Abstract
The study was carried out to find out predictors of axillary lymph node metastasis in breast cancer and to evaluate its significance in selecting the group of patients in whom axillary dissection could be avoided. Ninety-five breast cancer patients who underwent mastectomy and axillary dissection were included in the study. Factors like patient’s age, tumor size, histopathological type, histological grade and estrogen and progesterone receptor status were correlated with the axillary metastases. Out of 95 cases axillary metastasis was found in 47 (49.47%) cases. There was no correlation between patient’s age and tumor size with axillary metastasis (p > 0.05). Based on histopathological typing tumors like ductal carcinoma in situ, tubular carcinoma and mucinous carcinoma showed less tendency for axillary metastasis (p < 0.046). Association was found between histological grade and estrogen receptor and progesterone receptor positivity with presence of axillary metastasis (p < 0.001 and 0.002 respectively). The findings in this study indicate that breast cancer patients having favorable histological type, grade I tumors and estrogen and progesterone receptor negative tumor are good candidates to avoid axillary dissection.
Keywords: Breast cancer, Axillary metastases, Histological grade
Introduction
Axillary lymph node status is one of the most important prognostic indicators in breast cancer and the detection of nodal metastases is a key factor in recommending adjuvant chemotherapy after surgery [1]. Widespread use of mammography has resulted in marked increase in early detection of breast cancer, improvement in therapy and declining mortality [2]. The positive yield of axillary lymph node dissection also decreases and node negative patients do not benefit from axillary dissection but may suffer from its complications [3]. Axillary lymph node dissection is now no longer considered to be the standard treatment in all patients with invasive breast cancer [4]. The present study was undertaken to find out predictors of axillary lymph node metastasis in breast cancer which will help to select the group of patients in WHOM axillary dissection can be avoided.
Material and Methods
This was a retrospective study carried out in a tertiary care teaching hospital from June 2007 to May 2010. Study included 95 breast cancer patients who underwent mastectomy with axillary dissection. Patients with T4 tumor, history of previous lumpectomy and who had taken preoperative chemotherapy were excluded. Various factors like patient’s age, tumor size, histopathological type, histological grade and estrogen and progesterone receptor status were correlated with the axillary lymph node positivity. All the above mentioned data was collected from available clinical and histopathology records. The breast cancer was classified as per WHO recommendation into the following histological types—Ductal carcinoma insitu (DCIS), infiltrating duct carcinoma, infiltrating lobular carcinoma, medullary, mucinous, tubular and metaplastic carcinoma (Fig. 1). The Nottinghamm modification of the Bloom-Richardson Grading system [5] was used for histological grading of cancers (Fig. 2). Immunohistochemical staining for estrogen receptor (ER) [NOVACASTRA clone 6 F11] and progesterone receptor (PR) [NOVACASTRA clone PGR312] was performed using the Streptavidin-Biotin Immunoperoxidase procedure (Fig. 3).
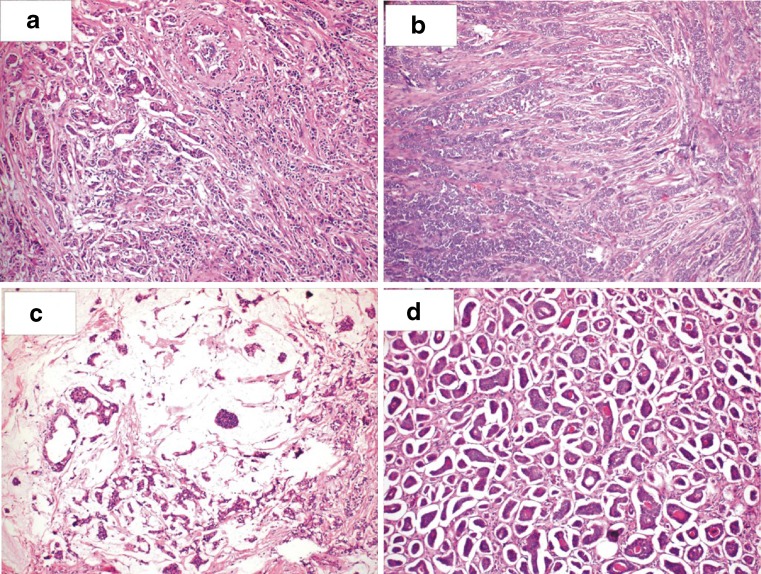
Fig. 1.
