Abstract
During development, epicardial cells act as progenitors for a large fraction of non-myocyte cardiac cells. Expression and function of molecules of the desmosome in the postnatal epicardium has not been studied. The objective of this study was to assess the expression of desmosomal molecules, and the functional importance of the desmosomal protein plakophilin-2 (PKP2), in epicardial and epicardium-derived cells. Epicardial explants were obtained from neonatal rat hearts. Presence of mechanical junction proteins was assessed by immunocytochemistry. Explants after PKP2 knockdown showed increased abundance of alpha smooth muscle actin-positive cells, increased abundance of lipid markers, enhanced cell migration velocity and increased abundance of a marker of cell proliferation. We conclude that a population of non-excitable, cardiac-resident cells express desmosomal molecules and, in vitro, show functional properties (including lipid accumulation) that depend on PKP2 expression. The possible relevance of our data to the pathophysiology of arrhythmogenic right ventricular cardiomyopathy, is discussed.
Keywords: Epicardial cells, Desmosome, Plakophilin-2, Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), Arrhythmogenic Cardiomyopathy (AC)
INTRODUCTION
During development, and after the formation of the primary heart tube, a group of cells originating in the splanchnic mesoderm reaches the forming heart. This “second heart field” constitutes a complex, mixed population of cells with both instructive and constructive functions (Viragh et al., 1993). The anterior heart field contributes to the formation of the outflow tract, while the posterior component contributes to the formation of the inflow tract myocardium. Of relevance, the posterior heart field also gives rise to the proepicardium, which delivers the epicardial progenitors to the forming heart, leading to the establishment of the epicardial layer. In addition to their protective function against mechanical stress, epicardial cells undergo an epithelial-mesenchymal transition (EMT) into various non-myocyte epicardium-derived cells (EPDC), including fibroblasts (Dettman et al., 1998). Both in vitro and in vivo studies have indicated that epicardial cells remain capable of migration and transformation in the adult heart (Bock-Marquette et al., 2009). Given their role as progenitors of a large fraction of non-myocyte heart cells (Dettman et al., 1998), and their potential as adjuvants in myocardial repair (Limana et al., 2011), these cells represent a population of fundamental importance in cardiac biology. Yet, their specific properties remain largely understudied. Here, we have focused on the role that expression of a particular desmosomal protein, plakophilin-2, has on the migration, proliferation and transdifferentiation of primary cultures of epicardial cells.
Desmosomes are intercellular adhesion structures involved in preservation of mechanical continuity between cells. Recent findings show that mutations in desmosomal proteins lead to arrhythmogenic right ventricular cardiomyopathy (ARVC), an inherited disease characterized by myocardial loss, replacement with fibrous and adipose tissue, as well as malignant ventricular arrhythmias and sudden death in the young (Gerull et al., 2004). To conform with modern nomenclature, we will refer to the disease as “arrhythmogenic cardiomyopathy,” or “ AC ” (Sen-Chowdhry et al., 2010). This disease is a leading cause of death among young athletes (Thiene et al., 1988) and has an overall prevalence of one in five thousand in the general population (Lahtinen et al., 2011). Though extensively characterized as a clinical and anatomo-pathological entity, (Sen-Chowdhry et al., 2010) the molecular/cellular mechanisms responsible for the clinical phenotype remain unclear. In particular, the identity of the cells that serve as progenitors of the fibrobalsts and adipocytes found in AC-afflicted hearts remains a subject of active investigation.
Recently, Lombardi et al (Lombardi et al., 2009) suggested that adipocytes found in mice deficient in the desmosomal protein desmoplakin (DP) derive from second heart field progenitors (Lombardi et al., 2009). The study did not differentiate among the various subsets of second-heart field derived cells, though it proposed that adipocytes resulted from a myogenic lineage. Here, we have used primary explants of epicardial cells to show that a deficiency in plakophilin-2, the protein most commonly associated with inherited cases of AC (Gerull et al., 2004), leads to an increased presence of alpha smooth muscle actin (αsma)-expressing cells. Cells in epicardial cultures treated with a PKP2-silencing (siRNA) construct also showed increased velocity of migration, increased proliferative rate, and increased adiposis. The possible relevance of our studies to the pathophysiology of AC, is discussed.
METHODS
Epicardial Explant Isolation and Culture
We followed procedures previously established in the literature (Oxford et al., 2007). Briefly, hearts were excised from 1–4 day old neonatal rats. Each ventricular piece was placed epicardium-side down on a 0.1% gelatin-coated surface. The tissue was removed after 4 days, and the cells continued to grow in culture for 3 additional days.
siRNA-mediated knockdown of Plakophilin-2 (PKP2) expression
The published protocol established by Oxford et. al (Oxford et al., 2007) was used to silence PKP2 in epicardial cultures. Briefly, epicardial cultures were treated with stealth RNAi™ siRNA from Invitrogen. The sequence of the silencing construct (siRNA-PKP2) was: CAGUCAAUGCGCGUACUUAUGAUCA. All control experiments were carried out in cells treated with a non-targeting construct (φsiRNA-PKP2) of sequence: CAGAGAACUGCGUAUGCAUUCUUCA. Lipofectamine RNAi™ Max (Invitrogen) was used for transfection in serum-free media. After 24 hours, the media was changed to 3% fetal bovine serum with 100 U/ml penicillin and 100μg/ml streptomycin in complete GIBCO® D-MEM Media. Cells were kept in culture for an additional 72 hours.
Immunocytochemistry
Western Blots
For detection of protein abundance, cell lysates were harvested with 4°C PBS and centrifuged at l0,000 rpm at 4°C for 10 minutes. The cell lysates were resuspended with an extraction buffer containing: 50 mM Tris (pH 8.0), 150 mM NaCl, 0.02% Sodium Azide, 1.0 PMSF, 1μg/mL Aprotinin, 1% Triton X-100, 25× Complete Protease Inhibitor, 1M NaF and 1 mM Na3VO4 and loaded on 4–20% gradient Tris-Glycine gels (Invitrogen). The proteins were transferred to nitrocellulose membranes (overnight transfer for Desmoplakin) and subsequently blocked with 5 % non-fat milk and 0.05% Tween-20 in PBS overnight at 4°C. The membranes were probed with the following antibodies (vendor, lot, dilution, reference): Desmoglein (Progen Biotechnik; lot # 611220; 1: 10; Suzuki et al., 2000), Desmocollin 2/3 (Invitrogen; lot # 745048A; 1:50; Sugimoto et al., 2008), PKP2a & b (Biodesign; lot #13B04809; 1:50; Oxford et al., 2007), β-catenin (BD Transduction Laboratories; lot # 70569; 1:1000; Eger et al., 2000) and Plakoglobin (BD Transduction Laboratories; lot # 97750; 1:1000; Aktary et al., 2010). A 4–12% gradient Tris-Glycine Gel (Invitrogen) was used for the Desmoplakin antibody (Fitzgerald Industries International; lot# 0331; 1:10; Nguyen et al., 2009). The secondary antibody used was anti-mouse IgG HRP (Sigma-Aldrich). Glyceraldehyde 3-phosphate dehydrogenase (Fitzgerald Industries International) was used for loading control for all primary antibodies.
