Abstract
Background
Nuclear factors of activated T cells (NFAT) are transcription factors that are central to cytokine production in activated T cells and regulate the development and differentiation of various tissues. NFATc2 is expressed in hematopoietic stem cells and regulated during myeloid commitment in a lineage-specific manner. The biological role of NFATc2 in hematopoiesis is, however, unclear.
Design and Methods
In the present study, we analyzed steady-state hematopoiesis in young (<3 months) and old (>12 months) mice lacking NFATc2. Complete blood counts were performed in the peripheral blood, bone marrow and spleen. Using cytological and histological analyses, the blood cell differential was determined. Colony-formation assays were used to determine the differentiation potential of hematopoietic cells. Bone cell cultures were derived from the bone marrow, and bone remodeling markers were determined in the serum.
Results
NFATc2−/− mice older than 12 months were anemic and thrombocytopenic. The bone marrows of these mice showed a markedly reduced number of hematopoietic cells, of which megakaryocytic and erythroid lineages were most affected. While the number of hematopoietic progenitor cells in NFATc2-deficent bone marrow was reduced, the myeloid differentiation potential of these cells remained intact. Aged NFATc2−/− mice showed ossification of their bone marrow space and developed extramedullary hematopoiesis in the spleen. Ex vivo differentiation assays revealed an intrinsic defect of NFATc2-deficient stromal cells, in which NFATc2−/− osteoblasts differentiated more efficiently than wild-type cells, whereas osteoclast differentiation was impaired.
Conclusions
Our data suggest that NFATc2 may play a role in the maintenance of steady-state hematopoiesis and bone remodeling in adult organisms.
Keywords: nuclear factor of activated T cells, anemia, thrombocytopenia, osteomyelosclerosis, extramedullary hematopoiesis
Introduction
Hematopoietic stem cells (HSC) are defined by their capacity to self-renew and differentiate into all types of mature blood cells.1 In an adult organism, bone marrow is the primary site of hematopoiesis, although extramedullary hematopoiesis may take place in the spleen and liver during periods of hematopoietic stress.2 Within the bone marrow, HSC are located in specialized niches (endosteal and perivascular niches) that provide an optimal microenvironment of cytokines, growth factors, and other signals that ensure long-term maintenance of the cells and regulate their functions.3–5
Various transcription factors have been identified that, during hematopoiesis, control the gradual steps of lineage-commitment and specifically change the gene expression profile of HSC. This allows the adaptation of a new set of genes which determines the specialized structure and function of each mature blood cell type.6 While some transcription factors are essential for commitment to certain lineages, such as globin transcription factor 1 (GATA-1) for the erythroid lineage or PU-1 for the granulocytic and monocytic lineages, others act in concert with more abundantly expressed transcription factors (e.g. AP-1 or STAT) to direct lineage-specificity.7–10
The nuclear factor of activated T cells (NFAT) family of transcription factors comprises five members (NFATc1-c4, NFAT5), of which NFATc1-c4 are regulated by the calcium-dependent phosphatase calcineurin, while NFAT5 reacts to osmotic stress.11 Activation of NFATc1-c4 usually occurs after binding of extracellular ligands to cell surface receptors that are able to induce a sustained increase in intracellular calcium levels. Upon dephosphorylation by calcineurin, a nuclear-localization signal is unmasked and allows the nuclear translocation and activation of NFAT proteins. Due to the strict dependence on calcineurin in the NFAT activation process and the fundamental role of NFAT in the activation of T cells, calcineurin inhibitors such as cyclosporine A and FK506 are potent immunosuppressants.12,13 Thus, calcineurin inhibitors are used as first-line therapy in patients after allogeneic stem cell or organ transplantation to prevent or treat organ rejection.
Although NFAT is best known for its function in T-cell activation, specific family members also regulate various developmental processes (e.g. heart valves, vasculature, central nervous system) as well as the differentiation of a multitude of cell types including cardiomyocytes, keratinocytes, chondrocytes, and osteoclasts.14 However, a potential role of NFAT in the maintenance of HSC properties and the regulation of hematopoiesis is largely unknown. Our previous studies show that all members of the NFAT family, except for NFATc4, are expressed in CD34+ HSC and that their expression is differentially regulated during the lineage-specific differentiation of myeloid cells.15,16 For example, while NFATc4 is not expressed in HSC and remains almost undetectable in differentiated neutrophil, eosinophil and erythroid cells, its expression is strongly induced during megakaryocyte differentiation. In contrast, the expression of NFATc2, which is abundantly found in HSC, is rapidly and almost completely suppressed upon their differentiation into neutrophil granulocytes, but is maintained in the megakaryocyte lineage. Thus, the exclusive expression pattern of NFAT members suggests a participation in the lineage commitment and/or differentiation of hematopoietic cells.
Design and Methods
Mice
NFATc2−/− and wild-type mice (C57BL/6 background) were held under pathogen-free conditions at the animal facility of the Technical University of Dresden.17,18 Twenty NFATc2-deficient mice and their age- and sex-matched wild-type littermates were divided into two age groups: younger than 3 months (n=9 pairs) and older than 12 months (n=11 pairs). Before sacrifice, animals were anesthetized by an intraperitoneal injection of a combination of ketamine and xylazine. All animal protocols were approved by the governmental and institutional animal care committees.
Hematologic analysis
Peripheral blood was collected by retro-orbital puncture into heparinized capillary tubes, and complete blood counts were performed (Sysmex, Norderstedt, Germany). The blood differential was assessed by cytomophological analysis of blood smears stained with May-Grünwald and confirmed by flow cytometry. Slides were examined and imaged using a light microscope (Nikon Eclipse E600 and Digital Sight DS-5M, Düsseldorf, Germany).