Photomicrograph showing a Infiltrating duct carcinoma b Infiltrating lobular carcinoma c Mucinous carcinoma and d Tubular carcinoma (H & E, ×100)
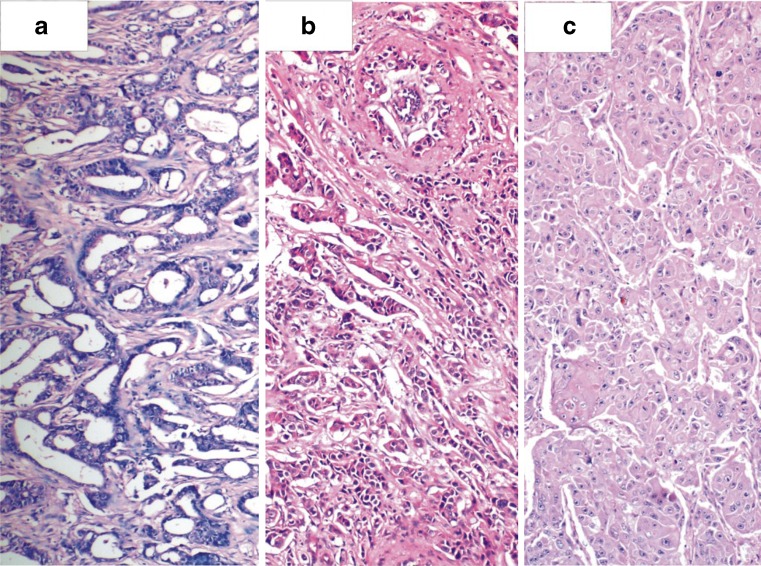
Fig. 2.
Photomicrograph showing a Grade I b Grade II and c Grade III infiltrating breast carcinoma (H & E, ×100)
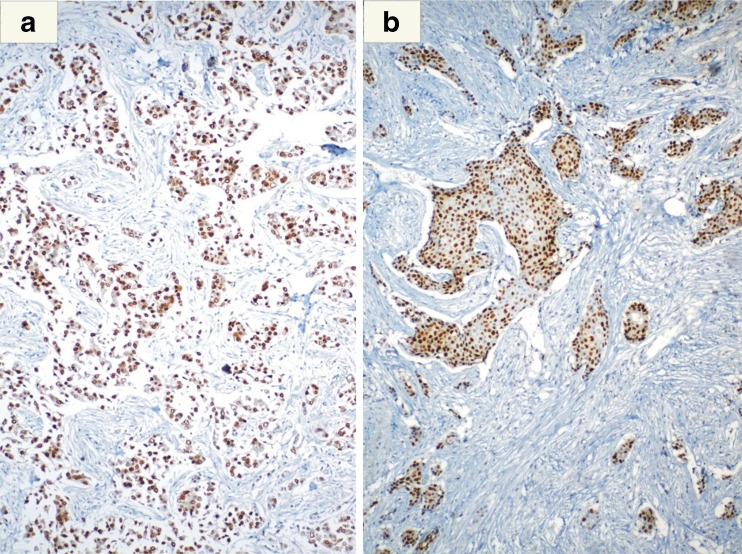
Fig. 3.
Breast cancer tumor nuclei showing a Estrogen Receptor (ER) and b Progesterone Receptor positivity (PR) on immunohistochemistry (IHC)
Statistical Analysis
Descriptive analysis and chi-square test was used as test of significance.
Results (Table 1)
Table 1.