Immunofluoresence of primary epicardial cultures
Epicardial cells were fixed 72 hours post-transfection. The coverslips were either fixed with ice-cold Methanol (Desmoplakin; Progen Biotechnik; lot # 909220; 1:10 dilution; 12 hours at 4°C; Eger et al., 1997) or 4% formaldehyde solution, and exposed to the following primary antibodies (vendor and lot # (if not yet listed) as well as dilution and time of incubation are given in parentheses): PKP2a&b (1:50 dilution; 12 hours at 4°C incubation), E-cadherin (BD Transduction Laboratories; lot # 42216; 1:50 dilution; 12 hours; Weng et al., 2002), β-catenin (1:100 dilution; 12 hours) and Plakoglobin (1:100 dilution, 12 hours). These monoclonal antibodies were co-incubated with a polyclonal αsma (AnaSpec; lot # JD2901; 1:100; 12 hours; Lewandowski et al., 2008). AlexaFluor® 488 donkey anti-rabbit IgG (H+L) and AlexaFluor® 594 donkey anti-mouse IgG (H+L) secondary antibodies were used in conjunction with the nuclear marker, 4′,6-diamidino-2-phenylindole (DAPI).
BrDU Incorporation
The 5-Bromo-2′-deoxy-uridine Labeling and Detecting Kit I (Roche) was used on cultures of epicardial cells 72 hours post-transfection. Briefly, media was aspirated and the cells were incubated with 10 μM of BrDU labeling reagent. Cells were fixed at -20°C with an Ethanol fixative containing 50 mM glycine. Coverslips were incubated with anti-BrDU working solution. The coverslips were then washed and incubated for 30 minutes at 37°C with DAPI and Anti-mouse Ig-fluorescein working solution. The number of BrDU-positive cells was interpreted as indicative of the proliferative activity of the cells in the culture, as in (Goldsworthy et al., 1993).
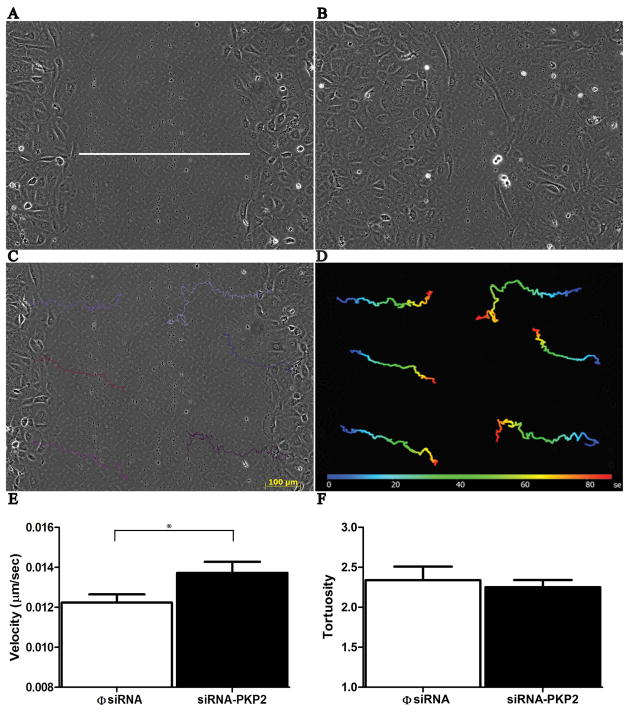
Wound healing and cell migration
A sterile pipette tip was used to scrape a longitudinal wound along an area of confluency in epicardial cultures 7 to 8 days after isolation of the explant. A Zeiss Axioobserver Z1 microscope connected to an AxioCam MRm camera was used to follow the migration of cells as they repopulated the area of the wound. Still frames were obtained at 4 minute intervals for 24 hours. Migration velocity and tortuosity were assessed using commercial software (Axiovision; Carl Zeiss, Inc.).
Induction of adipogenesis in epicardial cultures
Twenty-four hours after transfection with siRNA for PKP2 (or the control construct), epicardial cultures were treated with Preadipocyte Growth Medium-2 (Lonza Poietics®) containing 10% FBS, 2 mM L-Glutamine, 50 μg/mL GA-1000. One cell group was kept in media (as control) while a separate group was exposed to a combination of Insulin, Indomethacin, 3-Isobutyl-1-methylxanthine and Dexamethasone (Lonza SingleQuots®) to induce adipogenesis (Okada et al., 2011). Cells were incubated for 96 additional hours and then harvested for western blot analysis or fixed for microscopic analysis using a published protocol (Listenberger and Brown, 2007). Coverslips were fixed for 30 minutes with 4% PFA, and rinsed with PBS. A 1mg/ml working solution of BODIPY®493/503 Lipid Probe (Invitrogen) was diluted with 150 mM NaCl and incubated for 10 minutes at room temperature. DAPI was used as a nuclear marker.
Statistical Analysis
All experimental results were reported using descriptive statistics and Student’s t-test assuming unequal variances in Microsoft Excel. Results were also reported with mean +/− SEM. A value of p< 0.05 was used to determine significance.
RESULTS
Expression of desmosomal proteins in cells from epicardial explants
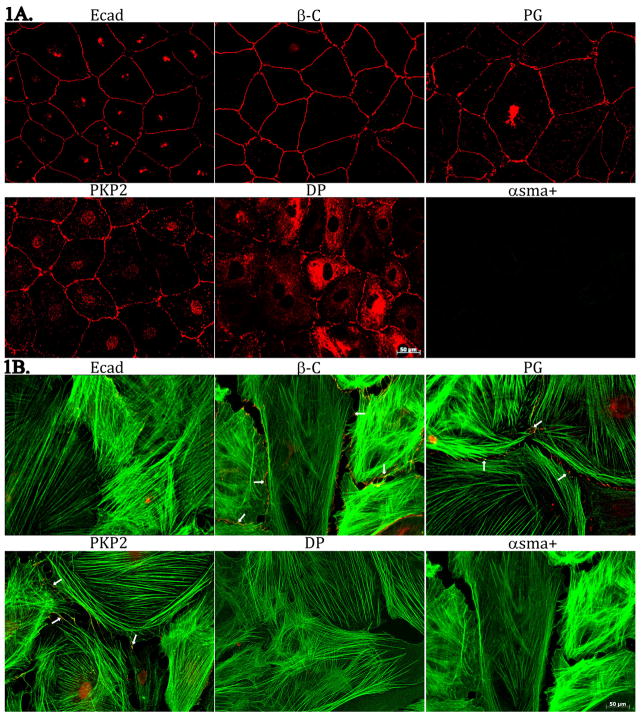
Epicardial explants were cultured for 7 days prior to characterization. Two predominant morphologies were observed. One, polygonal cobblestone-shaped, closely confluent cells forming a continuous monolayer (Figure 1A) and the other, large, flattened cells with irregular borders often free of contact at least in one of their sides (Figure 1B). To further characterize these phenotypes, we assessed the expression of E-cadherin (ECad; expected to be present in epicardial cells; (see Martinez-Estrada et al., 2010) and αsma, a protein found in vascular smooth muscle cells, and in myofibroblasts, both cell types a progeny of the epicardium (see Wessels and Perez-Pomares, 2004). Images in the upper left panel of Figure 1A and 1B were obtained from the same explant, treated with both E-cad, and αsma antibodies. Clearly, epicardial cells were abundantly positive for E-cad, and negative for αsma. In contrast, we observed cells showing strong staining of filamentous αsma and in these cells, E-cad staining was negative (see 1B, upper left corner). Mechanical junction proteins were clearly distinguishable in epicardial cells (Figure 1A). Interestingly, PKP2 abundance was significantly diminished, and desmoplakin (DP) was below detectable levels, in cells expressing filamentous αsma; yet, β-catenin (β-C) and plakoglobin (PG) were still abundantly present in these cells (see Figure 1B and Online Figure 1). Overall, the data show that both αsma-positive and αsma-negative cells were observed, the latter correlating with the morphology of an epicardial layer (Gan et al., 2007).
