressed in cells of the monocyte-macrophages and granulocyte lineages of hematopoietic differentiation (21, 22). The deletion of Exon 5 of the Nrf2 gene results in the loss of DNA-binding domain, whereas the deletion of Exons 2 and 3 of the Keap1 gene results in the loss of most of IVR domain (four of six of the kelch domains). PCR analysis of genomic DNA from LysM-Nrf2−/− or LysM-Keap1−/− BMDM, peritoneal neutrophils, and other vital organs revealed amplification of floxed allele (2,600 bp and 2,954 bp) and deleted allele (467 bp and 288 bp) (Figures 1B and 1E). The extent of deletion of Nrf2 or Keap1 floxed allele was complete in BMDM and neutrophils. The amplification of the deleted floxed allele was higher in spleen and lower in liver, kidney, and lung indicating the degree of presence of macrophages or neutrophils in these tissues, but there were no deleted floxed alleles in the flox mice (Figures 1B and 1E). LysM-Nrf2−/− or LysM-Keap1−/− mice were healthy and fertile. Basal mRNA levels of Keap1 were significantly reduced, whereas Nrf2-regulated genes NQO1 and GCLM were significantly up-regulated in peritoneal macrophages and peritoneal neutrophils from LysM-Keap1−/− mice compared with Keap1f/f. No significant difference was detected in Nrf2 mRNA expression between LysM-Keap1−/− and Keap1f/f (data not shown). However, the levels of nuclear Nrf2 protein were markedly higher in peritoneal macrophages of LysM-Keap1−/− compared with Keap1f/f (see Figure E1 in the online supplement). On the contrary, in LysM-Nrf2−/− mice, mRNA levels of Nrf2 and Nrf2-regulated genes (Nqo1 and Gclm) were markedly reduced constitutively (Figures 1C and 1F).
To gain a comprehensive view of Nrf2-regulated cytoprotective genes in response to Keap1 disruption in the macrophages, we performed microarray analysis in BMDM isolated from LysM-Keap1−/− and Keap1f/f mice. The level of Nrf2 regulated antioxidant and phase II genes including GSH-biosynthesizing enzymes, glutathione reductase, catalase, NQO1, peroxiredoxin, and thioredoxin reductase was significantly higher in macrophages from Lysm-Keap1−/− than in Keap1f/f (see Table E1). Concurrently, we also performed microarray analysis in BMDM isolated from wild-type and global Nrf2-knockout mice. The constitutive expression levels of these antioxidant genes were significantly lower in BMDM isolated from Nrf2-knockout mice compared with wild-type (see Table E1). Validation by quantitative PCR analysis showed similar but higher degree of up-regulation of selected Nrf2-regulated antioxidant genes (Nqo1, Gclm, and Gclc) (see Figure E2). Likewise, the level of these antioxidant genes was significantly lower in macrophages isolated from LysM-Nrf2−/− mice than in Nrf2f/f as analyzed by quantitative PCR (data not shown).
Myeloid Cell–Specific Deletion of Keap1 Protects against Mortality, whereas Deletion of Nrf2 Augments Mortality in Polymicrobial Sepsis
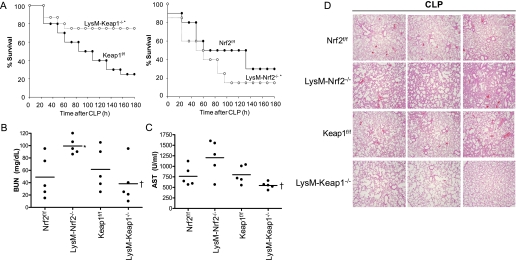
To determine whether disruption or enhancing Nrf2 pathway in myeloid cells (macrophages and neutrophils) affects mortality after sepsis, we subjected LysM-Keap1−/−, LysM-Nrf2−/−, Keap1f/f, and Nrf2f/f mice to CLP and monitored survival for 7 days. After CLP, no significant difference in the mortality between the Keap1f/f and Nrf2f/f mice groups was observed suggesting no change in phenotypes as a result of the insertion of loxP sites. LysM-Keap1−/− mice showed a dramatic improvement in the survival compared with Keap1f/f mice (P < 0.01). In contrast, LysM-Nrf2−/− mice showed poor survival compared with Nrf2f/f mice (P < 0.01). On Day 7, survival in LysM-Keap1−/−, LysM-Nrf2−/−, Keap1f/f, and Nrf2f/f mice was 75%, 15%, 30%, and 30%, respectively (Figure 2A). Sham surgery caused no death in any of the genotypes (data not shown). These data demonstrate that Nrf2 pathway plays a critical role in myeloid cells in regulating mortality after polymicrobial sepsis.
Figure 2.
