Abstract
Background
Classical Hodgkin’s lymphoma is characterized by Hodgkin and Reed Sternberg cells, which are of B-cell origin in many cases. We recently highlighted the adverse prognostic significance of cytotoxic molecule expression in patients with classical Hodgkin’s lymphoma. However, the clinical characteristics of cytotoxic molecule-positive classical Hodgkin’s lymphoma remain controversial.
Design and Methods
We investigated the clinicopathological profiles of 32 patients with cytotoxic molecule-positive Hodgkin’s lymphoma, comprising 23 with nodular sclerosis and 9 with mixed cellularity, and compared these profiles with those of 55 patients with cytotoxic molecule-positive nodal peripheral T-cell lymphoma, not otherwise specified and 439 patients with cytotoxic molecule-negative Hodgkin’s lymphoma.
Results
The patients with cytotoxic molecule-positive Hodgkin’s lymphoma consisted of 20 men and 12 women with a median age of 50 years (range, 19 to 81). All these patients had lymphadenopathy at presentation, and 14 showed mediastinal involvement. Physical findings included hepatomegaly and splenomegaly in six patients each. Four patients had a bulky mass, and nine showed stage IV disease. The tumor cells of patients with cytotoxic molecule-positive Hodgkin’s lymphoma had a prototypic immunophenotype of CD15+ CD30+ CD45RO− fascin+, with positivity for Epstein-Barr virus in 39% of cases. All patients were negative for Pax5. In comparison with patients with cytotoxic molecule-positive nodal peripheral T-cell lymphomas, not otherwise specified, patients with cytotoxic-positive Hodgkin’s lymphoma had relatively mild clinical symptoms, similar to those of patients with cytotoxic molecule-negative Hodgkin’s lymphoma. Regarding prognosis, the survival of patients with cytotoxic molecule-positive Hodgkin’s lymphoma was worse than that of patients with cytotoxic molecule-negative Hodgkin’s lymphoma (P=0.0003) but better than that of patients with cytotoxic molecule-positive peripheral T-cell lymphomas, not otherwise specified (P=0.002).
Conclusions
Cytotoxic molecule-positive Hodgkin’s lymphoma is characterized by an unfavorable prognosis, even if its clinicopathological features are within the boundaries of classical Hodgkin’s lymphoma. More effective chemotherapy for cytotoxic molecule-positive Hodgkin’s lymphoma is clearly required.
Keywords: Classical Hodgkin’s lymphoma, cytotoxic molecules, prognosis
Introduction
Classical Hodgkin lymphoma (CHL) is characterized by the presence of Hodgkin and Reed-Sternberg (H-RS) cells in varying numbers of background inflammatory cells. Monoclonal antibody and molecular methodologies have provided definitive evidence that H-RS cells in most cases of CHL are derived from germinal center B cells.1–7 Phenotypically, H-RS cells show positivity for characteristic markers, including CD30 and CD15, and a few are immunoreactive for cytotoxic molecules.8,9
Cytotoxic molecules are apoptosis-inducing proteins that are found in azurophilic cytoplasmic granules of T lymphocytes. Their presence is essential for the diagnosis of various T-cell lymphomas, and their expression profile (granzyme B,10 granzyme M,11 TIA-112 and perforin13) has accordingly attracted much attention.14–18 We recently reported that cytotoxic molecule expression is useful in predicting clinical outcome in patients with nodal peripheral T-cell lymphoma of not otherwise specified type (PTCL-N)19 and Hodgkin’s-like anaplastic large cell lymphoma (HD-like ALCL)20. We also recently highlighted that the expression of T-cell antigen and cytotoxic molecules, either singly or in combination, is an independent poor prognostic factor in CHL.21 To our knowledge, however, no comprehensive evaluation of the clinical characteristics of cytotoxic molecule-positive CHL has yet been reported.
Here, to further characterize cytotoxic molecule-positive CHL, we documented the clinicopathological profiles of 32 patients with this condition and compared these profiles with those of 55 patients with cytotoxic molecule-positive PTCL-N who showed the same cytotoxic phenotype and 439 patients with cytotoxic molecule-negative CHL.
Design and Methods
Selection of patients
Thirty-two patients with cytotoxic molecule-positive CHL diagnosed between 1986 and 2006 as part of the Hodgkin Lymphoma’s Multicenter Study Group were selected from the clinical records. None of the cases had human T-cell leukemia virus type 1 (HTLV1) antibody in their sera. HD-like ALCL under the REAL classification22 was excluded by confirming that the tumor cells showed no sinusoidal spread and grew separately from each other in all areas of the biopsy specimen, and, all cases were negative for ALK1. Nodular lymphocyte-predominant Hodgkin’s disease, which is now classified as a B-cell neoplasm, was also excluded. Cases positive for B-cell-specific activator protein (BSAP, also known as Pax5) were excluded on the grounds that they were B-cell-derived. Reactions for cytotoxic molecules, such as TIA-1, granzyme B and perforin, were considered positive when more than 10% of the H-RS cells stained positively. Cases with high positivity for cytotoxic molecules (>30%) were also excluded to distinguish HD-like ALCL with cytotoxic molecules.
