The brain iron overload disorders, collectively known as neurodegeneration with brain iron accumulation (NBIA), would seem to be tantalizing targets for chelation therapy. Efforts to lower brain iron in these disorders date back to the 1960s with deferoxamine mesylate, but systemic iron depletion limited its use.1 With the advent of chelators better able to specifically relieve central nervous system (CNS) iron overload, their clinical utility in this group of disorders with no other targeted therapy is again being explored.
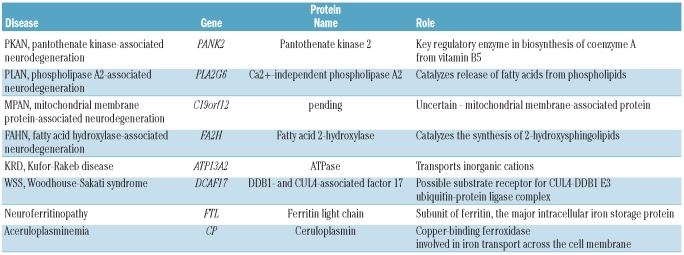
While chelating agents were evolving, so too was our understanding of the pathogenesis of these disorders. NBIA is a group of clinically and genetically heterogeneous neurological disorders in which iron accumulates in a subset of basal ganglia and is easily detected by MRI (Figure 1). While the pathophysiology of some forms of NBIA have a clear connection to metal homeostasis, for most the link to iron remains unclear.2 Despite this gap in knowledge, our insight into the primary pathogenesis of most forms of NBIA has progressed with the identification of each new disease gene (Table 1).3 While this group covers a diverse range of biological pathways, a feature common to many of the proteins defective in NBIA is their role in mitochondrial energy metabolism and membrane remodeling. Why the central nervous system should be selectively vulnerable to defects in these proteins, many of which have a wide pattern of tissue distribution, is another enigma.
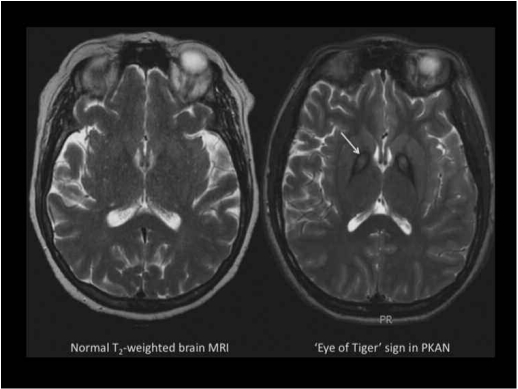
Figure 1.
(Left) Normal T2-weighted axial MRI. (Right) T2-weighted axial MRI from a patient with PKAN. In the globus pallidus, the region of central hyperintensity represents tissue edema and precedes the emergence of iron accumulation, which is seen as the surrounding hypointense region. This pattern is called the ‘eye of the tiger’ sign.
Table 1.
Causes of neurodegeneration with brain iron accumulation (NBIA).
How do defects in pathways that underlie major forms of NBIA, including coenzyme A biosynthesis and phospholipid turnover, lead to iron accumulation? Furthermore, is the observed brain iron necessarily toxic? For the moment, there are no convincing data to answer these questions. Despite the name, neurodegeneration with brain iron accumulation should not be assumed to be neurodegeneration due to brain iron accumulation. Nevertheless, high levels of free iron lead to free radical damage and tissue destruction in many disorders, so it is reasonable to hypothesize that iron contributes to disease pathogenesis and progression in NBIA as well. In pantothenate kinase-associated neurodegeneration (PKAN), the most common form of NBIA, neuroimaging evidence of abnormal basal ganglia iron deposition is generally evident prior to onset of clinical disease. But brain iron is variably present in other forms of NBIA and seemingly without a clear relationship to clinical disease severity or progression. Even in PKAN, there are cases of clinically evident illness prior to the appearance of the classic ‘eye of the tiger’ sign on brain MRI, further suggesting that iron accumulation is a secondary phenomenon in the pathophysiology of the disease. Thus it is worth asking how much iron contributes to morbidity, and as a corollary, what the therapeutic potential is of chelation: if we are able to normalize brain iron levels, will there still be a disease process in PKAN and other forms of NBIA?