Myeloid cell–specific deletion of Keap1 protects against cecal ligation and puncture (CLP)–induced sepsis pathogenesis. (A) Survival curves of mice (LysM-Nrf2−/−, Keap1f/f, Nrf2f/f, and LysM-Keap1−/−) after CLP (n = 10/gp); details are provided in the Methods section. Data were analyzed using log-rank test. *Significant compared with respective flox control mice; P < 0.01. (B and C) Serum level of blood urea nitrogen (BUN) and aspartate aminotransferase (AST) in LysM-Nrf2−/−, Keap1f/f, Nrf2f/f, and LysM-Keap1−/− mice 24 hours after CLP (n = 5/gp). (D) Histopathologic analysis of lung from Nrf2f/f, LysM-Nrf2−/−, Keap1f/f, and LysM-Keap1−/− mice 24 hours after CLP (n = 5/gp). Images (×40) from lung sections of three different mice are shown. Data were analyzed using Student t test. *Significant compared with respective flox control mice; †Significant compared with LysM-Nrf2−/− P < 0.05.
Lethality in sepsis is associated with vital organ failure. Therefore, we next examined pathology or function of major organs (lung, liver, and kidney) often injured in patients with sepsis. Serum levels of blood urea nitrogen, an indicator of renal dysfunction, was significantly elevated in LysM-Nrf2−/− compared with floxed controls and LysM-Keap1−/− mice (Figure 2B). Serum levels of aspartate aminotransferase, an indicator of liver injury, were significantly higher in LysM-Nrf2−/− mice compared with LysM-Keap1−/− mice (Figure 2C). The serum level of aspartate aminotransferase was high but not significant in the LysM-Nrf2−/− mice compared with floxed control (Figure 2C). Histopathologic analysis revealed greater lung injury as indicated by infiltration of inflammatory cells into lung parenchyma in LysM-Nrf2−/− compared with floxed controls and LysM-Keap1−/− mice (Figure 2D). Sham surgery showed no signs of organ injury in any of the genotypes (data not shown). Taken together, the results indicate that higher mortality in LysM-Nrf2−/− mice was associated with multiple organ failure.
LysM-Nrf2−/− Mice Showed Dysregulated Inflammatory Response Compared with LysM-Keap1−/− Mice
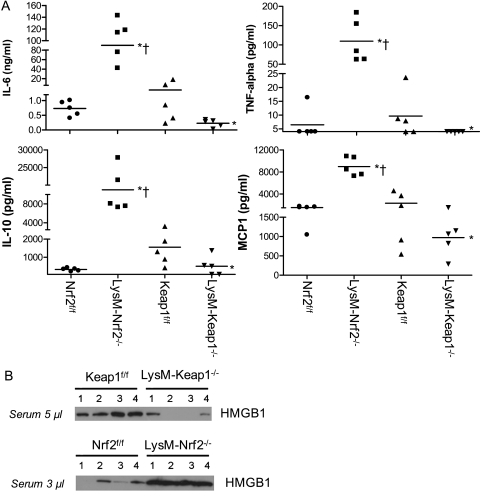
One of the hallmarks of sepsis pathogenesis is dysregulated host inflammatory response to infection that is reflected in circulating levels of proinflammatory and antiinflammatory cytokines. In a model of polymicrobial sepsis, high levels of proinflammatory mediators correlate with poor prognosis (23). Clinical and preclinical studies have reported a strong correlation between sepsis mortality and serum inflammatory mediators including IL-6, IL-1β, IL-10, HMGB1, and IL1-ra. Therefore, we analyzed the serum levels of proinflammatory and antiinflammatory mediators (IL-1β, IL-6, IL-12, IL-17, tumor necrosis factor [TNF]-α, IFN-γ, IL-1ra, IL-13, IL-4, IL-5, macrophage inflammatory protein [MIP]-2, MIP-1α, monocyte chemoattractant protein [MCP]-1, TNF-SRI, and TNF-SRII) that have been previously implicated in the pathogenesis of sepsis by microarray immunoassay. For assessing serum cytokines, we used a less severe CLP (22-gauge needle) model that produced approximately 20% mortality in LysM-Nrf2−/− mice but no mortality in LysM-Keap1−/− mice at 48 hours (data not shown). Sham surgery did not lead to any significant increase in cytokine levels in mice of all genotypes. Interestingly, the levels of proinflammatory and antiinflammatory mediators were markedly higher (∼ 5- to 10-fold) in LysM-Nrf2−/− mice compared with Nrf2f/f mice 24-hour after CLP (Figure 3A and Table 1). In contrast, these inflammatory mediators were significantly lower in the serum of LysM-Keap1−/− compared with LysM-Nrf2−/− or Keap1f/f mice. We also confirmed higher IL-6 levels in the serum of LysM-Nrf2−/− mice compared with LysM-Keap1−/− and floxed control at 6 hours in a severe CLP model by ELISA (see Figure E3). Immunoblot analysis showed that serum levels of HMGB1 were significantly higher in LysM-Nrf2−/− than in Nrf2f/f mice 24-hours after CLP. Serum levels of HMGB1 in LysM-Keap1−/− mice were markedly reduced compared with Keap1f/f and were lowest among all genotypes (Figure 3B). These data suggest that Nrf2 activity in macrophages and neutrophils is crucial in controlling lethal systemic inflammation in sepsis.
Figure 3.
