Abstract
Background
In preclinical studies the heat shock protein 90 (Hsp90) inhibitor tanespimycin induced down-regulation of checkpoint kinase 1 (Chk1) and other client proteins as well as increased sensitivity of acute leukemia cells to cytarabine. We report here the results of a phase I and pharmacological study of the cytarabine + tanespimycin combination in adults with recurrent or refractory acute leukemia.
Design and Methods
Patients received cytarabine 400 mg/m2/day continuously for 5 days and tanespimycin infusions at escalating doses on days 3 and 6. Marrow mononuclear cells harvested before therapy, immediately prior to tanespimycin, and 24 hours later were examined by immunoblotting for Hsp70 and multiple Hsp90 clients.
Results
Twenty-six patients were treated at five dose levels. The maximum tolerated dose was cytarabine 400 mg/m2/day for 5 days along with tanespimycin 300 mg/m2 on days 3 and 6. Treatment-related adverse events included disseminated intravascular coagulation (grades 3 and 5), acute respiratory distress syndrome (grade 4), and myocardial infarction associated with prolonged exposure to tanespimycin and its active metabolite 17-aminogeldanamycin. Among 21 evaluable patients, there were two complete and four partial remissions. Elevations of Hsp70, a marker used to assess Hsp90 inhibition in other studies, were observed in more than 80% of samples harvested 24 hours after tanespimycin, but down-regulation of Chk1 and other Hsp90 client proteins was modest.
Conclusions
Because exposure to potentially effective concentrations occurs only for a brief time in vivo, at clinically tolerable doses tanespimycin has little effect on resistance-mediating client proteins in relapsed leukemia and exhibits limited activity in combination with cytarabine.
Keywords: cytarabine, tanespimycin, phase I study, pharmacological study, acute leukemia
Introduction
Heat shock protein 90 (Hsp90) is an abundant and ubiquitous molecular chaperone that helps many key cellular polypeptides assume or maintain their active conformations.1–3 Extensive preclinical studies have examined this chaperone as a potential new drug target in hematologic malignancies.3–7 Among the more than 80 currently identified Hsp90 client proteins there are several kinases involved in myeloid neoplasms, including Bcr/abl,8,9 Flt3,4,10,11 and c-Kit,12 as well as the anti-apoptotic kinase Akt.13,14 When Hsp90 is inhibited, these polypeptides and other Hsp90 clients fail to fold properly during synthesis and, as a consequence, become less abundant as mature molecules turn over.15,16 This client protein down-regulation raises the possibility that Hsp90 inhibitors such as tanespimycin and alvespimycin might be used to target multiple potential resistance mechanisms simultaneously.1,3,6 In addition, tanespimycin selectively inhibits growth of aneuploid cells.17 Accordingly, there has been substantial interest in testing this class of agents in acute leukemia.
Although a recent phase I study of the Hsp90 inhibitor alvespimycin in patients with advanced myeloid leukemia produced only a modest 18% complete remission rate,18 the realization that pathways targeted by Hsp90 inhibitors contribute to drug resistance raises the question of whether Hsp90 inhibitors could be used as sensitizing agents in combination chemotherapy. Previous studies have also provided the preclinical rationale for combining tanespimycin with a number of other agents, including imatinib,19 Flt3 inhibitors,20 histone deacetylase inhibitors,21,22 etoposide23 and cytarabine.24 The present work focused on the cytarabine + tanespimycin combination because of its potential broad applicability to myeloid malignancies. Cytarabine triggers sequential activation of ataxia telangiectasia mutated/Rad3-related (ATR) kinase and its substrate checkpoint kinase 1 (Chk1) ex vivo,24–26 thereby activating the replication checkpoint.27 Conversely, Chk1 down-regulation by RNA interference or treatment with tanespimycin abrogates this checkpoint28 and sensitizes cells to cytarabine in vitro,24 suggesting that replication checkpoint signaling is protective.
We report here the results of a phase I study of cytarabine and tanespimycin in patients with relapsed/refractory acute leukemia or chronic myeloid leukemia in accelerated phase or blast crisis. The goals of this study were to assess the toxicities and establish the maximum tolerated dose of the cytarabine + tanespimycin combination, and also to examine the effect of tanespimycin on levels of Hsp90 clients in leukemic blasts in situ.
Design and Methods
Selection of patients
Adults (age ≥ 18 years) with acute myeloid leukemia (except M3) or acute lymphoblastic leukemia were eligible if they: (i) had failed to respond to intensive induction chemotherapy or relapsed after a first complete remission lasting 1 year or less; (ii) had a history of a prior myelodysplastic syndrome or myeloproliferative neoplasm; or (iii) presented with cytogenetic abnormalities of chromosome 5, 7, 8, or 11 or three or more karyotypic abnormalities. Patients with accelerated phase or blast crisis chronic myeloid leukemia were eligible only if prior imatinib therapy and one other induction regimen had failed.