The control group consisted of 55 patients consecutively diagnosed with cytotoxic molecule-positive PTCL-N during the same period at Aichi Cancer Center Hospital. Diagnostic criteria for nodal PTCL-N with cytotoxic molecule expression were established through histopathology and immunophenotyping as described previously.19 Additionally, clinicopathological data of 439 patients with cytotoxic molecule-negative CHL were simultaneously reviewed for comparison with the cytotoxic molecule-positive CHL cases. This series included 324 patients with CHL and 41 with cytotoxic molecule-positive PTCL-N who had been examined in our previous study.19,21 Approval for the study was provided by the institutional review board of Nagoya University.
Histological and immunohistochemical staining
Tissue samples were fixed in 10% formalin and embedded in paraffin, then sectioned at 5-μm intervals and stained with hematoxylin-eosin. Immunoperoxidase studies were performed on formalin-fixed paraffin sections. The monoclonal antibodies used were anti-CD2, CD3, CD8, UCHL-1/CD45RO, L26/CD20, 1F8/CD21, Ber-H2/CD30, fascin, EMA, and ALK1 (DAKO, Glostrup, Denmark); LeuM1/CD15, CXCR3, and CCR4 (Becton Dickinson, Sunnyvale, CA, USA); TIA-1 (Coulter Immunology, Hialeah, FL, USA); granzyme B (Monosan, Uden, the Netherlands); perforin, CD4, CD5 and CD56 (Novocastra, Newcastle, UK); TCRβ (Santa Cruz, Santa Cruz, CA, USA); and Pax5 (Biocare Medical, Walnut Creek, CA, USA). All antibodies were used after antigen retrieval following microwave oven heating treatment except Pax5, which required an autoclaving method.
In situ hybridization study
The presence of Epstein-Barr virus (EBV) small ribonucleic acids was determined by in situ hybridization using EBV-encoded small nuclear early region (EBER) oligonucleotides on formalin-fixed, paraffin-embedded sections as described previously.19
T-cell receptor-γ chain and immunoglobulin heavy chain gene rearrangement studies
DNA was extracted from formalin-fixed, paraffin-embedded tissue sections using standard techniques.23 T-cell receptor-γ (TCR-γ) chain genes and immunoglobulin heavy chain (IgH) genes were amplified using previously reported consensus primers specific for the framework determinants of the variable (V) region (Vγ1–8, Vγ9, Vγ10, and Vγ11) of the TCR-γ chain gene and the framework determinants of the variable (V) region (VH) of the IgH gene, respectively.24 Polymerase chain reaction (PCR) conditions included an initial 7-min denaturation step, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 7 min. Additional PCR assays were performed as controls for each sample using primers specific for a “housekeeping” gene, glyceraldehyde phosphate dehydrogenase (GPDH), and the products were analyzed by 6% acryl amide gel electrophoresis and ethidium bromide staining. Negative controls included water and DNA extraction without primers.
Statistical analysis
Differences in characteristics between the two groups were examined by the χ2 test, Fisher’s exact test, Student’s t test, or the Mann-Whitney U test as appropriate. Patients’ survival data were analyzed by the Kaplan-Meier method. Differences in survival were examined using the log-rank test. Survival for this study was evaluated in terms of disease-specific survival, measured from the date of diagnosis to the date of death due to a lymphoma-related cause. In the disease-specific survival analysis, patients were censored at the time of death if this was from a lymphoma-unrelated cause, while deaths from treatment-related causes were classified as death from lymphoma. All data were analyzed with the aid of STATA software (version 9.0; Stata Corp., College Station, TX, USA).