LysM–Keap1−/− mice are associated with markedly low systemic inflammation compared with LysM–Nrf2−/− and control floxed mice after CLP. (A) Serum cytokines (IL-6, tumor necrosis factor [TNF]-α, IL-10, and monocyte chemoattractant protein-1) in mice alive at 24 hours after CLP (22-guage, a less severe CLP model) as assessed by microarray immunoassay. Data were represented as scatter plot, and each point represents an individual mouse. The horizontal line represents mean value. Data were analyzed by U test and Student t test. *Significant compared with flox control group; P < 0.05. †Significant compared with LysM-Keap1−/− group; P < 0.01. (B) Serum high mobility group 1 levels in mice 24 hours after CLP as analyzed by immunoblot. Each band represents the level of HMGB1 in an individual mouse. For this analysis, 5 μl of serum from Keap1f/f and LysM-Keap1−/− mice and 3 μl of serum from Nrf2f/f and LysM-Nrf2−/− was used.
TABLE 1.
LEVELS OF INFLAMMATORY MEDIATORS IN THE SERUM OF LysM-Nrf2−/−, LysM-Keap1−/−, Nrf2f/f, AND Keap1f/f MICE 24 HOURS AFTER CLP
| Inflammatory mediators | Sham |
CLP |
||||||
| Nrf2f/f | LysM-Nrf2−/− | Keap1f/f | LysM-Keap1−/− | Nrf2f/f | LysM-Nrf2−/− | Keap1f/f | LysM-Keap1−/− | |
| IL-1β | BDL | BDL | BDL | BDL | 128 ± 166 | 294* ± 261 | 89 ± 47 | 45 ± 23 |
| IL-2 | 30 ± 14 | BDL | BDL | 32 ± 16 | 254 ± 124 | 333 ± 286 | 117 ± 194 | 66 ± 76 |
| L-4 | BDL | BDL | BDL | BDL | 59 ± 45 | 126* ± 70 | BDL | BDL |
| IL-5 | 11 ± 5 | 25 ± 25 | 19 ± 14 | 20 ± 12 | 31 ± 22 | 69 ± 35 | 27 ± 13 | 12† ± 7 |
| IL-1ra | BDL | 186 ± 69 | BDL | 198 ± 86 | 1,602 ± 947 | 8,337‡± 8,133 | 3,224 ± 2,312 | 566† ± 563 |
| IL-12p70 | BDL | BDL | BDL | BDL | 64 ± 32 | 195* ± 115 | BDL | BDL |
| IL-13 | BDL | BDL | BDL | BDL | 111 ± 70 | 350* ± 283 | 72 ± 27 | BDL† |
| IFN-γ | BDL | BDL | BDL | BDL | 58 ± 27 | 1,487‡ ± 1,136 | 190 ± 255 | BDL |
| MIP-2 | BDL | BDL | BDL | BDL | 822 ± 477 | 95,704‡ ± 54,811 | 1,662 ± 2,043 | 265† ± 182 |
| MIP-1α | BDL | BDL | BDL | BDL | 1,008 ± 442 | 26,835‡ ± 16,358 | 1,348 ± 1,076 | BDL |
| Eotaxin | 623 ± 596 | 4,310 ± 1,615 | 2,155 ± 1,443 | 2,216 ± 1,687 | 1,1432 ± 5,812 | 30,058‡ ± 14,467 | 6,957 ± 6,604 | 4,229 ± 3,875 |
| mEotaxin2 | 453 ± 275 | 905 ± 99 | 982 ± 179 | 513 ± 133 | 1,901 ± 1,395 | 3,510* ± 1,301 | 1,450 ± 1,066 | 989 ± 550 |
| mTNFsrI | 837 ± 130 | 735 ± 115 | 823 ± 118 | 744 ± 75 | 1,527 ± 110 | 7,987‡ ± 2,020 | 2,835 ± 1,428 | 1,630† ± 525 |
| mTNFsrII | 215 ± 105 | 303 ± 65 | 279 ± 55 | 209 ± 92 | 520 ± 83 | 4,486‡ ± 2,047 | 1,089 ± 551 | 479† ± 302 |
| mIL-17 | BDL | BDL | BDL | BDL | 12 ± 10 | 59‡ ± 34 | 7.1 ± 8.7 | BDL† |
| mICAM1 | 1,498 ± 393 | 1,575 ± 669 | 2,058 ± 581 | 2,311 ± 392 | 3,749 ± 1,712 | 5,742* ± 1,589 | 3,764 ± 914 | 4,239 ± 1,211 |
Definition of abbreviations: BDL = below detection limit; CLP = cecal ligation and puncture; ICAM = intercellular adhesion molecule; Keap1 = kelch-like ECH-associated protein; MIP = macrophage inflammatory protein; Nrf2 = nuclear factor–erythroid 2 p45-related factor 2; TNF = tumor necrosis factor.
Significant compared with Nrf2f/f at P < 0.05.
Significant compared with Keap1f/f at P < 0.05.
Significant compared with Nrf2f/f at P < 0.01.