Additional eligibility criteria included Eastern Cooperative Oncology Group performance status 0–2; total bilirubin less than 1.5 times the upper limit of normal; calculated creatinine clearance greater than 60 mL/min; QTc less than 450 msec (men) or 470 msec (women), determined from an electrocardiogram; cardiac ejection fraction greater than 40%; pulmonary CO diffusing capacity greater than 80% of normal; and recovery from toxicities of preceding therapy. Hydroxyurea or glucocorticoids administered to prevent impending leukostasis were stopped at least 48 h before initiation of cytarabine. Active central nervous system leukemia, uncontrolled infection, symptomatic pulmonary disease, or a requirement for a medication known to cause QTc prolongation precluded enrollment. Patients with a history of prior stem cell transplantation, pulmonary irradiation, chemotherapy-induced pulmonary toxicity, or egg allergy were also excluded. The study was approved by the Institutional Review Boards of the participating institutions. Written informed consent was obtained from all patients.
Evaluation of patients
Before treatment a history and physical examination, electrocardiogram, complete blood count, echocardiogram or multiple gated acquisition scan, pulmonary function tests and baseline pulse oximetry were performed and prothrombin time, activated partial thromboplastin time, fibrinogen, serum creatinine, sodium, potassium, uric acid, calcium, phosphate, magnesium, total protein, albumin, total bilirubin, direct bilirubin, aspartate transaminase and alkaline phosphatase were measured. The physical examination and chemistry tests were repeated at least twice weekly during each treatment cycle. Complete blood counts were performed daily until neutrophil and platelet counts were greater than 0.5×109/L and 20×109/L, respectively.
Dose, schedule and escalation scheme
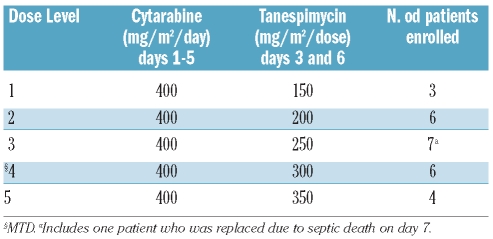
A cytarabine regimen previously employed for consolidation29 was utilized because the cytarabine dose (400 mg/m2/day for 5 days) was higher than that patients typically received as part of their prior induction therapy. Because tanespimycin induces G1 and G2 arrest,24 which could potentially inhibit the replication required for cytarabine-induced cytotoxicity,24,30,31 tanespimycin was administered for the first time beginning 48 h after the start of the 5-day cytarabine infusion (Figure 1). The starting dose of tanespimycin (150 mg/m2/dose on days 3 and 6), approximately 50% of the maximum tolerated dose of single-agent tanespimycin administered to patients with solid tumors in a weekly schedule,32 was chosen because of anticipated synergy of the agents.24 The tanespimycin dose was increased in 50 mg/m2 increments.
Figure 1.
Schematic representation of trial events. After pretreatment bone marrow aspirates were obtained, cytarabine was administered at a dose of 400 mg/m2/day by continuous infusion for 5 days. Approximately 48 h into this infusion, the day 3 bone marrow aspirate was obtained. Patients then received escalating doses of tanespimycin (repeated again on day 6 at the very end of the cytarabine infusion), followed 22±2 h later by the day 4 bone marrow aspirate to assess the impact on client protein expression
Drug formulation and administration
Cytarabine was reconstituted in sterile water at 50 mg/mL, diluted into D5W and administered as a continuous infusion at a dose of 400 mg/m2/day (rounded to the nearest 25 mg) in 500 mL D5W/day for 5 days. Tanespimycin, supplied by the Division of Cancer Treatment (NCI, Bethesda, MD, USA) as a 25 mg/mL solution in dimethyl sulfoxide, was diluted to 1 mg/mL in EPL egg phospholipid diluent and administered (rounded to the nearest 5 mg) as a 2-h infusion beginning 48 and 120 h after the start of cytarabine. Because of concerns about possible tanespimycin-induced QTc prolongation, serum potassium, calcium and magnesium levels were checked and repleted to the normal range within 24 h before the tanespimycin administration. Electrocardiograms were checked prior to, during the second half and within 6 h after completion of the first tanespimycin infusion; and QTc intervals were measured manually and corrected for rate using Bazett’s and Fridericia’s methods.
Ancillary treatment
All patients received allopurinol 300 mg/day on days 1–7 and oral valacyclovir 500 mg daily (or acyclovir 400 mg twice daily) beginning on day 9. Oral fluoroquinolones were administered pro-phylactically according to institutional practices. Patients with neutropenic fever initially received empiric antibiotics with coverage against Pseudomonas spp.
Retreatment and consolidation therapy
Patients who did not achieve a complete remission after one course were eligible for a second course on day 21 or later if the blast index (% cellularity x % blasts) decreased by more than 4-fold and all non-hematologic toxicities had resolved to grade 1 or less. Patients in complete remission were eligible for up to four courses of consolidation on the induction schedule beginning 30±10 days from hospital discharge after the preceding cycle. Dose reductions of one dose level were permitted for dose-limiting toxicity.
Definition of dose-limiting toxicity and maximum tolerated dose
Adverse events were graded by Common Terminology Criteria for Adverse Events, version 3.0. Dose-limiting toxicity was defined as: (i) grade 4 hematologic toxicity persisting beyond day 35 not attributable to persistent leukemia; (ii) grade 3 or higher QTc prolongation; (iii) grade 2 or higher allergic, non-QTc cardiac, pulmonary, genitourinary or neurocortical toxicity; (iv) grade 4 diarrhea, nausea or emesis despite maximal medical treatment; (v) grade 3 or higher ALT, AST, alkaline phosphatase or bilirubin elevation lasting 15 days or more; or (vi) any other grade 3 or higher nonhematologic toxicity that did not resolve with routine medical management.
