Abstract
The von Hippel-Lindau (VHL) tumor suppressor gene encodes a component of a ubiquitin ligase complex containing elongin B, elongin C, cullin 2, and Rbx1, which acts as a negative regulator of hypoxia inducible factor (HIF). VHL ubiquitinates and degrades the alpha subunits of HIF and this is proposed to suppress tumorigenesis and tumor angiogenesis. Several lines of evidence also suggest important roles for HIF-independent VHL functions in maintenance of primary cilium, extracellular matrix formation, and tumor suppression.
We undertook a series of proteomic analyses to gain a comprehensive picture of the VHL-interacting proteins. We found that the ARF tumor suppressor interacts with VHL30, a longer VHL isoform, but not with VHL19, a shorter VHL isoform. ARF was found to release VHL30 from the E3 ligase complex, promoting the binding of VHL30 to a protein arginine methyltransferase, PRMT3. Our analysis of the VHL19 interactome also uncovered that VHL19 displays affinity to collagens and their biosynthesis enzymes.
Keywords: von Hippel-Lindau tumor suppressor, ARF, PRMT3, p53, interactome, proteomics
INTRODUCTION
Germline mutation of the von Hippel-Lindau (VHL) tumor suppressor gene is the cause of a hereditary cancer syndrome called von Hippel-Lindau (VHL) disease, which is characterized by an increased risk of clear cell renal carcinoma, hemangioblastoma of the nervous system, and adrenal pheochromocytoma [for reviews see 1–4]. VHL disease patients harbor one wild-type and one defective VHL allele; the tumors arising in these patients display somatic inactivation of the remaining wild-type allele. Biallelic VHL inactivation is also common in sporadic clear cell renal carcinomas and hemangioblastomas. Using two different initiation codons, two isoforms of VHL are synthesized: VHL30, a 213-amino-acid protein in humans and VHL19, residues 54 – 213 of VHL30 [lacking the N-terminal acidic domain whose function is poorly defined (Figure 1A)]. Both VHL30 and VHL19 act as a substrate recognition subunit in the E3 ubiquitin ligase complex that also contains elongin B, elongin C, cullin 2, and Rbx1.
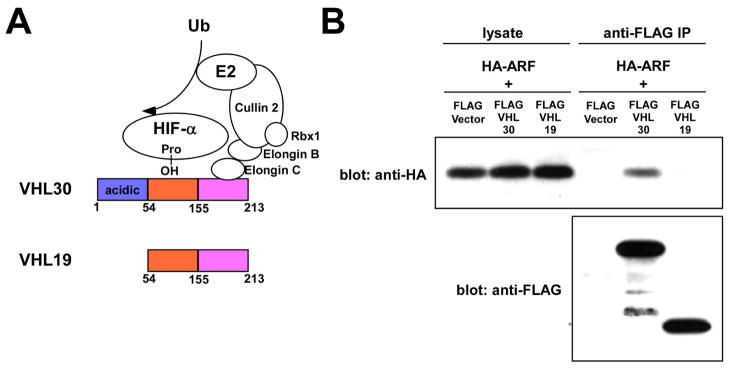
Figure 1.
VHL30 binds ARF.
(A) Structure of VHL30 and VHL19.
(B) VHL30 binds ARF.
U2OS cells were transfected with HA-ARF in conjunction with FLAG-vector, FLAG-VHL30, or FLAG-VHL19. Forty-eight hours after transfection, the binding of HA-ARF and FLAG-VHL30 or FLAG-VHL19 was examined by anti-FLAG immunoprecipitation followed by anti-HA immunoblotting.
VHL functions as a negative regulator of hypoxia inducible factors (HIFs), a family of transcription factors that regulate genes involved in the cellular response to hypoxia. In the presence of oxygen and iron, specific proline residues in HIF are hydroxylated and these hydroxylated prolines are recognized by VHL, resulting in ubiquitination and degradation of HIF. Hypoxia or depletion of iron inhibits the prolyl-hydroxylation of HIF, causing stabilization of HIF and induction of HIF target genes such as vascular endothelial growth factor (VEGF) and erythropoietin. Downregulation of HIF by VHL explains some of the phenotypes of tumors with VHL mutations. Hemangioblastomas and clear cell renal carcinomas are highly vascular tumors, due at least in part to VEGF overproduction. These tumors, along with pheochromocytomas, sometimes secrete erythropoietin, leading to overproduction of red blood cells.
It is also clear, however, that VHL has functions other than regulation of HIF 1–4. 1) VHL was shown to bind to other proteins including fibronectin, atypical PKC family proteins, SP1 transcription factor, RNA polymerase subunits Rpb1 and Rpb7, and a de-ubiquitinating enzyme VDU-1. Among these, VHL was shown to ubiquitinate Rpb1 5, 6 and Rpb7 7. 2) There is also evidence that VHL plays HIF-independent roles in extracellular matrix control 8, 9. 3) Type 2C VHL disease caused by specific VHL mutants such as L188V and V84L predispose mutation carriers to familial pheochromocytomas without hemangioblastomas or renal carcinomas. Importantly, these VHL mutants ubiquitinate and degrade HIF as efficiently as wild-type VHL, which suggests that HIF-independent function(s) of VHL play a role in tumorigenesis 9, 10. 4) Overexpression of constitutively active HIF in mice did not result in hemangioblastomas or renal carcinomas 11, suggesting that deregulation of HIF is not sufficient to initiate tumors in mice. 5) Finally, gain-of-function HIF-2α mutations were identified in familial erythrocytosis patients 12, 13, but these patients did not display predisposition to tumors, suggesting that activation of HIF is not sufficient to induce tumors in humans. These findings suggest that deregulation of HIF is not sufficient for tumorigenesis and that loss of HIF-independent function(s) of VHL plays a critical role in tumorigenesis.