Figure 1.
Immunostaining of cells derived from an epicardial explant. For both panels, cells were treated with antibodies for αsma (green) and for the protein identified on top of each frame. Notice the lack of αsma(+) signal in cells with a cobblestone morphology and abundant intercellular junctions (panel A) and the limited abundance of junctional proteins in cells positive for filamentous αsma (panel B). Small arrows in B highlight signal from the junctional protein. Separate frames for easier identification of the individual components, in Online Figure 1.
A different type of αsma(+) cell was observed in our cultures. In this case, staining was observed lining the periphery of the cell, rather than decorating stress filaments that traversed the cell from one extreme to another. The presence of this cortical αsma was often present in cells that were also positive for E-Cad. Figure 2 shows an example. Two cells marked with an asterisk particularly highlight the presence of both cortical αsma, and E-cad in the same cell. Interestingly, an alternating expression pattern of Ecad and αsma was apparent in cells that were initiating the transition into a fibroblast phenotype. As the cells dissociated from one another, they lost their cobblestone phenotype, and the intensity of the cortical αsma signal increased, followed by the loss of detectable Ecad. We speculate that this phenotype correspond to that of epicardial cells undergoing a transition into the filamentous αsma-positive, epicardial-derived cell. Next, we asked whether the expression of PKP2 is relevant to the behavior of epicardial cells.
Figure 2.
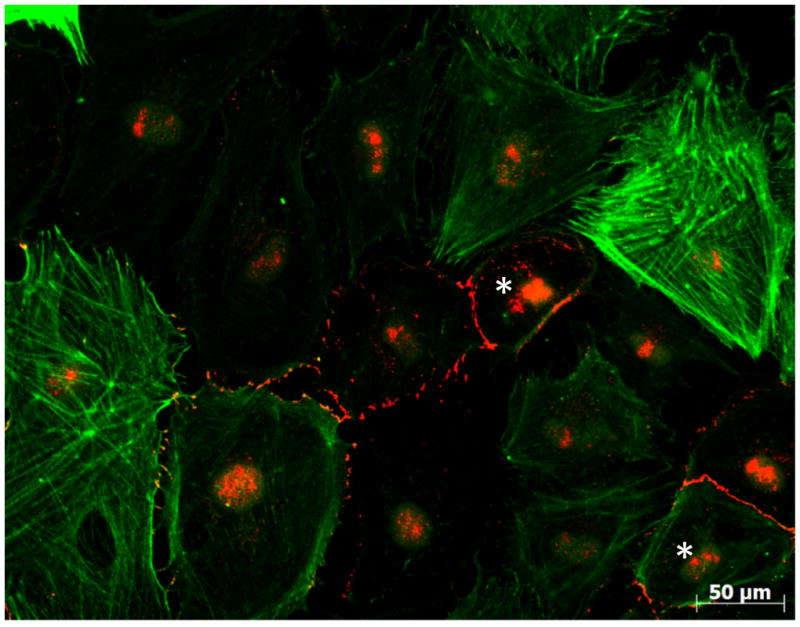
Localization of asma (green) and E-cad (red) in cells derived from an epicardial explant. Cells were kept in culture for 7 days before fixation. Asterisks denote cells co-expressing both markers. Notice the expression of αsma along filaments, or as a cortical protein. In the latter case, E-cad(+) regions can be observed.
Effect of PKP2 knockdown on desmosomal protein expression, αsma(+) cell abundance, and proliferative rate
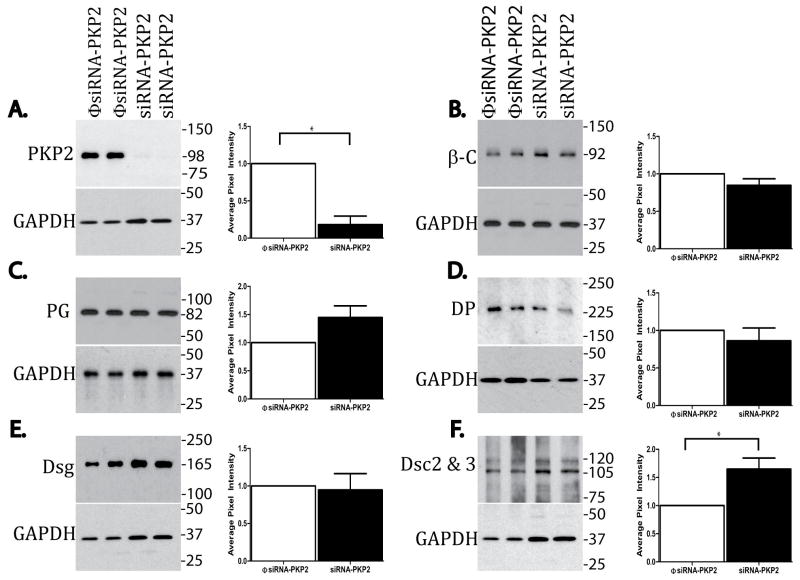
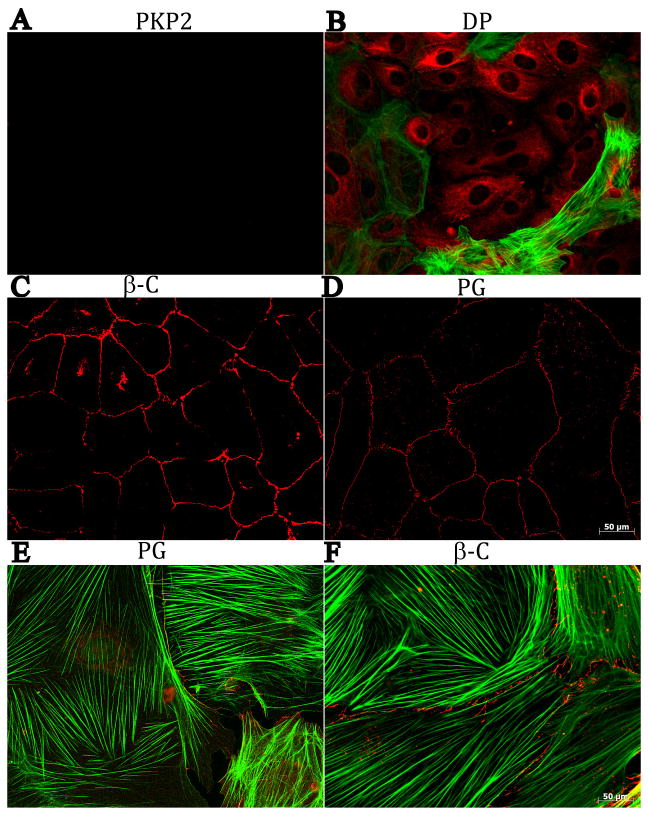
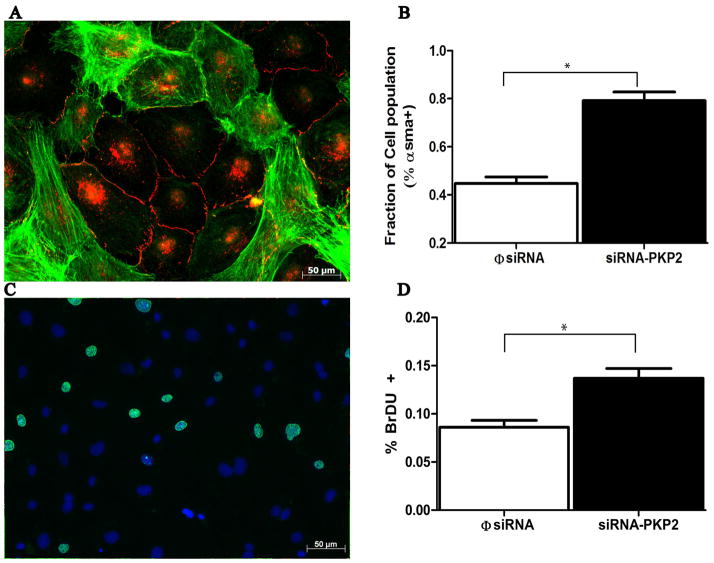
Epicardial explants were treated with siRNA for PKP2, or with a non-targeting construct. The Western blot data in Figure 3 show loss of expression of PKP2 consequent to siRNA treatment (panel A). The results also show that loss of PKP2 expression did not affect the abundance of β-C, DP or Dsg, whereas the abundance of Dsc was increased, and we noted a tendency, but not significant increase, in PG. Moreover, as shown in Figure 4, loss of PKP2 expression (panel A) was associated with loss of DP at sites of cell contact and an abundance of immunoreactive DP in the intracellular space (panel B), while both β-C (panel C) and PG (Panel D) were still present at sites of cell-cell apposition; in addition, both PG and β-C were still present in filamentous αsma(+) cells, as shown in panels E and F (see also online Figure 2). These results suggest that loss of PKP2 expression affected the molecular composition of the epicardial desmosome, but it did not cause a generalized loss of cell integrity or a complete loss of contact between the cells. Interestingly, the number of αsma positive cells (both cortical and filamentous), was significantly increased in cultures treated with siRNA for PKP2, when compared to control (Figure 5A–B). Separate studies revealed that the number of BrdU positive cells significantly increased in siRNA-PKP2-treated cultures (Figure 5C–D), thus suggesting an increase in proliferative activity following the loss of PKP2 expression.
Figure 3.
