Abstract
Alcohol drinking is a known risk factor for oral cancer in humans. However, previous animal studies on the promoting effect of ethanol on oral carcinogenesis were inconclusive. It is necessary to develop an animal model with which the molecular mechanism of ethanol-related oral carcinogenesis may be elucidated in order to develop effective prevention strategies. In this study, mice were first treated with 4-nitroquinoline-1-oxide (4NQO, 100μg/ml in drinking water) for 8 weeks, and then given water or ethanol (8%) as the sole drink for another 16 weeks. During the experiment, 8% ethanol was well tolerated by mice. The incidence of squamous cell carcinoma (SCC) increased from 20% (8/41) to 43% (17/40; p<0.05). Expression of 5-lipoxygenase (5-Lox) and cyclooxygenase 2 (Cox-2) was increased in dysplasia and SCC of 4NQO-treated tongues, and further enhanced by ethanol. Using this mouse model, we further demonstrated that fewer cancers were induced in Alox5−/− mice, as were cell proliferation, inflammation, and angiogenesis in the tongue, as compared with Alox5+/+ mice. Interestingly, Cox-2 expression was induced by ethanol in knockout mice, while 5-Lox and leukotriene A4 hydrolase (LTA4H) expression and leukotriene B4 (LTB4) biosynthesis were dramatically reduced. Moreover, ethanol enhanced expression and nuclear localization of 5-Lox and stimulated LTB4 biosynthesis in human tongue SCC cells (SCC-15 and SCC-4) in vitro. In conclusion, this study clearly demonstrated that ethanol promoted 4NQO-induced oral carcinogenesis, at least in part, through further activation of the 5-Lox pathway of arachidonic acid metabolism.
Keywords: Alcohol drinking, Ethanol, 4NQO, Oral cancer, 5-Lox
Introduction
Oral cancer is a common neoplasm worldwide, particularly in developing countries, where up to 25% of cancers are oral cancer (1). In recent decades, oral cancer incidence and mortality rates have been increasing in the developed countries, especially among young males (2). In the United States, approximately 36,540 new cases and 7,880 deaths are expected in 2010 (3). Survival of oral cancer patients has not improved significantly despite advances in radiotherapy and chemotherapy (4). Based on recent data from the NCI Surveillance Epidemiology and End Results, the overall 5-year relative survival in 1999–2006 from 17 geographic areas was 60.9%. At the distant stage, only 32.2% survive for 5 years. The surviving patients are usually left with severe functional compromise (5). A number of patients cured by primary treatment may develop a second cancer within a few years (6). Therefore, it is important to understand molecular mechanism of this disease and develop effective preventive strategies.
Epidemiological data strongly and consistently indicate the involvement of exogenous factors, such as tobacco use and alcohol drinking, in the development of over 75% of oral cancers in the US (7). Alcohol drinking has a stronger association with oral cancer than with cancers of other organ sites. According to a meta-analysis study on a total of 235 studies including over 117,000 human cases, strong direct trends in risk were observed for cancers of the oral cavity and pharynx (RR=6.0 for 100 g ethanol per day), esophagus (RR=4.2) and larynx (RR=3.9)(8). Oral cancer is associated with alcohol drinking in a dose-dependent manner with a RR increasing from 1.75 (25g/day), to 2.85 (50g/day) and 6.01 (100g/day), according to 26 studies of 7,954 cases. Even after adjusting for smoking, the dose-dependent effect was still significant (8). The total amount of ethanol and the duration of alcohol drinking are more important factors than the type or constitution of alcoholic beverage. Oral cancer risk was greater for those who drank straight (undiluted) liquor than for those who usually drank mixed (diluted) liquor (9). Alcohol drinking increases the risk of oral premalignant lesions in those who have never used tobacco as well as in past or current users. Alcohol drinking and tobacco smoking have additive or synergistic effects on oral carcinogenesis. Joint exposure leads to earlier onset and worse prognosis of oral cancer (10). However, it is still unclear through what mechanism alcohol drinking may promote oral cancer.
Previous animal studies on the cancer-promoting effects of ethanol were inconclusive. Ethanol feeding or painting on the oral epithelium produces hyperproliferation in animals (11). In one study, life-long exposure to 10% ethanol in drinking water significantly increased the incidence of cancer of the oral cavity in Sprague-Dawley rats. However, in this experiment ethanol treatment lasted for 179 weeks, which is too long for mechanistic studies. At this age, Sprague-Dawley rats spontaneously develop oral cancer even without any other treatment (12). Several studies in the literature suggested, but failed to confirm statistically, the cancer-promoting activity of ethanol feeding or painting in 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster cheek pouch carcinogenesis (13–16). Therefore, it is necessary to develop a proper animal model to investigate whether ethanol may promote oral carcinogenesis, and to use such a model to elucidate the molecular mechanism of ethanol-related oral cancer. 4-Nitroquinoline-1-oxide (4NQO) is a synthetic water-soluble carcinogen that induces tumors predominantly in the tongue. It produces all the stages of oral carcinogenesis and similar histological as well as molecular changes as seen in the human cancer (17). In the present study, with the 4NQO mouse model, we aimed to determine the long-term effect of ethanol on oral carcinogenesis.
Aberrant arachidonic acid metabolism plays an important role in oral carcinogenesis. Chemical inhibitors of cyclooxygenase 2 (Cox-2) and 5-lipoxygenase (5-Lox) had chemopreventive effects on chemically induced oral cancer (18, 19). Through the 5-Lox pathway, arachidonic acid is metabolized by 5-Lox and leukotrienes A4 hydrolase (LTA4H) into leukotrienes B4 (LTB4), a potent mediator of inflammation. In this study, we hypothesized that ethanol might promote 4NQO-induced oral carcinogenesis in mice by activating aberrant arachidonic acid metabolism, especially the 5-Lox pathway.
Materials and Methods
Animals and Treatment
Male wild-type C57BL/6J mice (B6129SF2/J, 8 weeks old) were housed 2 per cage in our animal facility in a 12-hr light-dark cycle. 5-Lox knockout mice (B6.129S2-Alox5tm1Fun/J) were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were bred in-house and PCR genotyped with an established protocol provided by the Jackson Laboratory. Animals were allowed free access to drink (tap water or ethanol) and laboratory rodent chow 5001 (LabDiet) at 20–22C and 50–60% relative humidity. All procedures involving the use of mice were in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Use and Care Committee.
