Abstract
This study determined the sensitivity of heart and brain arachidonic acid (ARA) and docosahexaenoic acid (DHA) to the dietary ARA level in a dose-response design with constant, high DHA in neonatal piglets. On day 3 of age, pigs were assigned to 1 of 6 dietary formulas varying in ARA/DHA as follows (% fatty acid, FA/FA): (A1) 0.1/1.0; (A2) 0.53/1.0; (A3-D3) 0.69/1.0; (A4) 1.1/1.0; (D2) 0.67/0.62; (D1) 0.66/0.33. At necropsy (day 28) higher levels of dietary ARA were associated with increased heart and liver ARA, while brain ARA remained unaffected. Dietary ARA had no effect on tissue DHA accretion. Heart was particularly sensitive, with pigs in the intermediate groups having different ARA (A2, 18.6 ± 0.7%; A3, 19.4 ± 1.0%) and a 0.17% increase in dietary ARA resulted in a 0.84% increase in heart ARA. Further investigations are warranted to determine the clinical significance of heart ARA status in developing neonates.
Keywords: Piglet, Arachidonic acid, Infant nutrition, Docosahexaenoic acid
Introduction
Arachidonic acid (20:4n-6; ARA) and docosahexaenoic acid (22:6n-3; DHA) are two long chain polyunsaturated fatty acids (LCPUFA; ≥2 double bonds, ≥20 carbon) that contribute to normal growth and development during the perinatal period. Both are natural components of breastmilk and since 2002, these LCPUFA have been added to infant formulas in the U.S. at target levels of 0.66% and 0.33% total fatty acids (FA), respectively (1). Mean worldwide levels of ARA and DHA in breastmilk are 0.47 ± 0.13% total FA (range: 0.24 – 1.0% FA) and 0.32 ± 0.22% FA (range: 0.06 – 1.4% FA), respectively, with individual variability in DHA attributed largely to maternal diet (2). Inevitably, the wide variation in breastmilk LCPUFA as well as the relative inaccessibility to human infant tissue presents a major challenge for optimizing levels of ARA and DHA in formula to support neonatal development.
It is well-established that the addition of ARA and DHA to formula enhances blood LCPUFA levels equivalent to those of breastfed infants and improves visual acuity and cognitive performance compared with infants fed LCPUFA-free formula (3–6). Functional outcome studies with infants provide clear evidence for the addition of DHA at 0.32% total FA (7,8), while higher DHA levels may offer further benefit for neural development, especially in target populations (9–11). Optimal levels of ARA remain to be determined at any DHA intake and are based largely on mean global levels in breastmilk (1). ARA comprises approximately 10 – 12% total FA in human infant central nervous tissue (e.g. cerebral cortex and retina) and appears to be influenced to a greater extent by postnatal age than dietary ARA supply (12). Studies with animal models suggest a relative resistance of brain and retina ARA to dietary ARA intake, possibly as a mechanism for regulating its potent bioactivity (13,14). ARA levels in the heart, liver and blood-borne pools vary with ARA intake and may compete with n-3 LCPUFA for tissue incorporation (3,15–22), especially in the liver (19,22). Further, in some regions of the neonatal brain, ARA declines when DHA intake is increased (22).
The sensitivity of heart ARA to dietary ARA content has been reported a few times but has not been systematically studied (16–18,20). In pigs (20), dietary ARA and DHA were maintained at a constant 2:1 ratio and total levels varied up to 5 times the target levels in formula. While liver ARA increased with only the highest ARA intakes, heart ARA responded in a direct dose-response manner to each incremental increase in dietary ARA. Further, heart ARA more than doubled with the highest ARA level compared with pigs fed LCPUFA-free formula.
We tested the hypothesis that heart and brain ARA is sensitive to dietary ARA in a dose-response design with DHA constant at a high physiological level. We examined four levels of ARA ranging from 0.09 to 1.0% total FA against a background of 1.0% DHA. Intermediate levels were 0.53 and 0.67% total FA, where 0.53% is slightly above the worldwide ARA mean in human breastmilk and 0.67% is commonly used in infant formulas, though it is among the higher values reported for human breastmilk (2). A DHA dose-response was also included to determine the influence of dietary DHA level on tissue ARA incorporation when ARA levels are comparable to those added in conventional formula (0.66% ARA). The range of DHA content in human milk is 0.06 to 1.4% (2). A randomized, maternal-reared (MR) reference group was included for comparison. The results of these experiments indicate that the heart and liver were sensitive to dietary ARA level, but the brain showed little response.
Materials and Methods
Animals and diets
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Cornell University. On day 3 of life, domestic piglets were matched for weight and gender and assigned to one of six milk replacer diets (n = 8 per diet). Piglets in the formula-reared (FR) groups were then transferred to the Large Animal Research and Teaching Unit, where they were housed individually in raised metal cages and maintained on a 16/8 hr light/dark cycle. The seventh group was MR and remained with the dam at the swine facility for the duration of the study. Diet intake and growth performance have been reported elsewhere (23).