Correlation between various factors and axillary lymphnode metastasis in breast cancer
| No of cases | Axillary lymphnode metastasis | P value | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Patient’s Age | NS | |||
| <50 years | 51 (53.68%) | 27 | 24 | |
| 50–70 years | 39 (41.05%) | 19 | 20 | |
| >70 years | 06 (6.31%) | 01 | 05 | |
| Tumor Size | NS | |||
| T1a | 04 (4.21%) | 01 | 03 | |
| T1b | 07 (7.36%) | 03 | 04 | |
| T1c | 15 (15.78%) | 05 | 10 | |
| T2 | 46 (48.42%) | 24 | 22 | |
| T3 | 25 (26.15%) | 14 | 09 | |
| Histological Type | P < 0.046 | |||
| DCIS | 04 (4.21%) | 00 | 04 | |
| Infiltrating duct carcinoma | 68 (71.57%) | 36 | 32 | |
| Infiltrating lobular carcinoma | 14 (14.73%) | 07 | 07 | |
| IDC & ILC | 03 (3.15%) | 02 | 01 | |
| Mucinous carcinoma | 02 (2.10%) | 00 | 02 | |
| Medullary carcinoma | 02 (2.10%) | 01 | 01 | |
| Tubular carcinoma | 01 (1.05%) | 00 | 01 | |
| Metaplastic carcinoma | 01 (1.05%) | 01 | 00 | |
| Histological Grade | P < 0.001 | |||
| I | 21 (22.10%) | 05 | 16 | |
| II | 49 (51.57%) | 24 | 25 | |
| III | 25 (26.31%) | 14 | 09 | |
| Hormonal Status | P < 0.002 | |||
| ER + & PR + | 37(38.94%) | 27 | 10 | |
| ER + & PR − | 21(22.10%) | 10 | 11 | |
| ER-& PR + | 08(8.42%) | 02 | 06 | |
| ER-& PR − | 29(30.52%) | 08 | 21 | |
The study included 95 cases of breast cancer, of which 47 (49.47%) showed axillary metastasis. Axillary lymph nodes dissected and examined in each case varied from 3–26 with average of 13.8. Mean age of the patient at the time of surgery was 49.32 years which ranged from 26–80 years. Fifty-one (53.68%) patients were below 50 years of age. The tumor size was T2 (2–5 cm) in 46 (48.42%) patients and T1 (<2 cm) in 27.23%. No correlation was observed between tumor size and patient’s age with axillary metastasis (p > 0.05). On histological typing of breast cancer, 68 (71.57%) cases were infiltrating duct carcinoma (IDC). Correlation was noted between histological type of tumor and axillary metastasis (p < ,0.046). Tumors like DCIS, tubular carcinoma and mucinous carcinoma showed less tendency for axillary metastasis compared to IDC. As regards histological grade, 49 (51.57%) patients had grade II tumors which were more common than others. There was strong association between histological grade and presence of axillary metastasis (p < 0.001). On ER and PR immunohistochemistry, ER and PR-negative status was significantly associated with low risk of axillary node metastasis (p < 0.002).
Discussion
In India, cancer of the breast is the most common cancer among women [6]. Standard treatment of carcinoma breast is mastectomy with axillary lymph node dissection (ALND). The dissection provides accurate prognostic information as well as excellent local control and improves the survival rate in the node positive group [7]. In addition, nodal status is one of the most important determining factors in recommending adjuvant chemotherapy for patients with breast carcinoma [1]. Axillary dissection can result in various early complications like infection, seroma and haematoma as well as late complications like oedema, paraesthesia, stiffness, pain and weakness of the upper extremity. Arm problems are frequent after operation for breast cancer, and these problems appear to increase the likelihood of psychological distress [8]. With increased emphasis on mammographic screening and early detection, the incidence of node positive breast cancer is decreasing. It must be noted that, in Western series, 30–40% of all invasive breast cancers are node positive. Therefore, two thirds of patients have histologically negative nodes [9]. In the present study axillary lymph nodes were negative in 48 (51.53%) cases. This indicates that more than half of the patients did not benefited from ALND instead they may suffer from its complications.
In the present study 53.68% patients were below 50 years of age and there was no correlation between patient’s age and axillary metastasis. There was no significant relationship between age and race and lymph node involvement [10]. Among patients affected with breast cancer, younger patients have a higher incidence of axillary nodal metastases than older patients [11].
Contrasting data have been published previously on the incidence of axillary lymph node metastases according to tumor size. Overall, the rate of axillary metastasis in patients with pT1 breast cancers is 18–38.5% [12]. Most of the patients in this study were of T2 and T3 tumor size only 27.23% cases were of T1 (<2 cm) tumor size. Possible reason for the larger size of tumor is that this study was conducted in a general hospital where patients come at the late stage of the disease. Results of our study failed to document the association between small tumor size and negative axillary lymph nodes. Various studies have shown that the gross size of tumor is one of the most significant prognostic factors in breast carcinoma and there is increased incidence of axillary lymph node metastases and decreased survival with increasing size of the tumor [1].
IDC showed high rate of axillary metastasis compared to DCIS, ILC, tubular carcinoma and mucinous carcinoma. This highlights the point that histological type of tumor can be used as useful marker for predicting axillary metastasis. It is documented that histological subtypes with favourable prognosis such as tubular, colloid, medullary and cribriform tumours are associated with node negativity in most of the cases [3].