Isolation and assessment of cells from the bone marrow, spleen and liver
Liver and spleen were mashed with the bottom-side of a 5 mL syringe, and bone marrow was flushed from both femora and tibiae. All cell lysates were passed through a 20 μm nylon mesh cell strainer to obtain single cell suspensions. Afterwards, the cells were subjected to cytospin and flow cytometry analysis to assess the cellular morphology, the lineage commitment and the differentiation stage of the hematopoietic cells. Flow cytometric analyses were essentially performed as described elsewhere.19,20 The flow cytometry details are provided in the Online Supplementary Design and Methods. Colony-formation assays were performed as also described in the Online Supplementary Design and Methods to determine the frequency of lineage-specific progenitors.
Differentiation of megakaryocytes
Megakaryocytes were differentiated from the blood of stem cell donors as described previously.21 The details are provided in the Online Supplementary Design and Methods. The study was approved by the institutional review board of the Medical Faculty of the Technical University of Dresden, and informed consent was obtained from the donors.
Histological analysis of bone marrow, liver and spleen
Spleens, livers and femora of NFATc2−/− and wild-type mice were fixed in 4% PBS-buffered formalin for 24 h. Femora were additionally decalcified in osteosoft (Merck, Darmstadt, Germany). Following dehydration with an ascending ethanol series and xylol, the tissues were embedded in paraffin. The sections were stained with hematoxylin-eosin (H&E), Turnbull blue for the detection of iron, Gomori for the visualization of reticular fibers, Giemsa for osteoblasts, and tartrate-resistant acid phosphatase (TRAP) for osteoclasts. Slides were examined and imaged using a light microscope (Nikon Eclipse E600 and Digital Sight DS-5M, Düsseldorf, Germany).
Generation and assessment of osteoblasts and osteoclasts, bone turnover markers
Bone cell differentiation assays were performed as described previously22 and are described in the Online Supplementary Design and Methods. Plasma levels of pro-collagen type I N-terminal peptide (P1NP) were determined using a commercially available enzyme-linked immunosorbent assay (ELISA; IDS Systems).
Statistical analysis
Data are presented as means ± standard error of the mean. Statistical significance was tested using an unpaired Student’s T-test. P-values less than 0.05 were considered statistically significant.
Results
Anemia, thrombocytopenia and lymphocytosis in aged NFATc2-deficient mice
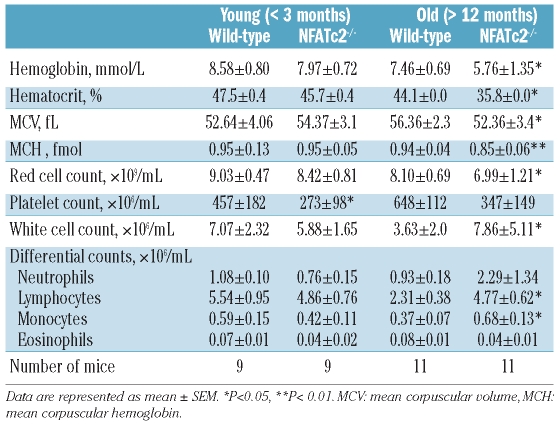
In order to assess the impact of NFATc2 on hematopoiesis, complete blood counts of young (<3 months) and aged (> 12 months) wild-type (WT) and NFATc2−/− (KO) mice were performed. The results are summarized in Table 1.
Table 1.
Hematologic parameters of age-matched wild-type and NFATc2−/− mice.
Aged KO mice were found to be consistently anemic with a significant reduction in all parameters of the erythroid lineage, namely erythrocyte number, hemoglobin, and hematocrit (Table 1). Likewise, the group of aged KO mice showed a strong reduction in their platelet number by about 50% (Table 1). Neutrophil counts were not significantly different between WT and KO mice (Table 1). As expected, a significant lymphocytosis was observed in the peripheral blood of aged KO mice, consistent with a previously described defect in the termination of lymphoproliferative responses in these mice.23,24 Peripheral blood abnormalities were less pronounced in the group of young KO mice than in aged mice, but significant thrombocytopenia as well as a tendency to anemia were noted (Table 1).
Anemia in aged NFATc2 KO mice was characterized by a decrease in the red blood cell indices mean corpuscular volume and mean corpuscular hemoglobin (Table 1). To exclude that iron deficiency was the cause of the anemia, bone marrow sections were stained with Turnbull blue. No differences were seen between WT and KO mice (data not shown). In contrast, the peripheral blood of KO mice revealed an increase in the number of reticulocytes (310±5×106 versus 488±68×106 cells/mL, P<0.05), confirmed by the polychromatic appearance of reticulocytes in peripheral blood smears (Online Supplementary Figure S1).
NFATc2 deficiency leads to bone marrow hypoplasia and osteomyelosclerosis
Because aged NFATc2−/− mice were anemic and thrombocytopenic, we next set out to investigate the bone marrow compartment as the origin of hematopoiesis in adult mice (Figure 1). Upon macroscopic examination, femora of aged KO mice already appeared paler than those of WT mice (Figure 1A), and significantly fewer bone marrow cells could be obtained (19.89±3.88×106 versus 35.81±4.31×106 cells/femur, P<0.001) (Figure 1B). Cytological differentiation of the extracted bone marrow cells revealed a significant decrease of all myeloid cells in the KO mice, which was most pronounced in erythroid and megakaryocytic lineages (Figure 1C). In contrast, the absolute number of lymphocytes, despite general marrow hypoplasia, remained largely unaffected. Upon cytomophological examination of the knockout cells, there was no evidence of the presence of cellular dysplasia (e.g. abnormalities in size or shape, nuclear or cytoplasmic atypia) or of an increase in blasts counts.
