Abstract
Background
Patients with β thalassemia intermedia can have substantial iron overload, irrespectively of their transfusion status, secondary to increased intestinal iron absorption. This study evaluates whether iron overload in patients with β thalassemia intermedia is associated with morbidity.
Design and Methods
This was a cross-sectional study of 168 patients with β thalassemia intermedia treated at two centers in Lebanon and Italy. Data on demographics, splenectomy status, transfusion status, and presence of co-morbidities were retrieved. Laboratory values of serum ferritin, fetal and total hemoglobin levels, as well as platelet and nucleated red blood cell counts were also obtained. Iron burden was determined directly by measuring liver iron concentration using magnetic resonance imaging. Patients were subdivided according to transfusion and splenectomy status into groups with phenotypes of different severity.
Results
The mean age of the patients was 35.2±12.6 years and 42.9% of them were male. The mean liver iron concentration was 8.4±6.7 mg Fe/g dry weight. On multivariate logistic regression analysis, after adjusting for age, gender, splenectomy status, transfusion status, and laboratory indices, an increase in 1 mg Fe/g dry weight liver iron concentration was independently and significantly associated with higher odds of thrombosis, pulmonary hypertension, hypothyroidism, osteoporosis, and hypogonadism. A liver iron concentration of at least 7 and at least 6 mg Fe/g dry weight were the best thresholds for discriminating the presence and absence of vascular and endocrine/bone morbidities, respectively (area under the receiver-operating characteristic curve: 0.72, P<0.001). Elevated liver iron concentration was associated with an increased rate of morbidity in patients with phenotypes of all severity, with a steeper increase in the rate of vascular morbidity being attributed to aging, and an earlier appearance of endocrine and bone disease.
Conclusions
Elevated liver iron concentration in patients with β thalassemia intermedia is a marker of increased vascular, endocrine, and bone disease.
Keywords: thalassemia intermedia, liver iron concentration, iron overload, vascular disease, endocrine disease, osteoporosis
Introduction
There is diversity in the severity of the phenotype of β thalassemia syndromes. The term β thalassemia intermedia was first suggested to describe patients who have milder anemia than patients with β thalassemia major, who usually present to medical attention later in childhood, and who remain largely transfusion-independent.1 However, it is now established that the diagnosis of β thalassemia intermedia spans a wide spectrum of severity and carries higher morbidity than previously recognized.2–3 Three main factors dominate the disease process in β thalassemia intermedia: ineffective erythropoiesis, chronic hemolytic anemia, and iron overload.3 The combination of ineffective erythropoiesis and chronic anemia leads to hepcidin suppression, increased iron absorption from the gut, and increased release of recycled iron from the reticuloendothelial system. This results in depletion of macrophage iron, relatively low levels of serum ferritin, and preferential portal and hepatocyte iron storage. This, in turn, leads to considerable hepatic iron overload and release of toxic iron species, such as non-transferrin-bound iron (NTBI), into the circulation.4–7 Iron overload can also be the consequence of transfusion therapy, which despite traditionally being an uncommon practice in β thalassemia intermedia, is now undertaken for many patients with severe disease after showing a potential role in ameliorating some disease complications.2,8,9 Moreover, age-related changes in adaptation to anemia by the bone marrow, alongside difficulty in maintaining a high output with normal vascular aging, cause many transfusion-independent patients with β thalassemia intermedia to become transfusion-dependent as they age.10,11 Several studies in patients with β thalassemia major have proven that uncontrolled iron overload is associated with significant morbidity and mortality, especially cardiac, highlighting the essential role of iron chelation therapy for survival.12 Studies on the morbidity or mortality from iron overload in patients with β thalassemia intermedia are lacking. Cardiac siderosis seems to be uncommon in β thalassemia intermedia, even in patients with severe iron overload.13–15 It does, therefore, remain essential to determine whether iron overload results in other clinical sequelae, before chelation therapy can be advised.
Liver iron concentration (LIC) has been regarded as the reference standard for estimating body iron load and has been shown to predict total body iron stores accurately.16 R2 and R2* magnetic resonance imaging (MRI) relaxation time techniques allow for non-invasive estimation of LIC in patients with hemoglobinopathies.17–19 The LIC cut-off points of 7 and 15 mg Fe/g dry weight (dw) have been used for the past two decades to categorize iron overload status, predict morbidity and mortality, and tailor iron chelation therapy in patients with β thalassemia major. However, these cut-off points were extrapolated from data on patients with hereditary hemochromatosis,20 and were only linked to liver pathology and cardiac disease in a few small studies on patients with β thalassemia major utilizing liver biopsy.21–24 There are no studies linking LIC or its cut-offs to morbidity or mortality in patients with β thalassemia intermedia.