Response evaluation
Bone marrow aspirates and biopsies were obtained within 48 h prior to initiation of therapy, on day 10–15, and every 7–14 days thereafter until counts recovered. Complete remission and partial remission were defined as previously reported,33 consistent with existing recommendations.34
Pharmacokinetic analysis
Blood samples were drawn on day 3 before tanespimycin infusion; 115 min into the 2-h infusion; and 5 min, 3 h, 9 h and 24 h after the end of infusion. Plasma concentrations of tanespimycin and its principal metabolite 17-aminogeldanamycin (17AG) were determined as described elsewhere.32 Tanespimycin and 17AG plasma concentration-time data were analyzed by non-compartmental methods using WINNONLIN version 4.1 (Pharsight Corp., Mountainview, CA, USA). Buffy coat DNA was genotyped as previously described32 for CYP3A5 polymorphisms, which are known to affect tanespimycin clearance.
Immunoblotting
Marrow mononuclear cells were isolated35 before treatment, on day 3 prior to tanespimycin administration, and on day 4 at 22±2 h after the start of tanespimycin (Figure 1). Whole cell lysates prepared in guanidine hydrochloride were processed for immunoblotting,35 which was performed using antibodies identified previously.24,32,36 Marrow mononuclear cells from pretreatment samples were also treated ex vivo as described elsewhere.24
Results
Patients’ characteristics
Twenty-six adult patients with leukemia (Table 1) received 30 courses of cytarabine + tanespimycin at five dose levels (Table 2). Of the 22 patients with acute myeloid leukemia enrolled, 17 had failed to enter remission with their preceding regimens; and five had relapsed after 1 year or less in first complete remission, several while still receiving consolidation therapy. Of the remaining patients, two had acute lymphoblastic leukemia and two had chronic myeloid leukemia in accelerated phase or blast crisis which had not responded to Bcr/abl inhibitor-containing therapy.
Table 1.
Characteristics of treated patients.
Table 2.
Summary of dose escalation.
Hematologic toxicities
All patients experienced prolonged grade 4 myelosuppression requiring platelet and red blood cell support. During induction, those patients with normal or elevated white blood cell counts typically experienced a decrease in white blood cells before day 5. The absolute neutrophil count reached a nadir of less than 0.1×109/L by a median of day 7 (range, 3–10 days) and, in those who subsequently achieved a complete remission, remained less than 0.5×109/L until day 24–34. The hematologic toxicities were less severe during second or later courses. Patients receiving the combination while in partial or complete remission did not become neutropenic until day 7–12 of therapy and had briefer periods of grade 4 neutropenia (generally 7–10 days), with recovery by day 21–28.
Non-hematologic toxicities
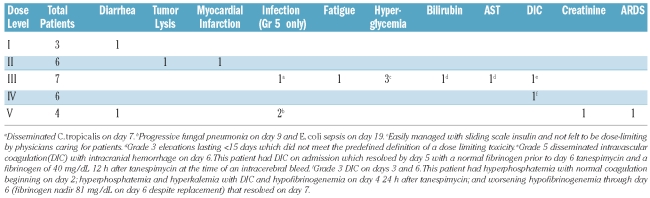
The non-haematologic toxicities are summarized in Table 3. Consistent with the observed myelosuppression, neutropenic fever occurred in all patients and was treated with empiric antimicrobial regimens. There were three infectious deaths, one at dose level 3 (on day 7 due to disseminated Candida tropicalis) and two at dose level 5 (one on day 9 with fungal pneumonia and one on day 19 with E. coli sepsis). A third patient at dose level 5 developed acute respiratory distress syndrome (ARDS) on day 7 leading to multi-organ failure and more than 2 weeks of mechanical ventilation before recovery. Patients treated at dose level 5 were also noted by their physicians to appear sicker and less tolerant of stresses such as fever and transient hypotension. Based on these observations, as well as the knowledge that 350 mg/m2/dose exceeds the weekly tanespimycin maximum tolerated dose in patients with solid tumors, dose level 4 (cytarabine 400 mg/m2/day x 5 days, tanespimycin 300 mg/m2/dose on days 3 and 6) was identified as the maximum tolerated dose and recommended phase 2 dose.
Table 3.
Non-hematologic adverse events of grade 3 or higher which were possibly, probably or definitely attributed to treatment.
Other severe adverse events at least possibly related to the treatment included a myocardial infarction on day 6 in a patient without a prior history of cardiac disease (dose level 2), an episode of grade 5 disseminated intravascular coagulopathy (DIC) leading to intracerebral hemorrhage on day 6 (dose level 3), and grade 3 DIC associated with tumor lysis on day 4 (dose level 4). As indicated in Table 3, both episodes of DIC occurred in patients with evidence of DIC earlier in their chemotherapy but were exacerbated by tanespimycin administration and, by definition, represent treatment-related adverse events. Notably, therapy-induced arrhythmias and QTc prolongations were not observed.