Results
Characteristics of cases of cytotoxic molecule-positive classical Hodgkin’s lymphoma
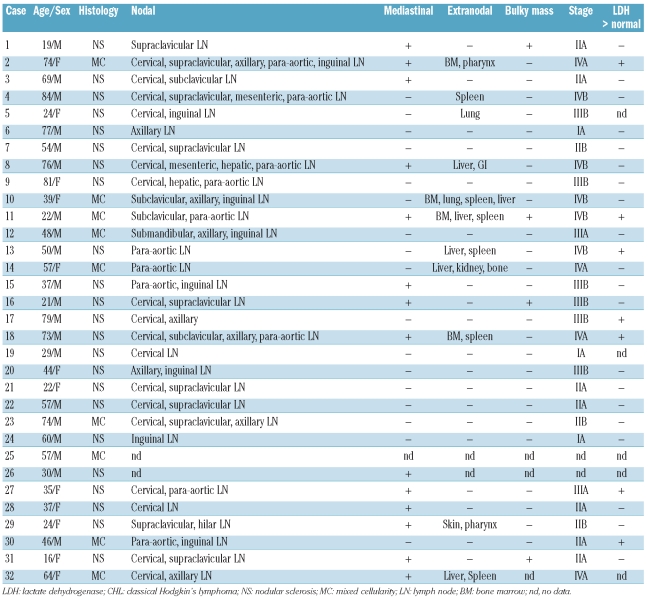
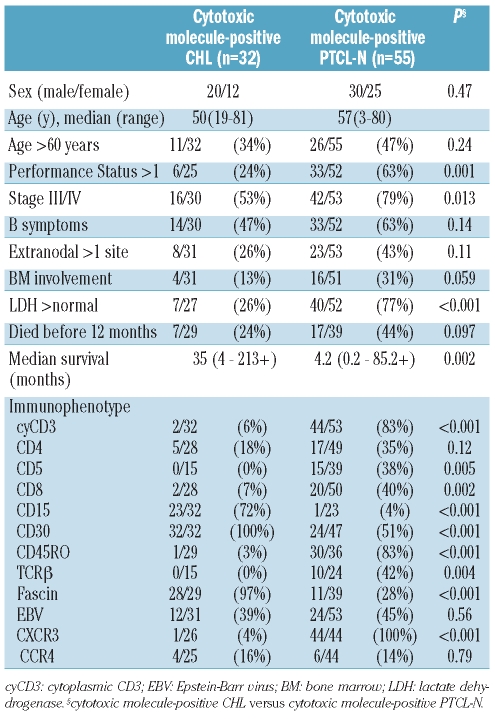
Table 1 summarizes the clinical characteristics of cytotoxic molecule-positive CHL patients. The 20 men and 12 women had a median age of 50 years (range, 19 to 81 years). All patients had lymphoadenopathy, and 14 showed mediastinal involvement at presentation. Physical findings included hepatomegaly and splenomegaly in six patients each. Four patients had a bulky mass, and nine showed stage IV disease. With regards to laboratory data at presentation, four patients had an elevated white blood cell count (greater than 15.0×109/L), five had anemia (hemoglobin level less than 10.5 g/dL), 16 had a decrease in serum albumin level, and seven showed abnormalities in lactate dehydrogenase (LDH) level.
Table 1.
Clinical characteristics of the patients with Hodgkin’s lymphoma with cytotoxic molecule-positive neoplastic cells.
Pathologic findings at presentation
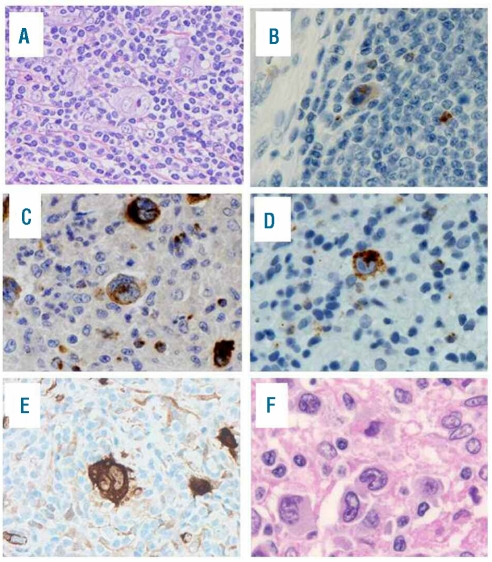
Lymph node biopsy samples were available in all cases. They contained typical H-RS cells, and were accompanied by a moderate to large number of background lymphocytes (Figure 1A). The diagnosis was the nodular sclerosis type in 23 cases and mixed cellularity in nine.
Figure 1.
Classical Hodgkin’s lymphoma with cytotoxic molecule expression. Case 9 shows the presence of Reed-Sternberg cells (A, original magnification x100) and immunoreactivity for TIA-1 (B, original magnification x400), granzyme B (C, original magnification x400), perforin (D, original magnification x400) and fascin (E, original magnification x400). Case 13 shows the presence of neoplastic cells with reniform nuclear indentation (F, original magnification x600).