The aim of this study was to evaluate the association between iron overload, as determined by LIC, and morbidity in a large cohort of patients with β thalassemia intermedia.
Design and Methods
This was a cross-sectional study of all patients with β thalassemia intermedia treated at two centers in Beirut, Lebanon and Milan, Italy, for whom LIC measurements were available (74/127 from Lebanon and 94/153 from Italy). The main criteria to define the β thalassemia intermedia phenotype on presentation in both centers was age more than 2 years at diagnosis and hemoglobin values maintained between 7 and 9 g/dL without the need for a regular transfusion regimen (at diagnosis) in patients with or without splenomegaly.25 Patients with Hb S, C, E/β or δβ thalassemia; or those who had co-inheritance of α thalassemia [α+ (-α3.7 and -α4.2) or α0 (--Med and --SEA)] or determinants associated with increased γ chain production [Xmn-I +/+ genotype at position –158 of HβG2] were excluded. All extracted data reflected the period of LIC measurement. Patients’ charts were reviewed to retrieve data on demographics (age and gender), splenectomy status, and transfusion history. None of the patients was receiving iron chelation therapy or any fetal hemoglobin-inducing agents at the time of LIC measurement. The data for transfusion history were categorized as follows: regularly transfused (patients transfused at regular intervals every 1–3 months), occasionally transfused (patients who required occasional transfusions for transient severe anemia secondary to infections, surgery, or pregnancy); and non-transfused. Laboratory data were retrieved and recorded as a mean of all measurements undertaken during the year of LIC measurement; the parameters of interest were serum ferritin level, fetal and total hemoglobin levels (before the scheduled transfusion in patients who were given transfusions), platelet count and nucleated red blood cell (NRBC) count. The iron burden in the liver (LIC) was determined directly by R2 MRI in Beirut and R2* MRI in Milan using established methodologies, calibrated to mg/g of iron by dry weight in fresh liver biopsy specimens.17–18 The study received Institutional Review Board approval.
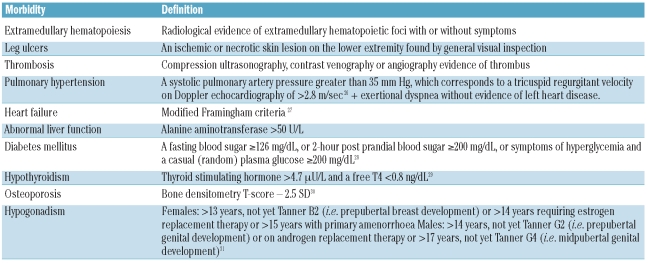
Data were also obtained on morbid conditions known to be common in patients with β thalassemia intermedia3 or that could be relevant in a state of iron overload. Complications were defined according to Table 1.26–31 The prevalence of other elements that could modify the rate of morbidities (family history of cardiovascular or endocrine disease, acquired or inherited thrombophilia, anticoagulant or antiplatelet use for reasons other than overt thrombosis, malignancy, orthopedic surgery, hepatitis C or B virus infection) was low and these elements were not, therefore, included in further analysis.
Table 1.
Definitions of morbidities.
Statistical analysis
Descriptive statistics are expressed as means (standard deviation, SD), medians (interquartile range, IQR) or percentages. Bivariate analysis was performed to determine the correlation between LIC and study variables using the independent samples t-test or the ANOVA test (for categorical variables) and the Pearson’s correlation coefficient (for continuous variables). Bivariate correlations between study variables and morbidities were evaluated by the independent samples t-test and the χ2 test except for heart failure and diabetes mellitus for which correlations were evaluated by the Mann-Whitney U test and the Fisher’s exact test. For bivariate analysis including LIC, we also double-checked and confirmed that statistical significance was maintained when geometric means or medians were compared instead of arithmetic means. Multivariate logistic regression analysis, using forward-stepwise selection, was used to determine which variables were independently associated with each morbidity. Transfusion history was categorized as transfused or non-transfused. A P value of 0.1 or less was used as the criterion for inclusion into the model to allow for correction of most confounders. Multicolinearity between variables in the model was evaluated using the variation inflation factor. All variation inflation factors were 3 or less (acceptable limit <10) indicating absence of multicolinearity. To determine the best LIC cut-offs for discriminating the presence and absence of morbidity, the maximum sum of sensitivity and specificity was calculated from receiver-operating characteristic (ROC) curve analysis. Retrieved cut-offs were also tested using the same multivariate logistic regression model. The effects of splenectomy and transfusion history on the association between LIC and morbidities was explored by grouping patients according to phenotypic severity: mild (neither splenectomized nor transfused), moderate (either splenectomized or transfused) and severe (both splenectomized and transfused). Logarithmic regression curves were used to determine the effect of age on the observed association between LIC and morbidities, as stratified for disease severity groups. All P-values are two-sided with values less than 0.05 considered statistically significant.