In the first animal experiment, mice were divided into four groups after one week of acclimation (Table 1). Group 1A (n=10) served as the negative control. The remaining mice were treated with 4NQO (Sigma, St. Louis, MO) in their drinking water at a concentration of 100 μg/ml for a period of 8 weeks. They were then randomly divided into a positive control group receiving no further treatment (Group B, n=45), and two experimental groups treated with 8% ethanol (Group 1C, n=40) or 35% ethanol (Group 1D, n=50). Ethanol (absolute) was made fresh to 8% and 35 % (v/v) solution every day. The 8% ethanol solution was given to Group 1C as the sole source of drinking fluid, and 0.1ml 35% ethanol solution was administered by gavage to Group 1D five days per week for the duration of the study. The overall experimental period was 24 weeks. The body weights were monitored biweekly and health status was examined daily until the end of the experiment.
Table 1.
Promotion of 4NQO-induced oral carcinogenesis in mice by ethanol
| Group | Treatment | No. of mice | Incidence of visible lesions (%) | Histopathology
|
|||
|---|---|---|---|---|---|---|---|
| Hyperplasia | Mild dysplasia (%) | Severe dysplasia (%) | SCC (%) | ||||
| 1A | Negative control | 10 | - | - | - | - | - |
| 1B | 4NQO | 41 | 19 (46%) | 10 (18%) | 22 (54%) | 1 (2%) | 8 (20%) |
| 1C | 4NQO and 8% Ethanol (as sole drink) | 40 | 16 (40%) | 8 (19%) | 13 (33%) | 2 (5%) | 17 (43%)* |
| 1D | 4NQO and 35% Ethanol (0.1ml, 1/day, gavage) | 49 | 15 (31%) | 3 (7%) | 29 (59%) | 7 (14%) | 10 (20%) |
Significantly higher than that of Group 1B (p<0.05) base on Fisher’s exact test.
In the second animal experiment, mice were divided into six groups after one week of acclimation (Table 2). Group 2A (n=10) served as the wild-type negative control. The remaining wild-type mice were treated with 4NQO in their drinking water at a concentration of 100μg/ml for a period of 8 weeks. They were then randomly divided into a positive control group receiving no further treatment (Group 2C, n=45), and an experimental group treated with 8% ethanol (Group 2D, n=45). Group 2B (n=10) served as the Alox5−/− negative control. The remaining Alox5−/− mice were treated with 4NQO as above. They were then randomly divided into an Alox5−/− positive control group receiving no further treatment (Group 2E, n=37), and an Alox5−/− experimental group treated with 8% ethanol (Group 2F, n=37). Ethanol was made fresh to 8% (v/v) solution every day and was given to Group 2D and Group 2F as the sole source of drinking fluid. Mice were monitored for their body weights biweekly and sacrificed at week 24.
Table 2.
Inhibition of 4NQO-induced and ethanol-related oral carcinogenesis in Alox5−/− mice
| Group | Genotype | Treatment | No. of mice | Incidence of visible lesions (%) | Histopathology
|
||
|---|---|---|---|---|---|---|---|
| Mild dysplasia (%) | Severe dysplasia (%) | SCC (%) | |||||
| 2A | Alox5+/+ | Negative control | 10 | - | - | - | - |
| 2B | Alox5−/− | Negative control | 10 | - | - | - | - |
| 2C | Alox5+/+ | 4NQO | 44 | 26 (59%) | 16 (36%) | 18 (41%) | 10 (23%) |
| 2D | Alox5+/+ | 4NQO + 8% Ethanol | 44 | 29 (66%) * | 7 (16%) * | 15 (34%) | 22 (50%) ** |
| 2E | Alox5−/− | 4NQO | 37 | 6 (16%) *** | 16 (43%) | 14 (38%) | 7 (19%) |
| 2F | Alox5−/− | 4NQO + 8% Ethanol | 36 | 3 (8%) | 16 (44%) | 13 (36%) | 7 (20%) |
Significantly different from that of Group 2F (p<0.01) base on Fisher’s exact test.
Significantly different from that of Group 2C (p<0.05) and Group 2F (p<0.01) base on Fisher’s exact test.
Significantly different from that of Group 2C (p<0.0001) base on Fisher’s exact test.
Tissue processing and histopathological analyses
At Week 24, all the animals were sacrificed at 2 hours after being given BrdU (50mg/kg, i.p.). The tongue was harvested and examined for the presence of macroscopic alterations, then split longitudinally. One half of the tongue was snap frozen in liquid nitrogen for Western blotting, and the half was fixed overnight in 10% neutral-buffered formalin, transferred to 70% ethanol, processed, and embedded in paraffin for histopathology.
Thirty sections (4μm) of each sample were cut and the 1st, 15th and 30th slides were stained with hematoxylin and eosin. Histopathological analysis was performed blind by our research pathologist without prior knowledge of the experiment. Mild dysplasia, severe dysplasia and squamous cell carcinoma (SCC) were diagnosed with established criteria (20). Severe dysplasia was characterized by irregular epithelial stratification, increased number of mitotic figures, increased nuclear-to-cytoplastic ratio, and loss of polarity of basal cells. SCC was diagnosed when dysplastic cells invaded underlying tissues.
Immunohistochemical staining of Cox-2, 5-Lox, BrdU, CD31, and histochemical staining of mast cells
After deparaffinization, the slides were submerged in methanol containing 0.3% hydrogen peroxide for 15 min at RT to inhibit endogenous peroxidase activity. Antigen retrieval was done for 5-Lox, Cox-2 and CD31 by incubating the sections in 0.01mol/L citrate buffer (pH 6) in a microwave oven for 18 minutes. Sections were incubated with a rabbit polyclonal anti-5-Lox antibody (1:100; Cayman Chemical, Ann Arbor, MI), a goat polyclonal anti-Cox-2 antibody (1:50; Cayman Chemical), a mouse monoclonal anti-BrdU antibody (1:500; Sigma), or a rabbit polyclonal anti-CD31 antibody (1:50; Abcam, Cambridge, MA), overnight at 4°C. Tissue sections were then washed again in PBS and incubated with suitable peroxidase-conjugated secondary antibodies for 30 minutes at 37°C. Detection of the antibody complex was done by the streptavidin-peroxidase reaction kit using DAB as a chromogen. To ensure the specificity of the primary antibody, control tissue sections were incubated in the absence of primary antibody.
Immunostaining of Cox-2 and 5-Lox was quantified using a computerized image analysis system (ImagePro Plus; Media Cybernetics, Silver Spring, MD). The area of positive staining and the mean optical density were measured for calculation of integrated optical density (IOD) by multiplying density by area.