The present study showed that there was strong association between histological grades of tumor with axillary metastasis. Grade II and III tumors had chances of positive axillary lymph nodes and grade 1 tumors showed low rate of axillary metastasis. Various studies have analyzed the importance of histological grade as a prognostic factor in carcinoma of the breast. It has been shown that patients with high grade tumors treated by mastectomy have significantly high frequency of lymph node metastases with four or more positive nodes; develop more systemic recurrences, and more of such patient’s die of metastatic disease compared to patients with low grade tumors [13, 14].
The findings of this study indicate that ER and PR-negative status was significantly associated with low risk of axillary node metastasis. Contrasting reports have been documented regarding this. Actually, the presence of steroid hormone receptors (ER and PR) represents a relatively weak prognostic factor for patients with breast cancer, but these receptors are the strongest predictive factors for response to hormonal therapy. Most of the tumors are obviously receptor positive [15]. Positive expression for ER and PR was significantly correlated with histological grade, mitotic score and nuclear pleomorphism [16]. In one of the series it has been documented that patients who underwent sentinel lymph node biopsy (SLNB), showed that the prevalence of SLN metastases had an inverse relationship with a lack of progesterone receptors [17]. The ER and PR levels do not contribute to the prediction of lymph node metastases since there was no correlation with node positivity [18].
With regard to the shift of diagnosis of breast cancer to earlier stage it is important to seek a less invasive staging method which will reduce the patient’s morbidity. The SLNB method seems perspective [19]. Radioguided sentinel node dissection offers a reliable way to assess nodal status in most breast cancer patients [20]. This explains the significance of intraoperative lymphatic mapping and sentinel lymphadenectomy to avert axillary dissection with its complications in almost two-thirds of the axillary negative patients. However, the difficulty of using prediction models of axillary node involvement should not be underestimated [21–23].
Histological type of tumor, histological grade and ER—PR status can predict the axillary metastasis while there was no association between patient’s age and tumor size with axillary metastasis in our study. Findings in this study indicate that, breast cancer patients having favorable histological type, grade I tumors and ER and PR negative tumor on biopsy are good candidates for SLNB to avoid axillary dissection. However, larger controlled trials are required to define the selection criteria of breast cancer patients in whom axillary dissection can be avoided by using SLNB.
Contributor Information
Amrut V. Ashturkar, Phone: +91-976-4524295, Email: amrutva@yahoo.com
Gayatri S. Pathak, Email: drgayatridp@yahoo.in
Sanjay D. Deshmukh, Email: drsanjay123in@yahoo.co.in
Harshal T. Pandave, Email: dr_harshalpandve@yahoo.co.in
References
- 1.Carter CL, Allen C, Henson DE. Relation of tumors size, lymph node status and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::AID-CNCR2820630129>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 2.Jatoi I, Miller AB. Why is breast cancer mortality declining? Lancet Oncol. 2003;4:251–254. doi: 10.1016/S1470-2045(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 3.Tan LGL, Tan YY, Heng D, Chan MY. Predictors of axillary lymph nodemetastases in women with early breast cancer in Singapore. Singapore Med J. 2005;46(12):693–697. [PubMed] [Google Scholar]
- 4.Haffty BG, Ward B, Pathare P, Salem R, McKhann C, Beinfield M, et al. Reappraisal of the role of axillary lymph node dissection in the conservative treatment of breast cancer. J Clin Oncol. 1997;15:691–700. doi: 10.1200/JCO.1997.15.2.691. [DOI] [PubMed] [Google Scholar]