Results
Patients’ characteristics
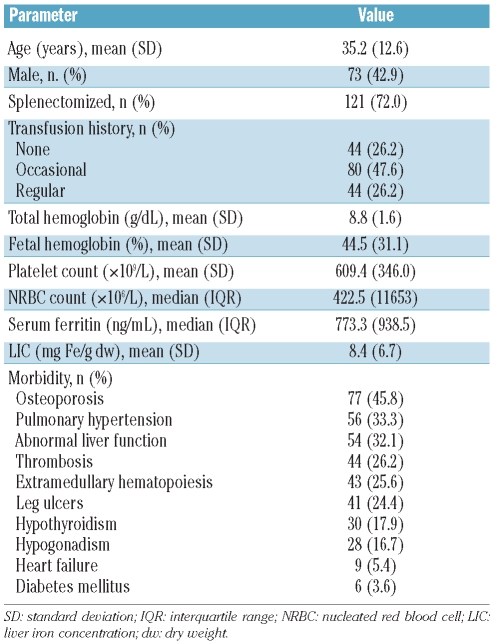
A total of 168 patients with β thalassemia intermedia were included in this analysis (Table 2). The mean LIC was 8.4±6.7 mg Fe/g dw (range, 0.5–32.1 mg Fe/g dw). Mean LIC was higher in splenectomized patients than in non-splenectomized ones (9.4±6.5 versus 5.8±6.6 mg Fe/g dw, respectively; P=0.001) and was higher in regularly (9.7±6.7 mg Fe/g dw) or occasionally (9.9±7.2 mg Fe/g dw) transfused patients than in non-transfused patients (4.3±3.1 mg Fe/g dw) (P<0.001). There was a weak positive correlation between LIC and serum ferritin level (r=0.53, P<0.001) as well as fetal hemoglobin level (r=0.22, P=0.008). There were no statistically significant correlations between LIC and age, gender, total hemoglobin level, platelet count or NRBC count.
Table 2.
Patients’ characteristics (n=168).
Liver iron concentration and morbidities
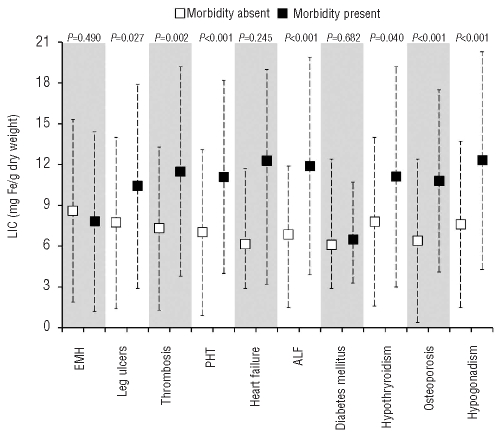
Mean LIC values were significantly higher in patients with leg ulcers, thrombosis, pulmonary hypertension, abnormal liver function, hypothyroidism, osteoporosis, and hypogonadism than in patients without these morbidities (Figure 1). Bivariate correlations between other study parameters and morbidities are summarized in Online Supplementary Table S1. On multivariate logistic regression analysis, and after adjusting for all study variables significant at the 0.1 level on bivariate analysis, a 1 mg Fe/g dw increase in LIC was significantly and independently associated with higher odds of thrombosis, pulmonary hypertension, hypothyroidism, osteoporosis, and hypogonadism (Online Supplementary Table S2).
Figure 1.