In order to dissect the HIF-independent function(s) of VHL as well as to better understand its HIF-dependent functions, it is important to comprehensively identify the VHL-interacting proteins. Therefore, we undertook a series of proteomic analyses of the VHL interactome by using immunoaffinity purification and quantitative proteomics. For this study, the results from pilot quantitative proteomic experiments were used to provide leads for subsequent immunoblotting analyses. We discovered that VHL30, but not VHL19, interacts with the ARF tumor suppressor. ARF was found to disrupt the VHL30 E3 ligase complex and instead enhance the interaction between VHL30 and a protein arginine methyltransferase, PRMT3. VHL30, ARF, and PRMT3 were shown to induce asymmetric arginine di-methylation of p53. Additionally, analysis of the VHL19 interactome revealed the association of VHL19 with collagens and enzymes involved in collagen biosynthesis.
EXPERIMENTAL PROCEDURES
Cell culture
293T cells were cultured in DMEM supplemented with 10% calf serum. U2OS cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum. Calcium phosphate co-precipitation was used for transfection.
Protein sample preparation, ICAT reagent labeling, and mass spectrometry
293T cells were transfected with FLAG-VHL or FLAG empty vector; 48 hours after transfection, the cells were lysed in TNE buffer (10 mM Tris pH 7.4/150 mM NaCl/1% NP-40/1 mM EDTA/1 mM AEBSF/10 μg/ml aprotinin/10 μg/ml Leupeptin/1 μg/ml Pepstatin A/20 mM sodium fluoride). Anti-FLAG immunoprecipitation was performed under non-denaturing conditions as described 14 and the immunoprecipitate was eluted with FLAG peptide. The two immunoprecipitates (FLAG-VHL and FLAG-vector) were labeled with isotopically light and heavy cleavable isotope-coded affinity tag (ICAT) reagent (Applied Biosystems), respectively. The two labeled samples were combined, digested with trypsin, and fractionated by strong cation exchange chromatography. ICAT reagent-labeled peptides were purified using an avidin affinity column to capture the biotin tag present in the reagent. The biotin tag was cleaved from the ICAT-labeled peptides by treatment with trifluoroacetic acid (TFA).
Released peptides were analyzed by capillary high performance liquid chromatography-tandem mass spectrometry (HPLC-ESI-MS/MS), using a Thermo Fisher LTQ linear ion trap mass spectrometer fitted with a New Objective PicoView 550 nanospray interface. On-line HPLC separation of the digests was accomplished with an Eksigent NanoLC micro HPLC: column, PicoFrit™ (New Objective; 75 μm i.d.) packed to 10 cm with C18 adsorbent (Vydac; 218MSB5, 5 μm, 300 Å); mobile phase A, 0.5% acetic acid (HAc)/0.005% TFA; mobile phase B, 90% acetonitrile/0.5% HAc/0.005% TFA; gradient 2 to 42% B in 1 hr; flow rate, 0.4 μl/min. MS conditions were: ESI voltage, 2.9 kV; isolation window for MS/MS, 3; relative collision energy, 35%; scan strategy, survey scan followed by acquisition of data dependent collision-induced dissociation (CID) spectra of the seven most intense ions in the survey scan above a set threshold.
Mass spectrometry data analysis
The MS files were converted to mzXML format using ReAdW and were searched against the IPI human protein database (v 3.24; 66,923 protein entries) using SEQUEST Cluster 3.1 SR1. For the VHL30+ARF dataset, the mouse ARF protein sequence (IPI00133446) was appended to the database. Variable modifications considered in the searches included methionine oxidation and addition of light (+227) and heavy (+236) ICAT tags to cysteine. Up to one missed tryptic cleavage was allowed. The peptide mass tolerance was set as 3.0 Da. The SEQUEST search results were analyzed by the Trans-Proteomic Pipeline [for review see 15] version 3.0. Peptide/protein identifications were validated by Peptide/ProteinProphet 16, 17. A ProteinProphet score of 0.8 was used as a cutoff, which corresponded to false identification rates of 2.0 %, 2.2%, and 2.0% in the VHL30, VHL30+ARF, and VHL19 datasets, respectively. Protein abundance ratios were calculated using ASAPRatio 18.
Immunoprecipitation and immunoblotting
Immunoprecipitation was performed as described 14. The cell lysates or immunoprecipitates were separated by SDS-PAGE and were analyzed by immunoblotting as described 19. The following antibodies were used: mouse monoclonal anti-FLAG (M2, Sigma-Aldrich); mouse monoclonal anti-HA (16B12, Covance); rabbit polyclonal anti-elongin C (Biolegend); rabbit polyclonal anti-cullin 2 (Thermo scientific); rabbit polyclonal anti-p53 (FL-393, Santa Cruz Biotechnology); mouse monoclonal anti-asymmetric dimethyl-arginine (7E6, Novus Biologicals); rabbit polyclonal anti-symmetric dimethyl-arginine (SYM11, Millipore); rabbit polyclonal anti-collagen IV (ab6586, Abcam); mouse monoclonal anti-procollagen prolyl-3-hydroxylase 1 (3C7, Abnova); and rabbit polyclonal anti-procollagen lysyl hydroxylase 3 (11027-1-AP, ProteinTech Group).
