The 46/1 haplotype of chromosome 9p, present in about 45% of the general population, is associated with a predisposition to mutations in the Janus Kinase 2 (JAK2) gene on the same allele and to chronic myeloproliferative neoplasms (MPN): polycythemia vera, essential thrombocythemia and primary myelofibrosis.1–4 The 46/1 haplotype is also associated with a predisposition to MPN with no mutation of JAK2, and with MPN with mutation in MPL, a gene located on a different chromosome (1p).5,6 An increased frequency of the 46/1 haplotype in patients with splanchnic vein thrombosis has also been reported but these findings remain controversial.7,8 The 46/1 haplotype has been studied in chronic myelogenous leukemia and in chronic myelomonocytic leukemia; no significant increase in frequency was noted (Table 1). Last year Andrikovics et al. reported that patients with acute myeloid leukemia (AML) with the 46/1 haplotype had a higher frequency of normal karyotype (NK).9 In the present issue of Haematologica, the same group found that the JAK2 46/1 haplotype is associated with an increased frequency of acute myelomonocytic leukemia and a tendency to reduced survival because of death from infection in patients with NK-AML.10 The latter findings need confirmation by other studies, for it is of great interest to establish whether the JAK2 46/1 haplotype is in fact a marker of myelomonocytic dysfunction and subsequently an unfavorable risk factor in NK-AML, as Nahajevszky et al. suggest, and/or in other disease categories.10 In fact, although the majority of studies have failed to detect any association of the 46/1 haplotype with hematologic and clinical parameters (Table 1), Tefferi et al. found that the 46/1 haplotype status influenced survival in primary myelofibrosis, and evolution toward myelofibrosis in polycythemia vera.5 Moreover, it has been reported that the frequency of the JAK2 46/1 haplotype is increased in the context of severe inflammation, for instance Crohn’s disease.11,12 Altogether, the literature (reviewed in Table 1) shows that the common 46/1 haplotype is associated with predisposition to several types of disorders of variable severity: rare myeloid malignancies with or without JAK2/MPL mutation, including MPN and perhaps also acute myelomonocytic leukemia and NK-AML; inflammatory diseases; and possibly, reduced defense against infection. The mechanisms that underlie the increased risk of acquisition of MPN and JAK2/MPL mutation in carriers of the 46/1 haplotype are not understood. Additionally, the mechanisms that make the V617F mutation occur preferentially in the JAK2 gene of the 46/1 allele remain largely unknown.