Comparison of LIC values in patients with and without morbidities. Data presented as means (squares) and standard deviations (whiskers), except for heart failure and diabetes mellitus for which data are presented as medians (square), 25th and 75th percentiles (whiskers). The P value was calculated using the independent samples t-test, except for heart failure and diabetes mellitus for which it was calculated using the Mann-Whitney U test. LIC: liver iron concentration; EMH: extramedullary hematopoiesis; PHT:pulmonary hypertension; ALF: abnormal liver function.
Liver iron concentration cut-offs
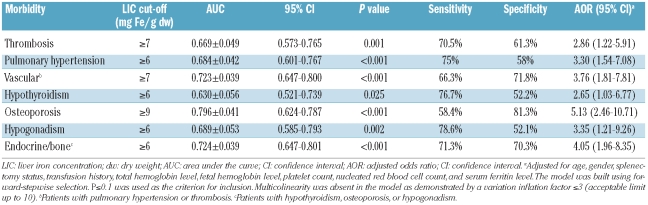
Using ROC curve analysis, a LIC of at least 7 mg Fe/g dw was found to be the best threshold for discriminating the presence and absence of vascular morbidity (thrombosis or pulmonary hypertension) with an area under the curve (AUC) of 0.723 (P<0.001). Patients with a LIC of at least 7 mg Fe/g dw were 3.76 times more likely to have vascular morbidity compared with patients with a LIC less than 7 mg Fe/g dw (Table 3). Similarly, a LIC of at least 6 mg Fe/g dw was found to be the best threshold for discriminating the presence and absence of endocrine or bone morbidity (hypothyroidism, osteoporosis, or hypogonadism) with an AUC of 0.724 (P<0.001). Patients with a LIC of at least 6 mg Fe/g dw were 4.05 times more likely to have endocrine morbidity than were patients with a LIC less than 6 mg Fe/g dw (Table 3).
Table 3.
Receiver operating characteristic (ROC) curve analysis to determine best LIC cut-offs for discriminating the presence and absence of morbidity.
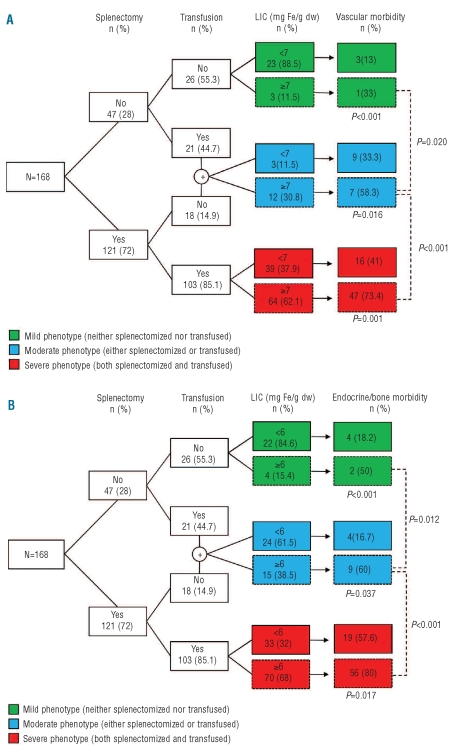
Effects of splenectomy and transfusion (phenotype severity)
Patients with a LIC of at least 7 mg Fe/g dw had a significantly higher rate of vascular morbidity than did patients with a LIC less than 7 mg Fe/g dw, in all groups of phenotype severity. Moreover, among the patients with a LIC of at least 7 mg Fe/g dw, the rate of vascular morbidity was significantly higher in those with a severe phenotype than in those with a moderate or mild phenotype (Figure 2A). Patients with a LIC of at least 6 mg Fe/g dw had a significantly higher rate of endocrine or bone morbidity than did patients with a LIC less than 6 mg Fe/g dw, in all phenotype severity groups. Moreover, among patients with a LIC of at least 6 mg Fe/g dw, the rate of endocrine or bone morbidity was significantly higher in those with a severe phenotype than in those with a moderate or mild phenotype (Figure 2B).
Figure 2.
Flow diagram showing the interplay between splenectomy, transfusion history, and elevated LIC and its effect on the rate of (A) vascular and (B) endocrine/bone morbidity. LIC, liver iron concentration; dw, dry weight. Data analyzed using the χ2 and Fisher’s exact tests.
