Abstract
Patients with chronic myeloid leukemia, treated with imatinib, who have a durable complete molecular response, might remain in complete molecular response after stopping treatment. Previous reports of patients stopping treatment in complete molecular response have included only patients with a good response to imatinib. We describe 3 patients with stable complete molecular response on dasatinib treatment following imatinib failure. Two of the 3 patients remain in complete molecular response more than 12 months after stopping dasatinib. In these 2 patients we used highly sensitive patient-specific BCR-ABL1 DNA PCR to show that the leukemic clone remains detectable, as we have previously shown in imatinib-treated patients. Dasatinib-associated immunological phenomena, such as the emergence of clonal T-cell populations, were observed both in one patient who relapsed and in one patient in remission. Our results suggest that the characteristics of complete molecular response on dasatinib treatment may be similar to that achieved with imatinib, at least in patients with adverse disease features.
Keywords: complete molecular response, CML, dasatinib, imatinib
Introduction
Complete molecular remission (CMR) of chronic myeloid leukemia (CML), defined as the absence of detectable BCR-ABL1 mRNA in real-time reverse transcriptase quantitative PCR (RQ-PCR), is not equivalent to eradication of leukemia since there may be minimal residual disease (MRD) below the lower limit of detection of the test.1,2 The withdrawal of imatinib treatment in carefully selected patients with chronic phase CML in a stable CMR for at least two years is followed by molecular relapse in around 60% of patients, whilst the remainder have a durable drug-free remission with follow up of up to four years.2,3 The more potent tyrosine kinase inhibitors (TKIs), dasatinib and nilotinib are now widely used and highly effective in first-line treatment of chronic phase CML,4,5 and may result in more patients achieving a CMR. The stability of CMR induced by a 2nd generation TKI after treatment withdrawal is not known. Here we report, for the first time, the outcome of cessation of dasatinib in patients in CMR. Two out of 3 patients who achieved a CMR on dasatinib treatment remain in CMR 18 and 27 months after stopping TKI therapy.
Design and Methods
Cases of 2nd generation TKI cessation were identified by a survey of members of the Australasian Leukaemia & Lymphoma Group (ALLG) involved in CML clinical trials. There were 3 cases of dasatinib cessation, but no patients stopping nilotinib in CMR were reported. RQ-PCR monitoring and assessment of T-cell receptor γ (TCR) clonality were performed in local diagnostic laboratories. BCR-ABL1 mRNA levels were measured in three different laboratories, all of which used the BCR control gene, and reported results on the BCR-ABL1 International Scale (IS). The lower limit of detection was in all cases over 4.0 log below the standardized baseline. Ethical approval for the study was obtained from the two institutions where the patients were treated. For 2 of the patients, the genomic BCR-ABL1 fusion sequence was identified in DNA extracted from cells obtained at the time of diagnosis, and patient-specific primers were designed for MRD analysis.6 Quantitative BCR-ABL1 DNA PCR was performed on peripheral blood samples collected after withdrawal of dasatinib whilst in CMR. The limit of detection of the DNA PCR assay was approximately 10−6.