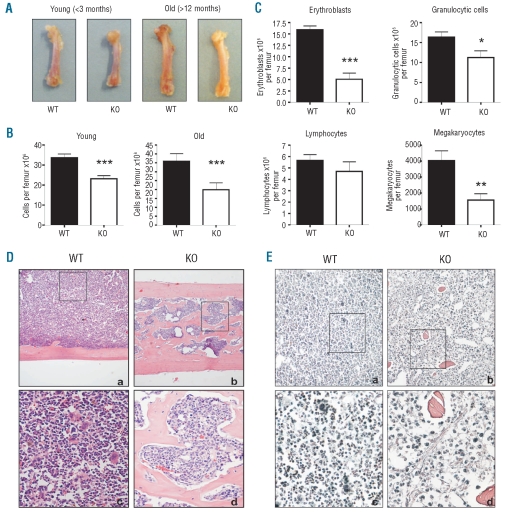
Figure 1.
Bone marrow hypoplasia and osteomyelosclerosis in aged NFATc2−/− mice. (A) Femora of young and aged wild-type (WT) and NFATc2 knock-out (KO) mice. Note that femora of aged KO mice appear pale compared to WT bones. (B) Numbers of bone marrow cells extracted from young and old WT and KO femora. (C) Bone marrow differentials. The numbers of erythroblasts, granulocytic cells, lymphocytes and megakaryocytes extracted from aged WT and KO femora are shown. Results are presented as means ± SEM. *P<0.05; **P<0.01; ***P<0.001. (D) Hematoxylin-eosin staining of femoral sections from an aged WT and KO mouse pair (a, c: WT; b,d: KO). Note the highly ossified bone marrow space in the KO femur. (E) Gomori staining of femoral sections from an aged WT and KO mouse pair (a, c: WT; b,d: KO). Note the presence of reticular fibers only in the KO bone marrow. Magnification, a,b: 100x; inserts c,d: 400x.
Examination of histological sections of KO femora confirmed the presence of bone marrow hypoplasia as well as the predominant reduction of erythroid cells and megakaryocytes (Figure 1D and E; compare panels a and c to panels b and d, respectively). Furthermore, it revealed that trabeculae had formed in the bone marrow space of aged KO mice (Figure 1D), which was in sharp contrast to the bone marrow space of all WT as well as of young KO mice (data not shown). Osteomyelosclerosis was found in all aged KO mice, albeit to a variable extent, and the degree of ossification appeared to correlate positively with the severity of the hematologic abnormalities observed in peripheral blood and marrow. To investigate whether the ossification of the bone marrow cavity in aged KO was accompanied by further alteration in the animals’ extracellular matrix, bone marrow sections of WT and KO mice were stained for the presence of reticular fibers. In addition to the presence of bone trabeculae, increased formation of reticular fibers was indeed found in the marrow space of four out of ten aged NFATc2-deficient mice (Figure 1E).
Femora of young KO mice were macroscopically indistinguishable from those of age-matched WT mice (Figure 1A), and histological analysis did not show signs of osteomyelosclerosis. However, as in aged mice, fewer cells could be extracted from femora of young KO compared to age-matched WT animals (23.19±4.82×106 versus 33.61±5.73×106 cells/femur, P<0.001) (Figure 1B), and these cells also showed a quantitative reduction of all myeloid lineages but not of lymphoid cells (data not shown).
Aged NFATc2-deficient mice display extramedullary hematopoiesis
Fibrosis and ossification of the bone marrow cavity along with peripheral blood anemia and thrombocytopenia, as detected in aged NFATc2−/− mice, are also found in the late (hypocellular) stage of human primary myelofibrosis, a clonal hematopoietic disorder, which is additionally characterized by splenomegaly and the presence of extramedullary hematopoiesis. We, therefore, next investigated whether extramedullary hematopoiesis was present in the spleens and livers of NFATc2−/− mice (Figure 2). Spleens were variably, but consistently enlarged in aged KO compared to WT mice. Notably, mice with highly ossified bone marrow also had drastically enlarged spleens (Figure 2A). On average, the relative weight of KO spleens was found to be significantly increased to about 200% of that of WT spleens (Figure 2B). Consistent with this observation, spleens of NFATc2−/− mice, upon homogenization, contained three times more cells than did WT spleens (218±40×106 versus 742±276×106 cells/spleen) (Figure 2C).
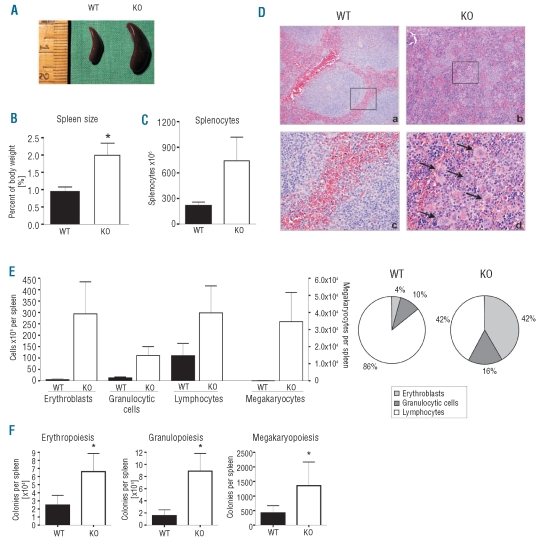
Figure 2.