Table 1.
JAK2 46/1 allele frequencies and clinical correlations reported in the literature.
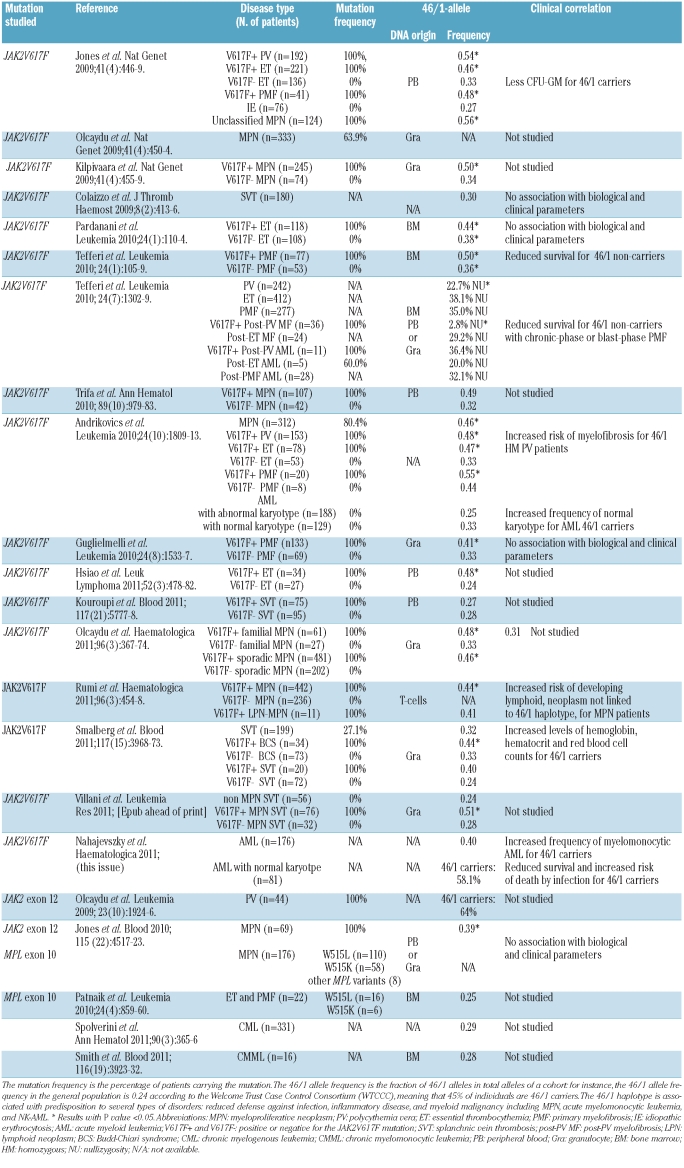
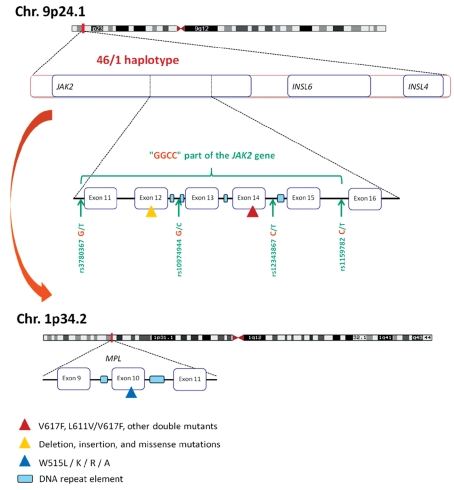
A haplotype (contraction of “haploid genotype”) is a set of closely linked genetic markers present on the same chromosome, which are not easily separable by recombination and thus tend to be inherited together, and can be identified by patterns of single nucleotide polymorphisms. The 46/1 haplotype, represented in Figure 1, is a 280 Kb-long region of chromosome 9p that includes three genes in their entirety: JAK2, INSL6 (Insulin-like 6) and INSL4. Of the three genes, only JAK2 is expressed in hematopoietic cells. The so-called “GGCC” part of the 46/1 haplotype begins in intron 10 and finishes in intron 15 of the JAK2 gene and is characterized by four single nucleotide polymorphisms located in introns 10, 12, 14 and 15. The four single nucleotide polymorphisms are in complete linkage disequilibrium, meaning that they are always inherited together. The four single nucleotide polymorphisms replace three thymidines (T) and one cytosine (C) by two guanosines (G) and two cytosines, resulting in a G G C C pattern, hence the phrase JAK2 “GGCC” haplotype (not to be confused with “GC-rich”). The “GGCC” part of the 46/1 haplotype is not “GC-rich” but it includes sequences frequently mutated in MPN: JAK2 exons 14 and 12, and to a lesser degree, exons 13 and 15. A plausible explanation for the high frequency of mutations in JAK2 exons 14 and 12 and in MPL exon 10 in association with MPN combines the presence of flanking DNA repeat elements (represented in Figure 1) and the fact that the three exons encode for protein domains critical for the function of the Jak2/Mpl couple. Activating mutations in these exons are likely to be detected as they confer growth advantages to the mutated clone, whereas silent mutations typically remain below the detection level of most diagnostic assays.13 DNA repeat elements include GC- or AT-rich sequences; they can be mutation “hot spots”, cause mismatching during DNA replication, or form fragile chromosomal break points – in short, these elements increase the risk of error when DNA is copied during cell division. DNA repeat elements can also influence gene expression by altering promoter accessibility, methylation or the chromatin structure. Our preliminary unpublished observations suggest that DNA repeat intronic sequences vary depending on individuals and MPN patients and that they can be present in the JAK2 gene independently of the 46/1 haplotype.
Figure 1.