Results and Discussion
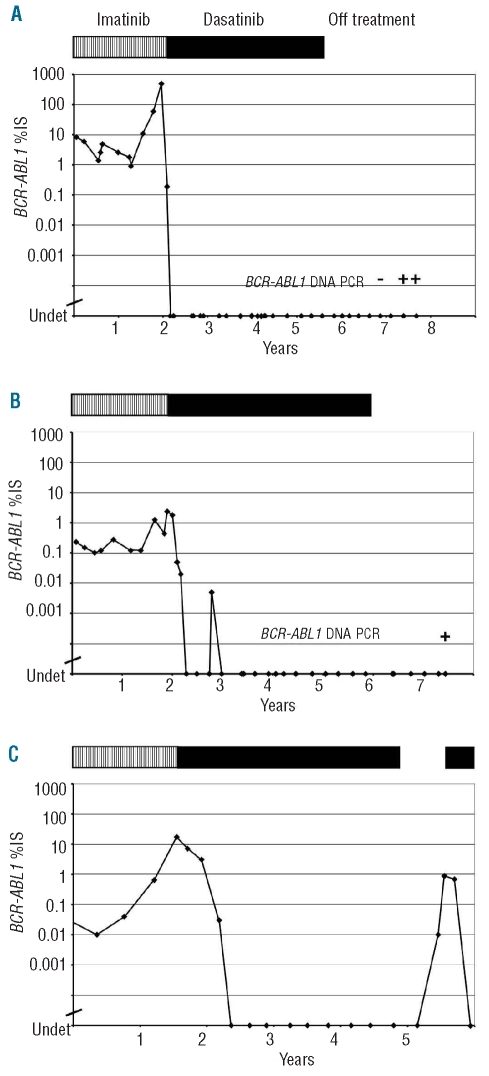
Patient 1 is a 44-year old female who had been diagnosed with chronic phase CML in 1995. She commenced treatment with interferon-α, but lost hematologic response in 2000. In November 2001, she commenced imatinib at a dose of 400 mg daily. She achieved a complete cytogenetic response (no BCR-ABL-positive interphase cells by fluorescence in situ hybridization) by February 2004, but later that year there was a rise in the BCR-ABL mRNA level, and the E355G kinase domain mutation was detected. She was treated with imatinib dose escalation but progressed to accelerated phase (hematologic and cytogenetic) in February 2005. Dasatinib was commenced at a dose of 50 mg twice daily and a CMR was achieved after only two months of treatment. After 12 months, a pleural effusion developed and was treated with dasatinib dose reduction and prednisolone. No peripheral blood lymphocytosis was detected. In January 2009, dasatinib was stopped due to recurrent pleural effusion after 35 months of stable CMR. The patient remains in a stable CMR after 27 months off treatment with no detectable BCR-ABL1 mRNA in any of 9 samples (Figure 1A) and normal peripheral blood counts. PCR analysis of the TCR gene identified a monoclonal T-cell population in the most recent peripheral blood sample. Quantitative BCR-ABL1 DNA PCR was performed on 3 samples collected 21–27 months after withdrawal of dasatinib. BCR-ABL1 DNA was detected in 2 of 3 samples at around 5.5 - 6.0 log below the level at diagnosis, confirming the presence of the original leukemic clone.
Figure 1.
BCR-ABL1 mRNA results of 3 CML patients who stopped dasatinib in CMR. Each patient’s treatment is indicated by the bar at the top of each graph: vertical hatching indicates imatinib treatment; solid bars indicate dasatinib treatment; no bar indicates off treatment. IS: International Scale for BCR-ABL1 RQ-PCR.
Patient 2 is a 37-year old male who had been diagnosed with chronic phase CML in January 2001. He initially declined therapy, but then in December 2001 commenced treatment with imatinib at a dose of 400 mg daily. He achieved a CCR but not an MMR, despite serial imatinib dose escalation to 800 mg daily. Thirty-two months after commencing treatment he had a significant rise in BCR-ABL1 mRNA and was found to have a cytogenetic relapse with the kinase domain mutations F359I and G250E. In October 2005, he commenced dasatinib at a dose of 70 mg twice daily. After four months a CMR was achieved. On one occasion whilst in CMR on dasatinib he had a slight lymphocytosis with normal T-cell subsets. TCR gene analysis showed a dominant band in a polyclonal background suggestive, but not diagnostic, of a small clonal expansion. After 35 months of stable CMR, the patient stopped dasatinib against medical advice, in the absence of any significant toxicity. He remains in a stable CMR after 18 months with no detectable BCR-ABL mRNA in any of 6 samples (Figure 1B). Follow-up TCR gene analysis (whilst off treatment) was polyclonal. Quantitative BCR-ABL1 DNA PCR was performed on one sample collected 17 months after withdrawal of dasatinib. BCR-ABL1 DNA was detected at around 5.5 log below the level at diagnosis.
Patient 3 is a 53-year old male who had been diagnosed with chronic phase CML in August 1997. He commenced treatment with interferon, and in May 2003 developed cytogenetic clonal evolution with a tetraploid karyotype and duplication of the Philadelphia chromosome. He commenced second-line treatment with imatinib at a dose of 400 mg daily. He achieved a major molecular response, but after three years on imatinib lost response with a kinase domain mutation, E292V. In May 2007, dasatinib treatment was commenced at a dose of 70 mg twice daily. After four months on dasatinib a CMR was achieved. The patient developed a T-cell lymphocytosis with an excess of CD8+ cells. In June 2010, the patient developed an interstitial pneumonitis for which no other cause could be found. A relationship to dasatinib was suspected and treatment was ceased in August 2010 after 33 months of stable CMR. Molecular relapse occurred after four months with the BCR-ABL1 mRNA level rising to 0.89%. Dasatinib treatment was resumed, without significant toxicity to date, and the patient regained CMR after three months (Figure 1C).