Western blot analysis of junctional proteins. In each panel, an example of a Western blot (with its loading control; GAPDH) is shown in the left, and the quantitative analysis is shown on the right. Each measurement of protein abundance in siRNA-PKP2 treated cultures was made relative to that observed in φsiRNA-PKP2-treated cells, after calibration to loading control. Panels A to F show data for (number of samples in parenthesis): PKP2 (26), β-C (8), PG (8); DP (9) Dsg (4), Dsc (6). Statistical significance was found for the decrease in PKP2 (p<0.001) and for an increase in the abundance of DSC2&3 (p<0.02). A tendency toward an increase was observed for PG, but did not reach significance.
Figure 4.
Staining of mechanical junction proteins (in red), and αsma (green), in cells treated with a PKP2-silencing construct. Panel A, PKP2, B: DP, C: β-C and D: PG. Panels E and F: PG and β-C in cells also expressing filamentous αsma.
Figure 5.
Functional effects of siRNA-PKP2 treatment in epicardial cell cultures. Panel A: Co-immunostaining of E-cadherin (red) and αsma (green) in an epicardial culture 3 days after treatment with siRNA-PKP2. Panel B: Abundance of αsma-positive sgnal in cells treated with siRNA-PKP2 or with the non-targeting constuct (φsiRNA). Each cell in a field was cataloged as αsma(+) or αsma(−), and their abundance measured relative to the number of cells counted for that field. A total of 11123 cells, and 10 separate cultures were included in the analysis of the PKP2-siRNA group. Data for the control (φsiRNA group) originated from 6014 cells and 6 Cultures. The difference in the results between the two groups was highly significant (p<0.001). Panel C: Fluorescence microscopy image of an siRNA-PKP2 treated epicardial explant exposed to BrDU (green). Cells were also treated with DAPI (blue) as a nuclear label. For each field, we quantified the number of BrDU-positive cells relative to the number of nuclei. Data were collected from 6471 cells, 79 fields in 6 φsiRNA-treated explants, and from 4879 cells in 58 fields in 6 explants treated with PKP2-siRNA (p<0.001 between the two groups).
PKP2 knockdown and cell migration
Previous studies have linked desmosomal integrity with cell migration (Setzer et al., 2004, South et al., 2003, Kundu et al., 2008, Yin et al., 2005). Here, we asked whether PKP2 expression is relevant to the motility of epicardial cells. A “wound” was inflicted (an area void of cells was created) by scraping a region of confuency with a sterile pipette tip (Figure 6A), and the process of “wound healing” was followed using time-lapse videomicroscopy. Figures 6A and 6B show still frames from one experiment, taken at time 5 hours (A) and 11.5 hours (B) after the scrape. Notice the decrease in the size of the acellular area, as the cells from the edges migrated into the center to repair the wound. Tracking software allowed us to monitor the movement of individual, non-dividing cells (see Figures 6C and 6D; see also online video I). As shown in Figures 6E and 6F, cells with reduced PKP2 expression migrated at a faster rate than cells treated with a non-silencing construct.
Figure 6.
Loss of PKP2 expression and cell motility. Panels A and B: Two frames acquired at 5 and 11.5 hours after inflicting a scrape through the center of a confluent culture of cells derived from an epicardial explant. White line in the center of panel A demarcates the width of the wound, which is repopulated with cells as they migrate toward the center. Software-aided tracking of individual cells (panel C) allowed us to generate time-space plots (D) that were translated into measurements of velocity (E; n = 48; p < .004) and tortuosity (F; φsiRNA, n = 72; siRNA-PKP2, n = 66).