Cell proliferation in the tongue epithelium was determined by counting the BrdU-labeling index. Three non-contiguous, randomly selected, high-power fields (x400) were photographed per sample. The BrdU-labeling index was calculated as the number of BrdU-positive cells divided by the total number of tongue epithelial cells.
Infiltration of inflammatory cells in the tongue was characterized by counting the number of mast cells after histochemical staining. Sections were stained with 0.5% toluidine blue for 30 seconds. Three non-contiguous and randomly selected areas of the tongue epithelium on each slide were selected for mast cell counting, and the average number per mm2 was calculated.
Angiogenesis in the tongue was characterized by counting CD31-positive microvessels. A CD31-positive endothelial cell cluster which was clearly separated from adjacent tissues was considered as a single microvessel. Three non-contiguous and randomly selected areas of the tongue epithelium on each slide were selected to count the number of microvessels. Microvessel density was calculated as the average number of microvessels per mm2.
Western blotting of 5-Lox, LTA4H and Cox-2
Mouse tongues were lysed for 30 minutes in RIPA buffer containing 1.0 mM phenylmethylsulfonyl fluoride and 1 μg/ml aprotinin. After homogenization on ice, the samples were centrifuged at 12,000rpm for 20 min at 4°C and the supernatants were used for western blotting. Protein concentration was measured by using BAC Protein Assay Kit (Pierce) according to the manufacturer’s instructions. Western blotting was performed with a standard protocol. In brief, 50μg of protein was resolved on a SDS-polyacrylamide gel and transferred to nitrocellulose membranes. 5-Lox, Cox-2, and LTA4H were detected with a rabbit polyclonal anti-5-Lox antibody (1:1000; Abcam), a goat polyclonal anti-Cox-2 antibody (1:1000; Abcam), and a rabbit polyclonal anti-LTA4H antibody (1:500; Cayman Chemical), respectively. Membranes were developed with ECL chemiluminescence and exposed on X-ray film. Quantification of the bands on the film was carried out by a densitometer (Gel Doc 1000; BioRad).
Enzyme immunoassay of LTB4
Mouse tongue was weighed and homogenized, and the supernatant was then analyzed with an ELISA kit (Cayman Chemical). All procedures were performed according to the manufacturer’s instructions. Each sample was analyzed in triple and results were expressed as pg of LTB4 per mg wet weight of tongue.
Culture of human tongue SCC cells, SCC-15 and SCC-4
Human tongue SCC cells, SCC-15 and SCC-4, were obtained from ATCC (Manassas, VA), and were cultured in DMEM/F12 1:1 medium supplemented with 15% fetal bovine serum at 37C in the presence of 5% CO2. These cell lines were characterized by ATCC (Catalogue no. CRL-1623 and CRL-1624) and used in our laboratory for fewer than 6 months after resuscitation. Cell growth was determined by MTT assay (Sigma). ~5,000 cells were placed in each well of three 96-well culture plates. After 24 hr, ethanol was added at various concentrations: 0, 25, 50,100, 250, or 500mM. Cells were cultured for another 24h, 48h, or 72h, depending on experimental group. Medium containing fresh ethanol was changed every 24h in order to minimize fluctuation of ethanol concentration due to evaporation. MTT reagent was added to each well at 5mg/ml in 20 μl and incubated for another 4h. Supernatants were discarded after termination of cell culture and 150μl of DMSO was added to each well. Plates were shaken for 10 min and the optical density was measured with a microplate reader at 490nm.
For 5-Lox expression and LTB4 biosynthesis, SCC-15 and SCC-4 were treated with 250mM ethanol for 0, 6, 12, or 24 hr. Medium was changed every 6 hr in order to maintain proper concentrations of the ethanol. Approximately 1×106 cells were lysed in RIPA buffer and total protein concentration determined with a BCA assay. Western blotting of 5-Lox was performed as mentioned before. LTB4 in cell lysate from 106 cells was analyzed as well as described above. All analyses were triplicated. The level of LTB4 was calculated as pg/106 cells.
Since nuclear localization of 5-Lox is known to be associated with its activation (21), we also determined protein localization with immunofluorescent staining. 5×104 SCC-15 and SCC-4 cells were cultured on a 24-well plate and treated with 250mM ethanol for 24h. Cells were fixed in cold 10% buffered neutral formalin for 15 min, stained with a rabbit polyclonal anti-5-Lox antibody (1:100; Abcam), and then a goat anti-rabbit IgG secondary antibody labeled with Alexa Fluor® 546 (Invitrogen; Carlsbard, CA). After counterstaining with DAPI, fluorescent signals were visualized under a fluorescent microscope.
Statistical analysis
Fisher’s exact test was used for evaluation of tumor incidence, Student’s t test was used for two-group comparisons, and one-way ANOVA was used for multiple-group comparisons. Values were expressed as mean± SD. P<0.05 was considered statistically significant.
Results
In this study, we conducted two animal experiments. The first experiment was aimed to determine whether ethanol may promote 4NQO-induced oral carcinogenesis in mice, 8% ethanol (as sole drink) or 35% ethanol (0.1ml once/day by gavage). The second experiment was aimed to determine whether loss of 5-Lox may attenuate the cancer-promoting effect of ethanol. Experiments with human tongue cancer cells were aimed to confirm the effect of ethanol on the 5-Lox pathway of arachidonic acid metabolism.
4NQO treatment (100μg/ml in water for 8 weeks) did not have significant impact on body weight and general health of mice in these two experiments. In the later time points after Week 20, those treated with 4NQO tended to have lower body weight as compared with negative control groups, probably due to tumor development. Alox5−/− mice had lower body weight than wild-type mice throughout the experiment (data not shown). In general, mice were healthy and active. 8% ethanol as the sole drink was well-tolerated by mice.
Promotion of 4NQO-induced oral carcinogenesis by ethanol
All 4NQO-treated animals had a roughened granular surface on the tongue mucosa with varying degrees of erythema and occasionally white plaque-like lesions. At Week 24, 8% ethanol (Group 1C) slightly reduced the incidence of visible lesions from 46% (19/41, Group 1B) to 40% (16/40). 35% ethanol (Group 1D) significantly decreased the incidence of visible lesions to 31% (15/49) (p<0.05) (Table 1). Under the microscope (Table 1), 8% ethanol shortened the duration of malignant transformation as Group 1C had a higher incidence of SCC (43 %, 17/40) than Group 1B (20%, 8/41) (p<0.05). Relative to SCC, the incidence of dysplasia was decreased by 8% ethanol. This suggested that 8% ethanol might promote the development of SCC from its precancerous lesion. Since 35% ethanol given by gavage once a day was very time-consuming and failed to promote 4NQO-induced oral carcinogenesis, this method of administration was not studied in the subsequent experiment.