Responses
Among the 21 evaluable patients, two obtained complete remissions. One occurred at dose level 4 in an patient with acute myeloid leukemia who had experienced complete remissions of 10 months and 6 months after two prior cytarabine-containing regimens. That patient received two consolidation cycles before declining further therapy and relapsing after a 10-month complete remission. The second complete remission occurred at dose level 5 in a patient with refractory Philadelphia-positive acute lymphoblastic leukemia but lasted only 1 month. In addition there were two partial remissions in patients with acute myeloid leukemia and two hematologic partial remissions in patients with chronic myeloid leukemia, all lasting less than 60 days.
Pharmacokinetics
The pharmacokinetics of tanespimycin and its metabolite 17AG, which also affects client protein stability,24 were evaluable for 20 patients (Table 4). Mean tanespimycin clearance was 17.8±6.4 L/h/m2. The half-lives for tanespimycin and 17AG were 4.5±2.7 h and 6.1±1.9 h, respectively. Moreover, tanespimycin and 17AG peak plasma concentrations and areas under the curves increased with doses over the 150–350 mg/m2 range. Importantly, tanespimycin mean peak plasma concentrations were greater than 3000 nM at all dose levels; and 17AG mean peak plasma concentrations exceeded 1500 nM.
Table 4.
Tanespimycin and 17AG pharmacokinetic parameters by dose levela.
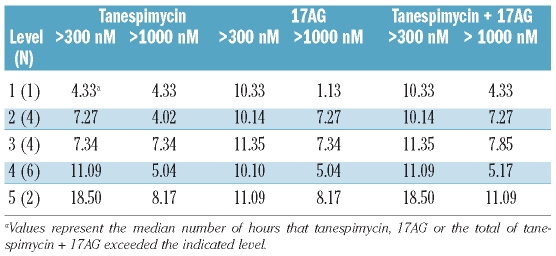
Because client protein down-regulation is known to require extended tanespimycin exposure,16,24 we also examined the duration of tanespimycin and 17AG plasma concentrations above 300 nM, a concentration previously shown to induce substantial client protein down-regulation after 24 h in leukemia cell lines in vitro,24 and 1000 nM, a concentration commonly used in preclinical studies. The sum of the tanespimycin and 17AG concentrations exceeded 300 nM for more than 10 h in all patients (Table 5). This sum exceeded 300 nM for more than 24 h in only three patients, none of whom had an obvious explanation based on cytochrome P450 polymorphisms. All three patients with prolonged exposure to higher drug levels experienced severe adverse events, including a myocardial infarction (dose level 2) and fatal sepsis (one patient each at dose level 3 and dose level 5).
Table 5.
Duration of plasma concentrations above 300 nM or 1000 nM by dose level.
Heat shock protein 90 client protein expression
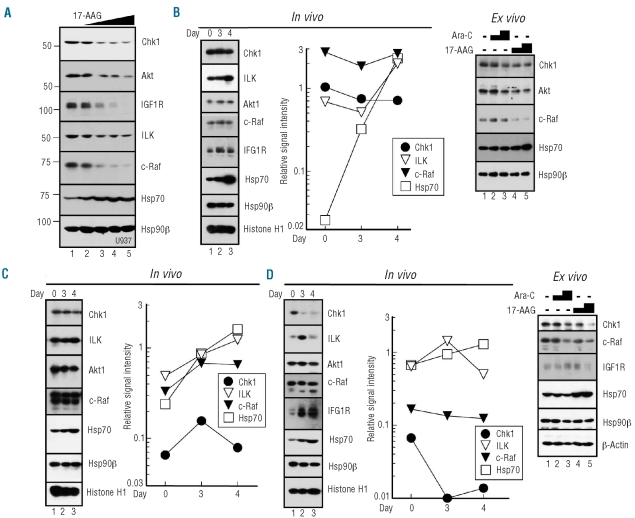
Correlative studies were designed to determine whether treatment affects Hsp90 function in situ. Bone marrow aspirates harvested prior to therapy, on day 3 before tanespimycin treatment, and on day 4 at 22±2 h after tanespimycin (Figure 1) were blotted for several Hsp90 clients that are down-regulated by tanespimycin in leukemia cell lines (Figure 2A and 24). Whenever possible, aliquots of pretreatment marrow were also exposed to cytarabine or tanespimycin ex vivo for comparison.
Figure 2.
Effects of treatment on client protein levels. (A). After U937 cells had been treated for 24 h with diluent (0.1% DMSO, lane 1) or tanespimycin at 30, 100, 300 or 1000 nM (lanes 2–5), whole cell lysates were subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting. Numbers on the left are molecular weight markers in kDa. (B-D) Marrow mononuclear cells from patients exposed to tanespimycin in vivo at (B) dose level 3, (C) dose level 4 or (D) dose level 1. In each case whole cell lysates were isolated from marrows harvested prior to treatment (day 0), after 48 h of cytarabine (day 3) and 22±2 h after tanespimycin administration (day 4). Polypeptides from 0.5–5x105 HL-60 cells were included on each gel to provide a standard curve for quantitating signals.35 Hsp90β and Histone H1 represent two loading controls. After immunoblotting, digitized signals were normalized for histone H1 content and compared to HL-60 cell dilutions. A value of 1.0 indicates the same antigen signal as an equal number of HL-60 cells. In panels (B) and (D), pretreatment marrow mononuclear cells from the same patients were also prospectively exposed ex vivo to diluent (0.1% DMSO), 300 nM cytarabine, 1000 nM cytarabine, 300 nM tanespimycin, or 1000 nM tanespimycin (lanes 1–5, respectively) for 24 h and subjected to immunoblotting. Cells for ex vivo exposure were not available from the patient in panel (C).