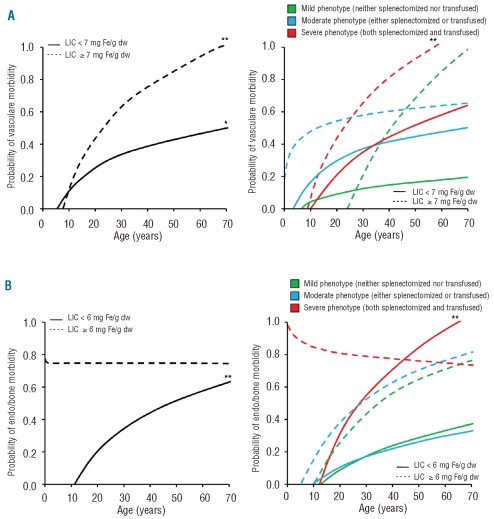
Effect of age
The probability of vascular morbidity significantly increased with age irrespectively of LIC, although reaching significantly higher values more steeply in patients with a LIC of at least 7 mg Fe/g dw than in those with a LIC less than 7 mg Fe/g dw (Figure 3A, left panel). When patients were stratified according to phenotype severity, the latter trend was maintained (Figure 3A, right panel). Moreover, the probability of endocrine or bone morbidity increased significantly with age in patients with LIC values less than 6 mg Fe/g dw; however, it showed a flat behavior starting with a high probability at young age in patients with values of at least 6 mg Fe/g dw (Figure 3B, left panel). When patients were stratified according to phenotype severity, the latter trend was maintained in patients with a severe phenotype (Figure 3B, right panel).
Figure 3.
Logarithmic regression curves demonstrating the effect of advancing age on (A) vascular and (B) endocrine/bone morbidity, in different subgroups of patients according to LIC and phenotype severity. LIC, liver iron concentration; dw, dry weight. *P<0.05; **P<0.01.
Discussion
Our study is the first to associate iron overload, reflected by LIC measurement, with vascular, endocrine, and bone morbidity in patients with β thalassemia intermedia. Elevated LIC was associated with an increased rate of vascular, endocrine, and bone morbidity in patients with phenotypes of all severity. Moreover, elevated LIC was associated with a steeper increase in the rate of vascular morbidity attributed to aging, and permitted endocrine and bone disease to appear at a younger age than in patients with low LIC. These novel findings have important clinical implications, although they need to be interpreted with caution.
A causal relationship between LIC and morbidity cannot yet be established. This is not because our study is cross-sectional in nature. Even if such an association were to be prospectively observed, the complexity of the disease process in β thalassemia intermedia makes it hard to determine whether elevated LIC is only a marker of disease severity (hence the increased morbidity) or a causative, modifiable risk factor. The definition and evaluation of severity in β thalassemia intermedia are challenging, especially given that hemoglobin level does not correlate with most morbidities,2 and markers of the severity of ineffective erythropoiesis have not been extensively evaluated. We undertook a practical approach and assumed that the need for splenectomy and transfusion therapy reflects a more severe phenotype. In such cases, elevated LIC was associated with an increased risk of complications in all severity groups, indicating that iron overload may be adding to any other causative factors attributed to a more severe disease. Moreover, elevated LIC worsened the observed effect of aging on complications, again indicating an additive role of iron overload to the established role of advancing age.11 Nevertheless, true evidence of target-organ iron toxicity can only be confirmed through radio-pathological studies, or through the observation of a beneficial effect of iron chelation therapy. In fact, evidence already exists regarding a protective role of iron chelation therapy, presumably necessitated in some of the severe cases, against several clinical complications in β thalassemia intermedia.2
If causation is hypothesized, how could iron toxicity be linked to the observed complications, especially vascular disease? Hypercoagulability in β thalassemia intermedia is attributed to several factors including ineffective erythropoiesis and secondary procoagulant activity of hemolysed circulating red blood cells, microparticles, increased platelet activation, thrombocytosis, coagulation factor defects, depletion of antithrombotic factors, and endothelial inflammation.32 Hypercoagulability leads to a high rate of thromboembolic events and probably pulmonary hypertension through multiple microthrombi in the pulmonary vasculature; especially in splenectomized and older patients with β thalassemia intermedia.2,11,33–36 Hemolysis and erythroid hyperplasia have also been linked to increased release of placental growth factor, endothelin-1, and pulmonary hypertension.37 Iron may contribute directly to hemolysis, or endothelial damage and vasculopathy. Iron-derived reactive oxygen species are implicated in the pathogenesis of several vascular disorders including atherosclerosis, microangiopathic hemolytic anemia, vasculitis, and reperfusion injury.38 Moreover, the relationship between iron overload and the severity of ineffective erythropoiesis seems to be bidirectional. Recent evidence suggests that managing iron overload with iron chelators or more novel therapeutics could improve the efficiency of erythropoiesis and the survival of the resulting reticulocytes and erythrocytes.39–42 Thus, iron overload may aggravate ineffective erythropoiesis and the secondary release into the circulation of damaged red blood cells with thrombogenic potential.43–44