- 5.Frierson HF, Jr, Wolber RA, Berean KW, Franquemont DW, Gaffey MJ, Boyd JC, Wilbur DC. Interobserver reproducibility of the Nottinghamm modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. Am J Clin Pathol. 1995;103:195–198. doi: 10.1093/ajcp/103.2.195. [DOI] [PubMed] [Google Scholar]
- 6.Murthy NS, Chaudhry K, Nadayil D, Agarwal UK, Saxena S. Changing trends in incidence of breast cancer: Indian scenario. Indian J Cancer. 2009;46:73–74. doi: 10.4103/0019-509X.48603. [DOI] [PubMed] [Google Scholar]
- 7.Moore MP, Kinne DW. Axillary lymphadenectomy:a diagnostic and therapeutic procedure. Surg Oncol. 1997;66(1):2–6. doi: 10.1002/(SICI)1096-9098(199709)66:1<2::AID-JSO2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Alastair M, Thompson Axillary node clearance for breast cancer. J R Coll Surg Edinb. 1999;44:111–117. [PubMed] [Google Scholar]
- 9.Jatoi I. Management of the axilla in primary breast cancer. Surg Clin N Am. 1999;79:1061–1073. doi: 10.1016/S0039-6109(05)70061-X. [DOI] [PubMed] [Google Scholar]
- 10.Yip CH, Taib NA, Tan GH, Ng KL, Yoong BK, Choo WY. Predictors of axillary lymph node metastases in breast cancer: is there a role for minimal axillary surgery? Word J Surg. 2009;33(1):54–57. doi: 10.1007/s00268-008-9782-7. [DOI] [PubMed] [Google Scholar]
- 11.Tatjana Ivkoviæ-Kapicl, Milana Panjkoviæ, Dejan Ninèiæ1, Slavica Kne¾eviæ-U¹aj.Factors correlating with lymph node metastases in patients with T1 ductal invasive breast cancer. Arch Oncol 2006;14(1–2):19–22
- 12.Iwasaki Y, Fukutomi T, Akashi-Tanaka S, Nanasawa T, Tsuda H. Axillary node metastasis from T1N0M0 breast cancer: possible avoidance of dissection in a subgroup. Jpn J Clin Oncol. 1998;28:601–603. doi: 10.1093/jjco/28.10.601. [DOI] [PubMed] [Google Scholar]
- 13.Henson DE. Histological grading of breast cancer: Significance of grade on recurrence and mortality. Arch Pathol Lab Med. 1988;112:1091–1096. [PubMed] [Google Scholar]
- 14.Hapton DS, Thorogood J, Clayden AD. Histological grading of breast cancer: significance of grade on recurrence and mortality. Eur J Surg Oncol. 1989;15:25–31. [PubMed] [Google Scholar]
- 15.Tot T. Assessment of the most important prognostic factors. In: Tot T, Tabar L, Dean P, editors. Practical breast pathology. New York: Thieme Medical Publishers; 2002. pp. 125–135. [Google Scholar]
- 16.Aye Thike; Mei Jiuan Chng; Stephanie Fook-Chong; Puay Hoon Tan Immunohistochemical expression of hormone receptors invasive breast carcinoma: correlation of results of H-score with pathological parameters. Pathology. 2001;33(1):21–25. [PubMed] [Google Scholar]
- 17.Viale G, Zurrida S, Maiorano E, et al. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer. 2005;103:492–500. doi: 10.1002/cncr.20809. [DOI] [PubMed] [Google Scholar]
- 18.Hlgreno J, Tal L, Estman G, Nesson L-G. Prediction of axillary lymph node metastases in screened breast cancer population. Acta Oncol. 1994;33(6):603–608. doi: 10.3109/02841869409121769. [DOI] [PubMed] [Google Scholar]
- 19.Pavlista D, Dusková M, Novotný J, Zikán M, Strunová M, Freitag P, Zivný J. Complications of axillary dissection in breast carcinoma. Ceská Gynekol. 2002;67(6):333–337. [PubMed] [Google Scholar]
- 20.Ponzone R, Biglia N, Maggiorotto F, Kubatzki F, Elia O, Rosa G, Sismondi P. Sentinel node dissection as definitive treatment fornode negative breast cancer patients. EJSO. 2003;29:703–706. doi: 10.1016/j.ejso.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Jaderborg JM, Harrison PB, Kiser JL. Maynard SL The feasibility and accuracy of the sentinel lymph node biopsy for breast carcinoma. Am Surg. 1999;65(8):699–705. [PubMed] [Google Scholar]
- 22.Rehman S, Sardi A, Spiegler E, Coandrea J, Frishberg D. Sentinel lymph node mapping for staging breast cancer: preliminary results of a prospective study. Md Med J. 1999;48(3):105–110. [PubMed] [Google Scholar]
- 23.Weaver DI, Karg DN, Ashikaga T, Harlow SP, O’Connell M. Pathologic analysis of sentinel and non-sentinel lymph nodes in breastcarcinoma: a multicenter study. Cancer. 2000;88(5):1099–1107. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1099::AID-CNCR22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]