Based on preclinical results, decreased levels of the Hsp90 clients and increased levels of Hsp70 [due to transcription mediated by heat shock factor-1 (HSF-1) released from Hsp90]1 were expected on day 4. Contrary to this prediction, decreases of the Hsp90 clients ILK, Akt, c-Raf and IGF1R were difficult to discern, although there were modest increases in Hsp70 (Figure 2B-2D, in vivo). Exposure of pretreatment blasts to tanespimycin for 24 h ex vivo confirmed that tanespimycin was able to down-regulate Chk1, Akt and c-Raf at 300 and 1000 nM (Figure 2B and 2D, ex vivo), albeit less extensively than in acute myeloid leukemia lines (Figure 2A, lane 4) or samples of previously untreated acute myeloid leukemia.24 In total, 17 sets of sequential samples were analyzed. Hsp70 showed a readily discernible increase between day 3 and day 4 in 10 of 12 (83%) samples that yielded an Hsp70 signal. In contrast, only three of 13 (23%) and one of 13 (8%) day 4 samples had decreased c-Raf or ILK, respectively, relative to baseline, indicating that effects on client proteins in leukemic blasts in situ were modest at the tanespimycin exposures achieved clinically. Moreover, there was no apparent relationship between these changes and clinical response.
Discussion
In the present study, we report the first results of a tanespimycin-containing combination in patients with relapsed/refractory acute leukemias and the first analysis of effects of these agents on Hsp90 clients in leukemia specimens in situ. Our results have potentially important implications for current efforts to modulate leukemia resistance mechanisms with Hsp90 inhibitors.
Several issues were considered in designing the present study. First, the trial was built on an established cytarabine regimen with defined activity and toxicity.29 Second, a cytarabine regimen that incorporates more than the usual 100–200 mg/m2/day was chosen to avoid treating refractory leukemias with a cytarabine dose that previously failed to induce remission. While we recognize that an even higher dose of cytarabine might, as a single-agent, be more effective in the setting of relapsed and refractory leukemia,37 high-dose cytarabine might also have further diminished the amount of tanespimycin that could be administered without dose-limiting toxicity. The present cytarabine dose represented a compromise between these two concerns. Finally, tanespimycin was delayed until day 3 to avoid ansamycin-induced inhibition of cell cycle progression,24,28 which antagonizes the effects of cytarabine.24
In addition to the typical hematologic toxicities of cytarabine infusion, we observed a number of dose-limiting toxicities that were attributed to the combination, including grade 3 and grade 5 DIC during therapy-induced tumor lysis, ARDS progressing to multi-organ failure, and a possibly higher than expected frequency of septic deaths. The occurrence of two grade 5 toxicities and grade 4 ARDS in three patients at dose level 5, combined with the information that the 350 mg tanespimycin/m2/dose exceeds the weekly single-agent maximum tolerated dose in patients with solid tumors,32 led to identification of the maximum tolerated dose as tanespimycin 300 mg/m2/dose on days 3 and 6 in combination with cytarabine 400 mg/m2/day in a 5-day infusion.
Among 22 patients with acute myeloid leukemia, one complete remission and two partial remissions were observed, all in patients with leukemia who relapsed soon after or were refractory to prior cytarabine-containing regimens. In addition, a complete remission was observed in a patient with primary refractory Philadelphia chromosome-positive acute lymphoblastic leukemia and two hematologic partial remissions were observed in patients with accelerated phase chronic myeloid leukemia, for an overall response rate of 23%. Enthusiasm for this regimen was, however, tempered by the short duration of all but one response and the seriousness of the adverse events.
Correlative studies were performed to determine whether tanespimycin had the expected impact on Hsp90 clients. Comparisons of samples harvested before and 22±2 h after tanespimycin treatment demonstrated discernible Chk1 decreases in six of 13 (46%) cases. The magnitude of Chk1 down-regulation was, however, relatively modest compared to that in tanespimycin-treated acute myeloid leukemia cell lines (Figure 2A and 24,28). Moreover, it was unclear whether Chk1 down-regulation reflected client protein degradation, as the Hsp90 client proteins c-Raf and ILK were down-regulated in only one and three of 13 serial samples, respectively, on day 4 (Figure 2B-D). An equally plausible explanation is that Chk1, which is expressed in a cell cycle-dependent manner,38 decreased due to tanespimycin-induced changes in cell cycle progression.24
A number of prior studies have examined Hsp70 up-regulation, which reflects increased activity of the transcription factor HSF-1 after release from Hsp90,1 as a marker of tanespimycin’s action.18,32,29 It is important to emphasize that Hsp70 up-regulation results in resistance to Hsp90 inhibitors, not sensitization.40,41 In our study Hsp70 was up-regulated after tanespimycin administration in blasts from 83% of patients. However, this Hsp70 up-regulation sometimes occurred before administration of tanespimycin (day 3, Figures 2B and 2D), possibly reflecting cytarabine-induced stress. Moreover, Hsp70 up-regulation did not predict client protein down-regulation. In preclinical studies published after the completion of our work, response to Hsp90 inhibitors in xenografts correlated best with prolonged Hsp90 active site occupancy, which in turn correlated with Hsp90 client down-regulation.16 Accordingly, some marker other than Hsp70 up-regulation might be needed to measure prolonged Hsp90 inhibition and sensitization in future combination studies.