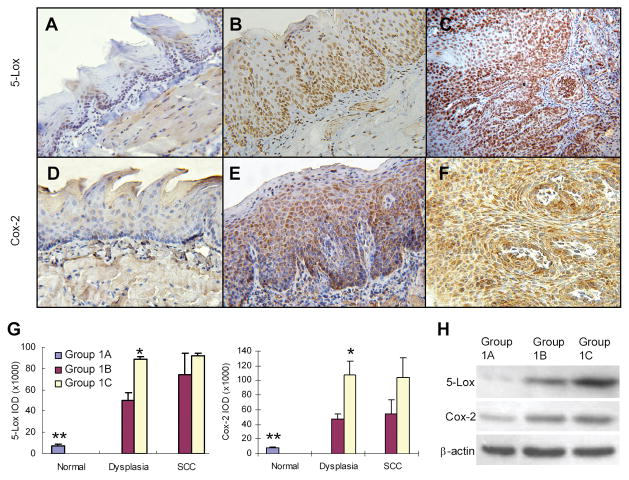
Since our previous studies have clearly shown the involvement of aberrant arachidonic acid metabolism in oral carcinogenesis (18, 19), we investigated such a possibility using tissue samples from this experiment. With immunohistochemical staining, 5-Lox and Cox-2 were detected at a low level of expression in the tongues of control mice (Group 1A). 4NQO treatment (Group 1B) up-regulated expression of both 5-Lox and Cox-2 in dysplasia and SCC. Strong expression was observed in both squamous epithelial cells and inflammatory cells in the stroma (Figure 1A to F). Semi-quantification of 5-Lox and Cox-2 expression in squamous epithelial cells showed that, as compared with control mice, 8% ethanol significantly enhanced expression of 5-Lox and Cox-2 in dysplasia (p<0.05), but not in SCC (Figure 1G).
Figure 1.
Overexpression of 5-Lox and Cox-2 in oral lesions induced by 4NQO/ethanol. (A, D) Normal epithelium; (B, E) Dysplasia; (C, F) SCC. Expression of 5-Lox and Cox-2 in the epithelial cells oral tissues was semi-quantified after immunohistochemical staining (G), and confirmed by Western blotting with frozen tissue samples (H). Protein samples of 3 representative tongues (one from each group) were shown. * Statistically different from Group 1B (p<0.05); ** Statistically different from dysplasia and SCC (p<0.01).
Using Western blotting, we further confirmed overexpression of 5-Lox and Cox-2 protein in mouse tongue treated with 4NQO and ethanol. In untreated mouse tongue, 5-Lox and Cox-2 were expressed at a fairly low level. Although 4NQO treatment itself up-regulated both proteins, combination of 4NQO and 8% ethanol greatly increased expression of 5-Lox (Figure 1H). It was interesting that ethanol was particularly effective in inducing 5-Lox expression as compared with Cox-2 expression.
Suppression of ethanol-related oral carcinogenesis in 5-Lox knockout mice
Using the same treatment regime as the first animal experiment, our second animal experiment aimed to determine whether loss of 5-Lox might make mice less susceptible to ethanol-related 4NQO-induced oral carcinogenesis (Table 2). Both Alox5+/+ and Alox5−/− mice tolerated 4NQO and ethanol well. The incidence of visible lesions in 4NQO-treated Alox5−/− mice (Group 2E) was significantly less than that in Alox5+/+ mice (Group 2C)(p<0.0001). Similar results were observed in mice treated with both 4NQO and ethanol (Group 2D vs. 2F) (p<0.0001). Ethanol did not further increase the incidence of visible lesions in either Alox5+/+ or Alox5−/− mice. Under the microscope, Alox5−/− mice had significantly lower incidence of SCC than Alox5+/+ mice when treated with both 4NQO and 8% ethanol (p<0.01). Meanwhile, there was an increased incidence of mild dysplasia (Group 2D vs. 2F). Interestingly, Alox5−/− mice had similar incidence of oral lesions as compared with Alox5+/+ mice, whether they were treated with ethanol and 4NQO, or with 4NQO alone (Group 2C vs. 2E). Similar to what we observed in the first animal experiment, ethanol shortened the duration of malignant transformation in 4NQO-treated Alox5+/+ mice (Group 2C vs. 2D; p<0.05). This cancer-promoting effect of ethanol was abolished in Alox5−/− mice (Group 2E vs. 2F).
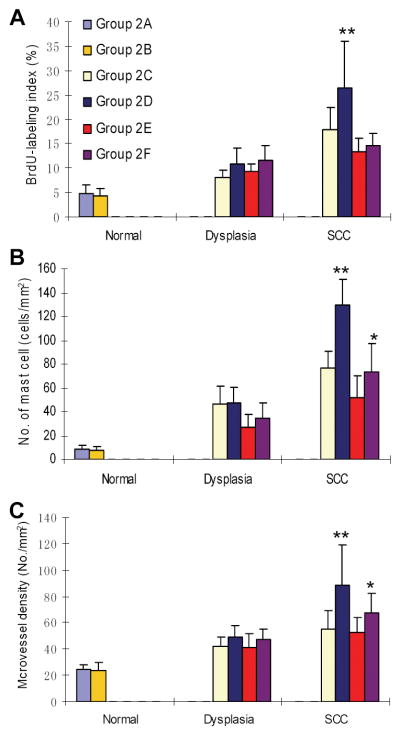
We then performed immunohistochemical staining of BrdU and CD31, and histochemical staining of mast cells. The percentage of BrdU-labeling index in tongue epithelium was counted as a marker of cell proliferation, the number of mast cells per area in the tongue epithelium as a marker of inflammation, and the microvessel density in the stroma (CD31-positive microvessel per area) as a marker of angiogenesis. As expected, cell proliferation, inflammation and angiogenesis all increased during 4NQO-induced oral carcinogenesis (Figure 2). Of note, the BrdU-labeling index in SCC of Alox5+/+ mice treated with both 4NQO and ethanol (Group 2D) was significantly higher than those of Alox5+/+ mice treated with 4NQO alone (Group 2C), and Alox5−/− mice treated with both 4NQO and ethanol (Group 2F) (p<0.01) (Figure 2A). There was a significant increase in the number of mast cells in SCC of Alox5+/+ mice treated with both 4NQO and ethanol (Group 2D) as compared to that of Alox5+/+ mice treated with 4NQO alone (Group 2C) (p<0.01). Ethanol treatment in Alox5−/− mice increased the number of infiltrating mast cells in mouse tongue as well (p<0.05) (Figure 2B). Microvessel density in the tongue changed in a manner similar to the number of mast cells (Figure 2C).