The 46/1 haplotype associated with a predisposition to mutation in the JAK2 and MPL genes and MPN. The 46/1 haplotype is a 280 Kb-long region of chromosome 9p which includes the entire JAK2, INSL6 and INSL4 genes. Most of the JAK2 mutations detected in MPN are localized in the “GGCC” part of the JAK2 gene, represented in green. The “GGCC” part, which begins in intron 10 and finishes in intron 15 of the JAK2 gene, is characterized by four single nucleotide polymorphisms: rs3780367 in intron 10, rs10974944 in intron 12, rs12343867 in intron 14, and rs1159782 in intron 15. These four single nucleotide polymorphisms are in complete linkage disequilibrium. The “GGCC” part of the 46/1 haplotype includes the most frequently mutated JAK2 exons: exon 14 (mainly the V617F mutation, represented by a red triangle), exon 12 (mutations and deletions are represented by a yellow triangle) and to a lesser degree, exons 13 and 15. In addition, the 46/1 haplotype has been reported to be associated with a predisposition to the acquisition of mutations in exon 10 of the MPL gene, located on a different chromosome. MPL exon 10 mutations are represented by a blue triangle. DNA repeat elements (represented by light blue rectangles) can be found close to JAK2 exons 12–15 and close to MPL exon 10. Such repeat sequences are known to increase the risk of DNA mutation or recombination. The presence of repeat elements being independent of the 46/1 haplotype, the mechanisms that make individuals carrying the 46/1 haplotype at higher risk of JAK2 and MPL mutation and MPN are currently not understood.
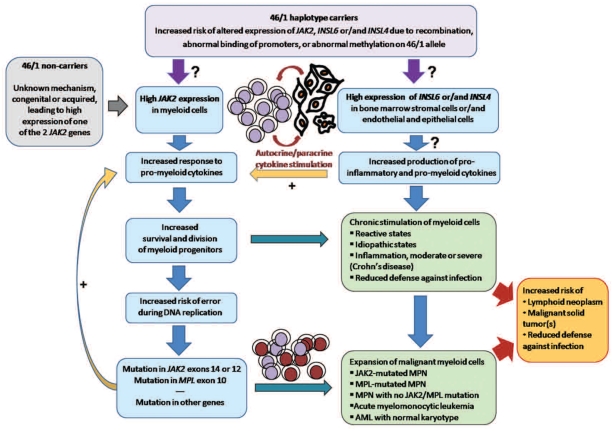
In fact, the JAK2V617F mutation and all three types of MPN occur in individuals who do not carry the 46/1 haplotype. In MPN patients who carry the 46/1 haplotype, the V617F and other JAK2 mutations can be found located in the other (TCTT) allele yet more than 80% of all V617F mutations occur on the 46/1 allele.4,14 Accordingly, the literature shows that the proportions of patients with essential thrombocythemia and primary myelofibrosis with the 46/1 haplotype are significantly higher in JAK2V617F-positive cohorts than in JAK2V617F-negative cohorts (Table 1). Moreover, the 46/1 haplotype can be associated with alterations other than mutations in the JAK2 gene, notably repeated recombination of JAK2 prior to and after the V617F mutation is associated with high JAK2 mRNA expression, as we recently described in patients with polycythemia vera.14 Hence one hypothesis is that the 46/1 haplotype may include unidentified intronic DNA repeat sequences that somehow facilitate DNA recombination and over-expression of the JAK2 gene located in the recombined allele. In such cases, as Jak2 transmits the proliferation signals from all the cytokines critical for myelopoiesis, the 46/1 haplotype would logically predispose carriers to chronically excessive stimulation of myelopoiesis, thereby exposing myeloid progenitors to more frequent cell division and further increased risk of error and mutation in genes more important for myelopoiesis, such as JAK2 and MPL but also TET2, CBL, LNK, EZH2, ASXL1 (list not exhaustive) (representation in Figure 2). We and others have begun to analyze JAK2 mRNA expression in relation to the 46/1 haplotype with discordant results likely due to the small numbers of patients studied.3,6,14,15 However, it is clear that mRNA expression of both wild-type and mutated JAK2 is frequently higher in leukocytes of MPN patients, and that high JAK2 expression is relevant to MPN pathogenesis since it is necessary to reproduce polycythemia vera and primary myelofibrosis phenotypes in murine models.15,16 Further studies specifically designed to analyze the relationship of JAK2 mRNA expression in function of the 46/1 haplotype status of MPN patients, associated with intron sequencing, will prove or disprove association of the 46/1 haplotype with high JAK2 expression.
Figure 2.