Milk replacer formula consisting of 60% experimental diet (Research Diets, Inc., New Brunswick, NJ) and 40% Birthright baby pig milk replacer (Ralco Nutrition, Inc., Marshall, MN) was fed to FR piglets on days 3 – 28 of age. The ingredient composition and nutrient analysis of the base experimental diet are presented in Table 1. A detailed nutrient composition and analysis of the Birthright baby pig milk replacer has been reported previously (24). As fed, the milk replacer formula had a caloric density of 0.7 kcal/mL and a nutrient composition as follows (% wt/wt): protein 27.0, fat 22.1, carbohydrate 39.5, fiber 0.7 and ash 10.7. The FA compositions of the milk replacer formulas and sow milk (day 14 in lactation) are presented in Table 2. Diets varied in the ratio of ARA/DHA as follows (% FA/FA): (A1) 0.1/1.0; (A2) 0.53/1.0; (A3-D3) 0.69/1.0; (A4) 1.1/1.0; (D2) 0.67/0.62; (D1) 0.66/0.33 (conventional infant formula). Diets A1 – A4 are a dose-response for ARA against DHA constant at 1.0%, while Diets D1 – D3 are a dose-response for DHA against ARA constant at 0.67%. The sow diets were a conventional pig ration consisting of corn and soybean as 96% of the total dry weight, and the PUFA content was comprised of 18:2n-6 (53.9 ± 0.2% FA) and 18:3n-3 (3.4 ± 0.1% FA). The excess 18:2 and low 18:3 apparently drove the very low sow milk DHA (0.01 ± 0.01% FA), while ARA was high compared to the human milk range (0.74 ± 0.02% FA).
TABLE 1.
Nutrient composition and analysis of experimental diets
| Composition | Content |
|---|---|
| Ingredient, g/kg dry | |
| Dried skim milk | 550 |
| Oil blend | 239 |
| Calcium sodium | 96 |
| Mineral mix1 | 48 |
| Vitamin mix2 | 48 |
| Xanthan gum | 14 |
| Methionine, DL | 5 |
| Nutrient analysis, % kcal | |
| Protein | 24.3 |
| Carbohydrate | 28.7 |
| Fat | 47 |
| Caloric density (kcal/g) | 4.67 |
The mineral mix contained (g/kg): 257.8 sucrose, 615.8 dibasic calcium phosphate, 106.7 sodium chloride, 11.8 ferric citrate, 4.0 zinc carbonate, 3.4 magnesium oxide, 0.4 cupric carbonate, 0.2 manganous carbonate, 0.1 potassium iodate, 0.01 sodium selenite.
The vitamin mix contained (g/kg): 910.1 sucrose, 75.0 choline bitartrate, 6.2 vitamin E acetate (50%), 3.4 vitamin A acetate (500,000 IU/g), 1.5 vitamin D3 (100,000 IU/g), 1.3 vitamin B12 (0.1% mannitol), 0.9 pantothenic acid (d, calcium), 0.9 niacin, 0.4 biotin (1%), 0.1 riboflavin, 0.1 thiamine HCl 0.03 menadione sodium bisulfite, 0.01 folic acid, 0.01 pyridoxine HCl.
TABLE 2.
FA composition of milk replacer formulas and sow milk1
| Diet | A1 | A2 | A3-D3 | A4 | D2 | D1 | MR |
|---|---|---|---|---|---|---|---|
| % total FA | |||||||
| ARA | 0.09 | 0.53 | 0.69 | 1.06 | 0.67 | 0.66 | 0.74 ± 0.02 |
| DHA | 1.00 | 1.02 | 1.001 | 1.04 | 0.62 | 0.33 | 0.01 ± 0.01 |
| ARA/DHA | 0.1 | 0.5 | 0.7 | 1.0 | 1.1 | 2.0 | 74.0 |
| Σ SFA + MUFA | 79.86 | 79.94 | 79.43 | 79.18 | 80.02 | 80.02 | 83.94 ± 3.16 |
| 18:2n-6 | 17.25 | 16.83 | 17.11 | 16.93 | 16.9 | 17.19 | 12.05 ± 2.98 |
| Σ n-6 | 17.5 | 17.52 | 17.95 | 18.15 | 17.76 | 18 | 15.21 ± 3.08 |
| 18:3n-3 | 1.53 | 1.41 | 1.48 | 1.47 | 1.48 | 1.53 | 0.57 ± 0.06 |
| Σ n-3 | 2.53 | 2.43 | 2.49 | 2.51 | 2.1 | 1.86 | 0.71 ± 0.04 |
| 18:2n-6/18:3n-3 | 11.3 | 12 | 11.6 | 11.5 | 11.4 | 11.3 | 21.1 |
Milk from two sows taken on day 14 of lactation; MR piglets remained with the sow for the duration of the study. The PUFA content of sow diets during gestation and lactation consisted of 53.90 ± 0.17% 18:2n-6 and 3.44 ± 0.04% 18:3n-3.
Sampling
On day 28 of age, piglets were killed via an intravenous injection of Fatal Plus (1 mL/4.54 kg body weight; Vortech Pharmaceuticals, Dearborn, MI, USA) followed by exsanguination. Necropsy was performed by a team to facilitate rapid removal and weighing of organs, and all samples were flash frozen in liquid nitrogen within 10 min of cessation of heart beat. The eyes and brain were removed by the attending veterinarian using bone cutters and surgical scissors. Retinas were submerged in physiologic saline prior to flash freezing. Cerebral cortex and cerebellum samples were harvested from the left hemisphere prior to submerging individual hemispheres in liquid nitrogen. Internal brain regions were sampled from partially thawed left hemispheres at a later time. Tissues were stored at −80°C until FA analysis.