Phenotypic features of cases of cytotoxic molecule-positive classical Hodgkin’s lymphoma
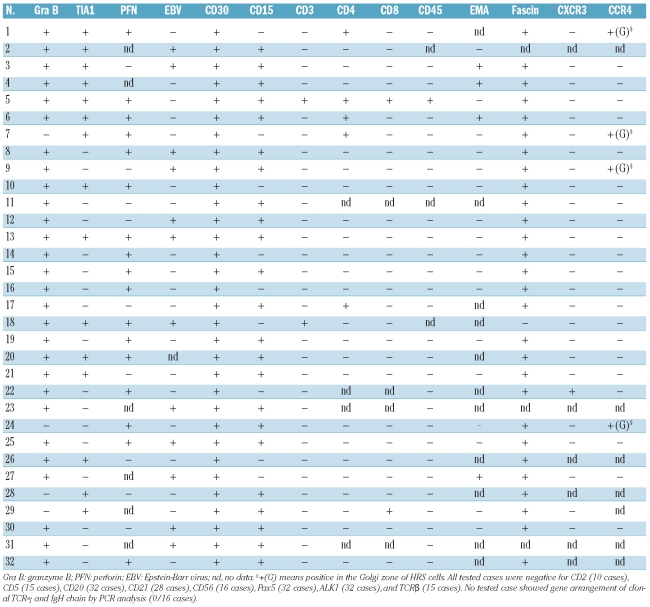
As shown in Table 2, all cases of cytotoxic molecule-positive CHL expressed at least one of TIA-1, granzyme B, and/or perforin on H-RS cells according to the study definitions (Figure 1B): 7 cases were positive for all three, 14 for two of the three molecules, and 11 for one. CD30 and CD15 were detected on H-RS cells in 32 and 23 of the present 32 cases, respectively. None of the cases showed any CD20 positivity on H-RS cells, while two and one expressed CD3ɛ and CD45RO, respectively. All tested cases were negative for CD2, CD5, and TCRβ. All cases were negative for Pax5, which is a pan pre-B marker. Fascin was detected in 28 of 29 patients tested (Figure 1C). Only one case showed expression of CXCR3, whereas four were positive for CCR4 within the Golgi area. In 12 of 31 cases studied by an in situ hybridization method, H-RS cells were positive for EBV RNA transcripts.
Table 2.
Phenotypic characteristics of cases of Hodgkin’s lymphoma with cytotoxic molecule-positive neoplastic cells.
Molecular features of cases of cytotoxic molecule-positive classical Hodgkin’s lymphoma
Molecular studies to assess T- or B-cell clonality via analysis of TCR-γ or IgH chain gene rearrangement by PCR were successful in 16 of 20 cases with available paraffin sections. No case which showed amplification of control GAPDH DNA by PCR analysis also showed gene arrangement of the clonal TCR-γ chain. Furthermore, no cases showed B-cell clonality.
Therapeutic response and outcome
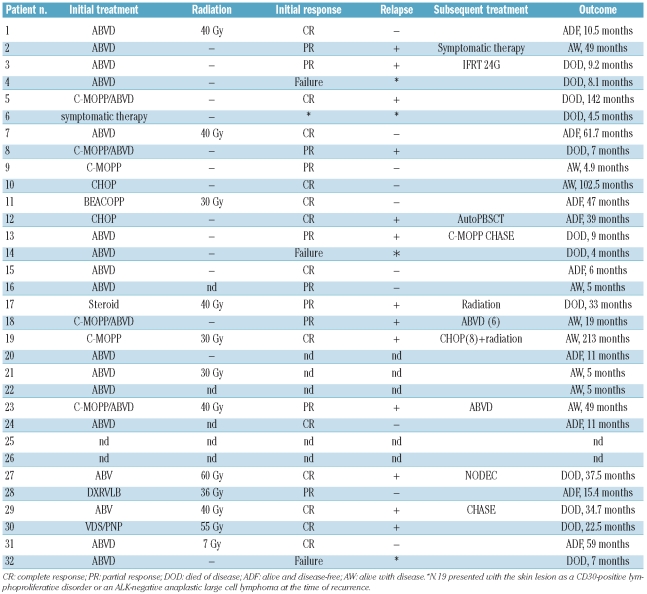
Twenty-five of 32 patients received systemic multiagent chemotherapy, with first-line regimens consisting of doxorubicin, bleomycin, vinblastine, and dacarbacin (ABVD) (21 patients); cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (2 patients); cyclophosphamide, vincristine, procarbazine, and prednisone (C-MOPP) (2 patients); and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) (1 patient) (Table 3). Two patients died due to disease progression within 6 months, before completion of the induction treatment. Overall, 23 patients responded to first-line treatment: 13 had a complete response and 10 had a good partial response. Thirteen of these 23 relapsed, and 8 died with a clinical course ranging from 7 to 142 months. All seven patients who died within 12 months were more than 50 years old. One patient (n. 19) presented with a skin lesion as a CD30+ lymphoproliferative disorder or ALK-negative ALCL at the time of recurrence.25
Table 3.
Treatment and response of patients with Hodgkin’s lymphoma harboring cytotoxic molecule-positive neoplastic cells.
Comparison of the clinicopathological characteristics of cases of cytotoxic molecule-positive and –negative classical Hodgkin’s lymphoma and cytotoxic molecule-positive peripheral T-cell lymphoma of not otherwise specified type
The clinicopathological characteristics of cytotoxic molecule-positive CHL and cytotoxic molecule-positive PTCL-N are summarized in Table 4. Compared with cytotoxic molecule-positive CHL, cytotoxic molecule-positive PTCL-N was associated with several aggressive clinical parameters, namely performance status more than 1 (P=0.001), advanced clinical stage (P=0.013), and elevated LDH levels (P<0.001) (Table 5). Immunophenotypically, cases of cytotoxic molecule-positive CHL showed significantly higher positivity for CD15, CD30, and fascin expression, while cytotoxic molecule-positive PTCL-N cases showed significantly higher positivity for CD3ɛ, CD5, CD8, CD45RO, TCRβ, and CXCR3.