The JAK2 46/1 haplotype as a marker of inappropriate myelomonocytic response to cytokine stimulation, leading to increased risk of inflammation, myeloid neoplasm, and impaired defense against infection. One hypothesis is that the 46/1 haplotype may be linked to high expression of the JAK2 gene and/or of other genes that constitute the haplotype: INSL6 and INSL4. The 46/1 haplotype could lead to high expression of the JAK2 gene on the recombined allele through DNA recombination, mutation, altered promoter accessibility or abnormal methylation. This hypothesis is supported by the fact that high mRNA expression of JAK2 is frequently observed in MPN patients, and required for certain MPN phenotypes in murine models. Thanks to high Jak2 levels, affected myeloid cells would be more likely to divide in response to Jak2-activating cytokines, making genes important for myelopoiesis, such as JAK2 and MPL, at greater risk of copy errors. Acquisition of mutations in JAK2, MPL or other genes would accelerate progression toward myeloid neoplasms. For patients who develop MPN, the presence of the 46/1 haplotype could influence disease evolution and survival. Carriers of the 46/1 haplotype who develop AML with normal karyotype (NK-AML) could have reduced survival due to death from infection, according to Nahajevszky et al. (this issue).10 In addition, the 46/1 haplotype may facilitate expression of INSL6 and INSL4 in non-hematopoietic cells, for instance in bone marrow stromal cells. Possible consequences could be excessive production of cytokines with dual action, pro-myeloid and pro-inflammatory, which would further stimulate expansion of myelomonocytic cells and thus facilitate an inflammatory response and perhaps impair defense against infection. Finally, patients with MPN, AML or severe chronic inflammation have been reported to be at greater risk of lymphoid neoplasms and malignant solid tumors.
However, JAK2 is only one of the three genes that constitute the 46/1 haplotype. Because they are presumably not expressed in hematopoietic cells, INSL6 and INSL4 have been disregarded so far. Yet INLS6 is expressed in rat bone marrow stromal cells, and INSL4 expression was reported as forming an autocrine loop in cancer cells.17,18 Thus we should perhaps not exclude that the 46/1 haplotype could be associated with altered expression of INSL6 and/or INSL4, for instance in bone marrow stromal cells, eventually leading to altered cytokine production. There is proof of yet unexplained JAK2V617F-independent cytokine over-production in MPN, by both bone marrow stromal cells and malignant hematopoietic progenitors.19,20 The cytokines that were found to be over-produced were all linked to inflammation as well as capable of stimulating myelopoiesis, an unsurprising finding since evidence of inflammation in MPN is abundant.21 Future studies investigating the possible expression and consequences of INSL6 and INLS4 expression in cell types relevant to myelopoiesis and their relationship to the 46/1 status of patients should bring new and important insights into the pathogenesis of myeloid neoplasms as well as of inflammatory diseases.
Finally, the model according to which the 46/1 haplotype could predispose carriers to increased myelomonocytic response to cytokine stimulation is consistent with the well established fact in MPN that the burden of JAK2V617F alleles (typically 46/1 alleles) correlates better with leukocyte cell counts than with hematocrit or platelet counts.15 A chronic tendency to myelomonocytic expansion would be consistent with increased risks of more severe inflammatory response compared to the risk in 46/1 non-carriers. It would also constitute fertile ground for the acquisition of mutations in genes critical for myelomonocytic expansion and differentiation, eventually leading to malignant transformation toward MPN or AML. It is to be hoped that new genome wide association technologies that investigate both exonic and intronic sequences will provide a detailed analysis of the consequences of the 46/1 haplotype in terms of expression of the JAK2, INSL6 and INSL4 genes and will allow progress in understanding the complex mechanisms that underlie the association of the 46/1 haplotype with predisposition to myeloid neoplasms and inflammation.