PKP2 knockdown and abundance of lipid droplets in cells derived from epicardial explants
The presence of αsma(+) cells in epicardial cultures has been previously reported (Zamora et al., 2007). Yet, whether these cells have the potential to be transformed into the adipocyte lineage, remains to be determined. As a first approach to this question, we assessed the presence of lipid accumulation in epicardial cells, either kept in control conditions, or treated with insulin, indomethacin, 3-isobutyl-1-methylxanthine and dexamethasone to induce adipogenesis (see also Okada et al., 2011). A small, yet detectable presence of cells immunoreactive to the lipid marker “BODIPY” was found in control conditions (Figure 7A). After induction, BODIPY-positive cells were readily observed (Figure 7B). Interestingly, the area occupied by a BODIPY positive signal (taken as an indication of lipid droplet abundance) was significantly larger in cells where PKP2 expression was knocked down (7C). Quantitative analysis is shown in Panel 7D. Overall, this is the first demonstration that epicardial cells can be driven into adiposis, and that the abundance of lipid-containing cells is larger in cultures deficient in a desmosomal protein.
Figure 7.
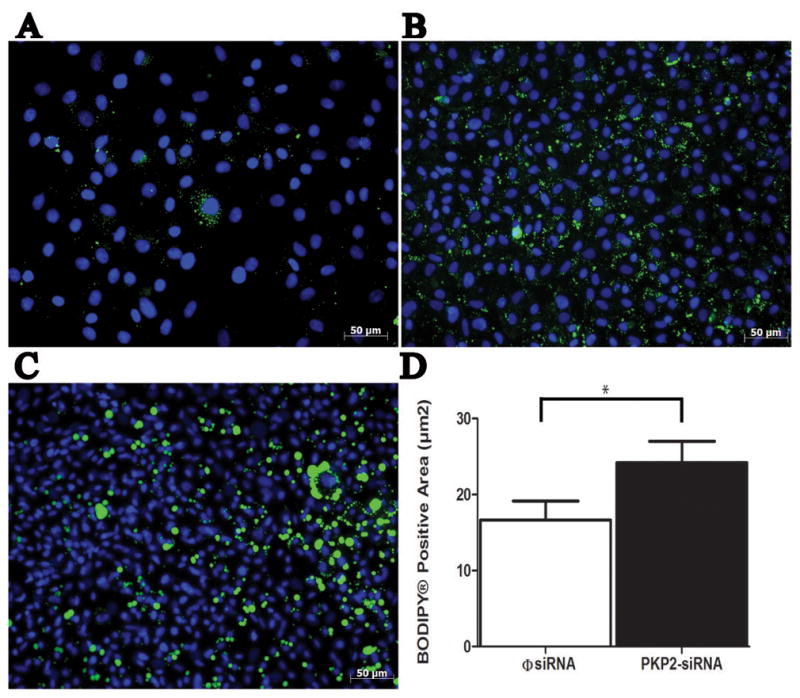
Lipid accumulation in epicardial cells. Cells treated with φsiRNA-PKP2 and cultured with pre-adipocyte growth media showed low levels of BODIPY®-labeled lipid droplets (A) compared to cultures treated with adipocyte differentiation medium (B). Epicardial cells treated both with siRNA-PKP2 and adipocyte differentiation medium (C) showed a significant increase in the area containing the BODIPY® signal (D). For φsiRNA, measurements were obtained from 74 fields, 7 separate explants. For siRNA-PKP2, data were collected from 98 separate fields, 8 separate explants. p< 0.05.
DISCUSSION
Previous studies have shown that the molecular integrity of mechanical junctions is relevant to the proliferation, migration and transformation of various cell types (Krusche et al., 2011, Kolegraff et al., 2011, Huang et al., 2008, Thiery, 2002). The presence of intercellular junctions including their associated molecules was discovered in a population of non-myocytes that include endothelial and valvular interstitial cells (VICs) (Noria et al., 1999, Xu et al., 2011, Noria et al., 2004, Lester et al., 1988, Mulholland and Gotlieb, 1996, Latif et al., 2006, Taylor et al., 2003, Chester and Taylor, 2007, McDouall et al., 2001, Latif et al., 2005, Filip et al., 1986, Lupu and Simionescu, 1985, Dejana et al., 2009, Grazia Lampugnani et al., 2003, Lampugnani et al., 1992). The expression of adherens junction molecules including PKP2 within this junction - a protein traditionally located within the desmosome - have also been detected in VICs (Barth et al., 2009). Studies evaluating the presence of E-cadherin in non-myocyte cells, specifically in the epicardium, have also been previously examined (Wada et al., 2001, Di Meglio et al., 2010, Martinez-Estrada et al., 2010). Yet, the presence, and functional relevance of desmosomal molecules in the epicardium has not been investigated. Here, we show that epicardial cells express molecules of the desmosome, which localize abundantly at sites of cell apposition. The expression of these molecules is different in cells that depart from the epicardial phenotype; in cells positive for filamentous αsma, immunoreactive PG and β-C could still be identified, PKP2 abundance was reduced and DP fell below the level of detection. Our data also show that loss of expression of PKP2 correlates with an increased abundance of αsma+ cells, increased migratory and proliferative rate, and increased abundance of cells expressing a marker of adiposis. We speculate that this subgroup of second heart field-derived cells may play a role in the origin of the fibrofatty infiltration characteristic of AC. However, before discussing the implications of our findings, methodological limitations should be considered.
Our observations were conducted in an in vitro system, more than seven days after the tissue was extracted from the living heart. Changes in the properties of the cells once in the artificial conditions of the culture dish are possible. Yet, it is worth noting that in vitro explants of epicardial cells have been extensively studied and their properties found to correlate with those of cells in the heart in situ (Chen et al., 2002, Smith et al., 2011, Kruithof et al., 2011). The presence of desmosomal proteins in epicardial cells is also consistent with other cell systems involved in tissue protection (Farquhar and Palade, 1963). Transformation of epicardial cells in vivo is also well documented (Wessels and Perez-Pomares, 2004). Moreover, studies on mice deficient for N-cadherin (Radice et al., 1997), or for connexin43 (Reaume et al., 1995), indicate that intercellular communication is relevant to the function of epicardial cells during development. Overall, our results indicate that fundamental properties of epicardial cells are PKP2-dependent; though we are unable to ascertain whether those properties are relevant to the behavior of epicardial cells in vivo, previous studies support the notion that loss of desmosomal integrity can affect the behavior of the epicardium in the living organism.