Nrf2 Activation in Innate Immune Cells Preserves Antibacterial Defenses
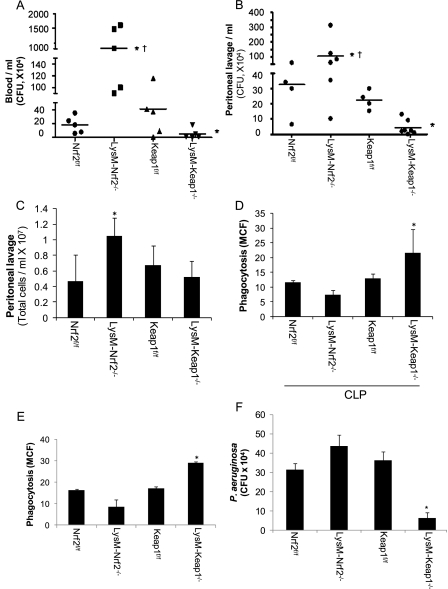
Inadequate immune-inflammatory response may impair antibacterial defenses and enhance risk to bacteremia. To address whether Nrf2 pathway in innate immune cells alters effective antibacterial defenses, bacterial burden was measured in LysM-Keap1−/−, LysM-Nrf2−/−, Keap1f/f, and Nrf2f/f mice 24-hours after CLP. Blood bacteremia (systemic bacterial burden) was significantly lower in LysM-Keap1−/− than in Keap1f/f, whereas it was higher in LysM-Nrf2−/− than in Nrf2f/f and LysM-Keap1−/− mice (Figure 4A). Bacterial burden between Keap1f/f and Nrf2f/f was not significant after CLP. Similarly, bacterial burden in peritoneal cavity (local bacterial burden) was markedly lower in LysM-Keap1−/− than in Keap1f/f, and it was higher in LysM-Nrf2−/− than in Nrf2f/f and LysM-Keap1−/− mice (Figure 4B). These results demonstrate that LysM-Keap1−/− mice showed improved ability to counter bacteremia.
Figure 4.
Keap1 deletion in myeloid cells augments antibacterial defenses, whereas Nrf2 deletion disposes to greater bacterial burden. (A) Blood CFUs in mice 24 hours after CLP. No blood CFU was detected in sham surgery group (data not shown); (B) Bacterial burden in peritoneal activity 24 hours after CLP; (C) Inflammatory cells in the peritoneal cavity 24 hours after CLP; (D) Bacterial phagocytic activity of peritoneal macrophages isolated 24 hours after CLP. (E and F) Phagocytosis and killing of bacteria by peritoneal macrophages isolated from healthy mice. Data were analyzed by Student t test; *Significant compared with flox control group; P < 0.05, †Significant compared with LysM-Keap1−/− group; P < 0.01.
To address whether greater bacterial burden in LysM-Nrf2−/− mice is caused by defective leukocyte migration, we analyzed inflammatory cells in the peritoneal cavity of mice of all genotypes after CLP. Leukocyte cell number (macrophages, neutrophils, and lymphocytes) was markedly higher in LysM-Nrf2−/− mice than in mice of flox control groups and LysM-Keap1−/−. No significant difference in the leukocyte cell number between LysM-Keap1−/− and floxed control groups was noted (Figure 4C). Macrophages from septic mice show impaired bacterial phagocytosis and are well correlated with bacterial burden and mortality (24). To determine whether Nrf2 signaling effects bacterial phagocytosis, we analyzed ex vivo bacterial phagocytic activity in peritoneal macrophages isolated from LysM-Keap1−/−, LysM-Nrf2−/−, Keap1f/f, and Nrf2f/f mice 24-hours after CLP. Macrophages from LysM-Keap1−/− mice showed greater phagocytosis of opsonized fluorescent-labeled (fluorescein isothiocyanate) P. aeruginosa, whereas macrophages from LysM-Nrf2−/− mice showed impaired phagocytosis (Figure 4D). Phagocytic ability was similar in Keap1f/f and Nrf2f/f (Figure 4D). We also examined bactericidal activity by peritoneal macrophages isolated from healthy mice. LysM-Keap1−/− macrophages showed enhanced bacterial phagocytosis and bactericidal activity compared with LysM-Nrf2−/− mice (Figures 4E and 4F) and floxed controls. The bactericidal activity was comparable among LysM-Nrf2−/−, Keap1f/f, and Nrf2f/f macrophages. These results suggest that enhancing Nrf2 activity in macrophages improves bactericidal activity and mediates appropriate innate immune-inflammatory response during sepsis.
Nrf2 Modulates Inflammatory Response by Redox Regulation of TLR4 Signaling
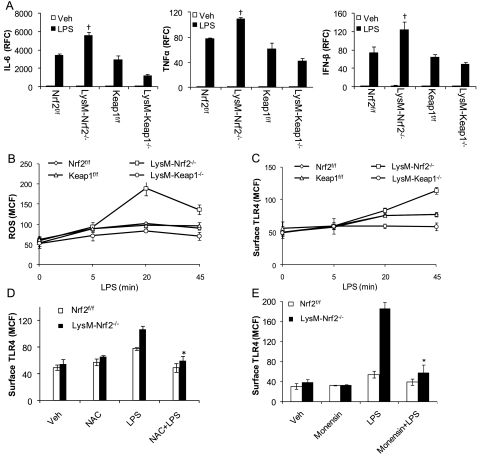
Several studies have shown that ROS modulates TLR signaling (25, 26). Because Nrf2 regulates several antioxidant defenses (see Table E1), we investigated inflammatory response in peritoneal macrophages isolated from LysM-Keap1−/−, LysM-Nrf2−/−, Keap1f/f, and Nrf2f/f toward different TLR ligands (TLR4-LPS, TLR3-Poly[I:C], TLR9-CpG, TLR2-LTA) and H2O2. The mRNA expression levels of IL-6, TNF-α, and IFN-β were significantly reduced in LysM-Keap1−/− macrophages, whereas the expression of these cytokines was highest in LysM-Nrf2−/− macrophages (Figure 5A; see Figure E4). We have previously shown that enhancing Nrf2 activity by disruption of Keap1 in lung epithelial cells dampens H2O2-induced ROS levels (27). As expected, higher antioxidant defenses attenuated H2O2-induced inflammatory response in LysM-Keap1−/− macrophages. On the contrary, H2O2 treatment augmented inflammatory response in LysM-Nrf2−/− macrophages compared with Nrf2f/f.