Analysis of tanespimycin pharmacokinetics (Tables 4 and 5) provided a potential explanation for the limited down-regulation of client proteins. The clearance of tanespimycin and 17AG in these leukemia patients (Table 4) was similar to that reported in earlier solid tumor trials.32,39 At the maximum tolerated dose, tanespimycin + 17AG exceeded 1000 nM, a concentration that induced substantial client protein down-regulation in some samples after 24 h (Figure 2B), for only 5 h. In contrast, our previous studies demonstrated that 6 h of incubation with tanespimycin is not sufficient to induce client protein down-regulation in vitro.24 Even though 350 mg/m2 tanespimycin resulted in more prolonged exposure to potentially effective concentrations of tanespimycin + 17AG (Table 5), this dose was not tolerable in combination with cytarabine. Accordingly, it appears that a single 2-h tanespimycin infusion at tolerable doses might not give sufficient exposure to induce substantial client protein down-regulation in leukemic blasts in vivo. Examination of the pharmacokinetic data in Table 4 and a graph of pooled mean plasma-concentration data for each dose level suggests that levels of tranespimycin + 17AG above 300 nM could be sustained in most patients for more than 24 h by administration of 150 mg/m2 tanespimycin every 12 hours for two doses on day 3 and again on day 6. It is, however, unclear whether such a schedule would be tolerable, given the adverse events observed in the present study.
In summary, the present study has defined the maximum tolerated dose of the cytarabine + tanespimycin regimen as cytarabine 400 mg/m2/day for 5 days and tanespimycin 300 mg/m2/dose on days 3 and 6. Despite the ability of tanespimycin to modulate multiple resistance mechanisms in vitro, disruption of these same pathways in leukemic cells in situ was difficult to demonstrate, likely reflecting drug exposure that was insufficient to down-regulate client proteins in leukemic blasts. It is possible that alternative Hsp90 inhibitors, especially those lacking a geldanamycin backbone,42 might exhibit a wider therapeutic index in this setting. Alternatively, in view of the recent development of highly effective and selective inhibitors of clients such as checkpoint kinases,43–45 Akt42,46,47 and IGFR,47 it will also be interesting to see whether these agents offer any benefit when combined with cytarabine in patients with acute myeloid leukemia.
Acknowledgments
The authors would like to thank the house staff, fellows, nurses, pharmacists and attending staff who helped to care for patients enrolled in this trial, the patients who participated, and Deb Strauss for secretarial assistance.
Footnotes
Funding: this work was supported in part by U01 CA69912 (to CE), U01 CA70095 (to JEK), P30 CA06973 (JEK, BDS), National Center for Research Resources grant M01 RR0052 supporting the Johns Hopkins University School of Medicine, General Clinical Research Center, and a Translational Research Grant from the Leukemia & Lymphoma Society of America (to SHK, JEK, and LMK).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–72. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 2.Pratt WB, Morishima Y, Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J Biol Chem. 2008;283(34):22885–9. doi: 10.1074/jbc.R800023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ. Targeting HSP90 for cancer therapy. Br J Cancer. 2009;100(10):1523–9. doi: 10.1038/sj.bjc.6605066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Shaer L, Walsby E, Gilkes A, Tonks A, Walsh V, Mills K, et al. Heat shock protein 90 inhibition is cytotoxic to primary AML cells expressing mutant FLT3 and results in altered downstream signalling. Br J Haematol. 2008;141(4):483–93. doi: 10.1111/j.1365-2141.2008.07053.x. [DOI] [PubMed] [Google Scholar]
- 5.Flandrin P, Guyotat D, Duval A, Cornillon J, Tavernier E, Nadal N, et al. Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones. 2008;13(3):357–64. doi: 10.1007/s12192-008-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reikvam H, Ersvaer E, Bruserud O. Heat shock protein 90 - a potential target in the treatment of human acute myelogenous leukemia. Curr Cancer Drug Targets. 2009;9(6):761–76. doi: 10.2174/156800909789271486. [DOI] [PubMed] [Google Scholar]
- 7.Napper JM, Sollars VE. 17-N-Allylamino-17-demethoxygeldanamycin induces a diverse response in human acute myelogenous cells. Leuk Res. 2010;34(11):1493–500. doi: 10.1016/j.leukres.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimmanapalli R, O’Bryan E, Bhalla K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res. 2001;61(5):1799–804. [PubMed] [Google Scholar]