RESULTS AND DISCUSSION
Proteomic analysis of the VHL30-interacting proteins
To dissect the VHL interactome, we expressed FLAG-tagged VHL30 in human embryonic kidney 293T cells and isolated the VHL-containing multi-protein complex by anti-FLAG immunoprecipitation under non-denaturing conditions followed by elution with FLAG peptide. As a control, 293T cells expressing FLAG empty vector were employed. The two immunoprecipitates (FLAG-VHL30 and FLAG-vector) were labeled with isotopically light and heavy cleavable isotope-coded affinity tag (ICAT) reagent, respectively. The two labeled samples were combined and processed for HPLC-ESI-MS/MS analysis as described in Experimental Procedures. Specific components of the complex would display enrichment in the FLAG-VHL30 immunoprecipitation sample whereas non-specific binding proteins would not. Therefore, by analyzing the protein abundance ratios between FLAG-VHL30 immunoprecipitate and FLAG-vector immunoprecipitate, specific interactors and non-specific contaminants can be distinguished. A partial list of proteins of interest that displayed a greater than two-fold enrichment in the FLAG-VHL30 sample compared to the FLAG-vector sample is shown in Table 1. (A complete list is provided in Supporting Information, Table S1.) As expected, the components of the VHL ubiquitin ligase complex (cullin 2, elongin B, and elongin C) co-immunoprecipitated with FLAG-VHL30. Previously reported VHL-interacting proteins such as p53 20 and components of chaperonin T-complex 1 21 were also identified, along with novel VHL30 interactors such as ARF.
Table 1.
Partial list of FLAG-VHL30 interacting proteins
| Protein | Relative abundancea |
|---|---|
| VHL E3 complex components | |
| VHL | 15 |
| Elongin B | 19 |
| Elongin C | 5 |
| Cullin 2 | 7 |
| Other VHL-interacting proteins | |
| p53 | * |
| ARF | 10 |
| T-complex 1 alpha | 4 |
| T-complex 1 beta | 3 |
| T-complex 1 gamma | 8 |
| T-complex 1 delta | 4 |
| T-complex 1 epsilon | 9 |
| T-complex 1 zeta | 3 |
| T-complex 1 eta | 7 |
| T-complex 1 theta | 4 |
Relative abundance in the FLAG-VHL30 immunoprecipitate compared to the FLAG-vector immunoprecipitate as determined by ASAPRatio
Only light-ICAT-labeled peptides (FLAG-VHL30) were detected in the immunoprecipitates, so a value for the ratio could not be determined.
ARF is a nucleolar tumor suppressor encoded by an alternative reading frame of the Ink4a locus. ARF inhibits the MDM2 E3 ubiquitin ligase, thereby stabilizing and activating p53 22. There is also evidence that ARF might perform p53-independent tumor suppressor functions 22.
ARF-VHL30 interaction releases VHL30 from the E3 ligase complex
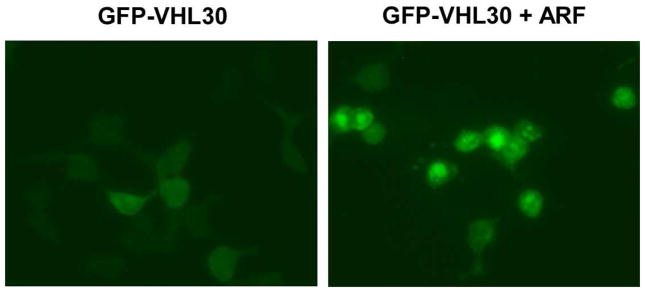
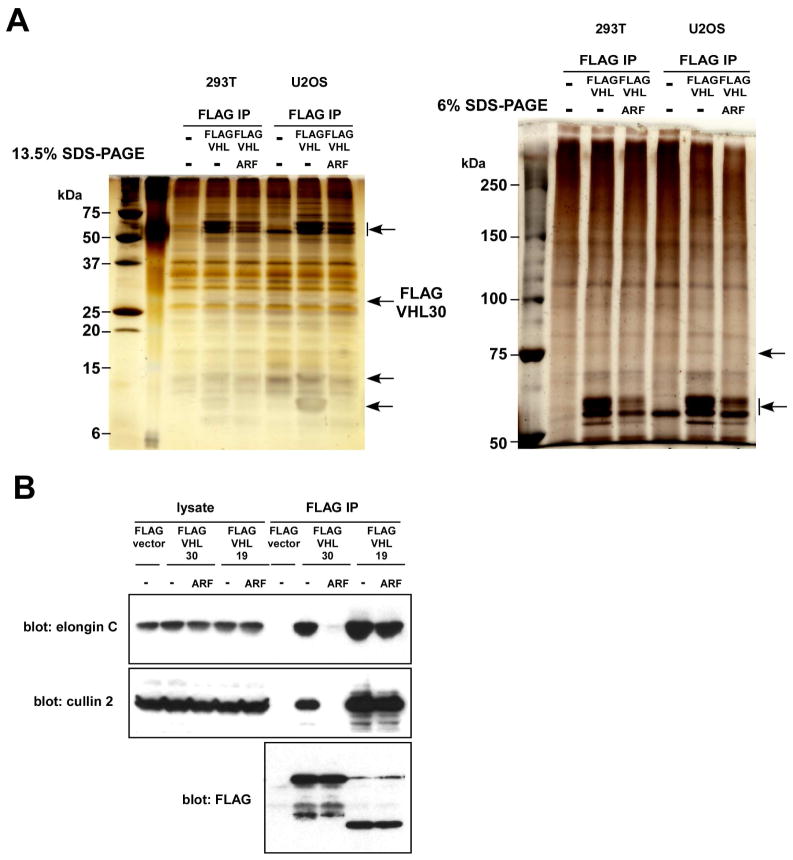
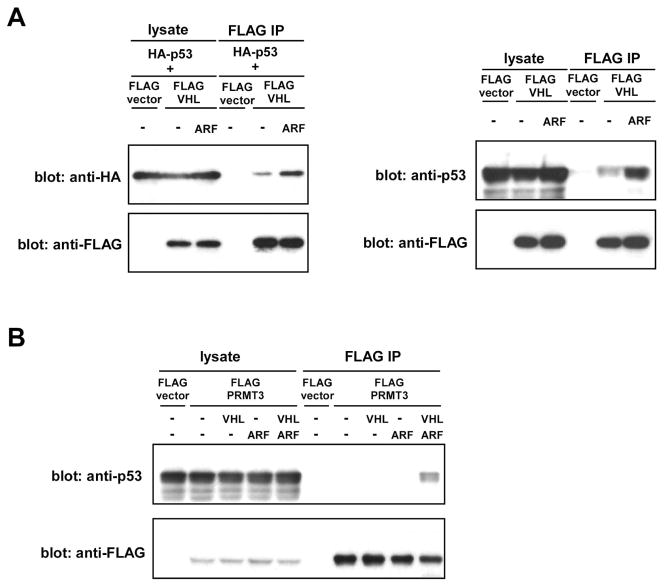
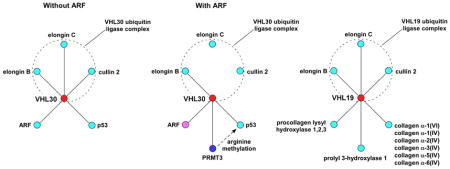