The need for iron chelation therapy in patients with β thalassemia intermedia who have never been transfused or have received only occasional transfusions has just recently started to emerge after documenting substantially high LIC and NTBI values in such patients.4,5 As for other aspects of the management of β thalassemia intermedia, clear guidelines on initiation of chelation therapy are not available. Current recommendations are based on expert opinion or are extrapolated from data on β thalassemia major.7 If the evidence of iron toxicity suggested here is confirmed, chelation therapy would be recommended to decrease toxic iron species such as NTBI. LIC measurements could be used to flag the hyperabsorption and increased labile iron and to avoid overchelation. Iron chelation therapy in patients with β thalassemia intermedia may not necessarily be life-long. Intermittent periods of iron chelation with careful assessment of LIC throughout the course of the disease could be sufficient in many cases. When LIC is lowered to desirable levels, low dose oral chelation may be of value in preventing further iron loading. Serum ferritin levels could not predict most morbidities in our study, and correlated weakly with LIC. The serum ferritin to LIC ratio was also shown to be lower relative to that in patients with β thalassemia major.5–6 Thus, reliance on serum ferritin to guide chelation therapy in β thalassemia intermedia may lead to delay in initiating treatment.
The main limitation of our study was the use of echocardiography instead of cardiac catheterization for the diagnosis of pulmonary hypertension which may increase the rate of false positive findings. However, our patients were mainly screened for pulmonary hypertension after presenting with exertional dyspnea with no evidence of left heart disease. Moreover, echocardiography is still the modality of choice used in many studies on tha-lassemia and sickle cell anemia, both because of the invasiveness and cost of cardiac catheterization, and because of the reports of good relationships between Doppler estimates and invasive measurements of pulmonary arterial pressure at baseline and after treatment.45–47 Moreover, we could not directly assess liver pathology in this study and relied on alanine aminotransferase levels to reflect liver abnormality. Although serum ferritin correlated better than LIC with liver enzyme levels in this study, associations with fibrosis and carcinoma through biopsy data should be evaluated. In our study, both R2 and R2* MRI techniques were used for the measurement of LIC. In a study of 384 observations in more than 200 patients, LIC measurements using R2* MRI were unbiased with respect to those using R2 MRI.48
In conclusion, our study demonstrated that elevated LIC in patients with β thalassemia intermedia is associated with significant vascular, endocrine, and bone morbidity. Further studies are needed to confirm the causative role of iron toxicity and evaluate the role of iron chelation therapy in preventing or reversing morbidity in β thalassemia intermedia. This may require collaborative efforts between international centers to ensure that a large sample from this heterogeneous population is evaluated.
Acknowledgments
We thank Professor Sir David J. Weatherall (Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, UK) for his review and thoughtful comments on this work.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Steinberg MH, Forget BG, Higgs DR, Weatherall DJ. Disorders of hemoglobin: genetics pathophysiology, and clinical management. 2nd ed. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 2.Taher AT, Musallam KM, Karimi M, El-Beshlawy A, Belhoul K, Daar S, et al. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115(10):1886–92. doi: 10.1182/blood-2009-09-243154. [DOI] [PubMed] [Google Scholar]
- 3.Taher AT, Musallam KM, Cappellini MD, Weatherall DJ. Optimal management of beta thalassaemia intermedia. Br J Haematol. 2011;152(5):512–23. doi: 10.1111/j.1365-2141.2010.08486.x. [DOI] [PubMed] [Google Scholar]