- 9.Blagosklonny MV, Fojo T, Bhalla KN, Kim JS, Trepel JB, Figg WD, et al. The Hsp90 inhibitor geldanamycin selectively sensitizes Bcr-Abl- expressing leukemia cells to cytotoxic chemotherapy. Leukemia. 2001;15(10):1537–43. doi: 10.1038/sj.leu.2402257. [DOI] [PubMed] [Google Scholar]
- 10.Minami Y, Kiyoi H, Yamamoto Y, Yamamoto K, Ueda R, Saito H, et al. Selective apoptosis of tandemly duplicated FLT3-transformed leukema cell inhibitors. Leukemia. 2002;16(8):1535–40. doi: 10.1038/sj.leu.2402558. [DOI] [PubMed] [Google Scholar]
- 11.Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9(12):4483–93. [PubMed] [Google Scholar]
- 12.Fumo G, Akin C, Metcalfe DD, Neckers L. 17-allylamino-17-demethoxygeldanamycin (17-AAG) is effective in down-regulating mutated, constitutively activated KIT protein in human mast cells. Blood. 2004;103(3):1078–84. doi: 10.1182/blood-2003-07-2477. [DOI] [PubMed] [Google Scholar]
- 13.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277(42):39858–66. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 14.Nimmanapalli R, O’Bryan E, Kuhn D, Yamaguchi H, Wang HG, Bhalla KN. Regulation of 17-AAG-induced apoptosis: role of Bcl-2, Bcl-XL, and Bax downstream of 17-AAG-mediated down-regulation of Akt, Raf-1, and Src kinases. Blood. 2003;102(1):269–75. doi: 10.1182/blood-2002-12-3718. [DOI] [PubMed] [Google Scholar]
- 15.Arlander SJ, Felts SJ, Wagner JM, Stensgard B, Toft DO, Karnitz LM. Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones. J Biol Chem. 2006;281(5):2989–98. doi: 10.1074/jbc.M508687200. [DOI] [PubMed] [Google Scholar]
- 16.Tillotson B, Slocum K, Coco J, Whitebread N, Thomas B, West KA, et al. Hsp90 (heat shock protein 90) inhibitor occupancy is a direct determinant of client protein degradation and tumor growth arrest in vivo. J Biol Chem. 2010;285(51):39835–43. doi: 10.1074/jbc.M110.141580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang YC, Williams BR, Siegel JJ, Amon A. Identification of aneuploidy-selective antiproliferation compounds. Cell. 2011;144(4):499–512. doi: 10.1016/j.cell.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24(4):699–705. doi: 10.1038/leu.2009.292. [DOI] [PubMed] [Google Scholar]
- 19.Radujkovic A, Schad M, Topaly J, Veldwijk MR, Laufs S, Schultheis BS, et al. Synergistic activity of imatinib and 17-AAG in imatinib-resistant CML cells over-expressing BCR-ABL--inhibition of P-glyco-protein function by 17-AAG. Leukemia. 2005;19(7):1198–206. doi: 10.1038/sj.leu.2403764. [DOI] [PubMed] [Google Scholar]
- 20.George P, Bali P, Cohen P, Tao J, Guo F, Sigua C, et al. Cotreatment with 17-allylamino-demethoxygeldanamycin and FLT-3 kinase inhibitor PKC412 is highly effective against human acute myelogenous leukemia cells with mutant FLT-3. Cancer Res. 2004;64(10):3645–52. doi: 10.1158/0008-5472.CAN-04-0006. [DOI] [PubMed] [Google Scholar]
- 21.Rahmani M, Reese E, Dai Y, Bauer C, Kramer LB, Huang M, et al. Cotreatment with suber-anoylanilide hydroxamic acid and 17-ally-lamino 17-demethoxygeldanamycin synergistically induces apoptosis in Bcr-Abl+ cells sensitive and resistant to STI571 (imatinib mesylate) in association with down-regulation of Bcr-Abl, abrogation of signal transducer and activator of transcription 5 activity, and Bax conformational change. Mol Pharmacol. 2005;67(4):1166–76. doi: 10.1124/mol.104.007831. [DOI] [PubMed] [Google Scholar]
- 22.Rao R, Fiskus W, Yang Y, Lee P, Joshi R, Fernandez P, et al. HDAC6 inhibition enhances 17-AAG--mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood. 2008;112(5):1886–93. doi: 10.1182/blood-2008-03-143644. [DOI] [PubMed] [Google Scholar]
- 23.Yao Q, Weigel B, Kersey J. Synergism between etoposide and 17-AAG in leukemia cells: critical roles for Hsp90, FLT3, topoisomerase II, Chk1, and Rad51. Clin Cancer Res. 2007;13(5):1591–600. doi: 10.1158/1078-0432.CCR-06-1750. [DOI] [PubMed] [Google Scholar]
- 24.Mesa RA, Loegering D, Powell HL, Flatten K, Arlander SAH, Dai NT, et al. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood. 2005;106(1):318–27. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21(13):4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho SH, Toouli CD, Fujii GH, Crain C, Parry D. Chk1 is essential for tumor cell viability following activation of the replication checkpoint. Cell Cycle. 2005;4(1):131–9. doi: 10.4161/cc.4.1.1299. [DOI] [PubMed] [Google Scholar]
- 27.O’Connell MJ, Cimprich KA. G2 damage checkpoints: what is the turn-on? J Cell Sci. 2005;118(Pt 1):1–6. doi: 10.1242/jcs.01626. [DOI] [PubMed] [Google Scholar]