Table 4.
Clinical and phenotypic characteristics of patients with cytotoxic molecule-positive classical Hodgkin’s lymphoma and PTCL-N.
Table 5.
Clinical and phenotypic characteristics of patients with cytotoxic molecule-positive and -negative classical Hodgkin lymphoma.
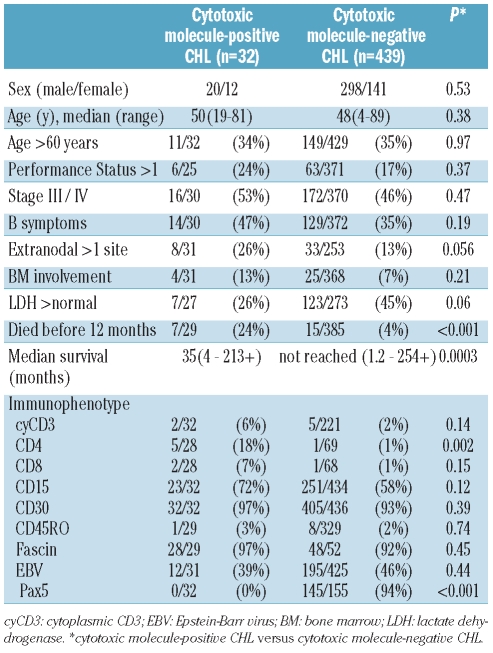
In comparison with cytotoxic molecule-negative CHL, there were no significant differences in clinical parameters between the two groups. Furthermore, no significant immunophenotypic differences were seen, except for a higher incidence of CD4 positivity (18%, P=0.002) and lower incidence of Pax5 positivity (0%, P<0.001) in the cytotoxic molecule-positive CHL cases.
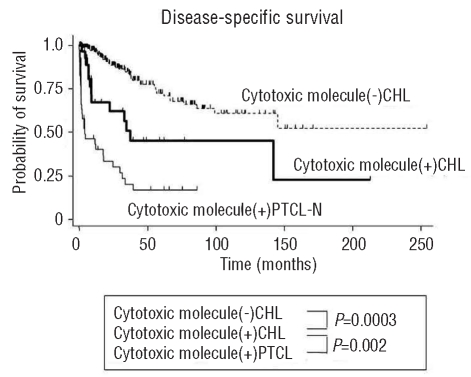
Outcome analysis showed that the disease-specific survival rate of patients with cytotoxic molecule-positive CHL was significantly poorer than that of patients with cytotoxic molecule-negative CHL (P=0.0003) and better than that of patients with cytotoxic molecule-positive PTCL-N (P=0.002, Figure 2).
Figure 2.
Survival data of patients with cytotoxic molecule-positive CHL compared with those with cytotoxic molecule-negative CHL and cytotoxic molecule-positive PTCL-N. Prognosis of patients with cytotoxic molecule-positive CHL (thick line) is significantly poorer than that of patients with cytotoxic molecule-negative CHL (dotted line), and better than that of patients with cytotoxic molecule-positive PTCL-N (thin line).
Discussion
In this clinicopathological characterization of 32 patients with cytotoxic molecule-positive CHL, we found that the histological appearance and immunophenotypic features of this condition were generally within the boundaries of CHL. Unlike typical CHL, however, we found no data to suggest that cytotoxic molecule-positive CHL cells originated from B cells. The clinical behavior of these cases of cytotoxic molecule-positive CHL was evidently more aggressive than that of cytotoxic molecule-negative CHL, and better than that of cytotoxic molecule-positive nodal PTCL-N.
The first report of CHL with cytotoxic molecules appeared in 1996.8,9 Muschen et al. then used single-cell PCR analysis to confirm the existence of rare cases of CHL derived from T cells.26 Seitz et al. also reported the existence of H-RS cells with a genotype and phenotype of T cells,27 and noted that H-RS cells from two of their 13 T-cell marker-positive CHL cases proved to contain clonally rearranged TCR-γ genes. These CHL cases with rearranged TCR-γ genes were positive for perforin and negative for BSAP. Here, to further characterize cytotoxic molecule-positive CHL, we documented the clinicopathological profiles of 32 patients with cytotoxic molecule-positive CHL and also compared these profiles with those of 55 patients with cytotoxic molecule-positive PTCL-N and 439 with cytotoxic molecule-negative CHL.