Under control conditions, our cultures were populated by at least two distinct cell populations: One, E-cadherin positive and negative for αsma and the other, with the reverse immunoreactivity (αsma positive; E-cad negative). The αsma positive cells may correspond to myofibroblasts, or smooth muscle cells derived directly from the explant. Alternatively, those cells may be epicardial-derived, consequent to the transformation of the progenitor epicardial cells, once in culture. The occurrence of EMT in epicardial cells has been shown before, both in vitro and in vivo (see Sridurongrit et al., 2008, Perez-Pomares et al., 1997, Martinez-Estrada et al., 2010, Di Meglio et al., 2010). In our experiments, we observed numerous cells that presented an intermediate phenotype: cortical αsma, dotted E-cad staining and a morphology transitory between the cobblestone shape of an epicardial cell, and the extended shape characteristic of a myofibroblast. The images of these cells are likely to be snapshots of cell transformation. In the quantification of αsma-positive cell abundance after PKP2 knockdown, we attempted to separate those that were also positive for E-cad and would thus correspond to an intermediate phenotype. Defining this intermediate group proved prone to inaccuracies, as E-cad antibodies can give variable background staining. We therefore chose to group cells as αsma positive or αsma negative. Our results show an increase in αsma positive cells after PKP2 silencing. Given the associated increase in proliferation observed, it is not possible at this point to discern whether the increased abundance of αsma positive cells is due only to proliferation of the αsma positive population, or whether an increase in the rate of EMT was also caused by PKP2. What we did observe is that loss of PKP2 expression led to an increase in the abundance of αsma positive cells (see also Munoz-Chapuli et al., 2002). The role of PKP2 in the control of transformation of epicardial cells into alternative phenotypes, remains a subject of future investigation. Of note, our studies are first to demonstrate the presence of cells positive for lipid markers in epicardial cultures. We have further shown that the abundance of adiposis increases in cells lacking PKP2. We speculate that epicardial cells can act as adipocyte progenitors, and that PKP2 abundance is relevant to the control of epicardium-derived adipogenesis; the latter may be relevant to the pathophysiology of fibrofatty infiltration in patients afflicted with AC.
Previous studies have shown that intercellular junction molecules are involved in the control of cell migration. In particular, the studies of Rhee et al showed that the migratory behavior of the proepicardial organ was affected by the loss of Cx43 (Rhee et al., 2009). The behavior of the proepicardial organ was also dependent on the expression of N-cadherin (Luo et al., 2006). Similar experiments remain to be conducted in mice deficient for PKP2. Our in vitro studies demonstrate that epicardial cell migration is dependent on PKP2 expression. Overall, we speculate that PKP2 deficiencies (such as those observed in patients with arrhythmogenic cardiomyopathy) may lead to dysfunctional migration, proliferation and/or transformation of cells in the forming epicardium, causing an excess of fibroblasts, adipocytes, or their progenitors, in the myocardial interstitium. From this perspective, we speculate that a latent “defect” in the abundance of second heart field cell progenitors may already exist at birth, and become manifest (or not) depending on the existence of epigenetic factors (Srivastava, 2006).
Second heart field progenitors give rise to a myogenic lineage (Waldo et al., 2001). These cells are also involved in the formation of the proepicardial organ and the epicardium. Epicardial cells are not primarily myogenic, though a potential for epicardial-myocyte transformation has been described (Cai et al., 2008). As such, the pathogenesis of AC may involve two different cell populations: the cardiac myocytes, in their dependence of desmosomal integrity for mechanical continuity and for proper ion channel function (Sato et al., 2009) and the epicardial cells, where desmosome integrity may be required for the control of migration, proliferation and transformation, starting during cardiogenesis. Given the accessibility of the epicardial space to medical intervention, it is tempting to propose that the epicardium could be a future target for gene therapy in patients afflicted with AC.
Supplementary Material
Acknowledgments
This work was supported by Supported by NIH grants RC1-HL100111-01, PO1-HL087226 and RO1-HL106632 (MD), and by a Foundation Leducq Transatlantic Network (MD).
ABBREVIATIONS
- αsma
alpha smooth muscle actin
- AC
Arrhythmogenic Cardiomyopathy
- β-C
β-catenin
- DSG
desmoglein
- DSC
desmocollin
- DP
desmoplakin
- E-cad
E-cadherin
- EMT
epithelial-mesenchymal transition
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- PG
plakoglobin
- PKP2
plakophilin-2
- φsiRNA-PKP2
control PKP2 silencing construct
Footnotes
Declaration of Interest
The authors report no declarations of interest.
References
- AKTARY Z, CHAPMAN K, LAM L, LO A, JI C, GRAHAM K, COOK L, LI L, MACKEY JR, PASDAR M. Plakoglobin interacts with and increases the protein levels of metastasis suppressor Nm23-H2 and regulates the expression of Nm23-H1. Oncogene. 2010;29:2118–29. doi: 10.1038/onc.2009.495. [DOI] [PubMed] [Google Scholar]
- BARTH M, SCHUMACHER H, KUHN C, AKHYARI P, LICHTENBERG A, FRANKE WW. Cordial connections: molecular ensembles and structures of adhering junctions connecting interstitial cells of cardiac valves in situ and in cell culture. Cell and tissue research. 2009;337:63–77. doi: 10.1007/s00441-009-0806-x. [DOI] [PubMed] [Google Scholar]
- BOCK-MARQUETTE I, SHRIVASTAVA S, PIPES GC, THATCHER JE, BLYSTONE A, SHELTON JM, GALINDO CL, MELEGH B, SRIVASTAVA D, OLSON EN, DIMAIO JM. Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. Journal of molecular and cellular cardiology. 2009;46:728–38. doi: 10.1016/j.yjmcc.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAI CL, MARTIN JC, SUN Y, CUI L, WANG L, OUYANG K, YANG L, BU L, LIANG X, ZHANG X, STALLCUP WB, DENTON CP, MCCULLOCH A, CHEN J, EVANS SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN TH, CHANG TC, KANG JO, CHOUDHARY B, MAKITA T, TRAN CM, BURCH JB, EID H, SUCOV HM. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Developmental biology. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- CHESTER AH, TAYLOR PM. Molecular and functional characteristics of heart-valve interstitial cells. Philosophical transactions of the Royal Society of London Series B. Biological sciences. 2007;362:1437–43. doi: 10.1098/rstb.2007.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEJANA E, ORSENIGO F, MOLENDINI C, BALUK P, MCDONALD DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell and tissue research. 2009;335:17–25. doi: 10.1007/s00441-008-0694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DETTMAN RW, DENETCLAW W, JR, ORDAHL CP, BRISTOW J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Developmental biology. 1998;193:169–81. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- DI MEGLIO F, CASTALDO C, NURZYNSKA D, ROMANO V, MIRAGLIA R, BANCONE C, LANGELLA G, VOSA C, MONTAGNANI S. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. Journal of molecular and cellular cardiology. 2010;49:719–27. doi: 10.1016/j.yjmcc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- EGER A, STOCKINGER A, SCHAFFHAUSER B, BEUG H, FOISNER R. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity. The Journal of cell biology. 2000;148:173–88. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGER A, STOCKINGER A, WICHE G, FOISNER R. Polarisation-dependent association of plectin with desmoplakin and the lateral submembrane skeleton in MDCK cells. Journal of cell science. 1997;110 ( Pt 11):1307–16. doi: 10.1242/jcs.110.11.1307. [DOI] [PubMed] [Google Scholar]