Figure 5.
Nrf2–dependent redox regulation of Toll-like receptor (TLR)4 surface expression in macrophages. (A) mRNA expression of IL-6, TNF-α, and IFN-β in LPS-stimulated peritoneal macrophages isolated from Nrf2f/f, LysM-Nrf2−/−, Keap1f/f, and LysM-Keap1−/− mice as assessed by quantitative polymerase chain reaction. (B) Levels of reactive oxygen species (ROS) in macrophages at, 5, 20, and 45 minutes after LPS challenge as assessed by fluorescence-activated cell sorter (FACS) analysis. (C) Surface expression of TLR4 on macrophages 5, 20, and 45 minutes after LPS treatment by FACS analysis. (D and E) LPS-induced surface expression of TLR4 in macrophages pretreated with N-acetylcysteine (NAC) (0.5 mM) (D) or monensin (10 μM) (E) for 30 minutes by FACS analysis. Surface TLR4 expression was assessed 45 minutes after LPS challenge. Data are presented as mean channel fluorescence (MCF) ± SD. All data presented are representative of three independent experiments. Data were analyzed by Student t test; †Significant compared with LysM-Keap1−/− group; *Significant compared with LPS alone; P < 0.05.
LPS responsiveness is partly regulated by the levels of TLR4 present on the plasma membrane. Recent studies have indicated that LPS stimulation increases surface expression of TLR4 on macrophages, which is mainly mediated by ROS (28). We hypothesize that Nrf2 modulates innate immune response by redox regulation of TLR signaling. In response to LPS, we found a time-dependent increase in intracellular ROS levels in peritoneal macrophages. The intracellular levels of ROS were quantified through dichlorofluorescin diacetate staining followed by flow cytometry analysis. The levels of intracellular ROS in LPS-stimulated macrophages from Keap1f/f and Nrf2f/f were similar. However, ROS levels in macrophages of LysM-Nrf2−/− were significantly elevated, whereas they were markedly reduced in LysM-Keap1−/− compared with floxed control (Figure 5B) at all the time-points tested. Concurrently, next we investigated TLR4 surface expression in peritoneal macrophages after LPS stimulation. Flow cytometry analysis revealed a significant increase in TLR4 surface trafficking after LPS stimulation in macrophages of all the genotypes. Constitutively, surface TLR4 expression in macrophages was comparable among the genotypes. However, macrophages from LysM-Nrf2−/− mice showed significantly higher surface expression of TLR4 compared with LysM-Keap1−/− and flox control 20 and 45 minutes after LPS stimulation. The TLR4 surface expression was comparable between Keap1f/f and Nrf2f/f after LPS stimulation (Figure 5C). No significant differences were observed in total TLR4 protein in vehicle- and LPS-treated macrophages from mice of all genotypes as revealed by immunoblot analysis (see Figure E5A). Similar results were obtained using immunocytochemistry analysis (see Figure E5B). To address that higher ROS levels in LysM-Nrf2−/− macrophages caused elevated TLR4 surface expression, we assessed TLR4 surface trafficking in presence of exogenous antioxidant scavenger, N-acetylcysteine. N-acetylcysteine significantly dampened LPS-induced TLR4 surface expression (Figure 5D) in LysM-Nrf2−/− macrophages and the levels were comparable with Nrf2f/f macrophages.
The cell surface level of TLR4 is determined by the amount of TLR4 trafficking from the Golgi to the plasma membrane and the amount of TLR4 internalized into endosomes (29). To determine whether LPS stimulation increases TLR4 trafficking from Golgi to plasma membrane, we examined the TLR4 surface expression and IL-6 levels (as a downstream indicator of TLR4 signaling) in macrophages from Nrf2f/f and LysM-Nrf2−/− mice in the presence of monensin (30), a specific inhibitor of trans-Golgi transport, after LPS stimulation. LPS stimulation induced approximately threefold increase in the surface expression of TLR4 in macrophages from LysMsM-Nrf2−/− compared with Nrf2f/f. However, in the presence of monensin, the surface expression of TLR4 was significantly reduced in macrophages from LysM-Nrf2−/− and was comparable with Nrf2f/f after LPS stimulation (Figure 5E). Concomitantly, the IL-6 levels were also significantly reduced in the presence of monensin after LPS stimulation (see Figures E6A and E6B).