Splenomegaly and extra medullary hematopoiesis in the spleen of NFATc2−/− mice. (A) Splenomegaly in an exemplary KO mouse with ossified bone marrow. (B) The spleens of KO mice were twice as heavy as those of WT mice. Data are presented as mean ± SEM. N = 6. *P<0.05. (C) The number of splenocytes was increased in KO mice. Data are presented as mean ± SEM. N = 6. (D) Exemplary hematoxylin-eosin stained sections of the spleen of WT and KO mice. The normal architecture of the parenchyma (a) is destroyed in NFATc2−/− mice (b). Magnification: 100x. (c, d) closer magnification (400x) of (a, b). Note the presence of megakaryocytes (arrows). (E) Differential of spleen cells. Cells of the erythroid, granulocytic, lymphocytic, and megakaryocytic lineages were increased in aged KO mice, whereas the percent increase of erythrocytes and granulocytes was greater than that of lymphocytes (pie chart). Results are given as mean ± SEM. N = 6. (F) Number of hematopoietic colonies (CFU-G, CFU-M, CFU-GM, BFU-E, CFU-MK) derived from 1x105 cells of the spleen. Three mouse pairs were analyzed. Results are given as mean ± SEM.
Histological examination of the spleen sections showed that the architecture of the parenchyma was destroyed in aged KO mice but intact in WT mice (Figure 2D). Moreover, sections of KO spleens revealed a notable prevalence of erythroid cells as well as the appearance of multiple megakaryocytes, which in comparison were rarely found in WT spleens (Figure 2D; compare panels b and d to panels a and c). Hematopoietic differentiation of homogenized spleen cells showed that, while cells of all myeloid and lymphoid lineages were present in increased numbers in KO spleens, a predominant expansion of erythroid and megakaryocytic cells was apparent (Figure 2E, left graph). As a result, spleens of aged KO mice displayed a profoundly altered relative proportion of myeloid (i.e. erythroid, granulocytic and megakaryocytic) to lymphoid cells (WT ~1:6 versus KO ~1:1), which in the KO had drastically shifted in favor of the myeloid compartment (Figure 2E, right graph).
To analyze the frequency of myeloid progenitor cells in spleens of aged WT and NFATc2−/− mice, colony-formation assays were performed. The frequencies of granulocytic/monocytic (CFU-GM/CFU-G/CFU-M), erythroid (BFU-E) and megakaryocytic (CFU-MK) progenitors were all markedly higher in the spleens of NFATc2-deficient than in WT mice (Figure 2F). Increased frequencies of granulocytic/monocytic and erythroid progenitors were also found in four out of five livers of aged NFATc2 KO animals (data not shown). Together, these results demonstrate the presence of extramedullary hematopoiesis in aged NFATc2−/− mice. As for the formation of trabeculae in the bone marrow, extramedullary hematopoiesis was not detected in young NFATc2−/− mice, suggesting that both phenomena were age-dependent and accumulated with some variability over the life span of NFATc2-deficient animals.
Reduced frequency but preserved differentiation potential of hematopoietic progenitor cells in the bone marrow of NFATc2-deficient mice
The term “hematopoietic niche” depicts the site of hematopoiesis in the bone marrow, in which a network of growth factors and membrane proteins, expressed by both hematopoietic cells and stromal cells, regulates the self-renewal of HSC and their differentiation into all hematopoietic lineages. The fact that the total number of hematopoietic cells was drastically reduced in the bone marrow of aged NFATc2−/− mice (Table 1), while intact hematopoiesis was clearly present in extramedullary organs (Figure 2), suggested that the bone marrow hematopoietic niche in NFATc2−/− mice may be disturbed. To further characterize the bone marrow hematopoietic compartment in these mice, we next analyzed the frequency of hematopoietic progenitors cells as well as their capacity for myeloid differentiation within their local bone marrow environment. As assessed by colony-formation assays, the numbers of granulocytic/monocytic, erythroid, and megakaryocytic precursor cells per femur was significantly lower in aged NFATc2−/− mice than in WT mice (Figure 3A), so that the reduction of the hematopoietic progenitor cells in the KO correlated with the reduction of all myeloid cells in the bone marrow. On the other hand, the local differentiation efficacy of the remaining progenitor cells was intact, as the relative distribution of morphologically more mature cells of both erythroid and granulocytic lineages was comparable in bone marrows of WT and NFATc2−/− mice (Figure 3B).
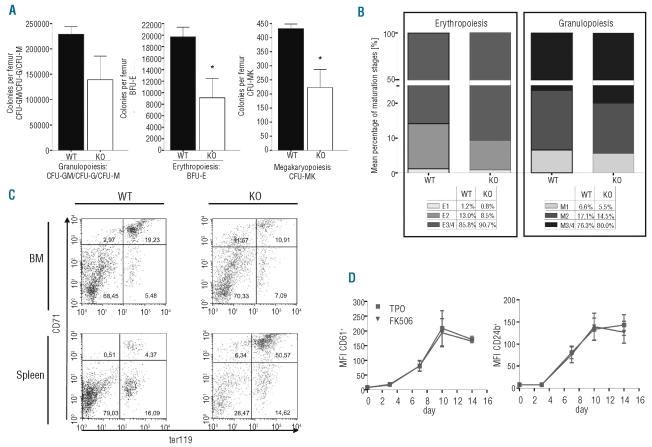
Figure 3.