Figure 2.
Cell proliferation, inflammation, and angiogenesis in 4NQO/ethanol-treated mouse tongue of Alox5+/+ and Alox5−/− mice. (A) Cell proliferation (BrdU-labeling index). ** Statistically different from the remaining groups (p<0.01). Values were expressed as mean ± SD. (B) Inflammation (number of infiltrating mast cells per mm2). ** Statistically different from Group 2C (p<0.01). * Statistically different from Group 2E (p<0.05). (C) Microvessel density (number of CD31-positive microvessels per mm2). ** Statistically different from Group 2C (p<0.01). * Statistically different from Group 2E (p<0.05).
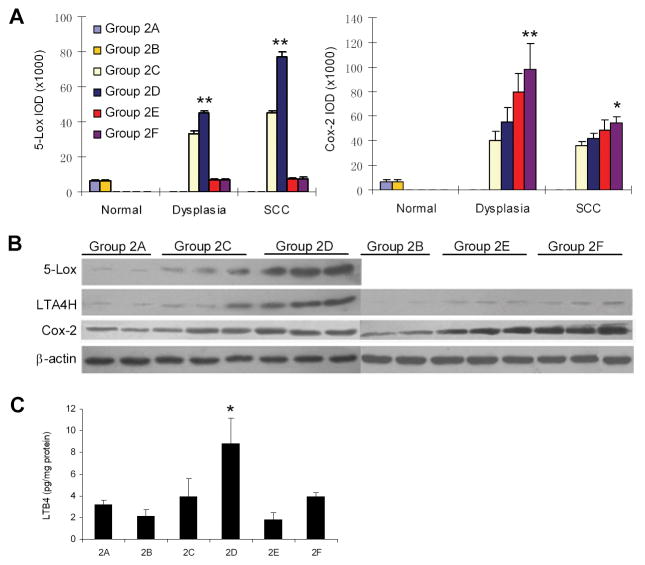
With immunohistochemical staining, we observed increasing expression of 5-Lox and Cox-2 in 4NQO-induced oral carcinogenesis (Figure 3A). As seen in the first animal experiment, ethanol enhanced 5-Lox expression in epithelial cells and inflammatory cells in dysplasia and SCC of Alox5+/+ mice. The increase of Cox-2 protein was not as dramatic as the increase in 5-Lox protein. However, as compared with Alox5+/+ mice, Alox5−/− mice had significantly higher expression of Cox-2 in both dysplasia and SCC (p<0.05), suggesting that activation of the Cox-2 pathway might be a potential shunting mechanism due to disruption of the 5-Lox pathway.
Figure 3.
5-Lox and Cox-2 expression and LTB4 biosynthesis in 4NQO/ethanol-treated mouse tongue of Alox5+/+ and Alox5−/− mice. (A) Overexpression of 5-Lox and Cox-2 in mouse tongue induced by ethanol as determined by immunohistochemical staining. ** Statistically different from Group 2C (p<0.01); * Statistically different from Group 2E (p<0.05), based on ANOVA test. (B) Overexpression of 5-Lox, LTA4H and Cox-2 in mouse tongue induced by ethanol as determined by Western blotting. Protein samples of representative tongues (2 from Group 2A, and 3 each from other groups) were shown. (C) Increased LTB4 biosynthesis in mouse tongue induced by ethanol as determined by enzyme immunoassay. * Statistically different from Group 2C (p<0.01).
Western blotting showed that ethanol significantly increased expression of 5-Lox and LTA4H in Alox5+/+ mice (Group 2C and 2D), but not in Alox5−/− mice (Group 2E, 2F). Consistent with our immunostaining data, Cox-2 expression was enhanced in Alox5−/− mice (Figure 2B). We also measured the level of LTB4 in mouse tongue. Ethanol was found to increase LTB4 significantly in Alox5+/+ mice (Figure 2C).
Activation of the 5-Lox pathway in human oral cancer cells by ethanol
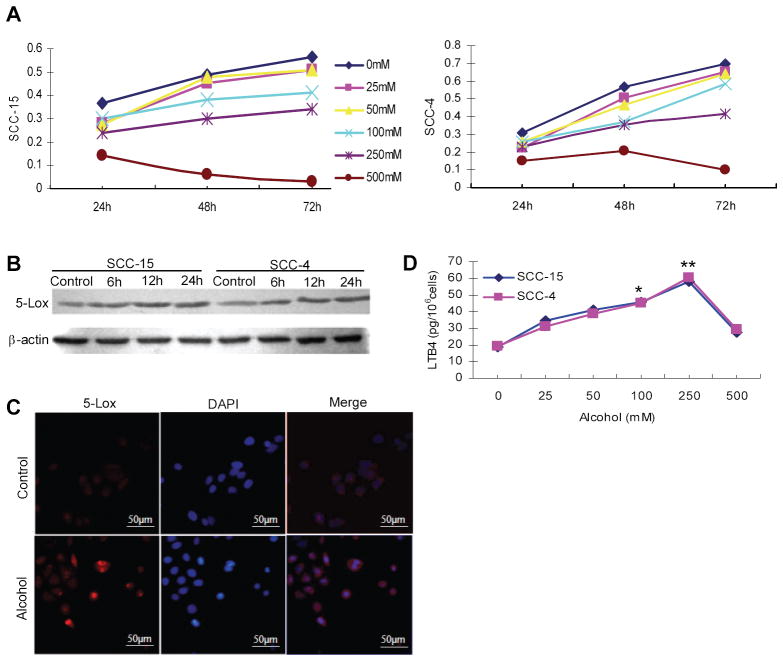
Two human tongue SCC cell lines, SCC-15 and SCC-4, were used to determine the effects of ethanol on the 5-Lox pathway in vitro. As shown in Figure 4A, there was a dose-dependent effect of ethanol on cell growth at lower concentrations. We set the upper limit of ethanol concentration at 500mM, which was toxic to cells. Western blotting showed a time-dependent increase of 5-Lox protein in both SCC-15 and SCC-4 cells (Figure 4B). Immunofluorescent staining of 5-Lox clearly demonstrated overexpression of 5-Lox protein in cells, as well as increased nuclear localization (Figure 4C). Corresponding to the changes of protein, the 5-Lox pathway was activated as evidenced by a dose-dependent increase of LTB4 in SCC-15 and SCC-4 cells (Figure 4D). It should be noted that normal tongue epithelial cells may respond to ethanol treatment differently from these SCC cells. Further studies with immortalized oral epithelial cells, when available, are needed to fully establish the association between ethanol treatment and 5-Lox activation at the early stage of oral carcinogenesis.