Using antibodies to these cytotoxic cell-restricted granular proteins of granzyme B, perforin, and TIA-1, we reconfirmed the presence of CHL with a cytotoxic cell-like phenotype. Immunophenotypically, 22% of the cytotoxic molecule-positive CHL cases co-expressed pan-T-cell antigens, of which most were CD4+ (5/28, 18%), whereas CD8 expression was seen in only two cases (7%). Although most cytotoxic T cells generally express CD8 antigen, a subset of CD4+ T cells has been reported to acquire cytotoxic activity on activation.28,29 Twenty-five of our cases of cytotoxic molecule-positive CHL did not display lineage-restricted markers. The frequency of T-cell marker-negative cases was higher among our cytotoxic molecule-positive CHL than cytotoxic molecule-positive PTCL-N cases. Moreover, most cytotoxic molecule-positive CHL cases were characterized by the lack of CXCR3 chemokine receptor. These results are concordant with those reported by Ohshima et al.30
Transformation of a cytotoxic T-cell to an H-RS cell may lead to the loss of T-lineage and chemokine receptor markers and the expression of CD30, CD15, and fascin as markers of Hodgkin’s lymphoma. Our cytotoxic molecule-positive CHL cases were negative for BSAP (Pax5), which is a pan-B and pan-pre-B marker. In contrast, Torlakovic reported that 97% of cases of CHL expressed Pax-5.31 In our study, cytotoxic molecule-positive CHL cases showed no clear evidence of either T- or B-cell origin. The Pax5 negativity of our cases of cytotoxic molecule-positive CHL may imply that the origin was not B cells. In support of this, Seitz reported that most Hodgkin’s lymphoma with T-cell phenotype H-RS cells, which were positive for BSAP, were genotypically derived from B cells.27 However, given that rare PTCL cases with Pax5 have been identified it would be an overstatement to say that Pax5 is a gold-standard marker for B-cell neoplasms, including CHL.32 No clonal IgH or TCR-gamma chain gene was detected in any of the patients following successful amplification of DNA by PCR analysis, although the limited number of cases hampered studies of IgH and TCR-γ rearrangement in H-RS cells. The origin of cytotoxic molecule-positive CHL does, therefore, warrant additional investigation, including single cell analysis.
We previously reported that expression of cytotoxic molecules is associated with a poorer prognosis in patients with nodal PTCL-N,19 and demonstrated that cytotoxic molecule and T-cell antigen expression, both singly and in combination, are useful in predicting an aggressive clinical outcome in CHL patients.21 These findings suggest that the expression of cytotoxic molecules on lymphoma cells might be associated with poorer overall survival among patients with nodal CHL and PTCL-N. We, therefore, also compared the clinicopathological profiles of patients with CHL and PTCL-N, who share cytotoxic molecule expression and nodal presentation. This analysis identified several significant clinical differences between the two groups. Compared to patients with cytotoxic molecule-positive PTCL-N, patients with cytotoxic molecule-positive CHL had relatively mild clinical symptoms on first admission, similar to those in cytotoxic molecule-negative CHL. However, the survival of the cytotoxic molecule-positive CHL patients was poorer than that of those with cytotoxic molecule-negative CHL and better than that of patients with cytotoxic molecule-positive PTCL-N. Of note, seven patients with cytotoxic molecule-positive CHL died within 12 months despite systemic multiagent chemotherapy; all seven of these patients were more than 50 years old. Furthermore, among the 13 relapsed cases, the only disease-free survivor was the patient treated with autologous peripheral blood stem cell transplantation. More effective chemotherapy for cytotoxic molecule-positive CHL is, therefore, clearly required.
Barry et al. reported 11 cases of PTCL co-expressing CD30 and CD15 on at least a subset of tumor cells, which morphologically overlapped with CHL.33 Moreover, Gorczyca et al. reported PTCL cases with histological features mimicking CHL with Reed-Sternberg-like cells in an inflammatory background. Although the co-expression of CD30 and CD15 is typical of CHL, it may also be present in a subset of peripheral T-cell neoplasms.34 The distinction between CHL and PTCL is further obfuscated by recent reports of rare cases of CHL containing RS cells of either or both the T-cell phenotype and genotype.26,27 These ambiguous cases, including the present cytotoxic molecule-positive CHL cases, may indicate the presence of abundant overlap between the morphological and immunophenotypic features of CHL and PTCL.
In conclusion, the histological and immunophenotypic features of cytotoxic molecule-positive CHL were generally within the boundaries of the CHL category, but differed from typical CHL cases in the following two respects: (i) there were no data to suggest that cytotoxic molecule-positive CHL cells originated from B cells; and (ii) the clinical behavior of cytotoxic molecule-positive CHL was evidently more aggressive than that of typical CHL without cytotoxic molecule expression. The question of whether cytotoxic molecule-positive CHL should be categorized as Hodgkin’s lymphoma or T-cell lymphoma warrants ongoing discussion. Moreover, novel therapeutic approaches to the treatment of cytotoxic molecule-positive CHL patients should be explored.
Acknowledgments
The authors thank staff members in all collaborating institutions and Mr. H. Ishida in Aichi Cancer Center and Mr. Yamada in Nagoya University for technical assistance. Our deepest appreciation goes to Dr. Masao Seto (Division of Molecular Medicine, Aichi Cancer Center Institute) whose many comments and suggestions throughout the course of our study were critical.