Dasatinib is a dual Src/ABL1 inhibitor which inhibits ABL1 with a potency in vitro of approximately 300 times that of imatinib.7 It is associated with the emergence, in a significant proportion of patients, of lymphocytosis with evidence of T-cell clonality by PCR.8 The emergence of these aberrant lymphoid or NK cell populations has been reported to be associated with an improvement in molecular response, and possibly also with immune-mediated side effects, such as pleural effusion.8 These features led us to speculate that patients in CMR on dasatinib who manifest these immunological responses might be more likely to remain in CMR when the kinase inhibitor was withdrawn. We found a clonal T-cell population in one of the 2 patients in stable CMR. The one patient who had a dasatinib-induced lymphocytosis relapsed when treatment was stopped. The impact of dasatinib-related immunological phenomena on the stability of CMR after dasatinib treatment remains to be determined in a larger number of patients.
All 3 dasatinib-treated patients had evidence of MRD whilst in CMR (defined by conventional RQ-PCR): in 2 cases this was demonstrated by patient-specific DNA PCR, whereas in the third patient the presence of MRD was proven by molecular relapse. Despite the higher potency of dasatinib the level of MRD measured by sensitive DNA PCR was similar to that seen in patients in the ALLG CML8 study on patients in CMR.2 The presence of BCR-ABL1-positive cells in the blood of a patient in a stable drug-free CMR appears to indicate that eradication of the leukemic clone is not a pre-requisite for the achievement of an operational cure of CML.
These results in 3 dasatinib-treated patients show close parallels to what has previously been reported in patients undergoing a trial of imatinib cessation. As in the imatinib data, if relapse occurred, it did so within several months of therapy withdrawal, and was responsive to re-institution of the TKI.3 The number of patients in this series is too small for us to determine whether the risk of relapse after dasatinib cessation differs from that after imatinib cessation. The patients that we describe all started dasatinib after imatinib failure in accelerated phase and/or with kinase domain mutations and, therefore, constitute a group of higher risk patients than those in the STIM3 or ALLG CML82 studies of imatinib cessation in CMR. These preliminary results suggest that the nature of the molecular remission on dasatinib is not qualitatively different from imatinib-induced CMR, at least in these patients with advanced disease features, despite the observation of immunological responses that appear to be relatively specific to dasatinib treatment. A prospective clinical trial of dasatinib withdrawal will be required to estimate accurately the probability of drug-free CMR and to identify factors that influence the risk of molecular relapse. The cases described here establish that durable disease control is possible following dasatinib cessation in CMR, despite prior imatinib failure and adverse disease features, including BCR-ABL1 kinase domain mutations.
Acknowledgments
The authors would like to thank the patients concerned. The authors would also like to thank Ms Rosemary Hoyt, Royal Melbourne Hospital, and Dr David Westerman, Peter MacCallum Cancer Centre, for their assistance with specimen collection, Mr Brad Budgen, Flinders University, for technical assistance, and Ms Lesley Snell, SA Pathology, Flinders Medical Centre, for TCR gene analysis.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Sobrinho-Simoes M, Wilczek V, Score J, Cross NC, Apperley JF, Melo JV. In search of the original leukemic clone in chronic myeloid leukemia patients in complete molecular remission after stem cell transplantation or imatinib. Blood. 2010;116(8):1329–35. doi: 10.1182/blood-2009-11-255109. [DOI] [PubMed] [Google Scholar]
- 2.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Bartley PA, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24(10):1719–24. doi: 10.1038/leu.2010.185. [DOI] [PubMed] [Google Scholar]
- 3.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 5.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 6.Bartley PA, Ross DM, Latham S, Martin-Harris MH, Budgen B, Wilczek V, et al. Sensitive detection and quantification of minimal residual disease in chronic myeloid leukaemia using nested quantitative PCR for BCR-ABL DNA. Int J Lab Hematol. 2010;32(6 Pt 1):e222–8. doi: 10.1111/j.1751-553X.2010.01236.x. [DOI] [PubMed] [Google Scholar]
- 7.O'Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500–5. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 8.Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23(8):1398–405. doi: 10.1038/leu.2009.46. [DOI] [PubMed] [Google Scholar]