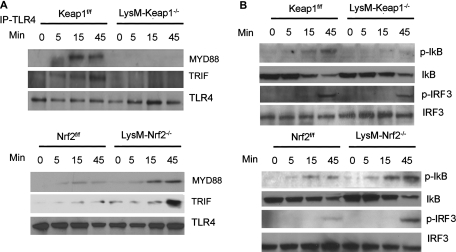
Next, we examined whether higher surface levels of TLR4 led to increase in the recruitment of downstream adapter molecules and signaling pathway. We found greater recruitment of Myd88 and TRIF to TLR4 and activation of Nf-κB pathway (as indicated by phosphorylation of IκB and degradation of IkB) and IRF3 pathway (phosphorylated-IRF3) in peritoneal macrophages from LysM-Nrf2−/− mice compared with LysM-Keap1−/− mice (Figures 6A and 6B). In agreement, we observed significantly higher expression of Myd88-dependent cytokine, TNF-α and TRIF-dependent cytokine, IFN-β in LPS-treated LysM-Nrf2−/− macrophages compared with LysM-Keap1−/− macrophages (Figure 5A). Taken together, these results suggest that the amplified inflammatory response in Nrf2-deficient macrophages is mediated by ROS-dependent regulation of TLR4 signaling partly by controlling the translocation of TLR4 from trans-Golgi network to plasma membrane.
Figure 6.
Nrf2–dependent regulation of TLR4–Nf-κB and TLR4-IRF3 signaling in macrophages. (A) Immunoblot analysis of MYD88, TRIF, and TLR4 in the macrophage cell lysates immunoprecipitated with anti-TLR4 antibody after LPS treatment. (B) Immunoblot analysis of phosphorylated IκB and IRF3 and total IκB and IRF3 in macrophage cell lysate after LPS challenge. Cell lysates were prepared from macrophages 0, 5, 15, and 45 minutes after LPS challenge. All immunoblots shown are representative of three independent experiments.
Discussion
Myeloid lineage phagocytic leukocytes, macrophages, and neutrophils are essential for host defenses against bacterial infection and play an important role in the pathogenesis of sepsis. Aberrant activation and impaired bacterial killing ability of macrophage and neutrophils is well correlated with severity and poor outcome in patients with sepsis. ROS may play a pivotal role in aberrant functions of macrophages and neutrophils and pathogenesis of sepsis. Excess intracellular ROS function as a signal messenger and may mediate hyperactivation of innate immune cells by affecting signal transduction. Extracellular ROS released from leukocytes (hypochloric acid, superoxide, hydrogen peroxide, and peroxynitrate) mediates tissue injury subsequent to activation of innate immune responses. Nrf2 is a ubiquitous transcription factor that regulates pleiotropic cytoprotective defenses including several antioxidants and electrophile detoxification proteins, which collectively protect against oxidative-mediated inflammatory process and tissue injury. We report that increasing Nrf2 regulated antioxidants in myeloid leukocytes improves septic survival by balancing inflammatory responses via redox regulation of TLR4–nuclear factor-κB (NF-κB) signaling while preserving antibacterial defenses.
We previously reported that Nrf2 is essential for survival in LPS shock- and CLP-induced sepsis using Nrf2−/− global knockout mice (16). However, it was unclear whether Nrf2 affected immune-inflammatory response or tissue injury that helped in survival of mice after sepsis and whether increasing Nrf2-regulated endogenous antioxidants in macrophages and neutrophils would be protective or detrimental in sepsis. To address these questions, we used mice with deletion of Nrf2 or Nrf2 inhibitor, Keap1 specifically in myeloid lineage leukocytes (macrophages and neutrophils). The constitutive expression of Nrf2-regulated antioxidant genes (GSH biosynthesizing enzymes and GSH [data not shown]) was significantly higher in macrophages and neutrophils from LysM-Keap1−/− compared with LysM-Nrf2−/− mice, whereas the expression of these genes was comparable between Nrf2f/f and Keap1f/f. We found that the deletion of Nrf2 in myeloid leukocytes augmented mortality, whereas the deletion of Keap1 dramatically protected against CLP-induced mortality. The mortality between Nrf2f/f and Keap1f/f after CLP was not significant. In agreement with mortality data, the cytokine array data showed that the levels of both the proinflammatory (e.g., IL-6, TNF, and MIP-2) and antiinflammatory (e.g., IL-10 and IL-1ra) cytokines were markedly higher in serum from LysM-Nrf2−/− compared with LysM-Keap1−/− mice after CLP. In a recent study, IL-17 has been demonstrated to promote mortality in sepsis by increasing proinflammatory response, neutrophil mobilizing cytokines, and bacteremia (31). The level of IL-17 was significantly higher in LysM-Nrf2−/− mice than in LysM-Keap1−/− mice. HMGB1, a well-characterized cytokine with pleiotropic function, is a crucial late mediator of mortality in sepsis (32). Secretory HMGB1 augments proinflammatory response and promotes inflammation and tissue injury (33).The level of HMGB-1, was also significantly higher in LysM-Nrf2−/− compared with LysM-Keap1−/−. Both mice and clinical studies have documented that the balance of proinflammatory and antiinflammatory cytokines is a better predictive marker of severity and mortality in sepsis rather than a single mediator (23, 34). The analysis of proinflammatory and antiinflammatory cytokines after increasing severity of CLP in mice models showed that the higher expression of antiinflammatory cytokine, such as IL-10, restrains the proinflammatory responses and improves the survival of septic mice (23). In this study, the ratio of antiinflammatory cytokine (IL-10 or IL-1ra) versus proinflammatory cytokine (IL-6, TNF, or MIP2) was markedly higher (greater than fivefold) in LysM-Keap1−/− compared with LysM-Nrf2−/−. Taken together, these data suggest that inadequate antiinflammatory responses may be partly responsible for severe systemic inflammatory response syndrome and mortality of LysM-Nrf2−/− mice. On the contrary, robust antiinflammatory defenses improved survival of LysM-Keap1−/− mice after CLP.