Reduced number but maintained differentiation capacity of NFATc2-deficient bone marrow hematopoietic progenitor cells. (A) Colony-forming assays (CFU-G, CFU-M, CFU-GM, BFU-E, CFU-MK) of aged wild-type (WT) and knock-out (KO) mice. The number of colonies per femur of three WT and KO mouse pairs are shown. Results are expressed as means ± SEM. (B) Exemplary distribution of maturation stages of erythroid and granulocytic cells in the bone marrow of aged WT and KO mice, according to morphological criteria. E1, proerythroblasts; E2, basophilic erythroblasts; E3/4, polychromatic and orthochromatic erythroblasts; M1, myeloblasts and promyelocytes; M2, myelocytes and metamyelocytes; M3/4, bands and segmented granulocytes. (C) Flow cytometric analysis of erythroblast maturation stages in the bone marrow (BM) and spleen of aged WT and KO mice. Upper right quadrant (Ter119+ CD71high): basophilic erythroblasts; lower right quadrant (Ter119+ CD71low): polychromatic and orthochromatic erythroblasts (E3/4) and reticulocytes. (D) Megakaryocytic differentiation was induced from CD34+ hematopoietic progenitor cells in the presence or absence of FK506. At different time-points, the expression of megakaryocyte-specific surface markers was analyzed. Results are represented as mean fluorescence intensities (MFI) ± SEM from three individual experiments.
We confirmed this observation by flow cytometric analysis of erythroid cells, as reported by Asari et al.,19 which can determine the maturation stage of erythroblasts (Ter119+) by the loss of CD71 expression. While the total fraction of Ter119+ erythroblasts within the bone marrow of NFATc2-deficient mice was reduced, the differentiation of mature Ter119+ CD71low erythroblasts (ratios of lower right to upper right quadrants) was maintained and even a trend towards increased maturation was observed in NFATc2−/− mice (Figure 3C, upper panel). In contrast, in NFATc2−/− spleens there was a substantial increase in the total number of erythroblasts, as expected, although with a left shift towards more immature cells (Ter119+ CD71high) (Figure 3C, lower panel). The latter observation is consistent with massively stimulated extramedullary erythropoiesis in NFATc2 KO spleens, and explains the aforementioned reticulocytosis in the peripheral blood of these mice (Online Supplementary Figure S1).
NFATc2 is expressed in hematopoietic progenitor cells, and its expression is maintained throughout megakaryopoiesis.15,16 We, therefore, set out to determine whether NFATc2 was necessary for the intrinsic megakaryocytic differentiation potential of hematopoietic progenitor cells, and also extended this question to other NFAT family members. Of note, activation of NFAT proteins strictly requires post-translational modification (i.e. dephosphorylation) by calcineurin and can, therefore, be effectively blocked by the pharmacological calcineurin inhibitors cyclosporine and tacrolimus (FK506).13 For this purpose, we cultured human hematopoietic progenitor cells in the presence of thrombopoietin to induce their differentiation into megakaryocytic cells, and analyzed their differentiation efficacy in the presence or absence of FK506. As shown in Figure 3D, the continuous presence of FK506 during the culture did not impair the intrinsic megakaryocytic differentiation efficacy of the progenitor cells. Together, these results suggest that the hematopoietic compartment of the NFATc2−/− bone marrow is characterized by a reduced frequency of hematopoietic progenitor cells, but that these cells maintain their full potential to differentiate along erythroid, granulocytic, and megakaryocytic lineages.
Lack of NFATc2 leads to increased bone formation
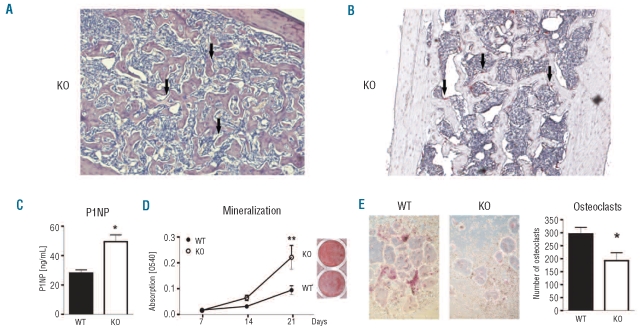
Within the bone marrow hematopoietic niche, stromal cells and hematopoietic cells are part of a complex network, in which both cell types critically influence each other.25 For example, the production of osteogenic factors by megakaryocytes in the hypercellular phase of human primary myelofibrosis induces the proliferation of osteoblasts and excessive deposition of extracellular matrix, which is then followed by the hypocellular/fibrotic stage of the disease with progressive hematopoietic insufficiency. To further characterize the stromal abnormalities (i.e. osteomyelosclerosis and fibrosis) in aged NFATc2−/− mice, sections of WT and KO femora were first analyzed for the presence of osteoblasts and osteoclasts. The femoral shaft, which is usually devoid of trabecular structures (Online Supplementary Figure S2), of aged NFATc2-deficient mice contained a highly connected trabecular network. Numerous osteoblasts (Figure 4A) and osteoclasts (Figure 4B) were found adjacent to the bone surface, indicating the presence of active bone remodeling sites. Additionally, plasma levels of pro-collagen type I N-terminal peptide (P1NP) were increased 2-fold in NFATc2−/− mice, thus, further pointing towards an enhanced production of bone tissue (Figure 4C). To further assess the intrinsic differentiation potential of the bone cells, ex vivo cell cultures were performed. Osteoblasts derived from NFATc2-deficient mice deposited more mineralized matrix after 21 days of culture compared to WT osteoblasts (Figure 4D) and displayed a 2.5-3-fold higher activity of alkaline phosphatase (data not shown), indicating a higher osteogenic potential. The proliferation rate of osteoblast progenitor cells was not, however, changed (data not shown). In contrast, significantly fewer osteoclasts formed from the bone marrow of NFATc2-deficient mice as compared to WT mice (192±62 versus 296±60 osteoclasts/well, P<0.05) (Figures 4E).