Lipid analysis
FAME were prepared from ~50 mg of tissue or dry diet and 50 μL sow milk according to the one-step hydrolysis, extraction and methylation procedure (25) with previous modifications (26). Retinas, which were suspended in physiological saline at necropsy, were first lyophilized using a Savant SpeedVac Concentrator (Thermo Fisher Scientific, Waltham, MA) to prevent excess water molecules from hindering the methylation reaction. FAME were quantified on a 5890 Series II gas chromatograph (Hewlett-Packard, Palo Alto, CA) equipped with a BPX70 fused silica column (25 m × 0.22 mm i.d. × 0.25 m film; SGE Inc, Austin, TX). FAME were structurally identified by covalent-adduct chemical ionization tandem mass spectrometry on a Saturn 2000 ion trap mass spectrometer (Varian, Inc., Walnut Creek, CA) (27). An equal weight FAME mixture was used daily to verify response factors.
Statistical analysis
Values are reported as means ± SD. Statistical analysis was carried out using the Fit Model platform of JMP (2008 SAS Institute, 8.0) to fit mixed models and tested the hypothesis that all mean treatment values were not equal. Fixed effects were diet, gender, day 3 of age body weight and the full factorial of interactions. The random effect of litter was included in the model. Interaction effects were considered significant at P < 0.10 and fixed effects at P < 0.05. For each parameter analyzed, effects not considered significant were removed from the final model in a stepwise manner. Significance of pairwise comparisons was determined using the Student's t-test. Linear regression analysis was performed to determine the dose-response of tissue LCPUFA to the dietary ARA and DHA level. Figures are presented with best-fit curves to summarize the relationship between LCPUFA intake and tissue composition.
Results
Intake and growth
Formula intake and growth performance have been reported elsewhere (23). Formula intake was similar among all FR groups and averaged 51.6 ± 0.1 L across the entire study. Mean body weight for the FR groups on day 28 of age was 10.3 ± 0.6 kg, while MR piglets weighed significantly less with a mean weight of 7.9 ± 0.9 kg. Relative organ weights were similar for the FR groups. Absolute brain weights were similar among all piglets.
LCPUFA accretion in liver and heart
Table 3 presents selected LCPUFA from the heart and liver of pigs fed varying levels of ARA and DHA from day 3 – 28 of age. A detailed FA profile of these tissues is presented in Supplemental Tables 1 – 2. Heart ARA increased with increasing dietary ARA. Heart ARA levels were higher in the A3 group compared with Diet A2 (P = 0.04) such that a 0.17% increase in dietary ARA corresponded to an increase of 0.84% in heart ARA between the two treatments. Mean heart ARA was 40% higher in the A4 group compared with A1 pigs. The A3 and A4 diets were not significantly different from the MR heart ARA levels and the mean value (20.3% FA) was only reached at an ARA intake of 1.06% FA in the A4 diet. Liver ARA increased with increasing dietary ARA, although the greatest increase in liver ARA was observed between Diets A1 and A2. The low level of ARA intake in the A1 diet did not support the same level of liver ARA as that observed in the MR piglets.
TABLE 3.
Selected LCPUFA of heart and liver from piglets fed varying levels of ARA and DHA from days 3 – 28 of age1
| Diet ARA/DHA2 | A1 0.09/1.00 | A2 0.53/1.02 | A3-D3 0.69/1.01 | A4 1.06/1.04 | D2 0.67/0.62 | D1 0.66/0.33 | MR 0.74/0.01 | P |
|---|---|---|---|---|---|---|---|---|
| Heart | % total FA | |||||||
| ARA | 14.76 ± 1.07a | 18.57 ± 0.67b | 19.41 ± 1.00c | 20.90 ± 0.93d | 19.98 ± 1.38cd | 19.09 ± 1.88bc | 20.26 ± 1.20cd | <.0001 |