Upon co-expression in U2OS cells (Figure 1B) or in 293T cells (data not shown), HA-ARF co-immunoprecipitated with FLAG-VHL30, but, interestingly, not with FLAG-VHL19, a naturally occurring VHL isoform that lacks the 53-residue N-terminal acidic domain (Figure 1A). GFP-tagged VHL30 expressed in U2OS cells was located in both the cytoplasm and the nucleus, but co-expression of ARF resulted in accumulation of GFP-VHL30 in the nucleus or nucleolus (Figure 2), suggesting that ARF induces nuclear/nucleolar translocation of VHL. We then analyzed the effect of ARF on the VHL30 E3 ligase complex. As shown in Figure 3A, co-expression of ARF resulted in an altered composition of the proteins co-immunoprecipitating with FLAG-VHL30. Further, we found that ARF abolishes the interaction between VHL30 and two E3 ligase components, elongin C and cullin 2 (Figure 3B). Consistent with the lack of interaction between VHL19 and ARF (Figure 1B), ARF did not affect the interaction of VHL19 with elongin C and cullin 2 (Figure 3B), suggesting that ARF disrupts the VHL30-containing E3 ligase complex, but the VHL19-containing E3 ligase complex remains intact.
Figure 2.
ARF recruits VHL30 to the nucleus and nucleolus.
U2OS cells were transfected with GFP-VHL30 alone or together with ARF. The subcellular location of GFP-VHL30 was examined by fluorescence microscopy.
Figure 3.
ARF alters the composition of the VHL30 complex.
(A) Protein components of the FLAG-VHL30 complex in the presence or absence of ARF co-expression.
293T or U2OS cells were transfected with FLAG-VHL30 alone or together with ARF. Forty-eight hours after transfection, the FLAG-VHL30 complex was purified by anti-FLAG immunoprecipitation and was analyzed by SDS-PAGE and silver staining. Notable differences are indicated by the arrows.
(B) ARF disrupts the binding of VHL30 and elongin C or cullin 2.
U2OS cells were transfected with FLAG-VHL30, FLAG-VHL19, and ARF, as indicated. Forty-eight hours after transfection, the interaction of FLAG-VHL with elongin C or cullin 2 was examined by anti-FLAG immunoprecipitation followed by anti-elongin C or anti-cullin 2 immunoblotting.
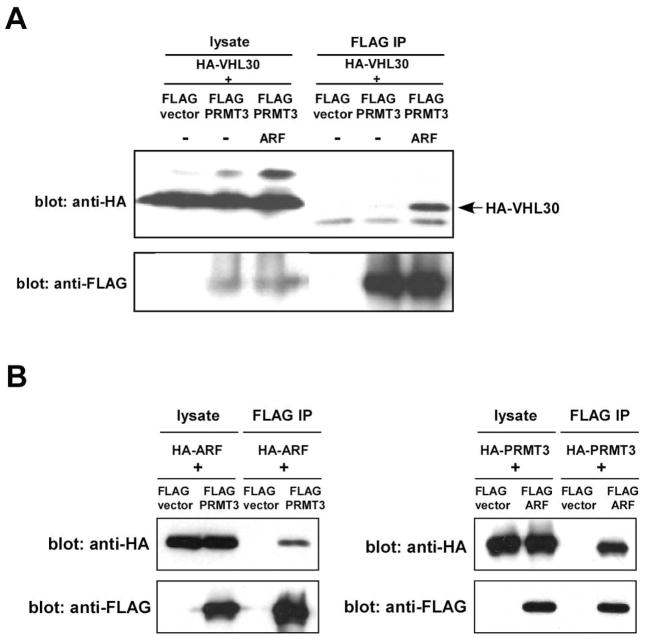
ARF enhances VHL30-PRMT3 interaction
To determine which proteins associate with VHL30 when VHL30 is released from the E3 ligase complex by ARF, we repeated the interactome analysis described above, but added ARF co-expression. As shown in Table 2 and Table S2, in the presence of co-expressed ARF, two of the VHL E3 ligase complex components, elongin C and cullin 2, did not interact with FLAG-VHL30. However, a new protein, PRMT3, was now detected in the FLAG-VHL30 immunoprecipitate (Table 2); this protein was not identified in the absence of ARF co-expression (Table 1). This suggested that ARF releases VHL30 from the E3 ligase complex, facilitating the VHL30–PRMT3 interaction. We tested this possibility by co-expressing ARF, VHL, and PRMT3 in U2OS cells. As shown in Figure 4A, ARF co-expression enhanced the co-immunoprecipitation of HA-VHL30 with FLAG-PRMT3. We were also able to demonstrate that ARF interacts with PRMT3 (Figure 4B).