Figure 4.
NFATc2-deficiency leads to an intrinsic bone defect that favors bone formation. (A) Bone marrow section of the femoral shaft of an aged NFATc2−/− mouse stained with Giemsa. Note the presence of multiple osteoblasts along the trabeculae (arrows). Magnification: 200x. (B) Bone marrow section of the femoral shaft of an aged NFATc2−/− mouse stained for tartrate-resistant acid phosphatase (TRAP). Note the abundant presence of osteoclasts (stained in pink, arrows). Magnification: 200x. (C) Plasma levels of the bone formation marker P1NP were analyzed in aged wild-type and NFATc2−/− mice using an ELISA. N=10. (D) Alizarin red S staining of osteoblast cultures (day 21) derived from wild-type (WT) or NFATc2 knock-out (KO) bone marrow stromal cells. Data are represented as means ± SEM. N = 5. **P<0.01. (E) Generation of TRAP-positive osteoclasts from WT and KO mice. Magnification: 100x. (F) Quantification of osteoclasts shown in (E). TRAP-positive, multi-nucleated cells were counted as osteoclasts. Results are expressed as means ± SEM of four mouse pairs. *P<0.05.
Discussion
NFAT transcription factors are involved in the development of several tissues and regulate the differentiation of a multitude of specialized cells, such as cardiomyocytes and skeletal cells.14 However, their role in the differentiation of hematopoietic lineage cells is currently unclear. Our previous studies showed that members of the NFAT family are expressed in hematopoietic progenitor cells and are regulated during myeloid commitment in a lineage-specific manner.15,16 These results prompted us to investigate the role of NFATc2 in hematopoiesis.
Here we report that NFATc2-deficient mice older than 12 months acquire anemia, thrombocytopenia, and lymphocytosis. The bone marrow of aged NFATc2 KO mice proved to be hypoplastic and displayed a significant loss of erythroid, granulocytic, and megakaryocytic lineage cells. Moreover, NFATc2-deficient mice developed osteomyelosclerosis and, in some cases, osteomyelofibrosis. In parallel, splenomegaly due to extramedullary hematopoiesis was observed in all aged NFATc2-deficient mice.
An apparent cause for the observed peripheral blood anemia and thrombocytopenia was a production defect of these cells in the bone marrow, which was only partially compensated for by the extramedullary hematopoiesis in the spleen and liver. The combination of bone marrow hypoplasia with reticulocytosis and the presence of intact hematopoiesis in extramedullary organs strongly argues against a general defect of hematopoiesis in NFATc2-deficient animals (e.g., by nutrient deficiency or mechanisms intrinsic to hematopoietic stem cells) but rather points towards a selective dysregulation of the hematopoiesis within the local bone marrow microenvironment. The detection of extramedullary, left-shifted erythropoiesis in the spleens of NFATc2-deficient mice also explained the (at first sight contradictory) reticulocytosis in the blood of the mice, as erythropoietic precursor cells are prematurely released from the spleen into the periphery.
We further specified the functional defect in the NFATc2-deficient bone marrow and first focused on the hematopoietic part. Hematopoiesis in the bone marrow is dependent on the capability of hematopoietic progenitor cells to self-renew and differentiate into various myeloid lineages, and both functions are critically influenced by adjacent stromal cells (e.g. osteoblasts and mesenchymal stem cells). Of note, NFATc2 is expressed by both hematopoietic stem cells and stromal cells.15,26,27 and thus both direct and indirect mechanisms have to be considered as causes for the hematopoietic insufficiency in the bone marrow of NFATc2-deficient animals. Our results suggest that NFATc2 is neither directly nor indirectly involved in the myeloid differentiation of hematopoietic progenitor cells, as the capacity for erythroid and granulocytic differentiation of these cells was maintained in the absence of NFATc2, both within the local bone marrow environment and in extramedullary organs. Furthermore, NFAT activation was not required for megakaryocytic differentiation in ex vivo cultures of normal progenitors. These results are supported by those of a recent study by Gallo et al., in which bone marrow transfer experiments showed that the development of lymphocytes, but not the differentiation of cells of various myeloid lineages, is dependent on the activation of NFAT signaling by the calcineurin subunit B1.28
On the other hand, we found that the total number of hematopoietic progenitor cells in the femora of aged NFATc2 KO mice was reduced. At the same time, these mice consistently exhibited ossification of their bone marrow (osteomyelosclerosis), although to a variable degree. Notably, the extent of osteomyelosclerosis correlated with the severity of hematologic defects and the extent of extramedullary hematopoiesis. Therefore, dysregulation of the stroma might represent the primary cause for the reduced frequency of hematopoietic progenitor cells in the bone marrow of NFATc2 KO mice. The precise mechanism remains unclear: simple “outspacing” by excessive deposition of extracellular material (bone, fibers) is one possibility; interference with chemotaxis or adhesion of hematopoietic progenitors to the stroma are conceivable alternatives.