Excess ROS production in leukocytes in response to bacterial pathogen can alter redox signaling cascade affecting membrane receptors, protein kinases, phosphatases, and transcription factors resulting in aberrant inflammatory responses and phagocytic activity (35). ROS augment onset of inflammatory response by effecting NF-κB signaling (36, 37). Conversely, inhibition of ROS using exogenous antioxidant scavengers, such as N-acetylcysteine and vitamin E, attenuates inflammation (36, 37). Therefore, antioxidant defenses are critical to leash inflammatory response; however, it could be hypothesized that excess antioxidants may predispose to immune suppression. The fact that patients with sepsis are associated with immune suppression rather than immune activation further raises this skepticism (38). In this study, we found that increasing endogenous antioxidant defenses regulated by Nrf2 in effector cells of innate immunity, macrophages, and neutrophil attenuated inflammation without compromising host antibacterial defense mechanisms. In in vitro studies, we found that Keap1−/− macrophages showed a diminished inflammatory response after stimulation by a variety of TLR agonists (TLR2, TLR3, TLR4, and TLR9) compared with wild-type macrophages. On the contrary, deficiency of Nrf2 enhanced inflammatory response after stimulation with these TLR agonists. In in vivo studies, we found a markedly lower number of leukocytes in the peritoneum of LysM-Keap1−/− compared with LysM-Nrf2−/− or Keap1f/f after CLP. Concomitantly, the bacterial colonization was significantly lower in the peritoneal cavity and blood of LysM-Keap1−/− mice compared with LysM-Nrf2−/− or Keap1f/f. Peritoneal macrophages from septic mice are associated with impaired bacterial phagocytic ability (24). We found greater bacterial phagocytic ability of peritoneal macrophages isolated from LysM-Keap1−/− compared with LysM-Nrf2−/− or Keap1f/f after CLP. At present, the underlying molecular mechanisms responsible for improved bacterial phagocytic ability by Keap1−/− macrophages after CLP are unclear and further investigations are needed. Lower peritoneal and systemic inflammation in LysM-Keap1−/− mice compared with LysM-Nrf2−/− or Keap1f/f after CLP may be partly caused by the lower bacterial colonization and a diminished inflammatory response.
LPS, a constituent of outer membrane of gram-negative bacteria, is recognized by the surface receptor TLR4. On recognition, TLR4 dimerizes and activates two downstream signaling pathway mediated by MYD88 and TRIF. Through cascade of events, Myd88 and TRIF signaling pathway leads to the activation of NF-κB and IRF3 (39). Disruption or defective TLR4 dampens LPS-induced inflammation in mice (40). Similarly, a weak surface expression of TLR4 on phagocytes suppresses inflammatory response. Several studies have suggested that ROS modulate TLR4 activation by augmenting TLR4 trafficking to surface (28, 41). Inhibition of ROS generation by disruption of NADP reduced oxidase activity dampens TLR4 activation partly by reducing TLR4 surface trafficking. Previously, we reported that excess ROS generation caused by NADP reduced oxidase activity augments TLR4 surface trafficking and downstream signaling in Nrf2−/− macrophages resulting in enhanced inflammatory response (18). In agreement with the previous study, we found greater intracellular ROS levels and concurrent TLR4 surface expression and activation of downstream signal transducers (Myd88, TRIF, phosphorylated-IkB, and phosphorylated-IRF3) in peritoneal macrophages isolated from LysM-Nrf2−/− compared with Nrf2f/f after LPS stimulation. In contrast, Keap1−/− macrophages (high Nrf2 activity) were associated with reduced ROS and TLR4 signaling compared with Keap1f/f or LysM-Nrf2−/− after LPS challenge. These results suggest that Nrf2 modulates TLR4 activation by regulating intracellular ROS levels. Although ROS affect TLR4 surface trafficking, the underlying molecular regulators are unknown. In normal unstimulated cells, TLR4 is primarily located in the Golgi and plasma membrane (42). Using a specific inhibitor of trans-Golgi transport, we demonstrated that Nrf2 deficiency augments TLR4 surface expression partly by increasing TLR4 trans-Golgi transport to cell surface.
Because of the dynamics and complexity of the sepsis syndrome, clinical trials designed to limit inflammation either by specific anticytokine therapy, antiinflammatory drugs, or antioxidant therapy have failed (1, 5). Our data suggest that the Nrf2 pathway functions as a critical nodal point in redox modulation of TLR signaling in innate immune cells, limits dysregulation of inflammatory response, and preserves host antibacterial defenses in sepsis. Pharmacologic activators of Nrf2 may function as an “immunomodulator” for therapeutic intervention in sepsis.