| 22:4n-6 | 0.63 ± 0.09a | 0.71 ± 0.09a | 0.71 ± 0.06ab | 0.82 ± 0.09bc | 0.88 ± 0.13cd | 0.96 ± 0.12d | 1.33 ± 0.13e | <.0001 |
| 22:5n-6 | 0.00 ± 0.01a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.02 ± 0.03a | 0.08 ± 0.01b | <.0001 |
| 20:5n-3 | 0.75 ± 0.09a | 0.54 ± 0.09b | 0.49 ± 0.04b | 0.41 ± 0.04c | 0.42 ± 0.04c | 0.32 ± 0.03d | 0.28 ± 0.06d | <.0001 |
| 22:5n-3 | 0.59 ± 0.07a | 0.59 ± 0.08a | 0.57 ± 0.04a | 0.57 ± 0.04a | 0.71 ± 0.07b | 0.83 ± 0.13c | 1.24 ± 0.07d | <.0001 |
| DHA | 4.81 ± 0.24a | 5.20 ± 0.49b | 4.78 ± 0.43a | 5.01 ± 0.25ab | 4.20 ± 0.64c | 3.00 ± 0.50d | 0.75 ± 0.08e | <.0001 |
| Σ 22C LCPUFA3 | 6.04 ± 0.25ab | 6.50 ± 0.51c | 6.06 ± 0.40abc | 6.40 ± 0.23ac | 5.78 ± 0.72b | 4.81 ± 0.59d | 3.40 ± 0.15e | <.0001 |
| Liver | ||||||||
| ARA | 13.92 ± 1.49a | 16.14 ± 0.87b | 16.12 ± 1.10bc | 16.88 ± 1.50c | 17.36 ± 0.63c | 17.87 ± 1.46d | 17 ± 1.36bcd | <.0001 |
| 22:4n-6 | 1.08 ± 0.29a | 1.15 ± 0.22ab | 1.27 ± 0.22b | 1.36 ± 0.22bc | 1.28 ± 0.20bc | 1.50 ± 0.10c | 2.27 ± 0.35d | <.0001 |
| 22:5n-6 | 0.03 ± 0.06a | 0.06 ± 0.05a | 0.04 ± 0.04a | 0.06 ± 0.07a | 0.2 ± 0.09b | 0.37 ± 0.11c | 1.89 ± 0.53d | <.0001 |
| 20:5n-3 | 0.61 ± 0.14a | 0.42 ± 0.15bc | 0.45 ± 0.10b | 0.36 ± 0.11cd | 0.34 ± 0.05cd | 0.31 ± 0.04d | 0.18 ± 0.06e | <.0001 |
| 22:5n-3 | 0.94 ± 0.11a | 0.91 ± 0.09a | 0.94 ± 0.12a | 0.94 ± 0.14a | 1.01 ± 0.07a | 1.11 ± 0.16b | 1.35 ± 0.20c | <.0001 |
| DHA | 10.77 ± 1.77a | 11.43 ± 1.22a | 10.49 ± 1.41a | 10.45 ± 1.79a | 9.65 ± 1.01b | 7.52 ± 0.69c | 2.37 ± 0.30d | <.0001 |
| Σ 22C LCPUFA | 12.82 ± 1.86a | 13.55 ± 1.26a | 12.74 ± 1.44ab | 12.81 ± 1.90ab | 12.14 ± 1.21b | 10.51 ± 0.81c | 7.88 ± 1.22d | <.0001 |
Values represent mean ± SD, n = 8 per diet. P < 0.05 indicates that all means were not equal; NS, not significant. Pairwise comparisons determined using Student's t-test; means not sharing a common superscript were significantly different (P < 0.05).
ARA/DHA, dietary content of ARA/DHA (% FA/FA).
Σ 22C LCPUFA = Σ 22:4n-6, 22:5n-6, 22:5n-3, DHA.
DHA accretion in the liver reflected dietary intake with A1 – A4 pigs having the highest liver levels, D1 and D2 pigs having intermediary levels and MR pigs having the lowest liver DHA (Table 3). Similarly, DHA levels in the heart reflected dietary DHA content, with A1 – A4 pigs having the highest, D1 and D2 pigs having intermediary and MR pigs having the lowest heart DHA levels (P < 0.0001); although for Diets A1 – A4, A2 pigs had higher heart DHA levels than the A1 and A3 groups (P < 0.05). MR pigs had a strikingly low heart DHA content (0.75 ± 0.08%) that was 6-fold lower than DHA levels in the A2 group, reflecting the low DHA content of the sow milk.
In both the heart and liver, the sum of 22-carbon LCPUFA reflected tissue DHA content and was lowest in D1 and MR pigs (P < 0.0001; Table 3). Compared with the D3 group, D1 pigs had lower liver DHA and higher liver levels of 22:4n-6, 22:5n-6 and 22:5n-3 (P < 0.001). In the heart, however, D1 pigs showed increases in 22:4n-6 and 22:5n-3 compared with D3 pigs (P < 0.0001), while trace levels of 22:5n-6 were detected in all samples.
LCPUFA accretion in neural tissues
Tables 4 & 5 present selected LCPUFA from neural tissue of pigs fed varying levels of ARA and DHA from day 3 – 28 of age. A detailed FA composition of each neural tissue is presented in Supplemental Tables 3 – 8. ARA levels in the cerebral cortex, hippocampus, globus pallidus and inferior colliculus were similar across all dietary treatments. ARA levels in the cerebellum were significantly influenced by diet (P = 0.007) but showed no distinct pattern associated with ARA or DHA intake. A1 pigs had the lowest retinal ARA levels of the FR groups, although differences among the means only reached significance for the A4, D1 and D2 groups (P < 0.05).
TABLE 4.