Table 2.
Partial list of FLAG-VHL30 interacting proteins upon ARF co-expression
| Protein | Relative abundancea |
|---|---|
| VHL E3 complex components | |
| VHL | 23 |
| Elongin B | 40 |
| Other VHL-interacting proteins | |
| p53 | 3 |
| ARF | 25 |
| PRMT3 | 27 |
| T-complex 1 alpha | 11 |
| T-complex 1 beta | 10 |
| T-complex 1 gamma | 8 |
| T-complex 1 delta | 17 |
| T-complex 1 epsilon | 10 |
| T-complex 1 eta | 17 |
| T-complex 1 theta | 5 |
Relative abundance in the FLAG-VHL30 immunoprecipitate compared to the FLAG-vector immunoprecipitate as determined by ASAPRatio
Figure 4.
ARF enhances the interaction of VHL30 and PRMT3.
(A) U2OS cells were transfected with HA-VHL30, FLAG-PRMT3, and ARF, as indicated. Forty-eight hours after transfection, the interaction of HA-VHL30 with FLAG-PRMT3 was examined by anti-FLAG immunoprecipitation followed by anti-HA immunoblotting.
(B) U2OS cells were transfected with HA-ARF and FLAG-PRMT3 (left) or with HA-PRMT3 and FLAG-ARF (right) as indicated. Forty-eight hours after transfection, the interaction of ARF and PRMT3 was examined by anti-FLAG immunoprecipitation followed by anti-HA immunoblotting.
ARF and VHL30 promote p53 arginine methylation by PRMT3
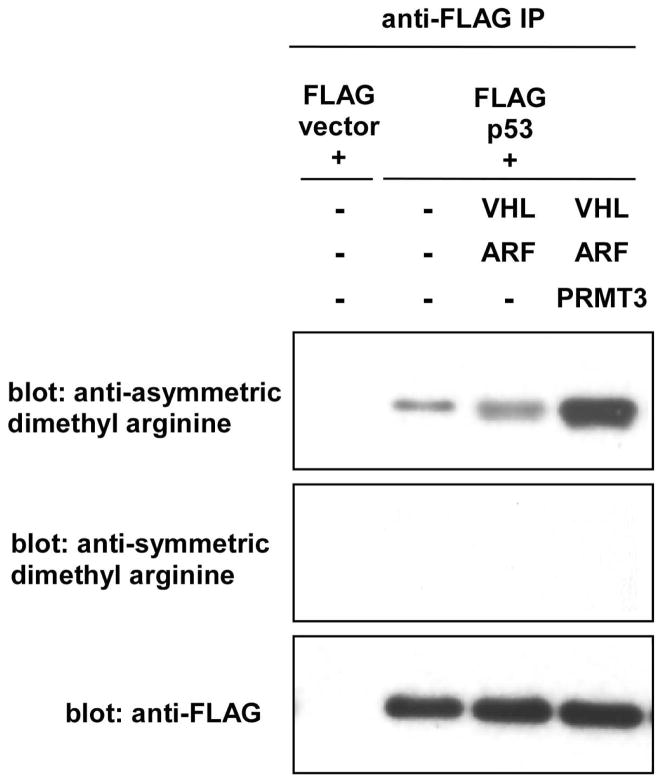
PRMT3 is a type I arginine methyltransferase that catalyzes the mono-methylation and asymmetric di-methylation of arginine residues in proteins. Known substrates of PRMT3 include ribosomal protein S2 and an uncharacterized 40-kDa protein associated with ribosomes 23, 24. Among the proteins identified in the FLAG-VHL30 immunoprecipitate upon ARF co-expression (Table 2), p53 is known to be methylated on multiple arginine residues 25. In agreement with previous report20, we were able to verify the interaction of FLAG-VHL30 with p53 (Figure 5A). Furthermore, co-expression of VHL30 and ARF resulted in the recruitment of PRMT3 to p53 (Figure 5B). We, therefore, tested the possibility that PRMT3, in conjunction with VHL30 and ARF, methylates p53. As shown in Figure 6, co-expression of VHL30 and ARF with PRMT3 resulted in enhanced asymmetric arginine di-methylation of p53. These results suggest that VHL30 and ARF recruit PRMT3 to p53 and induce asymmetric arginine di-methylation of p53.
Figure 5.
VHL30 and ARF recruit PRMT3 to p53.
(A) VHL30 binds p53.
Left, Cells were transfected with HA-P53, FLAG-VGL30, and ARF, as indicated. The interaction of HA-P53 with FLAG-VHL30 was examined by anti-FLAG immunoprecipitation followed by anti-HA immunoblotting.
Right, Cells were transfected with FLAG-VHL30 with or without ARF, as indicated. The interaction of endogenous p53 and FLAG-VHL30 was examined by anti-FLAG immunoprecipitation followed by anti-HA immunoblotting.
(B) VHL30 and ARF recruit PRMT3 to p53.
Cells were transfected with FLAG-PRMT3, VHL30, and ARF as indicated. The interaction of FLAG-PRMT3 and endogenous p53 was examined by anti-FLAG immunoprecipitation followed by anti-p53 immunoblotting.