Within the bone marrow hematopoietic niche, hematopoietic and stroma cells influence each other, in that dysfunction of hematopoietic cells (i.e. aberrant production of cytokines) may cause stromal abnormalities, and vice versa. For example, human primary myelofibrosis (WHO 2008) is a chronic myeloproliferative disease in which hematopoietic insufficiency of the bone marrow is accompanied by the presence of bone marrow fibrosis/sclerosis and extramedullary hematopoiesis. Primary myelofibrosis is a clonal disorder of hematopoietic stem cells, which can be experimentally transmitted and cured by allogeneic stem cell transplantation.29–31 About 50 % of patients with primary myelofibrosis carry a mutation of the JAK2 gene, which confers cytokine-independent proliferation and expansion of the hematopoietic clone. In turn, cytokines and growth factors produced by these cells (including osteoprotegerin,32,33 transforming growth factor-β,34,35 and platelet-derived growth factor36) alter the bone marrow microenvironment in such a way that large deposits of fibrous and osseous tissue are formed.
The central role of hematopoietic cells in stromal homeostasis is also supported by a number of transgenic mouse models. For example, mice that over-express the megakaryocytic growth factor thrombopoietin,37 or mice that express low levels of the megakaryocytic transcription factor GATA-1,38 develop a primary myelofibrosis-like phenotype and, like NFAT2-deficient mice, do not display any gross abnormalities below the age of 12 months. As in human primary myelofibrosis, the genetic defect of these mice points towards a hematopoietic cell (especially the megakaryocyte) as the origin of the stromal abnormalities.
Our ex vivo experiments with NFATc2-deficient osteoblasts and osteoclasts suggest the presence of a cell-intrinsic stromal defect in these mice, in which the differentiation of both bone cell types is altered, with osteoblastic differentiation or function being enhanced and osteoclastic functions being suppressed. The in vivo consequence of this observation is unclear; the presence of multiple osteoblasts and osteoclasts at the sites of aberrant bone production (bone marrow trabeculae) may indicate a disturbed balance of bone remodeling. Whether alterations in the stromal compartment in NFAT2-deficient mice cause the hematopoietic abnormalities, or vice versa, is presently unclear. Reciprocal transplantation chimera experiments, in which purified NFATc2-deficient HSC are transplanted into lethally irradiated wild-type recipients, and vice versa, as well as ex vivo co-culture experiments with NFATc2-deficient stromal and hematopoietic cells, will help to define the respective contribution of hematopoietic and stromal cells for the observed phenotype in these mice.
Our results are in accordance with those of the study by Ikeda et al., which showed that mice over-expressing NFATc2 in osteoclasts develop an osteopenic phenotype.39 They are in seeming contrast to those reported by Koga et al., who did not observe increased bone formation in the absence of NFATc2; however, those mice were young and had a different genetic background.27 The in vivo role of NFAT in bone remodeling is complex, because the contribution of different NFAT family members, the function of osteoblasts and osteoclasts, as well as indirect effects of hematopoietic cells have to be considered.
In conclusion, our data suggest that NFATc2 may play a role in the maintenance of steady-state hematopoiesis and bone remodeling in adult organisms. Further experiments will be necessary to elucidate the exact mechanism and will help to define the role of NFATc2 within the bone marrow hematopoietic niche.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG)/Sonderforschungsbereich 655 to AK, a grant from the DFG (RA 1923/1-1), the MeDDrive program of the Medical Faculty of the TU Dresden, and the European Calcified Tissue Society/AMGEN fellowship to MR. LCH holds grants from the DFG (TR67-project B2, RA 1923/1-1 and the Research Center and Cluster of Excellence for Regenerative Therapy Dresden).
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet. 2000;1(1):57–64. doi: 10.1038/35049577. [DOI] [PubMed] [Google Scholar]
- 2.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8(4):290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 3.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 4.Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97(10):3075–85. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 6.Akashi K, He X, Chen J, Iwasaki H, Niu C, Steenhard B, et al. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood. 2003;101(2):383–9. doi: 10.1182/blood-2002-06-1780. [DOI] [PubMed] [Google Scholar]
- 7.Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9(10):1250–62. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- 8.Ward AC, Loeb DM, Soede-Bobok AA, Touw IP, Friedman AD. Regulation of granulopoiesis by transcription factors and cytokine signals. Leukemia. 2000;14(6):973–90. doi: 10.1038/sj.leu.2401808. [DOI] [PubMed] [Google Scholar]
- 9.Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1(4):416–27. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Liebermann DA, Gregory B, Hoffman B. AP-1 (Fos/Jun) transcription factors in hematopoietic differentiation and apoptosis. Int J Oncol. 1998;12(3):685–700. doi: 10.3892/ijo.12.3.685. [DOI] [PubMed] [Google Scholar]
- 11.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 12.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357(6380):695–7. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 13.Kiani A, Rao A, Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity. 2000;12(4):359–72. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 15.Kiani A, Habermann I, Haase M, Feldmann S, Boxberger S, Sanchez-Fernandez MA, et al. Expression and regulation of NFAT (nuclear factors of activated T cells) in human CD34+ cells: down-regulation upon myeloid differentiation. J Leukoc Biol. 2004;76(5):1057–65. doi: 10.1189/jlb.0404259. [DOI] [PubMed] [Google Scholar]