Selected LCPUFA of cerebral cortex, cerebellum and retina from piglets fed varying levels of ARA and DHA from days 3 – 28 of age1
| Diet ARA/DHA2 | A1 0.09/1.00 | A2 0.53/1.02 | A3-D3 0.69/1.01 | A4 1.06/1.04 | D2 0.67/0.62 | D1 0.66/0.33 | MR 0.74/0.01 | P |
|---|---|---|---|---|---|---|---|---|
| Cerebral cortex | % total FA | |||||||
| ARA | 11.95 ± 0.29 | 11.82 ± 0.60 | 11.80 ± 0.35 | 12.04 ± 0.63 | 11.93 ± 0.40 | 11.77 ± 0.68 | 12.33 ± 0.34 | NS |
| 22:4n-6 | 4.03 ± 0.52 | 4.06 ± 0.16 | 4.09 ± 0.35 | 4.21 ± 0.60 | 4.21 ± 0.32 | 4.19 ± 0.26 | 4.81 ± 0.30 | NS |
| 22:5n-6 | 4.49 ± 0.40a | 4.44 ± 0.47a | 4.68 ± 0.29a | 4.61 ± 0.56a | 5.52 ± 0.64b | 5.49 ± 0.25b | 8.26 ± 0.18c | <.0001 |
| 22:5n-3 | 0.20 ± 0.02a | 0.19 ± 0.02a | 0.19 ± 0.04a | 0.19 ± 0.03a | 0.20 ± 0.02a | 0.25 ± 0.05b | 0.32 ± 0.03c | <.0001 |
| DHA | 12.10 ± 0.83a | 11.91 ± 0.74ab | 11.98 ± 0.69ab | 11.90 ± 0.69ab | 11.30 ± 0.70b | 10.56 ± 0.67c | 6.88 ± 0.55d | <.0001 |
| Σ 22C LCPUFA3 | 20.82 ± 0.81 | 20.60 ± 0.61 | 20.94 ± 1.01 | 20.91 ± 0.99 | 21.23 ± 0.88 | 20.49 ± 0.79 | 20.27 ± 0.72 | NS |
| Cerebellum | ||||||||
| ARA | 10.40 ± 0.30a | 10.76 ± 0.26ab | 11.03 ± 0.31bc | 10.44 ± 0.44a | 10.95 ± 0.62bc | 10.78 ± 0.43ab | 11.48 ± 0.73c | 0.007 |
| 22:4n-6 | 3.29 ± 0.41a | 3.23 ± 0.38a | 3.39 ± 0.32ab | 3.43 ± 0.32ab | 3.55 ± 0.19b | 3.97 ± 0.25c | 4.66 ± 0.31d | <.0001 |
| 22:5n-6 | 2.12 ± 0.26ab | 2.19 ± 0.34a | 2.18 ± 0.19ab | 2.17 ± 0.26a | 2.70 ± 0.37bc | 3.34 ± 1.09c | 4.82 ± 0.23d | <.0001 |
| 22:5n-3 | 0.35 ± 0.06ab | 0.31 ± 0.05bc | 0.32 ± 0.05bc | 0.32 ± 0.05c | 0.31 ± 0.02bc | 0.36 ± 0.05a | 0.47 ± 0.04d | <.0001 |
| DHA | 11.29 ± 1.61a | 11.08 ± 1.68a | 11.29 ± 1.19a | 11.19 ± 1.44a | 10.37 ± 0.75ab | 9.83 ± 0.76b | 6.10 ± 0.28c | <.0001 |
| Σ 22C LCPUFA | 17.47 ± 1.79 | 17.19 ± 2.23 | 17.56 ± 1.51 | 17.47 ± 1.54 | 17.36 ± 0.70 | 17.88 ± 1.37 | 16.53 ± 0.30 | NS |
| Retina | ||||||||
| ARA | 9.48 ± 0.23a | 9.70 ± 0.61ab | 9.66 ± 0.32ab | 9.81 ± 0.31b | 9.96 ± 0.37b | 9.95 ± 0.25b | 10.74 ± 0.50c | 0.0004 |
| 22:4n-6 | 1.66 ± 0.15a | 1.72 ± 0.12ab | 1.77 ± 0.12b | 1.81 ± 0.17b | 1.98 ± 0.19c | 2.13 ± 0.12d | 2.98 ± 0.17e | <.0001 |
| 22:5n-6 | 1.48 ± 0.30a | 1.48 ± 0.32a | 1.45 ± 0.16a | 1.55 ± 0.30a | 2.03 ± 0.64b | 2.29 ± 0.26c | 5.24 ± 0.39d | <.0001 |
| 22:5n-3 | 0.64 ± 0.04a | 0.60 ± 0.05b | 0.63 ± 0.06ab | 0.60 ± 0.03b | 0.55 ± 0.03c | 0.60 ± 0.05b | 0.60 ± 0.05abc | 0.0001 |
| DHA | 20.42 ± 1.20ab | 20.70 ± 0.54a | 20.07 ± 1.47ab | 20.49 ± 1.31ab | 19.50 ± 1.50bc | 18.68 ± 0.96c | 14.16 ± 1.37d | <.0001 |
| Σ 22C LCPUFA | 24.29 ± 1.34 | 24.61 ± 0.49 | 24.03 ± 1.49 | 24.55 ± 1.48 | 24.20 ± 1.31 | 23.81 ± 0.90 | 23.10 ± 0.98 | NS |
Values represent mean ± SD, n = 8 per diet. P < 0.05 indicates that all means were not equal; NS, not significant. Pairwise comparisons determined using Student's t-test; means not sharing a common superscript were significantly different (P < 0.05).
ARA/DHA, dietary content of ARA/DHA (% FA/FA).
Σ 22C LCPUFA = Σ 22:3(n-9), 22:4n-6, 22:5n-6, 22:5n-3, DHA.
TABLE 5.