Supplementary Material
Footnotes
Supported by NIH grants GM079239 (S.B.) and HL081205 (S.B.); NHLBI SCCOR grants P50HL084945 (S.B.), 5P50ES015903 (S.B.), and GM50401 (D.R.); NIEHS Center grant ES03819; Clinical Innovator Award from FAMRI (S.B.); Young Clinical Scientist Award from FAMRI (R.T.); and HL66109 (S.P.R.) and ES11863 (S.P.R.).
Author's contribution: X.K. performed the experiments and analyzed the data. R.T. and S.B. conceived the study, designed the experiments, analyzed and interpreted the data, and wrote the manuscript. A.S. helped in design and generation of conditional knockout mice. P.K. helped in development and crossing of mice. C.H. performed bacterial phagocytosis experiments. S.P.R. contributed in generation of Nrf2 flox/flox mice. D.R. and F.C. helped in cytokine immunoassay.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201102-0271OC on July 28, 2011
Author Disclosure: X.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.T. received institutional grant support from the Flight Attendant Medical Research Institute. F.C., C.H., A.S., P.K., S.P.R., D.R., and S.B. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med 2003;9:517–524 [DOI] [PubMed] [Google Scholar]
- 2.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med 2002;28:108–121 [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 4.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez, Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344:699–709 [DOI] [PubMed] [Google Scholar]
- 5.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 2008;8:776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves-Filho JC, Freitas A, Souto FO, Spiller F, Paula-Neto H, Silva JS, Gazzinelli RT, Teixeira MM, et al. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci U S A 2009;106:4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med 2008;205:2609–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med 2008;205:1277–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiller S, Elson G, Ferstl R, Dreher S, Mueller T, Freudenberg M, Daubeuf B, Wagner H, Kirschning CJ. TLR4-induced IFN-gamma production increases TLR2 sensitivity and drives Gram-negative sepsis in mice. J Exp Med 2008;205:1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007;47:89–116 [DOI] [PubMed] [Google Scholar]
- 11.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 2004;114:1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 2002;62:5196–5203 [PubMed] [Google Scholar]
- 13.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 2005;202:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 2008;178:592–604 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Paupe V, Dassa EP, Goncalves S, Auchere F, Lonn M, Holmgren A, Rustin P. Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia. PLoS ONE 2009;4:e4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 2006;116:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 1999;8:265–277 [DOI] [PubMed] [Google Scholar]
- 18.Kong X, Thimmulappa R, Kombairaju P, Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol 2010;185:569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun 2006;351:883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 2011;3:78ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonifer C, Bosch FX, Faust N, Schuhmann A, Sippel AE. Evolution of gene regulation as revealed by differential regulation of the chicken lysozyme transgene and the endogenous mouse lysozyme gene in mouse macrophages. Eur J Biochem 1994;226:227–235 [DOI] [PubMed] [Google Scholar]
- 22.Cross M, Mangelsdorf I, Wedel A, Renkawitz R. Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci USA 1988;85:6232–6236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol 2006;177:1967–1974 [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA 2009;106:6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biswal S, Remick DG. Sepsis: redox mechanisms and therapeutic opportunities. Antioxid Redox Signal 2007;9:1959–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolls JK. Oxidative stress in sepsis: a redox redux. J Clin Invest 2006;116:860–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blake DJ, Singh A, Kombairaju P, Malhotra D, Mariani TJ, Tuder RM, Gabrielson E, Biswal S. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am J Respir Cell Mol Biol 2010;42:524–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers KA, Szaszi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A, Rotstein OD. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med 2006;203:1951–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGettrick AF, O'Neill LA. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr Opin Immunol 2010;22:20–27 [DOI] [PubMed] [Google Scholar]
- 30.Mollenhauer HH, Morre DJ, Rowe LD. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta 1990;1031:225–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, et al. Adverse functions of IL-17A in experimental sepsis. FASEB J 2008;22:2198–2205 [DOI] [PubMed] [Google Scholar]
- 32.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 2006;441:513–517 [DOI] [PubMed] [Google Scholar]
- 33.Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol 2004;173:3467–3481 [DOI] [PubMed] [Google Scholar]
- 34.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 2000;181:176–180 [DOI] [PubMed] [Google Scholar]
- 35.Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 2007;42:153–164 [DOI] [PubMed] [Google Scholar]
- 36.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in toll-like receptor 4-dependent activation of NF-kappaB. J Immunol 2004;172:2522–2529 [DOI] [PubMed] [Google Scholar]
- 37.Mitra S, Abraham E. Participation of superoxide in neutrophil activation and cytokine production. Biochim Biophys Acta 2006;1762:732–741 [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150 [DOI] [PubMed] [Google Scholar]
- 39.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511 [DOI] [PubMed] [Google Scholar]
- 40.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci USA 2009;106:2348–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Sasidhar M, Nabel EG, Takahashi T, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med 2006;203:2377–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2–CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem 2002;277:47834–47843 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.