- FARQUHAR MG, PALADE GE. Junctional complexes in various epithelia. The Journal of cell biology. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIP DA, RADU A, SIMIONESCU M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circulation research. 1986;59:310–20. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- GAN Q, YOSHIDA T, LI J, OWENS GK. Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha-actin expression. Circulation research. 2007;101:883–92. doi: 10.1161/CIRCRESAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- GERULL B, HEUSER A, WICHTER T, PAUL M, BASSON CT, MCDERMOTT DA, LERMAN BB, MARKOWITZ SM, ELLINOR PT, MACRAE CA, PETERS S, GROSSMANN KS, DRENCKHAHN J, MICHELY B, SASSE-KLAASSEN S, BIRCHMEIER W, DIETZ R, BREITHARDT G, SCHULZE-BAHR E, THIERFELDER L. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nature genetics. 2004;36:1162–4. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- GOLDSWORTHY TL, BUTTERWORTH BE, MARONPOT RR. Concepts, labeling procedures, and design of cell proliferation studies relating to carcinogenesis. Environmental health perspectives. 1993;101(Suppl 5):59–65. doi: 10.1289/ehp.93101s559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAZIA LAMPUGNANI M, ZANETTI A, CORADA M, TAKAHASHI T, BALCONI G, BREVIARIO F, ORSENIGO F, CATTELINO A, KEMLER R, DANIEL TO, DEJANA E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. The Journal of cell biology. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG H, ASIMAKI A, LO D, MCKENNA W, SAFFITZ J. Disparate effects of different mutations in plakoglobin on cell mechanical behavior. Cell motility and the cytoskeleton. 2008;65:964–78. doi: 10.1002/cm.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLEGRAFF K, NAVA P, HELMS MN, PARKOS CA, NUSRAT A. Loss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/beta-catenin signaling. Molecular biology of the cell. 2011;22:1121–34. doi: 10.1091/mbc.E10-10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUITHOF BP, XU J, FRITZ DT, CABRAL CS, GAUSSIN V, ROGERS MB. An in vivo map of bone morphogenetic protein 2 post-transcriptional repression in the heart. Genesis. 2011 doi: 10.1002/dvg.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUSCHE CA, HOLTHOFER B, HOFE V, VAN DE SANDT AM, ESHKIND L, BOCKAMP E, MERX MW, KANT S, WINDOFFER R, LEUBE RE. Desmoglein 2 mutant mice develop cardiac fibrosis and dilation. Basic research in cardiology. 2011;106:617–33. doi: 10.1007/s00395-011-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDU ST, GOSAVI P, KHAPARE N, PATEL R, HOSING AS, MARU GB, INGLE A, DECAPRIO JA, DALAL SN. Plakophilin3 downregulation leads to a decrease in cell adhesion and promotes metastasis. International journal of cancer Journal international du cancer. 2008;123:2303–14. doi: 10.1002/ijc.23797. [DOI] [PubMed] [Google Scholar]
- LAHTINEN AM, LEHTONEN E, MARJAMAA A, KAARTINEN M, HELIO T, PORTHAN K, OIKARINEN L, TOIVONEN L, SWAN H, JULA A, PELTONEN L, PALOTIE A, SALOMAA V, KONTULA K. Population-prevalent desmosomal mutations predisposing to arrhythmogenic right ventricular cardiomyopathy. Heart rhythm: the official journal of the Heart Rhythm Society. 2011;8:1214–21. doi: 10.1016/j.hrthm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- LAMPUGNANI MG, RESNATI M, RAITERI M, PIGOTT R, PISACANE A, HOUEN G, RUCO LP, DEJANA E. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. The Journal of cell biology. 1992;118:1511–22. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATIF N, SARATHCHANDRA P, TAYLOR PM, ANTONIW J, BRAND N, YACOUB MH. Characterization of molecules mediating cell-cell communication in human cardiac valve interstitial cells. Cell biochemistry and biophysics. 2006;45:255–64. doi: 10.1385/CBB:45:3:255. [DOI] [PubMed] [Google Scholar]
- LATIF N, SARATHCHANDRA P, TAYLOR PM, ANTONIW J, YACOUB MH. Molecules mediating cell-ECM and cell-cell communication in human heart valves. Cell biochemistry and biophysics. 2005;43:275–87. doi: 10.1385/CBB:43:2:275. [DOI] [PubMed] [Google Scholar]
- LESTER W, ROSENTHAL A, GRANTON B, GOTLIEB AI. Porcine mitral valve interstitial cells in culture. Laboratory investigation; a journal of technical methods and pathology. 1988;59:710–9. [PubMed] [Google Scholar]
- LEWANDOWSKI R, PROCIDA K, VAIDYANATHAN R, COOMBS W, JALIFE J, NIELSEN MS, TAFFET SM, DELMAR M. RXP-E: a connexin43-binding peptide that prevents action potential propagation block. Circulation research. 2008;103:519–26. doi: 10.1161/CIRCRESAHA.108.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIMANA F, CAPOGROSSI MC, GERMANI A. The epicardium in cardiac repair: from the stem cell view. Pharmacology & therapeutics. 2011;129:82–96. doi: 10.1016/j.pharmthera.2010.09.002. [DOI] [PubMed] [Google Scholar]
- LISTENBERGER LL, BROWN DA. Fluorescent detection of lipid droplets and associated proteins. In: Bonifacino Juan S, et al., editors. Current protocols in cell biology/editorial board. Unit 24. Chapter 24. 2007. p. 2. [DOI] [PubMed] [Google Scholar]
- LOMBARDI R, DONG J, RODRIGUEZ G, BELL A, LEUNG TK, SCHWARTZ RJ, WILLERSON JT, BRUGADA R, MARIAN AJ. Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circulation research. 2009;104:1076–84. doi: 10.1161/CIRCRESAHA.109.196899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO Y, HIGH FA, EPSTEIN JA, RADICE GL. N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Developmental biology. 2006;299:517–28. doi: 10.1016/j.ydbio.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUPU F, SIMIONESCU M. Organization of the intercellular junctions in the endothelium of cardiac valves. Journal of submicroscopic cytology. 1985;17:119–32. [PubMed] [Google Scholar]
- MARTINEZ-ESTRADA OM, LETTICE LA, ESSAFI A, GUADIX JA, SLIGHT J, VELECELA V, HALL E, REICHMANN J, DEVENNEY PS, HOHENSTEIN P, HOSEN N, HILL RE, MUNOZ-CHAPULI R, HASTIE ND. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nature genetics. 2010;42:89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDOUALL RM, FARRAR MW, KHAN S, YACOUB MH, ALLEN SP. Unique sensitivities to cytokine regulated expression of adhesion molecules in human heart-derived endothelial cells. Endothelium: journal of endothelial cell research. 2001;8:25–40. doi: 10.3109/10623320109063155. [DOI] [PubMed] [Google Scholar]