- 16.Kiani A, Kuithan H, Kuithan F, Kyttala S, Habermann I, Temme A, et al. Expression analysis of nuclear factor of activated T cells (NFAT) during myeloid differentiation of CD34+ cells: regulation of Fas ligand gene expression in megakaryocytes. Exp Hematol. 2007;35(5):757–70. doi: 10.1016/j.exphem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Kiani A, Garcia-Cozar FJ, Habermann I, Laforsch S, Aebischer T, Ehninger G, et al. Regulation of interferon-gamma gene expression by nuclear factor of activated T cells. Blood. 2001;98(5):1480–8. doi: 10.1182/blood.v98.5.1480. [DOI] [PubMed] [Google Scholar]
- 18.Kiani A, Viola JP, Lichtman AH, Rao A. Down-regulation of IL-4 gene transcription and control of Th2 cell differentiation by a mechanism involving NFAT1. Immunity. 1997;7(6):849–60. doi: 10.1016/s1074-7613(00)80403-3. [DOI] [PubMed] [Google Scholar]
- 19.Asari S, Sakamoto A, Okada S, Ohkubo Y, Arima M, Hatano M, et al. Abnormal erythroid differentiation in neonatal bcl-6-deficient mice. Exp Hematol. 2005;33(1):26–34. doi: 10.1016/j.exphem.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 21.Kyttala S, Habermann I, Minami T, Ehninger G, Kiani A. Regulation of Down syndrome critical region 1 expression by nuclear factor of activated T cells in megakaryocytes. Br J Haematol. 2009;144(3):395–408. doi: 10.1111/j.1365-2141.2008.07490.x. [DOI] [PubMed] [Google Scholar]
- 22.Rauner M, Sipos W, Goettsch C, Wutzl A, Foisner R, Pietschmann P, et al. Inhibition of lamin A/C attenuates osteoblast differentiation and enhances RANKL-dependent osteoclastogenesis. J Bone Miner Res. 2009;24(1):78–86. doi: 10.1359/jbmr.080902. [DOI] [PubMed] [Google Scholar]
- 23.Hodge MR, Ranger AM, Charles de la Brousse F, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4(4):397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 24.Xanthoudakis S, Viola JP, Shaw KT, Luo C, Wallace JD, Bozza PT, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272(5263):892–5. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 25.Kacena MA, Gundberg CM, Horowitz MC. A reciprocal regulatory interaction between megakaryocytes, bone cells, and hematopoietic stem cells. Bone. 2006;39(5):978–84. doi: 10.1016/j.bone.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Muller MR, Sasaki Y, Stevanovic I, Lamperti ED, Ghosh S, Sharma S, et al. Requirement for balanced Ca/NFAT signaling in hematopoietic and embryonic development. Proc Natl Acad Sci USA. 2009;106(17):7034–9. doi: 10.1073/pnas.0813296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, Nakashima K, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11(8):880–5. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 28.Gallo EM, Ho L, Winslow MM, Staton TL, Crabtree GR. Selective role of calcineurin in haematopoiesis and lymphopoiesis. EMBO \Rep. 2008;9(11):1141–8. doi: 10.1038/embor.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guardiola P, Esperou H, Cazals-Hatem D, Ifrah N, Jouet JP, Buzyn A, et al. Allogeneic bone marrow transplantation for agnogenic myeloid metaplasia. French Society of Bone Marrow Transplantation. Br J Haematol. 1997;98(4):1004–9. doi: 10.1046/j.1365-2141.1997.3073124.x. [DOI] [PubMed] [Google Scholar]
- 30.Villeval JL, Cohen-Solal K, Tulliez M, Giraudier S, Guichard J, Burstein SA, et al. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90(11):4369–83. [PubMed] [Google Scholar]
- 31.Yan XQ, Lacey D, Hill D, Chen Y, Fletcher F, Hawley RG, et al. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88(2):402–9. [PubMed] [Google Scholar]
- 32.Wang JC, Hemavathy K, Charles W, Zhang H, Dua PK, Novetsky AD, et al. Osteosclerosis in idiopathic myelofibrosis is related to the overproduction of osteoprotegerin (OPG) Exp Hematol. 2004;32(10):905–10. doi: 10.1016/j.exphem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Chagraoui H, Tulliez M, Smayra T, Komura E, Giraudier S, Yun T, et al. Stimulation of osteoprotegerin production is responsible for osteosclerosis in mice overexpressing TPO. Blood. 2003;101(8):2983–9. doi: 10.1182/blood-2002-09-2839. [DOI] [PubMed] [Google Scholar]
- 34.Martyre MC, Romquin N, Le Bousse-Kerdiles MC, Chevillard S, Benyahia B, Dupriez B, et al. Transforming growth factor-beta and megakaryocytes in the pathogenesis of idiopathic myelofibrosis. Br J Haematol. 1994;88(1):9–16. doi: 10.1111/j.1365-2141.1994.tb04970.x. [DOI] [PubMed] [Google Scholar]
- 35.Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100(10):3495–503. doi: 10.1182/blood-2002-04-1133. [DOI] [PubMed] [Google Scholar]
- 36.Zauli G, Visani G, Catani L, Vianelli N, Gugliotta L, Capitani S. Reduced responsiveness of bone marrow megakaryocyte progenitors to platelet-derived transforming growth factor beta 1, produced in normal amount, in patients with essential thrombocythaemia. Br J Haematol. 1993;83(1):14–20. doi: 10.1111/j.1365-2141.1993.tb04624.x. [DOI] [PubMed] [Google Scholar]
- 37.Kakumitsu H, Kamezaki K, Shimoda K, Karube K, Haro T, Numata A, et al. Transgenic mice overexpressing murine thrombopoietin develop myelofibrosis and osteosclerosis. Leuk Res. 2005;29(7):761–9. doi: 10.1016/j.leukres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Vannucchi AM, Bianchi L, Cellai C, Paoletti F, Rana RA, Lorenzini R, et al. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice) Blood. 2002;100(4):1123–32. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda F, Nishimura R, Matsubara T, Hata K, Reddy SV, Yoneda T. Activation of NFAT signal in vivo leads to osteopenia associated with increased osteoclastogenesis and bone-resorbing activity. J Immunol. 2006;177(4):2384–90. doi: 10.4049/jimmunol.177.4.2384. [DOI] [PubMed] [Google Scholar]