The promise of iron chelation in NBIA is under active scrutiny, including a pilot trial reported by Abbruzzese et al. in this issue of the Journal.4 An individual case report5 and a separate pilot phase II study by Zorzi et al.6 have suggested that deferiprone is safe, well-tolerated and effective in lowering iron levels in brain as measured by MRI; conclusions also supported by the current study. Clinical benefit, however, is less clear from these combined data. Both studies evaluated clinical status but neither was powered for this outcome; nor were they of a duration likely to show a difference. In the Abbruzzese study, 3 of 6 patients showed improvement in a 12-month period; 2 of these 3 had PKAN. However, another patient with PKAN showed a trend towards worsening and the remaining patients did not change. In the Zorzi study of 10 PKAN patients, no change in clinical status was demonstrated despite a robust decrease in brain iron measurements.
There are several possible reasons for this apparent lack of a connection between a decrease in brain iron levels and an improvement in clinical status. The most obvious is that brain iron accumulation is not, in fact, key to the pathogenesis of the disease. But other considerations underscore the challenges of performing clinical trials in a rare disorder. The phenotypic spectrum of PKAN spans age and disease severity, ranging from rapidly progressive, profoundly disabling dystonia in early childhood to mild Parkinsonism beginning in adulthood and advancing slowly to disability in the eighth decade. No clinical scale effectively captures this range of features across the lifespan; indeed, no clinical scale reasonably could. The natural history of PKAN poses a further challenge. Progression may be explosive and episodic with periods of rapid decline over weeks, or it may be exceedingly slow, with clinical stability over years. None of the clinical rating scales is likely to be sensitive enough to detect differences over the time course of either of these pilot studies for most patients with PKAN.
An additional challenge of the Abbruzzese study is the inclusion of a genetically heterogeneous group of study participants. Though all are reported to have some form of NBIA, only 4 of 6 have PKAN. By including much older participants with other primary disorders affecting different brain regions, the authors have limited the conclusions that can be drawn from their work since the underlying disease processes and the specific role iron plays in each will differ.
Clearly what is needed is a large multi-center placebo-controlled study of deferiprone in a genetically homogeneous population over a longer duration with greater statistical power to detect differences in this challenging disorder. Fortunately, such a trial has now been funded by the European Commission and is set to begin in 2012. By designing a trial that addresses many of the recognized challenges and obstacles of rare disease clinical research, investigators have the best chance to answer the question of whether chelation improves clinical outcomes in patients with PKAN. Families affected by NBIA have been encouraged by these pilot studies as well as by individual experiences and have waited patiently for a definitive study. The international community is now poised to answer this question in PKAN.
Footnotes
(Related Original Article on page 1708)
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Dooling EC, Schoene WC, Richardson EP., Jr Hallervorden-Spatz syndrome. Arch Neurol. 1974;30(1):70–83. doi: 10.1001/archneur.1974.00490310072012. [DOI] [PubMed] [Google Scholar]
- 2.Gregory A, Polster BJ, Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet. 2009;46(2):73–80. doi: 10.1136/jmg.2008.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregory A, Hayflick SJ. Genetics of neurodegeneration with brain iron accumulation. Curr Neurol Neurosci Rep. 2011;11(3):254–61. doi: 10.1007/s11910-011-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbruzzese G, Cossu G, Balocco M, Marchese R, Murgia D, Melis M, et al. A pilot trial of deferiprone for neurodegeneration with brain iron accumulation. Haematologica. 2011 Jul 26; doi: 10.3324/haematol.2011.043018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forni GL, Balocco M, Cremonesi L, Abbruzzese G, Parodi RC, Marchese R. Regression of symptoms after selective iron chelation therapy in a case of neurodegeneration with brain iron accumulation. Mov Disord. 2008;23(6):904–7. doi: 10.1002/mds.22002. [DOI] [PubMed] [Google Scholar]
- 6.Zorzi G, Zibordi F, Chiapparini L, Bertini E, Russo L, Piga A, et al. Iron-related MRI images in patients with pantothenate kinase-associated neurodegeneration (PKAN) treated with deferiprone: Results of a phase II pilot trial. Mov Disord. 2011;26(9):1755–9. doi: 10.1002/mds.23751. [DOI] [PubMed] [Google Scholar]