Selected LCPUFA of hippocampus, globus pallidus and inferior colliculus from piglets fed varying levels of ARA and DHA from days 3 – 28 of age1
| Diet ARA/DHA2 | A1 0.09/1.00 | A2 0.53/1.02 | A3–D3 0.69/1.01 | A4 1.06/1.04 | D2 0.67/0.62 | D1 0.66/0.33 | MR 0.74/0.01 | P |
|---|---|---|---|---|---|---|---|---|
| Hippocampus | % total FA | |||||||
| ARA | 10.40 ± 0.89 | 10.78 ± 0.93 | 10.57 ± 1.09 | 10.61 ± 1.57 | 11.10 ± 0.97 | 10.85 ± 1.23 | 11.77 ± 0.96 | NS |
| 22:4n-6 | 4.57 ± 0.36 | 4.68 ± 0.43 | 4.63 ± 0.57 | 4.72 ± 0.63 | 4.78 ± 0.44 | 5.03 ± 0.51 | 5.37 ± 0.43 | NS |
| 22:5n-6 | 2.89 ± 0.22a | 2.96 ± 0.39a | 3.06 ± 0.39ab | 2.68 ± 0.67b | 3.71 ± 0.40c | 3.31 ± 0.53ac | 5.58 ± 0.60d | <.0001 |
| 22:5n-3 | 0.24 ± 0.03ab | 0.22 ± 0.05bc | 0.22 ± 0.02bc | 0.20 ± 0.05bc | 0.19 ± 0.02c | 0.24 ± 0.04ab | 0.27 ± 0.04a | 0.009 |
| DHA | 9.89 ± 0.26a | 9.74 ± 1.43a | 9.66 ± 0.68ab | 9.63 ± 1.73a | 9.44 ± 1.03a | 8.27 ± 1.48b | 6.12 ± 0.68c | 0.0002 |
| Σ 22C LCPUFA3 | 17.20 ± 1.41 | 17.60 ± 1.87 | 17.13 ± 1.99 | 17.23 ± 2.89 | 18.12 ± 1.44 | 16.84 ± 2.23 | 17.34 ± 0.64 | NS |
| Globus pallidus | ||||||||
| ARA | 10.33 ± 0.68 | 9.33 ± 1.28 | 10.07 ± 0.44 | 9.92 ± 0.76 | 10.02 ± 0.88 | 10.09 ± 0.96 | 10.73 ± 0.82 | NS |
| 22:4n-6 | 3.81 ± 0.32ab | 3.59 ± 0.73b | 4.25 ± 0.51bc | 3.98 ± 0.87ab | 3.72 ± 0.37ab | 4.04 ± 0.58ab | 4.78 ± 0.65c | 0.02 |
| 22:5n-6 | 2.93 ± 0.37a | 2.24 ± 0.77b | 2.81 ± 0.40ab | 2.64 ± 0.58ab | 3.16 ± 0.73a | 3.04 ± 0.78a | 5.40 ± 0.92c | <.0001 |
| 22:5n-3 | 0.15 ± 0.12 | 0.12 ± 0.04 | 0.13 ± 0.03 | 0.13 ± 0.02 | 0.11 ± 0.04 | 0.14 ± 0.06 | 0.19 ± 0.06 | NS |
| DHA | 9.45 ± 0.97a | 8.09 ± 2.61ab | 8.80 ± 0.94ab | 8.18 ± 1.36ab | 7.91 ± 1.95b | 7.66 ± 1.68bc | 6.08 ± 0.35c | 0.01 |
| Σ 22C LCPUFA | 16.74 ± 1.12 | 14.49 ± 3.72 | 16.44 ± 1.37 | 15.41 ± 2.20 | 15.37 ± 2.68 | 15.34 ± 2.50 | 16.92 ± 1.43 | NS |
| Inferior colliculus | ||||||||
| ARA | 8.58 ± 0.35 | 8.55 ± 0.75 | 8.85 ± 0.84 | 8.61 ± 0.52 | 8.63 ± 0.50 | 8.97 ± 0.73 | 9.45 ± 1.25 | NS |
| 22:4n-6 | 3.54 ± 0.21a | 3.36 ± 0.19a | 3.55 ± 0.31a | 3.56 ± 0.29a | 3.42 ± 0.30a | 3.70 ± 0.35a | 4.68 ± 1.16b | <.0001 |
| 22:5n-6 | 1.58 ± 0.49ab | 1.50 ± 0.35a | 1.60 ± 0.45ab | 1.62 ± 0.52ab | 1.93 ± 0.53b | 1.91 ± 0.32b | 3.76 ± 0.45c | <.0001 |
| 22:5n-3 | 0.19 ± 0.02ab | 0.18 ± 0.04abc | 0.19 ± 0.04ab | 0.15 ± 0.02c | 0.16 ± 0.03bc | 0.18 ± 0.03bc | 0.21 ± 0.06a | 0.02 |
| DHA | 10.71 ± 0.94a | 10.32 ± 1.88a | 10.84 ± 1.50a | 9.58 ± 1.21a | 10.18 ± 1.27a | 10.53 ± 0.85a | 6.27 ± 1.14b | <.0001 |
| Σ 22C LCPUFA | 16.35 ± 1.10 | 15.66 ± 2.18 | 16.46 ± 2.03 | 15.28 ± 1.60 | 16.02 ± 1.46 | 16.61 ± 1.23 | 15.40 ± 1.74 | NS |
Values represent mean ± SD, n = 8 per diet. P < 0.05 indicates that all means were not equal; NS, not significant. Pairwise comparisons determined using Student's t-test; means not sharing a common superscript were significantly different (P < 0.05).