Figure 6.
VHL30, ARF, and PRMT3 induce asymmetric arginine di-methylation of p53.
Cells were transfected with FLAG-p53, VHL30, ARF, and PRMT3, as indicated. Arginine methylation of FLAG-p53 was examined by anti-FLAG immunoprecipitation under denaturing conditions followed by anti-asymmetric dimethyl arginine or anti-symmetric dimethyl arginine immunoblotting.
Preferential interaction of VHL19 with collagens and collagen biosynthesis enzymes
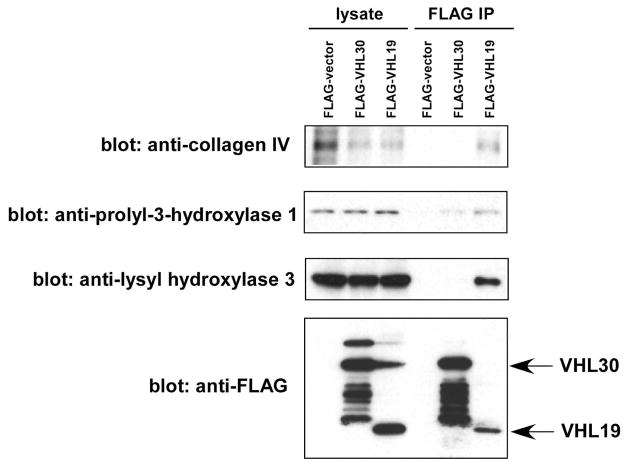
Although both VHL30 and VHL19 can interact with components of the VHL E3 ubiquitin ligase complex and function as a substrate recognition subunit of the E3 ligase complex, there is some evidence suggesting that there are partly overlapping, yet distinct functions of VHL30 and VHL19 26, 27. The above-mentioned physical and functional interaction of ARF with VHL30, but not VHL19, also supports this notion. To gain insight into the functional differences between VHL30 and VHL19, we conducted a proteomic analysis of the FLAG-VHL19 interacting proteins and compared the VHL30 and VHL19 interactomes. Whereas both VHL30 and VHL19 interacted with the components of the E3 ligase complex, VHL19 was found to associate with collagens and enzymes involved in collagen fiber biosynthesis such as procollagen lysyl hydroxylases and procollagen prolyl 3-hydroxylase 1 (Table 3). VHL has been reported to interact with collagen 28, 29 when the N-terminal portion of collagen protrudes from the ER to the cytosol 29, but the relative affinity of VHL30 and VHL19 with collagen has not been examined. The preferential interaction of VHL19 with collagen and collagen biosynthesis enzymes was further verified by immunoprecipitation – immunoblotting approach. As shown in Figure 7, compared with FLAG-VHL30, FLAG-VHL19 interacted more efficiently with collagen, procollagen prolyl-3-hydroxylase 1, and procollagen lysyl hydroxylase 3. Taken together, these results suggest that while VHL30 and VHL19 can both participate in an E3 ligase complex, these two VHL isoforms also display differential interactions with cellular proteins.
Table 3.
Partial list of FLAG-VHL19 interacting proteins
| Protein | Relative abundancea |
|---|---|
| VHL E3 complex components | |
| VHL | 18 |
| Elongin B | 23 |
| Elongin C | 21 |
| Cullin 2 | 13 |
| Other VHL-interacting proteins | |
| Collagen α-1(IV) | 67 |
| Collagen α-2(IV) | 23 |
| Collagen α-3(IV) | 15 |
| Collagen α-5(IV) | 30 |
| Collagen α-6(IV) | 10 |
| Collagen α-1(VI) | * |
| Procollagen lysyl hydroxylase 1 | 6 |
| Procollagen lysyl hydroxylase 2 | 4 |
| Procollagen lysyl hydroxylase 3 | 8 |
| Prolyl 3-hydroxylase | 3 |
| T-complex 1 alpha | 7 |
| T-complex 1 beta | 8 |
| T-complex 1 gamma | 19 |
| T-complex 1 delta | 21 |
| T-complex 1 epsilon | 7 |
| T-complex 1 zeta | 15 |
| T-complex 1 eta | 24 |
| T-complex 1 theta | 15 |
Relative abundance in the FLAG-VHL19 immunoprecipitate compared to the FLAG-vector immunoprecipitate as determined by ASAPRatio
Only light-ICAT-labeled peptides (FLAG-VHL19) were detected in the immunoprecipitates, so a value for the ratio could not be determined.
Figure 7.
Preferential interaction of VHL19 and collagen and collagen biosynthesis enzymes
293T cells were transfected with FLAG-vector, FLAG-VHL30, or FLAG-VHL19. Forty-eight hours after transfection, the interaction of VHL isoforms with collagen IV, procollagen prolyl-3-hydroxylase-1, and procollagen lysyl hydroxylase 3 was examined by anti-FLAG immunoprecipitation followed by immunoblotting with indicated antibodies.
The VHL interactome
The analysis of the VHL30-interacting proteins presented here identified the interaction of a nucleolar tumor suppressor ARF and VHL30. This interaction appears to be specific to VHL30, and VHL19 did not detectably interact with ARF (Figure 1). ARF disrupted the VHL30 E3 ligase complex and instead promoted the binding of VHL30 and a protein arginine methyltransferase PRMT3 (Figure 3 and 4). Further analysis demonstrated that VHL and ARF recruit PRMT3 to p53 and together induce asymmetric arginine di-methylation of p53 (Figure 5 and 6). Comparison of the VHL30 and VHL19 interactomes also revealed the preferential interaction of VHL19 with collagen and collagen biosynthesis enzymes, (Figure 7).