Figure 4.
Effect of ethanol on cell growth, 5-Lox expression, cellular localization of 5-Lox, and LTB4 biosynthesis, in SCC-15 and SCC-4 cells. (A) Dose-dependent effect of ethanol on cell proliferation of SCC-15 and SCC-4 cells as determined by MTT assay. (B) Increased expression of 5-Lox in SCC-15 and SCC-4 after ethanol treatment (250mM) for 6h, 12h and 24h as determined by Western blotting. (C) Increased nuclear localization of 5-Lox in SCC-15 cells after ethanol treatment (250mM) as determined by immunofluoresent staining with DAPI counterstaining. (D) Increased LTB4 biosynthesis in SCC-15 and SCC-4 cells after ethanol treatment (25–500mM) for 24 hr as determined by enzyme immunoassay. * Significantly different from control (p<0.05) ** Significantly different from control (p<0.01)
Discussion
Although epidemiological studies have shown clearly that alcohol drinking is a major risk factor for human oral cancer, a proper animal model has not been developed to study ethanol-related oral carcinogenesis. Several previous animal studies failed to statistically confirm the cancer-promoting activity of ethanol on DMBA-induced hamster cheek pouch carcinogenesis (13–16). In our study, mice were given 8% ethanol ad libitum as the sole drink based on the following rationale: a) drinking is a method of administration best resembling human behavior (22). Humans consume alcoholic beverages of varying concentrations of ethanol, such as beer (3–8%), wine (12–14%), and liquor (35–70%); b) delivering ethanol in drink or diet is always limited by poor tolerance of animals to concentrated ethanol (>10%)(Table 1). Carcinogen and ethanol were given separately in this study in order to understand the cancer-promoting effect of ethanol at the post-initiation stage.
Although many studies have been published on the mechanism of ethanol-related carcinogenesis, it is still unclear how ethanol may promote oral cancer (23). It is known that ethanol interferes with arachidonic acid metabolism at multiple levels. It has been shown in human neuroblastoma cells that ethanol exposure up-regulated activity of cPLA2 for arachidonic acid release in a time-dependent manner (24). It is well established that ethanol increases the levels of leukotrienes and reduces the levels of prostaglandins in rodent stomach (25). Ethanol not only stimulates biosynthesis of leukotrienes, but also inhibits their degradation (26). Ethanol-induced hemorrhagic lesions were significantly reduced by 5-Lox inhibitors, EP2/EP4 agonists, and leukotriene receptor antagonists (27, 28). Previous studies by others and us have clearly demonstrated that aberrant arachidonic acid metabolism plays a critical role in oral carcinogenesis. Apart from the Cox-2 pathway, the 5-Lox pathway is a critical metabolism pathway in oral carcinogenesis (18, 19). In this study, we found that ethanol up-regulated expression of Cox-2 and 5-Lox in dysplasia and SCC (Figure 1G, 3A). Western blotting confirmed overexpression of 5-Lox in ethanol-treated mouse tongue (Figure 1H, 3B). These data suggested that 5-Lox overexpression induced by ethanol was an early event in ethanol-related oral carcinogenesis. In addition, LTA4H expression and LTB4 biosynthesis in mouse tongue were also enhanced by ethanol (Figure 3B, 3C). In vitro ethanol up-regulated 5-Lox expression in human tongue SCC cells, stimulated nuclear localization of 5-Lox, and promoted LTB4 biosynthesis (Figure 3B, C, D).
The most convincing data of this study were obtained in the second animal experiment using Alox5−/− mice. Although these mice reproduced and lived normally, they were more resistant to inflammation or certain inflammation-associated diseases, but more susceptible to certain infections (29–31). Recent studies have shown that 5-Lox deficiency reduces hepatic inflammation and associated damage in hyperlipidemic mice (32), and impairs leukemia stem cells (33). We demonstrated that the promoting effect of ethanol on 4NQO-induced oral carcinogenesis depended, at least in part, on 5-Lox (Table 2). In Alox5+/+ mice (Group 2C and 2D), 8% ethanol shortened the duration of malignant transformation. However in Alox5−/− mice, ethanol had no such an effect. This cancer-promoting effect of ethanol was further associated with its effects on cell proliferation (BrdU-labeling index), inflammation (number of mast cells per area) and angiogenesis (microvessel density) in mouse tongue (Figure 2).
Our data were in agreement with a recent study showing lack of chemopreventive effect of a 5-Lox inhibitor (Zileuton) on 4NQO-induced oral carcinogenesis in rats (34). In this study, loss of 5-Lox only slightly reduced the incidence of SCC when mice were treated with 4NQO alone (Group 2C vs. 2E in Table 2). However, our previous studies on the DMBA-induced hamster cheek pouch model have shown chemopreventive efficacy of topically applied Zileuton (18). Differences in experimental designs, animal species (mouse, rat, or hamster), methods of disruption (genetic or pharmacological), and routes of administration (topical or oral), may contribute to the discrepancy. A more probable explanation, however, may lie with different mechanisms of the 4NQO model and the DMBA model. Although both models produce lesions similar to those found in humans, the 4NQO model is known to produce less severe inflammation than the DMBA model. Populations of infiltrating inflammatory cells in the oral epithelium are also different in these two models (35). The 4NQO model has been more and more widely used for studying the roles of genes/pathways in, and preventive/therapeutic effects on, oral carcinogenesis because of its convenience of use and potential use of genetically modified mice (36–41). Parallel studies with both the 4NQO model and the DMBA model may generate novel findings, which may provide insights on different pathologies of human oral cancer.
Two observations in this study were interesting: a) Shunting between the 5-Lox pathway and the Cox-2 pathway: In Alox5−/− mice, Cox-2 was overexpressed as compared with Alox5+/+ mice when treated with 4NQO and ethanol (Figure 3A, 3B). This is consistent with previous studies showing that genetic or pharmacological disruption of Cox-2 activated the 5-Lox pathway (41, 42), and knockout of 5-Lox or 5-Lox activating protein increased the Cox metabolites produced by inflammatory cells (43, 44). Our data support the idea of combination therapy or chemoprevention. b) Involvement of mast cells in ethanol-related 4NQO-induced oral carcinogenesis: It is known that mast cells are not only producers of pro-inflammatory metabolites of arachidonic acid, but also regulated by arachidonic acid metabolites in an autocrine or paracrine manner (45). In the DMBA model, mast cell activation was associated with the increased cell proliferation in hamster cheek pouch (46). A recent study showed that mast cell 5-Lox promoted intestinal polyposis in APCΔ468 mice (47). In this study, fewer mast cells infiltrated SCC in Alox5−/− mice than in Alox5+/+ mice (Figure 2B). It would be interesting to further investigate how mast cells interact with epithelial cells in the process of ethanol-related oral carcinogenesis.