Footnotes
Funding: this work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Young Scientists 20790279).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Tzankov A, Zimpfer A, Pehrs AC, Lugli A, Went P, Maurer R, et al. Expression of B-cell markers in classical Hodgkin lymphoma: a tissue microarray analysis of 330 cases. Mod Pathol. 2003;16(11):1141–7. doi: 10.1097/01.MP.0000093627.51090.3F. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Taylor CR. Apoptosis and cell cycle related gene and proteins in classical Hodgkin lymphoma. Appl Immuno-histochem Mol Morphol. 2003;11(3):206–13. doi: 10.1097/00129039-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Küppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histologic sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci USA. 1994;91(23):10962–6. doi: 10.1073/pnas.91.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cossman J, Annunziata CM, Barash S, Staudt L, Dillon P, He WW, et al. Reed-Sternberg cell genome expression supports a B-cell lineage. Blood. 1999;94(2):411–6. [PubMed] [Google Scholar]
- 5.Taylor CR, Riley CR. Evolving concepts of the nature of Hodgkin’s disease: a history. Ann Diagn Pathol. 2000;4(5):337–46. doi: 10.1053/adpa.2000.17893. [DOI] [PubMed] [Google Scholar]
- 6.Taylor CR, Riley CR. Molecular morphology of Hodgkin lymphoma. Appl Immuno-histochem Mol Morphol. 2001;9(3):187–202. doi: 10.1097/00129039-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Thomas RK, Re D, Wolf J, Diehl V. Part I: Hodgkin’s lymphoma-molecular biology of Hodgkin and Reed-Sternberg cells. Lancet Oncol. 2004;5(1):11–8. doi: 10.1016/s1470-2045(03)01319-6. [DOI] [PubMed] [Google Scholar]
- 8.Oudejans JJ, Kummer JA, Jiwa M, van der Valk P, Ossenkoppele GJ, Kluin PM, et al. Granzyme B expression in Reed-Sternberg cells of Hodgkin’s disease. Am J Pathol. 1996;148(1):233–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Krenacs L, Wellmann A, Sorbara L, Himmelmann AW, Bagdi E, Jaffe ES, et al. Cytotoxic cell antigen expression in anaplastic large cell lymphomas of T- and null-cell type and Hodgkin’s disease: evidence for distinct cellular origin. Blood. 1997;89(3):980–9. [PubMed] [Google Scholar]
- 10.Garcia-Sanz JA, Velotti F, MacDonald HR, Masson D, Tschopp J, Nabholz M. Appearance of granule-associated molecules during activation of cytolytic T-lymphocyte precursors by defined stimuli. Immunology. 1988;64(1):129–34. [PMC free article] [PubMed] [Google Scholar]
- 11.Pilat D, Fink T, Obermaier-Skrobanek B, Zimmer M, Wekerle H, Lichter P, et al. The human Met-ase gene (GZMM): structure, sequence, and close physical linkage to the serine protease gene cluster on 19p13.3. Genomics. 1994;24(3):445–50. doi: 10.1006/geno.1994.1651. [DOI] [PubMed] [Google Scholar]
- 12.Tian Q, Streuli M, Saito H, Schlossman SF, Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991;67(3):629–39. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- 13.Masson D, Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T- lymphocytes. J Biol Chem. 1985;260(16):9069–72. [PubMed] [Google Scholar]
- 14.de Bruin PC, Kummer JA, van der Valk P, van Heerde P, Kluin PM, Willemze R, et al. Granzyme B expressing peripheral T-cell lymphomas: neoplastic equivalents of activated cytotoxic T cells with preference for mucosa-associated lymphoid tissue localization. Blood. 1994;84(11):3785–91. [PubMed] [Google Scholar]
- 15.Krenacs L, Smyth MJ, Bagdi E, Krenacs T, Kopper L, Rudiger T, et al. The serine protease granzyme M is preferentially expressed in NK-cell, γδ T-cell, and intestinal T-cell lymphomas: evidence of origin from lymphocytes involved in innate immunity. Blood. 2003;101(9):3590–3. doi: 10.1182/blood-2002-09-2908. [DOI] [PubMed] [Google Scholar]
- 16.Felgar RE, Macon WR, Kinney MC, Roberts S, Pasha T, Salhany KE. TIA-1 expression in lymphoid neoplasms: identification of subsets with cytotoxic T lymphocyte or natural killer cell differentiation. Am J Pathol. 1997;150(6):1893–900. [PMC free article] [PubMed] [Google Scholar]
- 17.Foss HD, Anagnostopoulos I, Araujo I, Assaf C, Demel G, Kummer JA, et al. Anaplastic large-cell lymphomas of T-cell and null-cell phenotype express cytotoxic molecules. Blood. 1996;88(10):4005–11. [PubMed] [Google Scholar]