Previous work has shown the physical and functional interaction of VHL and p53 20. VHL directly associates with and stabilizes p53 by suppressing Mdm2-mediated ubiquitination and nuclear export of p53. Upon DNA damage, VHL induces the acetylation of p53 through p300, which results in an enhanced p53 transcriptional activity and p53-mediated cell cycle arrest and apoptosis. p53 is also well known to be regulated by ARF. ARF binds and inhibits MDM2, thereby stabilizing p53 22. Our study uncovered a novel aspect of the VHL-p53-ARF interaction, arginine methylation of p53 by VHL, ARF, and PRMT3.
p53 was recently shown to be methylated at Arg 333, 335 and 337 by PRMT5 25. PRMT5-mediated p53 arginine methylation stimulated p53-dependent G1 arrest upon DNA damage, but it did not affect the p53-dependent apoptotic response. The cell-cycle effect was the consequence of p21 activation while p53 apoptosis target gene expression was barely affected by PRMT5. These results suggested that PRMT5-mediated arginine methylation of p53 modulates p53 target gene specificity. Our study identified PRMT3 as a second arginine methyltransferase that can methylate p53. Unlike PRMT5, PRMT3 appears to be recruited to p53 by VHL and ARF. Whereas PRMT5 is a type II arginine methyltransferase which catalyzes symmetric di-methylation as well as mono-methylation of protein arginine residues, PRMT3 is a type I arginine methyltransferase which catalyze asymmetric di-methylation and mono-methylation of arginine residues. It is possible that PRMT5 and PRMT3 regulate p53 differentially by catalyzing symmetric and asymmetric arginine di-methylation, respectively. Further work is needed to elucidate the functional significance of p53 arginine methylation by PRMT3 in complex with VHL and ARF.
Comparison of the VHL30 and VHL19 interactomes suggested that VHL19 preferentially interacts with collagen and collagen biosynthesis enzymes (Figure 7). Previous subcellular fractionation experiments suggested that VHL30 and VHL19 exhibit different subcellular localization 26: VHL19 was present in both the cytoplasm and nucleus while VHL30 was mainly cytoplasmic. VHL30 was also found in the cell membrane fraction whereas VHL19 was not. Moreover, VHL19 was identified in the insoluble nuclear pellet while VHL30 was not. The partly overlapping, yet distinct interactomes of VHL30 and VHL19 revealed by our analyses together with the differential subcellular location of VHL30 and VHL19 support the idea that VHL30 and VHL19 perform common as well as isoform-specific functions.
Conclusions
The analysis of the VHL30 interactome led to a discovery of an unexpected physical and functional interaction of VHL30, ARF, PRMT3, and p53. We found that p53 was asymmetrically di-methylated by PRMT3 in the presence of VHL and ARF. Moreover, VHL19 was found to associate with collagen and collagen biosynthesis enzymes, which may have implications for control of the extracellular matrix by different VHL isoforms. Future work should more precisely clarify the functional significance of the protein-protein interactions involving VHL that were uncovered by the present study.
Supplementary Material
Table S1. FLAG-VHL30 interacting proteins
Table S2. FLAG-VHL30 interacting proteins upon ARF co-expression
Table S3. FLAG-VHL19 interacting proteins
Figure S1. Tandem mass spectra of peptides derived from the proteins that were further characterized in this paper.
Synopsis.
We undertook a series of proteomic analyses to gain a comprehensive picture of the VHL-interacting proteins. We found that ARF interacts with VHL30, a longer VHL isoform. ARF was found to release VHL30 from the E3 ligase complex, promoting the binding of VHL30 to PRMT3. Our analysis of the interactome for VHL19, a shorter VHL isoform, also uncovered that VHL19 displays affinity to collagens and their biosynthesis enzymes.
Acknowledgments
We thank Dr. William Kaelin for VHL cDNA and Mr. Barron Blackman for assistance with proteomics informatics. We are grateful to Dr. Sara Hook for critical reading of the manuscript. This work was supported by NIH grants CA125020 (to Y.S.) and CA054174 (Cancer Therapy and Research Center at UTHSCSA - Mass Spectrometry Shared Resource).
Footnotes
The supplementary tables list the proteins identified and quantified in the FLAG-VHL immunoprecipitate compared with the FLAG-vector immunoprecipitate.
The supplementary figure shows the tandem mass spectra of peptides derived from the proteins that were further characterized in this paper.