- 28.Arlander SJH, Eapen AK, Vroman BT, McDonald RJ, Toft DO, Karnitz LM. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem. 2003;278(52):52572–7. doi: 10.1074/jbc.M309054200. [DOI] [PubMed] [Google Scholar]
- 29.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia in the Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 30.Major PP, Egan EM, Beardsley GP, Minden MD, Kufe DW. Lethality of human myeloblasts correlates with the incorporation of arabinofuranosylcytosine into DNA. Proc Natl Acad Sci USA. 1981;78(5):3235–9. doi: 10.1073/pnas.78.5.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelicano H, Carew JS, McQueen TJ, Andreeff M, Plunkett W, Keating MJ, et al. Targeting Hsp90 by 17-AAG in leukemia cells: mechanisms for synergistic and antagonistic drug combinations with arsenic trioxide and Ara-C. Leukemia. 2006;20(4):610–9. doi: 10.1038/sj.leu.2404140. [DOI] [PubMed] [Google Scholar]
- 32.Goetz MP, Toft DO, Reid JM, Ames MM, Stensgard B, Safgren S, et al. A phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23(6):1078–87. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann SH, Karp JE, Letendre L, Kottke TJ, Safgren S, Greer J, et al. Phase I and pharmacological study of infusional topotecan and carboplatin in relapsed and refractory leukemia. Clin Cancer Res. 2005;11(18):6641–9. doi: 10.1158/1078-0432.CCR-05-0817. [DOI] [PubMed] [Google Scholar]
- 34.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann SH, Svingen PA, Gore SD, Armstrong DK, Cheng Y-C, Rowinsky EK. Altered formation of topotecan-stabilized topoisomerase I-DNA adducts in human leukemia cells. Blood. 1997;89(6):2098–104. [PubMed] [Google Scholar]
- 36.Wahner Hendrickson D, Haluska P, Schneider PA, Loegering DA, Peterson KL, Attar RM, et al. Expression of insulin receptor isoform a and insulin-like growth factor-1 receptor in human acute myeloid leukemia: Effect of the dual receptor inhibitor BMS-536924 in vitro. Cancer Res. 2009;69(19):7635–43. doi: 10.1158/0008-5472.CAN-09-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern W, Aul C, Maschmeyer G, Schonrock-Nabulsi R, Ludwig WD, Bartholomaus A, et al. Superiority of high-dose over intermediate-dose cytosine arabinoside in the treatment of patients with high-risk acute myeloid leukemia: results of an age-adjusted prospective randomized comparison. Leukemia. 1998;12(7):1049–55. doi: 10.1038/sj.leu.2401066. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko YS, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, et al. Cell-cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene. 1999;18(25):3673–81. doi: 10.1038/sj.onc.1202706. [DOI] [PubMed] [Google Scholar]
- 39.Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, et al. Phase I pharmaco-kinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23(18):4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 40.Guo F, Rocha K, Bali P, Pranpat M, Fiskus W, Boyapalle S, et al. Abrogation of heat shock protein 70 induction as a strategy to increase antileukemia activity of heat shock protein 90 inhibitor 17-allylamino-demethoxy geldanamycin. Cancer Res. 2005;65(22):10536–44. doi: 10.1158/0008-5472.CAN-05-1799. [DOI] [PubMed] [Google Scholar]
- 41.McCollum AK, TenEyck CJ, Stensgard B, Morlan BW, Ballman KV, Jenkins RB, et al. P-Glycoprotein-mediated resistance to Hsp90-directed therapy is eclipsed by the heat shock response. Cancer Res. 2008;68(18):7419–27. doi: 10.1158/0008-5472.CAN-07-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Montfort RL, Workman P. Structure-based design of molecular cancer therapeutics. Trends Biotechnol. 2009;27(5):315–28. doi: 10.1016/j.tibtech.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Zabludoff SD, Deng C, Grondine MR, Sheehy AM, Ashwell S, Caleb BL, et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol Cancer Ther. 2008;7(9):2955–66. doi: 10.1158/1535-7163.MCT-08-0492. [DOI] [PubMed] [Google Scholar]
- 44.Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res. 2010;16(2):376–83. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guzi TJ, Paruch K, Dwyer MP, Labroli M, Shanahan F, Davis N, et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol Cancer Ther. 2011;10(4):591–602. doi: 10.1158/1535-7163.MCT-10-0928. [DOI] [PubMed] [Google Scholar]
- 46.Cheng GZ, Park S, Shu S, He L, Kong W, Zhang W, et al. Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr Cancer Drug Targets. 2008;8(1):2–6. [PubMed] [Google Scholar]
- 47.Hixon ML, Paccagnella L, Millham R, Perez-Olle R, Gualberto A. Development of inhibitors of the IGF-IR/PI3K/Akt/mTOR pathway. Rev Recent Clin Trials. 2010;5(3):189–208. doi: 10.2174/157488710792007329. [DOI] [PubMed] [Google Scholar]