- 18.Yamashita Y, Yatabe Y, Tsuzuki T, Nakayama A, Hasegawa Y, Kojima H, et al. Perforin and granzyme expression in cytotoxic T-cell lymphomas. Mod Pathol. 1998;11(4):313–23. [PubMed] [Google Scholar]
- 19.Asano N, Suzuki R, Kagami Y, Ishida F, Kitamura K, Fukutani H, et al. Clinico-pathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T-cell lymphoma, unspecified. Am J Surg Pathol. 2005;29(10):1284–93. doi: 10.1097/01.pas.0000173238.17331.6b. [DOI] [PubMed] [Google Scholar]
- 20.Asano N, Suzuki R, Matsuo K, Kagami Y, Ishida F, Tamaru JI, et al. Cytotoxic molecule expression is predictive of prognosis in Hodgkins-like anaplastic large cell lymphoma. Histopathology. 2007;50(7):962. doi: 10.1111/j.1365-2559.2007.02674.x. [DOI] [PubMed] [Google Scholar]
- 21.Asano N, Oshiro A, Matsuo K, Kagami Y, Ishida F, Suzuki R, et al. Prognostic significance of T-cell or cytotoxic molecules phenotype in classical Hodgkin’s lymphoma: a clinicopathologic study. J Clin Oncol. 2006;24(28):4626–33. doi: 10.1200/JCO.2006.06.5342. [DOI] [PubMed] [Google Scholar]
- 22.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International lymphoma Study Group. Blood. 1994;84(5):1361–92. [PubMed] [Google Scholar]
- 23.Shibata DK, Arnheim N, Martin WJ. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988;167(1):225–30. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon M, Kind P, Kaudewitz P, Krokowski M, Graf A, Prinz J, et al. Automated high-resolution polymerase chain reaction fragment analysis: a method for detecting T-cell receptor gamma-chain gene rearrangements in lymphoproliferative diseases. Am J Pathol. 1998;152(1):29–33. [PMC free article] [PubMed] [Google Scholar]
- 25.Kiniwa Y, Ide Y, Fukushima M, Asano N, Saida T. A case of systemic anaplastic large cell lymphoma with 'Hodgkin-like' appearance and skin involvement mimicking lymphomatoid papulosis. J Cutan Pathol. 2011;38(1):38–42. doi: 10.1111/j.1600-0560.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- 26.Müschen M, Rajewsky K, Bräuninger A, Baur AS, Oudejans JJ, Roers A, et al. Rare occurrence of classical Hodgkin's disease as a T cell lymphoma. J Exp Med. 2000;191(2):387–94. doi: 10.1084/jem.191.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seitz V, Hummel M, Marafioti T, Anagnostopoulos I, Assaf C, Stein H. Detection of clonal T-cell receptor gamma-chain gene rearrangements in Reed-Sternberg cells of classic Hodgkin disease. Blood. 2000;95(10):3020–4. [PubMed] [Google Scholar]
- 28.Grossi CE, Zicca A, Cadoni A, Mingari MC, Moretta A, Moretta L. Ultrastructural characteristics of human T cell clones with various cytolytic activities. Eur J Immunol. 1983;13(8):670–7. doi: 10.1002/eji.1830130812. [DOI] [PubMed] [Google Scholar]
- 29.Nakata M, Kawasaki A, Azuma M, Tsuji K, Matsuda H, Shinkai Y, et al. Expression of perforin and cytolytic potential of human peripheral blood lymphocyte subpopulations. Int Immunol. 1992;4(9):1049–54. doi: 10.1093/intimm/4.9.1049. [DOI] [PubMed] [Google Scholar]
- 30.Ohshima K, Karube K, Hamasaki M, Suefuji H, Tutiya T, Yamaguchi T, et al. Imbalances of chemokines, chemokine receptors and cytokines in Hodgkin lymphoma: classical Hodgkin lymphoma vs. Hodgkin-like ATLL. Int J Cancer. 2003;106(5):706–12. doi: 10.1002/ijc.11301. [DOI] [PubMed] [Google Scholar]
- 31.Torlakovic E, Torlakovic G, Nguyen PL, Brunning RD, Delabie J. The value of anti-pax-5 immunostaining in routinely fixed and paraffin-embedded sections: a novel pan pre-B and B-cell marker. Am J Surg Pathol. 2002;26(10):1343–50. doi: 10.1097/00000478-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Tzankov AS, Went PT, Münst S, Papadopoulos T, Jundt G, Dirnhofer SR. Rare expression of BSAP (PAX-5) in mature T-cell lymphomas. Mod Pathol. 2007;20(6):632–7. doi: 10.1038/modpathol.3800778. [DOI] [PubMed] [Google Scholar]
- 33.Barry TS, Jaffe ES, Sorbara L, Raffeld M, Pittaluga S. Peripheral T-cell lymphomas expressing CD30 and CD15. Am J Surg Pathol. 2003;27(12):1513–22. doi: 10.1097/00000478-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Gorczyca W, Tsang P, Liu Z, Wu CD, Dong HY, Goldstein M, et al. CD30-positive T-cell lymphomas co-expressing CD15: an immunohistochemical analysis. Int J Oncol. 2003;22(2):319. [PubMed] [Google Scholar]