ARA/DHA, dietary content of ARA/DHA (% FA/FA).
Σ 22C LCPUFA = Σ 22:3(n-9), 22:4n-6, 22:5n-6, 22:5n-3, DHA.
Pigs fed 1.0% DHA had significantly higher DHA levels in the cerebral cortex, cerebellum and retina than the D1 and MR groups, while D2 pigs showed intermediary levels (P < 0.0001; Table 4 & 5). DHA accretion in the hippocampus, globus pallidus and inferior colliculus was greatest for pigs fed 1.0% DHA and lowest for the MR group (P < 0.05). For these tissues, DHA levels were 17%, 15% and 3% higher, respectively, in the D3 compared with D1 pigs, although differences were not statistically significant.
The sum of the 22-carbon LCPUFA, corresponding to the sum of 22:3(n-9), 22:4n-6, 22:5n-6, 22:5n-3 and DHA, was similar across all dietary treatments in all neural tissues reported (Tables 4 & 5). For these tissues, the magnitude of the increase in non-DHA 22-carbon LCPUFA was greatest for 22:5n-6. Differences in the composition of 22-carbon LCPUFA between Diets D1 and D3 were significant and apparent, with D1 pigs having higher 22:5n-6 in cerebral cortex, cerebellum and retina (P < 0.01), and higher 22:4n-6 in cerebellum and retina than D3 pigs (P < 0.001).
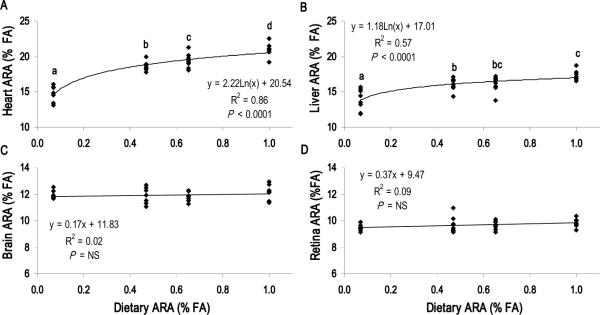
Tissue ARA and DHA dose-response to diet
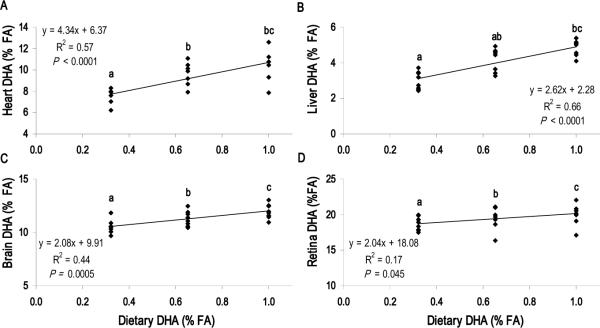
Figure 1 summarizes the linear regression analysis for the tissue ARA dose-response to the dietary ARA level (Diets A1 – A4). ARA levels in the heart and liver were highly responsive to the dietary ARA content (P < 0.0001), whereas ARA levels in the cerebral cortex and retina were not responsive to ARA intake. Regression analysis for the tissue DHA dose-response to dietary DHA content (Diets D1 – D3) is presented in Figure 2. Cerebral cortex, retina, liver and heart DHA levels increased with dietary DHA content (P < 0.05). For heart and liver, however, differences were most pronounced between Diets D1 and D3, as increasing the DHA content from 0.62 to 1.0% total FA (Diets D2 vs. D3) did not significantly increase heart or liver DHA levels. Cerebellum and hippocampus DHA levels also increased with dietary DHA content (P < 0.05), while globus pallidus and inferior colliculus DHA levels did not correlate with dietary DHA content (Supplemental Figure 1).
FIGURE 1.
ARA tissue dose-response to dietary ARA with constant DHA (1.0% total FA). Dietary ARA content for A1, A2, A3 and A4 was 0.09, 0.53, 0.69 and 1.06% total FA, respectively. (A) Heart and (b) Liver responded significantly (P < 0.0001) while (C) Brain (cerebral cortex) and (D) Retina did not. Each point represents one animal (n = 32 per panel). P < 0.05 indicates significant correlation between dietary ARA content and tissue ARA level; NS, not significant. Student's t-test used to determine significance of pairwise comparisons of mean tissue ARA content; means not sharing a common letter were significantly different (P < 0.05).
FIGURE 2.
DHA tissue dose-response to dietary DHA with constant ARA (0.67% total FA). Dietary DHA content for D1, D2 and D3 was 0.32, 0.62 and 1.01% total FA, respectively. Tissue DHA rises with dietary DHA in all tissues presented, (A) Heart, (B) Liver, (C) Brain (Cerebral cortex) and (D) Retina. Each point represents one animal (n = 24 per panel). P < 0.05 indicates significant correlation between dietary DHA content and tissue DHA level; NS, not significant. Student's t-test used to determine significance of pairwise comparisons of mean tissue DHA content; means not sharing a common letter were significantly different (P < 0.05).