- 4.Taher A, Musallam KM, El Rassi F, Duca L, Inati A, Koussa S, et al. Levels of non-transferrin-bound iron as an index of iron overload in patients with thalassaemia intermedia. Br J Haematol. 2009;146(5):569–72. doi: 10.1111/j.1365-2141.2009.07810.x. [DOI] [PubMed] [Google Scholar]
- 5.Taher A, El Rassi F, Isma’eel H, Koussa S, Inati A, Cappellini MD. Correlation of liver iron concentration determined by R2 magnetic resonance imaging with serum ferritin in patients with thalassemia intermedia. Haematologica. 2008;93(10):1584–6. doi: 10.3324/haematol.13098. [DOI] [PubMed] [Google Scholar]
- 6.Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583–8. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 7.Taher A, Hershko C, Cappellini MD. Iron overload in thalassaemia intermedia: reassessment of iron chelation strategies. Br J Haematol. 2009;147(5):634–40. doi: 10.1111/j.1365-2141.2009.07848.x. [DOI] [PubMed] [Google Scholar]
- 8.Aessopos A, Kati M, Meletis J. Thalassemia intermedia today: should patients regularly receive transfusions? Transfusion. 2007;47(5):792–800. doi: 10.1111/j.1537-2995.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 9.Taher AT, Musallam KM, Nasreddine W, Hourani R, Inati A, Beydoun A. Asymptomatic brain magnetic resonance imaging abnormalities in splenectomized adults with thalassemia intermedia. J Thromb Haemost. 2010;8(1):54–9. doi: 10.1111/j.1538-7836.2009.03651.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell A, Premawardhena A, Arambepola M, Allen SJ, Peto TE, Fisher CA, et al. Age-related changes in adaptation to severe anemia in childhood in developing countries. Proc Natl Acad Sci USA. 2007;104(22):9440–4. doi: 10.1073/pnas.0703424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taher AT, Musallam KM, El-Beshlawy A, Karimi M, Daar S, Belhoul K, et al. Age-related complications in treatment-naive patients with thalassaemia intermedia. Br J Haematol. 2010;150(4):486–9. doi: 10.1111/j.1365-2141.2010.08220.x. [DOI] [PubMed] [Google Scholar]
- 12.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364(2):146–56. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roghi A, Cappellini MD, Wood JC, Musallam KM, Patrizia P, Fasulo MR, et al. Absence of cardiac siderosis despite hepatic iron overload in Italian patients with thalassemia intermedia: an MRI T2* study. Ann Hematol. 2010;89(6):585–9. doi: 10.1007/s00277-009-0879-3. [DOI] [PubMed] [Google Scholar]
- 14.Taher AT, Musallam KM, Wood JC, Cappellini MD. Magnetic resonance evaluation of hepatic and myocardial iron deposition in transfusion-independent thalassemia intermedia compared to regularly transfused thalassemia major patients. Am J Hematol. 2010;85(4):288–90. doi: 10.1002/ajh.21626. [DOI] [PubMed] [Google Scholar]
- 15.Origa R, Barella S, Argiolas GM, Bina P, Agus A, Galanello R. No evidence of cardiac iron in 20 never- or minimally-transfused patients with thalassemia intermedia. Haematologica. 2008;93(7):1095–6. doi: 10.3324/haematol.12484. [DOI] [PubMed] [Google Scholar]
- 16.Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343(5):327–31. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 17.St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105(2):855–61. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 18.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–5. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22(23):2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 20.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89(3):739–61. [PubMed] [Google Scholar]
- 21.Angelucci E, Muretto P, Nicolucci A, Baronciani D, Erer B, Gaziev J, et al. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood. 2002;100(1):17–21. doi: 10.1182/blood.v100.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Telfer PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110(4):971–7. doi: 10.1046/j.1365-2141.2000.02298.x. [DOI] [PubMed] [Google Scholar]
- 23.Jensen PD, Jensen FT, Christensen T, Nielsen JL, Ellegaard J. Relationship between hepatocellular injury and transfusional iron overload prior to and during iron chelation with desferrioxamine: a study in adult patients with acquired anemias. Blood. 2003;101(1):91–6. doi: 10.1182/blood-2002-06-1704. [DOI] [PubMed] [Google Scholar]
- 24.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331(9):567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 25.Camaschella C, Cappellini MD. Thalassemia intermedia. Haematologica. 1995;80(1):58–68. [PubMed] [Google Scholar]