In conclusion, we demonstrated that ethanol promoted 4NQO-induced oral carcinogenesis, at least in part, through further activation of the 5-Lox pathway of arachidonic acid metabolism. To our knowledge, this study is the first to demonstrate a critical role of the 5-Lox pathway in ethanol-related oral carcinogenesis. This animal model may be used for future mechanistic and chemopreventive studies on ethanol-related oral carcinogenesis. With recent elucidation of the structure of human 5-Lox (48), novel and potent inhibitors of 5-Lox may be developed and potentially used for oral cancer prevention in the future.
Acknowledgments
This work was supported by the National Institutes of Health (U54 AA019765, U54 CA156735) and the National Natural Science Foundation of China (No. 30772421).
Abbreviations
- DMBA
7,12-dimethylbenz[a]anthracene
- 4NQO
4-nitroquinoline 1-oxide
- SCC
squamous cell carcinoma
- 5-Lox
5-lipoxygenase
- Cox-2
cyclooxygenase-2
- PGE2
prostaglandin E2
- LTA4H
leukotriene A4 hydrolase
- LTB4
leukotriene B4
- IOD
integrated optical density
References
- 1.Magrath I, Litvak J. Cancer in developing countries: opportunity and challenge. J Natl Cancer Inst. 1993;85:862–74. doi: 10.1093/jnci/85.11.862. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie J, Ah-See K, Thakker N, Sloan P, Maran AG, Birch J, et al. Increasing incidence of oral cancer amongst young persons: what is the etiology? Oral Oncol. 2000;36:387–9. doi: 10.1016/s1368-8375(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Levi F, Randimbison L, Te VC, Franceschi S, La Veccchia C. Trends in cancer survial in Vaud, Switzerland. Eur J Cancer. 1992;28:1490–5. doi: 10.1016/0959-8049(92)90551-c. [DOI] [PubMed] [Google Scholar]
- 5.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review. 1975–2007 http://seercancergov/csr/1975_2007/2010.
- 6.Vecchia CL, Tavani A, Franceschi S, Levi F, Corrao G, Negri E. Epidemiology and prevention of oral cancer. Oral Oncol. 1997;33:302–12. doi: 10.1016/s1368-8375(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 7.Johnson N. Tobacco use and oral cancer: a global perspective. J Dent Educ. 2001;65:328–39. [PubMed] [Google Scholar]
- 8.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85:1700–5. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang WY, Winn DM, Brown LM, Gridley G, Bravo-Otero E, Diehl SR, et al. Alcohol concentration and risk of oral cancer in Puerto Rico. Am J Epidemiol. 2003;157:881–7. doi: 10.1093/aje/kwg055. [DOI] [PubMed] [Google Scholar]
- 10.Maserejian NN, Joshipura KJ, Rosner BA, Giovannucci E, Zavras AI. Prospective study of alcohol consumption and risk of oral premalignant lesions in men. Cancer Epidemiol Biomarkers Prev. 2006;15:774–81. doi: 10.1158/1055-9965.EPI-05-0842. [DOI] [PubMed] [Google Scholar]
- 11.Maier H, Weidauer H, Zoller J, Seitz HK, Flentje M, Mall G, et al. Effect of chronic alcohol consumption on the morphology of the oral mucosa. Alcohol Clin Exp Res. 1994;18:387–91. doi: 10.1111/j.1530-0277.1994.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 12.Soffritti M, Belpoggi F, Cevolani D, Guarino M, Padovani M, Maltoni C. Results of long-term experimental studies on the carcinogenicity of methyl alcohol and ethyl alcohol in rats. Ann N Y Acad Sci. 2002;982:46–69. doi: 10.1111/j.1749-6632.2002.tb04924.x. [DOI] [PubMed] [Google Scholar]
- 13.Henefer EP. Ethanol, 30 per cent, and hamster pouch carcinogenesis. J Dent Res. 1966;45:838–44. doi: 10.1177/00220345660450035401. [DOI] [PubMed] [Google Scholar]
- 14.Elzay RP. Effect of alcohol and cigarette smoke as promoting agents in hamster pouch carcinogenesis. J Dent Res. 1969;48:1200–5. doi: 10.1177/00220345690480061801. [DOI] [PubMed] [Google Scholar]
- 15.Elzay RP. Local effect of alcohol in combination with DMBA on hamster cheek pouch. J Dent Res. 1966;45:1788–95. doi: 10.1177/00220345660450063401. [DOI] [PubMed] [Google Scholar]
- 16.Nachiappan V, Mufti SI, Eskelson CD. Ethanol-mediated promotion of oral carcinogenesis in hamsters: association with lipid peroxidation. Nutr Cancer. 1993;20:293–302. doi: 10.1080/01635589309514297. [DOI] [PubMed] [Google Scholar]
- 17.Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–13. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Sood S, Wang S, Fang M, Wang P, Sun Z, et al. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res. 2005;11:2089–96. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Sood S, Li N, Ramji D, Yang P, Newman RA, et al. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7, 12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis. 2006;27:1902–8. doi: 10.1093/carcin/bgl039. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z, Guan X, Li N, Liu X, Chen X. Chemoprevention of oral cancer in animal models and human patients with ZengShengPing, a mixture of medicinal herbs. Oral Oncol. 2010;45:105–10. doi: 10.1016/j.oraloncology.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Luo M, Jones SM, Peters-Golden M, Brock TG. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc Natl Acad Sci U S A. 2003;100:12165–70. doi: 10.1073/pnas.2133253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegmund S, Haas S, Schneider A, Singer MV. Animal models in gastrointestinal alcohol research-a short appraisal of the different models and their results. Best Pract Res Clin Gastroenterol. 2003;17:519–42. doi: 10.1016/s1521-6918(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 23.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 24.Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Activation of arachidonic acid-specific phospholipase A2 in human neuroblastoma cells after chronic alcohol exposure: prevention by GM1 ganglioside. Alcohol Clin Exp Res. 1997;21:1199–203. [PubMed] [Google Scholar]