Footnotes
(Related Original Article on page 1613)
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Pardanani A, Fridley BL, Lasho TL, Gilliland DG, Tefferi A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood. 2008;111(5):2785–9. doi: 10.1182/blood-2007-06-095703. [DOI] [PubMed] [Google Scholar]
- 2.Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41(4):455–9. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41(4):446–9. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olcaydu D, Harutyunyan A, Jäger R, Berg T, Gisslinger B, Pabinger I, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41(4):450–4. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A, Lasho TL, Patnaik MM, Finke CM, Hussein K, Hogan WJ, et al. JAK2 germline genetic variation affects disease susceptibility in primary myelofibrosis regardless of V617F mutational status: nullizygosity for the JAK2 46/1 haplotype is associated with inferior survival. Leukemia. 2010;24(1):105–9. doi: 10.1038/leu.2009.225. [DOI] [PubMed] [Google Scholar]
- 6.Jones AV, Campbell PJ, Beer PA, Schnittger S, Vannucchi AM, Zoi K, et al. The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood. 2010;115(22):4517–23. doi: 10.1182/blood-2009-08-236448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smalberg JH, Koehler E, Darwish Murad S, Plessier A, Seijo S, Trebicka J, et al. European Network for Vascular Disorders of the Liver (EN-Vie) The JAK2 46/1 haplotype in Budd-Chiari syndrome and portal vein thrombosis. Blood. 2011;117(15):3968–73. doi: 10.1182/blood-2010-11-319087. [DOI] [PubMed] [Google Scholar]
- 8.Kouroupi E, Kiladjian JJ, Chomienne C, Dosquet C, Bellucci S, Valla D, et al. The JAK2 46/1 haplotype in splanchnic vein thrombosis. Blood. 2011;117(21):5777–8. doi: 10.1182/blood-2011-03-343657. [DOI] [PubMed] [Google Scholar]
- 9.Andrikovics H, Nahajevszky S, Koszarska M, Meggyesi N, Bors A, Halm G, et al. JAK2 46/1 haplotype analysis in myeloproliferative neoplasms and acute myeloid leukemia. Leukemia. 2010;24(10):1809–13. doi: 10.1038/leu.2010.172. [DOI] [PubMed] [Google Scholar]
- 10.Nahajevszky S, Andrikovics H, Batai A, Adam E, Bors A, Csomor J, et al. The prognostic impact of germline 46/1 haplotype of Janus kinase 2 in cytogenetically normal acute myeloid leukemia. Haematologica. 2011;96(11):1613–8. doi: 10.3324/haematol.2011.043885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40(8):955–62. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR, et al. Genetic factors in chronic inflammation: single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn’s disease in a New Zealand population. Mutat Res. 2010;690(1–2):108–15. doi: 10.1016/j.mrfmmm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Cleyrat C, Jelinek J, Girodon F, Boissinot M, Ponge T, Harousseau J-L, et al. JAK2 mutation and disease phenotype: A double L611V/V617F in cis mutation of JAK2 is associated with isolated erythrocytosis and increased activation of AKT and ERK1/2 rather than STAT5. Leukemia. 2010;24(5):1069–73. doi: 10.1038/leu.2010.23. [DOI] [PubMed] [Google Scholar]
- 14.Vilaine M, Olcaydu D, Harutyunyan A, Bergeman J, Mourad T, Ramée J-F, et al. Homologous recombination of wild-type JAK2, a novel early step in he development of myeloproliferative neoplasm. Blood. doi: 10.1182/blood-2011-08-372813. in press. [DOI] [PubMed] [Google Scholar]
- 15.Lippert E, Boissinot M, Kralovics R, Girodon F, Dobo I, Praloran V, et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006;108(6):1865–7. doi: 10.1182/blood-2006-01-013540. [DOI] [PubMed] [Google Scholar]
- 16.Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, Skoda RC. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111(8):3931–40. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 17.Qi X, Shao M, Peng H, Bi Z, Su Z, Li H. In vitro differentiation of bone marrow stromal cells into neurons and glial cells and differential protein expression in a two-compartment bone marrow stromal cell/neuron co-culture system. J Clin Neurosci. 2010;17(7):908–13. doi: 10.1016/j.jocn.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Brandt B, Kemming D, Packeisen J, Simon R, Helms M, Feldmann U, et al. Expression of early placenta insulin-like growth factor in breast cancer cells provides an autocrine loop that predominantly enhances invasiveness and motility. Endocr Relat Cancer. 2005;12(4):823–37. doi: 10.1677/erc.1.00975. [DOI] [PubMed] [Google Scholar]
- 19.Corre-Buscail I, Pineau D, Boissinot M, Hermouet S. Erythropoietin-independent erythroid colony formation by bone marrow progenitors exposed to interleukins 11 and 8. Exp Hematol. 2005;33(11):1299–308. doi: 10.1016/j.exphem.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Boissinot M, Cleyrat C, Vilaine M, Jacques Y, Corre I, Hermouet S. Anti-inflammatory hepatocyte growth factor and interleukin-11 are overexpressed in polycythemia vera and contribute to the growth of mutated erythroblasts independently of JAK2V617F. Oncogene. 2011;30(8):990–1001. doi: 10.1038/onc.2010.479. [DOI] [PubMed] [Google Scholar]
- 21.Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E, et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96(2):315–8. doi: 10.3324/haematol.2010.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]