- 26.Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):40S–7S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 27.Kleber FX, Niemoller L, Doering W. Impact of converting enzyme inhibition on progression of chronic heart failure: results of the Munich Mild Heart Failure Trial. Br Heart J. 1992;67(4):289–96. doi: 10.1136/hrt.67.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8(6):457–69. [PubMed] [Google Scholar]
- 30.El-Hajj Fuleihan G, Baddoura R, Awada H, Arabi A, Okais J. First update of the Lebanese guidelines for osteoporosis assessment and treatment. J Clin Densitom. 2008;11(3):383–96. doi: 10.1016/j.jocd.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Fung EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, et al. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135(4):574–82. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 32.Cappellini MD, Motta I, Musallam KM, Taher AT. Redefining thalassemia as a hypercoagulable state. Ann NY Acad Sci. 2010;1202:231–6. doi: 10.1111/j.1749-6632.2010.05548.x. [DOI] [PubMed] [Google Scholar]
- 33.Taher A, Isma’eel H, Mehio G, Bignamini D, Kattamis A, Rachmilewitz EA, et al. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost. 2006;96(4):488–91. [PubMed] [Google Scholar]
- 34.Taher AT, Musallam KM, Karimi M, El-Beshlawy A, Belhoul K, Daar S, et al. Splenectomy and thrombosis: the case of thalassemia intermedia. J Thromb Haemost. 2010;8(10):2152–8. doi: 10.1111/j.1538-7836.2010.03940.x. [DOI] [PubMed] [Google Scholar]
- 35.Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli G, Mannucci AP. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol. 2000;111(2):467–73. doi: 10.1046/j.1365-2141.2000.02376.x. [DOI] [PubMed] [Google Scholar]
- 36.Atichartakarn V, Likittanasombat K, Chuncharunee S, Chandanamattha P, Worapongpaiboon S, Angchaisuksiri P, et al. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. Int J Hematol. 2003;78(2):139–45. doi: 10.1007/BF02983382. [DOI] [PubMed] [Google Scholar]
- 37.Sundaram N, Tailor A, Mendelsohn L, Wansapura J, Wang X, Higashimoto T, et al. High levels of placenta growth factor in sickle cell disease promote pulmonary hypertension. Blood. 2010;116(1):109–12. doi: 10.1182/blood-2009-09-244830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balla J, Vercellotti GM, Nath K, Yachie A, Nagy E, Eaton JW, et al. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transplant. 2003;18(Suppl 5):v8–12. doi: 10.1093/ndt/gfg1034. [DOI] [PubMed] [Google Scholar]
- 39.Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin Invest. 2010;120(12):4466–77. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos P, Melchiori L, Gardenghi S, Van-Roijen N, Grady RW, Ginzburg Y, et al. Iron metabolism and ineffective erythropoiesis in beta-thalassemia mouse models. Ann NY Acad Sci. 2010;1202:24–30. doi: 10.1111/j.1749-6632.2010.05596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliva EN, Ronco F, Marino A, Alati C, Pratico G, Nobile F. Iron chelation therapy associated with improvement of hematopoiesis in transfusion-dependent patients. Transfusion. 2010;50(7):1568–70. doi: 10.1111/j.1537-2995.2010.02617.x. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Rybicki AC, Suzuka SM, von Bonsdorff L, Breuer W, Hall CB, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16(2):177–82. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 43.Borenstain-Ben Yashar V, Barenholz Y, Hy-Am E, Rachmilewitz EA, Eldor A. Phosphatidylserine in the outer leaflet of red blood cells from beta-thalassemia patients may explain the chronic hypercoagulable state and thrombotic episodes. Am J Hematol. 1993;44(1):63–5. doi: 10.1002/ajh.2830440114. [DOI] [PubMed] [Google Scholar]
- 44.Gardenghi S, Grady RW, Rivella S. Anemia, ineffective erythropoiesis, and hepcidin: interacting factors in abnormal iron metabolism leading to iron overload in beta-thalassemia. Hematol Oncol Clin North Am. 2010;24(6):1089–107. doi: 10.1016/j.hoc.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, et al. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97(11):3411–6. doi: 10.1182/blood.v97.11.3411. [DOI] [PubMed] [Google Scholar]
- 46.Aessopos A, Farmakis D, Deftereos S, Tsironi M, Tassiopoulos S, Moyssakis I, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127(5):1523–30. doi: 10.1378/chest.127.5.1523. [DOI] [PubMed] [Google Scholar]
- 47.Aessopos A, Kati M, Farmakis D. Heart disease in thalassemia intermedia: a review of the underlying pathophysiology. Haematologica. 2007;92(5):658–65. doi: 10.3324/haematol.10915. [DOI] [PubMed] [Google Scholar]
- 48.Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol. 2007;14(3):183–90. doi: 10.1097/MOH.0b013e3280d2b76b. [DOI] [PMC free article] [PubMed] [Google Scholar]