- 25.Bode C, Maute G, Bode JC. Prostaglandin E2 and prostaglandin F2 alpha biosynthesis in human gastric mucosa: effect of chronic alcohol misuse. Gut. 1996;39:348–52. doi: 10.1136/gut.39.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boughton-Smith NK, Whittle BJ. Inhibition by 16,16-dimethyl PGE2 of ethanol-induced gastric mucosal damage and leukotriene B4 and C4 formation. Prostaglandins. 1988;35:945–57. doi: 10.1016/0090-6980(88)90118-9. [DOI] [PubMed] [Google Scholar]
- 27.Gyomber E, Vattay P, Szabo S, Rainsford KD. Effect of lipoxygenase inhibitors and leukotriene antagonists on acute and chronic gastric haemorrhagic mucosal lesions in ulcer models in the rat. J Gastroenterol Hepatol. 1996;11:922–7. [PubMed] [Google Scholar]
- 28.Hattori Y, Ohno T, Ae T, Saeki T, Arai K, Mizuguchi S, et al. Gastric mucosal protection against ethanol by EP2 and EP4 signaling through the inhibition of leukotriene C4 production. Am J Physiol Gastrointest Liver Physiol. 2008;294:G80–7. doi: 10.1152/ajpgi.00292.2007. [DOI] [PubMed] [Google Scholar]
- 29.Funk CD, Chen XS, Johnson EN, Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002;68–69:303–12. doi: 10.1016/s0090-6980(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Sood S, Yang CS, Li N, Sun Z. Five-lipoxygenase pathway of arachidonic acid metabolism in carcinogenesis and cancer chemoprevention. Curr Cancer Drug Targets. 2006;6:613–22. doi: 10.2174/156800906778742451. [DOI] [PubMed] [Google Scholar]
- 31.Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, Strouch M, et al. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14:6525–30. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Clemente M, Ferre N, Gonzalez-Periz A, Lopez-Parra M, Horrillo R, Titos E, et al. 5-lipoxygenase deficiency reduces hepatic inflammation and tumor necrosis factor alpha-induced hepatocyte damage in hyperlipidemia-prone ApoE-null mice. Hepatology. 2010;51:817–27. doi: 10.1002/hep.23463. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Hu Y, Zhang H, Peng C, Li S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet. 2009;41:783–92. doi: 10.1038/ng.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick DL, Phillips JM, Horn TL, Johnson WD, Steele VE, Lubet RA. Overexpression of cyclooxygenase-2 in rat oral cancers and prevention of oral carcinogenesis in rats by selective and nonselective COX inhibitors. Cancer Prev Res (Phila) 2010;3:73–81. doi: 10.1158/1940-6207.CAPR-09-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews JB, Mason GI, Scully CM, Prime SS. In situ characterisation of the oral mucosal inflammatory cell response of rats induced by 4-nitroquinoline-N-oxide. Carcinogenesis. 1986;7:783–8. doi: 10.1093/carcin/7.5.783. [DOI] [PubMed] [Google Scholar]
- 36.Shirai K, Kaneshiro T, Wada M, Furuya H, Bielawski J, Hannun YA, et al. A role of sphingosine kinase 1 in head and neck carcinogenesis. Cancer Prev Res (Phila) 2011;4:454–62. doi: 10.1158/1940-6207.CAPR-10-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leeman-Neill RJ, Seethala RR, Singh SV, Freilino ML, Bednash JS, Thomas SM, et al. Inhibition of EGFR-STAT3 signaling with erlotinib prevents carcinogenesis in a chemically-induced mouse model of oral squamous cell carcinoma. Cancer Prev Res (Phila) 2011;4:230–7. doi: 10.1158/1940-6207.CAPR-10-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila) 2009;2:27–36. doi: 10.1158/1940-6207.CAPR-08-0147. [DOI] [PubMed] [Google Scholar]
- 39.Hasina R, Martin LE, Kasza K, Jones CL, Jalil A, Lingen MW. ABT-510 is an effective chemopreventive agent in the mouse 4-nitroquinoline 1-oxide model of oral carcinogenesis. Cancer Prev Res (Phila) 2009;2:385–93. doi: 10.1158/1940-6207.CAPR-08-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang XH, Albert M, Scognamiglio T, Gudas LJ. A DNA methyltransferase inhibitor and all-trans retinoic acid reduce oral cavity carcinogenesis induced by the carcinogen 4-nitroquinoline 1-oxide. Cancer Prev Res (Phila) 2009;2:1100–10. doi: 10.1158/1940-6207.CAPR-09-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fong LY, Jiang Y, Riley M, Liu X, Smalley KJ, Guttridge DC, et al. Prevention of upper aerodigestive tract cancer in zinc-deficient rodents: inefficacy of genetic or pharmacological disruption of COX-2. Int J Cancer. 2008;122:978–89. doi: 10.1002/ijc.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffield-Lillico AJ, Boyle JO, Zhou XK, Ghosh A, Butala GS, Subbaramaiah K, et al. Levels of prostaglandin E metabolite and leukotriene E(4) are increased in the urine of smokers: evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res (Phila) 2009;2:322–9. doi: 10.1158/1940-6207.CAPR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romano M, Claria J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. Faseb J. 2003;17:1986–95. doi: 10.1096/fj.03-0053rev. [DOI] [PubMed] [Google Scholar]
- 44.Fan XM, Tu SP, Lam SK, Wang WP, Wu J, Wong WM, et al. Five-lipoxygenase-activating protein inhibitor MK-886 induces apoptosis in gastric cancer through upregulation of p27kip1 and bax. J Gastroenterol Hepatol. 2004;19:31–7. doi: 10.1111/j.1440-1746.2004.03194.x. [DOI] [PubMed] [Google Scholar]
- 45.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–85. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 46.Aromando RF, Perez MA, Heber EM, Trivillin VA, Tomasi VH, Schwint AE, et al. Potential role of mast cells in hamster cheek pouch carcinogenesis. Oral Oncol. 2008;44:1080–7. doi: 10.1016/j.oraloncology.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Cheon EC, Khazaie K, Khan MW, Strouch MJ, Krantz SB, Phillips J, et al. Mast Cell 5-Lipoxygenase Activity Promotes Intestinal Polyposis in APC{Delta}468 Mice. Cancer Res. 2011;71:1627–36. doi: 10.1158/0008-5472.CAN-10-1923. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert NC, Bartlett SG, Waight MT, Neau DB, Boeglin WE, Brash AR, et al. The structure of human 5-lipoxygenase. Science. 2011;331:217–9. doi: 10.1126/science.1197203. [DOI] [PMC free article] [PubMed] [Google Scholar]