These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kaelin WG., Jr The von Hippel-Lindau protein, HIF hydroxylation, and oxygen sensing. Biochem Biophys Res Commun. 2005;338(1):627–38. doi: 10.1016/j.bbrc.2005.08.165. [DOI] [PubMed] [Google Scholar]
- 2.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22(24):4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 3.Barry RE, Krek W. The von Hippel-Lindau tumour suppressor: a multi-faceted inhibitor of tumourigenesis. Trends Mol Med. 2004;10(9):466–72. doi: 10.1016/j.molmed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Czyzyk-Krzeska MF, Meller J. von Hippel-Lindau tumor suppressor: not only HIF’s executioner. Trends Mol Med. 2004;10(4):146–9. doi: 10.1016/j.molmed.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Kuznetsova AV, Meller J, Schnell PO, Nash JA, Ignacak ML, Sanchez Y, Conaway JW, Conaway RC, Czyzyk-Krzeska MF. von Hippel-Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc Natl Acad Sci U S A. 2003;100(5):2706–11. doi: 10.1073/pnas.0436037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikhaylova O, Ignacak ML, Barankiewicz TJ, Harbaugh SV, Yi Y, Maxwell PH, Schneider M, Van Geyte K, Carmeliet P, Revelo MP, Wyder M, Greis KD, Meller J, Czyzyk-Krzeska MF. The von Hippel-Lindau tumor suppressor protein and Egl-9-Type proline hydroxylases regulate the large subunit of RNA polymerase II in response to oxidative stress. Mol Cell Biol. 2008;28(8):2701–17. doi: 10.1128/MCB.01231-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Na X, Duan HO, Messing EM, Schoen SR, Ryan CK, di Sant’Agnese PA, Golemis EA, Wu G. Identification of the RNA polymerase II subunit hsRPB7 as a novel target of the von Hippel-Lindau protein. Embo J. 2003;22(16):4249–59. doi: 10.1093/emboj/cdg410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop T, Lau KW, Epstein AC, Kim SK, Jiang M, O’Rourke D, Pugh CW, Gleadle JM, Taylor MS, Hodgkin J, Ratcliffe PJ. Genetic analysis of pathways regulated by the von hippel-lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol. 2004;2(10):e289. doi: 10.1371/journal.pbio.0020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG., Jr von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet. 2001;10(10):1019–27. doi: 10.1093/hmg/10.10.1019. [DOI] [PubMed] [Google Scholar]
- 10.Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER. Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Genet. 2001;10(10):1029–38. doi: 10.1093/hmg/10.10.1029. [DOI] [PubMed] [Google Scholar]
- 11.Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev. 2001;15(19):2520–32. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, Lee FS. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358(2):162–8. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Percy MJ, Beer PA, Campbell G, Dekker AW, Green AR, Oscier D, Rainey MG, van Wijk R, Wood M, Lappin TR, McMullin MF, Lee FS. Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood. 2008;111(11):5400–2. doi: 10.1182/blood-2008-02-137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci U S A. 2003;100(23):13225–30. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deutsch EW, Mendoza L, Shteynberg D, Farrah T, Lam H, Tasman N, Sun Z, Nilsson E, Pratt B, Prazen B, Eng JK, Martin DB, Nesvizhskii AI, Aebersold R. A guided tour of the Trans-Proteomic Pipeline. Proteomics. 10(6):1150–9. doi: 10.1002/pmic.200900375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74(20):5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 17.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75(17):4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 18.Li XJ, Zhang H, Ranish JA, Aebersold R. Automated statistical analysis of protein abundance ratios from data generated by stable-isotope dilution and tandem mass spectrometry. Anal Chem. 2003;75(23):6648–57. doi: 10.1021/ac034633i. [DOI] [PubMed] [Google Scholar]
- 19.Shiio Y, Donohoe S, Yi EC, Goodlett DR, Aebersold R, Eisenman RN. Quantitative proteomic analysis of Myc oncoprotein function. Embo J. 2002;21(19):5088–96. doi: 10.1093/emboj/cdf525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roe JS, Kim H, Lee SM, Kim ST, Cho EJ, Youn HD. p53 stabilization and transactivation by a von Hippel-Lindau protein. Mol Cell. 2006;22(3):395–405. doi: 10.1016/j.molcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Feldman DE, Thulasiraman V, Ferreyra RG, Frydman J. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol Cell. 1999;4(6):1051–61. doi: 10.1016/s1097-2765(00)80233-6. [DOI] [PubMed] [Google Scholar]
- 22.Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer. 2006;6(9):663–73. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- 23.Swiercz R, Person MD, Bedford MT. Ribosomal protein S2 is a substrate for mammalian PRMT3 (protein arginine methyltransferase 3) Biochem J. 2005;386(Pt 1):85–91. doi: 10.1042/BJ20041466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swiercz R, Cheng D, Kim D, Bedford MT. Ribosomal protein rpS2 is hypomethylated in PRMT3-deficient mice. J Biol Chem. 2007;282(23):16917–23. doi: 10.1074/jbc.M609778200. [DOI] [PubMed] [Google Scholar]
- 25.Jansson M, Durant ST, Cho EC, Sheahan S, Edelmann M, Kessler B, La Thangue NB. Arginine methylation regulates the p53 response. Nat Cell Biol. 2008;10(12):1431–9. doi: 10.1038/ncb1802. [DOI] [PubMed] [Google Scholar]
- 26.Iliopoulos O, Ohh M, Kaelin WG., Jr pVHL19 is a biologically active product of the von Hippel-Lindau gene arising from internal translation initiation. Proc Natl Acad Sci U S A. 1998;95(20):11661–6. doi: 10.1073/pnas.95.20.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol. 2003;5(1):64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- 28.Grosfeld A, Stolze IP, Cockman ME, Pugh CW, Edelmann M, Kessler B, Bullock AN, Ratcliffe PJ, Masson N. Interaction of hydroxylated collagen IV with the von hippel-lindau tumor suppressor. J Biol Chem. 2007;282(18):13264–9. doi: 10.1074/jbc.M611648200. [DOI] [PubMed] [Google Scholar]
- 29.Kurban G, Duplan E, Ramlal N, Hudon V, Sado Y, Ninomiya Y, Pause A. Collagen matrix assembly is driven by the interaction of von Hippel-Lindau tumor suppressor protein with hydroxylated collagen IV alpha 2. Oncogene. 2007 doi: 10.1038/sj.onc.1210709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. FLAG-VHL30 interacting proteins
Table S2. FLAG-VHL30 interacting proteins upon ARF co-expression
Table S3. FLAG-VHL19 interacting proteins
Figure S1. Tandem mass spectra of peptides derived from the proteins that were further characterized in this paper.