- MULHOLLAND DL, GOTLIEB AI. Cell biology of valvular interstitial cells. The Canadian journal of cardiology. 1996;12:231–6. [PubMed] [Google Scholar]
- MUNOZ-CHAPULI R, GONZALEZ-IRIARTE M, CARMONA R, ATENCIA G, MACIAS D, PEREZ-POMARES JM. Cellular precursors of the coronary arteries. Texas Heart Institute journal/from the Texas Heart Institute of St. Luke’s Episcopal Hospital, Texas Children’s Hospital. 2002;29:243–9. [PMC free article] [PubMed] [Google Scholar]
- NGUYEN B, DUSEK RL, BEAUDRY VG, MARINKOVICH MP, ATTARDI LD. Loss of the desmosomal protein perp enhances the phenotypic effects of pemphigus vulgaris autoantibodies. The Journal of investigative dermatology. 2009;129:1710–8. doi: 10.1038/jid.2008.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORIA S, COWAN DB, GOTLIEB AI, LANGILLE BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circulation research. 1999;85:504–14. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- NORIA S, XU F, MCCUE S, JONES M, GOTLIEB AI, LANGILLE BL. Assembly and reorientation of stress fibers drives morphological changes to endothelial cells exposed to shear stress. The American journal of pathology. 2004;164:1211–23. doi: 10.1016/S0002-9440(10)63209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA E, YAMANAKA M, ISHIKAWA O. New insights into the mechanism of abnormal calcification in nephrogenic systemic fibrosis - gadolinium promotes calcium deposition of mesenchymal stem cells and dermal fibroblasts. Journal of dermatological science. 2011;62:58–63. doi: 10.1016/j.jdermsci.2011.01.009. [DOI] [PubMed] [Google Scholar]
- OXFORD EM, MUSA H, MAASS K, COOMBS W, TAFFET SM, DELMAR M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circulation research. 2007;101:703–11. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- PEREZ-POMARES JM, MACIAS D, GARCIA-GARRIDO L, MUNOZ-CHAPULI R. Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Developmental dynamics: an official publication of the American Association of Anatomists. 1997;210:96–105. doi: 10.1002/(SICI)1097-0177(199710)210:2<96::AID-AJA3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- RADICE GL, RAYBURN H, MATSUNAMI H, KNUDSEN KA, TAKEICHI M, HYNES RO. Developmental defects in mouse embryos lacking N-cadherin. Developmental biology. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- REAUME AG, DE SOUSA PA, KULKARNI S, LANGILLE BL, ZHU D, DAVIES TC, JUNEJA SC, KIDDER GM, ROSSANT J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–4. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- RHEE DY, ZHAO XQ, FRANCIS RJ, HUANG GY, MABLY JD, LO CW. Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development. 2009;136:3185–93. doi: 10.1242/dev.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO PY, MUSA H, COOMBS W, GUERRERO-SERNA G, PATINO GA, TAFFET SM, ISOM LL, DELMAR M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circulation research. 2009;105:523–6. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN-CHOWDHRY S, MORGAN RD, CHAMBERS JC, MCKENNA WJ. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annual review of medicine. 2010;61:233–53. doi: 10.1146/annurev.med.052208.130419. [DOI] [PubMed] [Google Scholar]
- SETZER SV, CALKINS CC, GARNER J, SUMMERS S, GREEN KJ, KOWALCZYK AP. Comparative analysis of armadillo family proteins in the regulation of a431 epithelial cell junction assembly, adhesion and migration. The Journal of investigative dermatology. 2004;123:426–33. doi: 10.1111/j.0022-202X.2004.23319.x. [DOI] [PubMed] [Google Scholar]
- SMITH CL, BAEK ST, SUNG CY, TALLQUIST MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circulation research. 2011;108:e15–26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUTH AP, WAN H, STONE MG, DOPPING-HEPENSTAL PJ, PURKIS PE, MARSHALL JF, LEIGH IM, EADY RA, HART IR, MCGRATH JA. Lack of plakophilin 1 increases keratinocyte migration and reduces desmosome stability. Journal of cell science. 2003;116:3303–14. doi: 10.1242/jcs.00636. [DOI] [PubMed] [Google Scholar]
- SRIDURONGRIT S, LARSSON J, SCHWARTZ R, RUIZ-LOZANO P, KAARTINEN V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Developmental biology. 2008;322:208–18. doi: 10.1016/j.ydbio.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRIVASTAVA D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–48. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO M, INOKO A, SHIROMIZU T, NAKAYAMA M, ZOU P, YONEMURA S, HAYASHI Y, IZAWA I, SASOH M, UJI Y, KAIBUCHI K, KIYONO T, INAGAKI M. The keratin-binding protein Albatross regulates polarization of epithelial cells. The Journal of cell biology. 2008;183:19–28. doi: 10.1083/jcb.200803133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI K, TANAKA T, ENOKI M, NISHIDA T. Coordinated reassembly of the basement membrane and junctional proteins during corneal epithelial wound healing. Investigative ophthalmology & visual science. 2000;41:2495–500. [PubMed] [Google Scholar]
- TAYLOR PM, BATTEN P, BRAND NJ, THOMAS PS, YACOUB MH. The cardiac valve interstitial cell. The international journal of biochemistry & cell biology. 2003;35:113–8. doi: 10.1016/s1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- THIENE G, NAVA A, CORRADO D, ROSSI L, PENNELLI N. Right ventricular cardiomyopathy and sudden death in young people. The New England journal of medicine. 1988;318:129–33. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- THIERY JP. Epithelial-mesenchymal transitions in tumour progression. Nature reviews. Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- VIRAGH S, GITTENBERGER-DE GROOT AC, POELMANN RE, KALMAN F. Early development of quail heart epicardium and associated vascular and glandular structures. Anatomy and embryology. 1993;188:381–93. doi: 10.1007/BF00185947. [DOI] [PubMed] [Google Scholar]
- WADA AM, REESE DE, BADER DM. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development. 2001;128:2085–93. doi: 10.1242/dev.128.11.2085. [DOI] [PubMed] [Google Scholar]
- WALDO KL, KUMISKI DH, WALLIS KT, STADT HA, HUTSON MR, PLATT DH, KIRBY ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–88. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- WENG Z, XIN M, PABLO L, GRUENEBERG D, HAGEL M, BAIN G, MULLER T, PAPKOFF J. Protection against anoikis and down-regulation of cadherin expression by a regulatable beta-catenin protein. The Journal of biological chemistry. 2002;277:18677–86. doi: 10.1074/jbc.M105331200. [DOI] [PubMed] [Google Scholar]
- WESSELS A, PEREZ-POMARES JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- XU S, LIU AC, KIM H, GOTLIEB AI. Cell density regulates in vitro activation of heart valve interstitial cells. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2011 doi: 10.1016/j.carpath.2011.01.004. [DOI] [PubMed] [Google Scholar]
- YIN T, GETSIOS S, CALDELARI R, KOWALCZYK AP, MULLER EJ, JONES JC, GREEN KJ. Plakoglobin suppresses keratinocyte motility through both cell-cell adhesion-dependent and -independent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5420–5. doi: 10.1073/pnas.0501676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMORA M, MANNER J, RUIZ-LOZANO P. Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18109–14. doi: 10.1073/pnas.0702415104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.