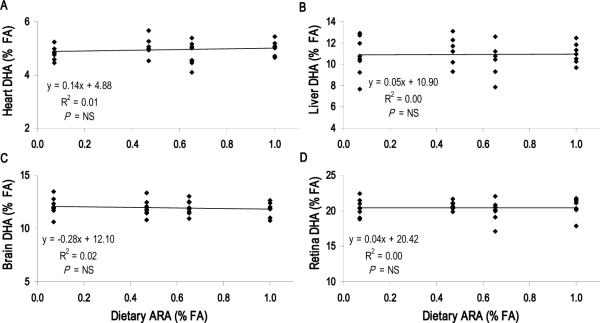
ARA and DHA competition for tissue incorporation
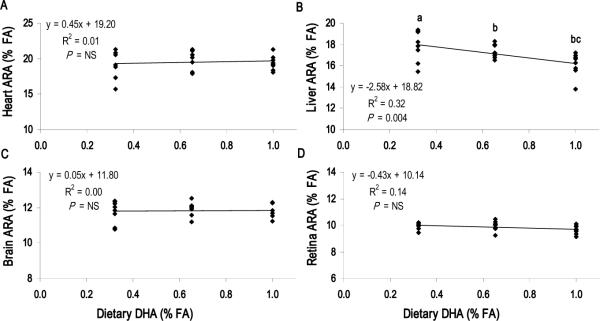
Dietary ARA level had no effect on DHA accretion in the heart, liver or neural tissues (P > 0.05) (Figure 3, Supplemental Figure 2). Conversely, there was an apparent competition between liver ARA and diet-derived DHA (Diets D1 – D3), with D3 pigs showing lower liver ARA levels than D1 pigs (P = 0.004) (Figure 4). This competition was not detected in the heart, brain or retina, indicating that the dietary DHA content has no effect on ARA levels in these tissues (Figure 3).
FIGURE 3.
DHA tissue dose-response to dietary ARA content with constant DHA (1.0% total FA). Dietary ARA content for A1, A2, A3 and A4 was 0.09, 0.53, 0.69 and 1.06% total FA, respectively. Increasing ARA had no effect on DHA levels in (A) Heart, (B) Liver, (C) Brain (Cerebral cortex) or (D) Retina. Each point represents one animal (n = 24 per panel). P < 0.05 indicates significant correlation between dietary ARA content and tissue DHA level; NS, not significant. Student's t-test used to determine significance of pairwise comparisons of mean tissue DHA content; means not sharing a common letter were significantly different (P < 0.05).
FIGURE 4.
ARA tissue dose-response to dietary DHA content with constant ARA (0.67% total FA). Dietary DHA content for D1, D2 and D3 was 0.32, 0.62 and 1.01% total FA, respectively. Increasing DHA reduced ARA levels in (B) Liver but not (A) Heart, (C) Brain (Cerebral cortex) or (D) Retina. Each point represents one animal (n = 24 per panel). P < 0.05 indicates significant correlation between dietary DHA content and tissue ARA level; NS, not significant. Student's t-test used to determine significance of pairwise comparisons of mean tissue ARA content; means not sharing a common letter were significantly different (P < 0.05).
Discussion
The neonatal pig serves as a practical biomedical model of human infant development. Pigs are similar to humans in their metabolic responses, genetics of the FA desaturases and rates of perinatal brain growth (28,29). Their rapid postnatal growth enables facile collection of data relevant to humans over a relatively short study duration. The work presented here employed the neonatal pig model and clearly demonstrated that the heart and liver were sensitive to dietary ARA level.
The sensitivity of heart ARA to the dietary ARA content has been reported from time to time in pigs (18,20) and rats (16,17). However, the underlying metabolic mechanisms are unknown and the clinical significance largely unstudied. In the present study, heart ARA levels reflected dietary ARA content and were significantly different for pigs in all ARA groups including those with intermediate intakes, indicating that heart ARA is limited by intake, at least at high infant DHA intake levels. These observations are particularly relevant to ARA recommendations for infant formula as 0.53% (A2) is slightly above the worldwide ARA mean in human breastmilk and 0.67% (A3) is the level currently added in formula and is near the higher end of breastmilk values reported (2). ARA serves several known functions in cardiac physiology, such as providing precursor for eicosanoid signaling molecules and acting directly to modulate voltage-gated ion channel activity and cellular excitability (30). The degree to which heart ARA status influences cardiac physiology in the developing neonate, as well as the potential long term implications, remains to be determined. Nonetheless, our findings suggest that the level of ARA in formula may not yet be fully optimized.
Biosynthesis of ARA and DHA from the nutritionally essential linoleic and alpha-linolenic acids occurs through a series of elongation and desaturation reactions catalyzed by a common set of enzymes (31). These reactions occur primarily in the liver. Accordingly, liver lipids typically reflect dietary FA content, but the lipids are also influenced by the expression and regulation of the lipid-related enzymes (31,32). The brain and heart also express the FA desaturases and elongases (Elovl-1, -5 and -6) for synthesizing ARA and DHA, but these organs are not generally considered major sites of LCPUFA biosynthesis (33). Indeed, previous findings and the data presented here strongly suggest that dietary DHA content is important for the developing brain and that dietary ARA content is important for the developing heart. Emerging evidence points to a number of important roles for ARA related to brain function (34–37). In addition, considerable amounts of preformed ARA is elongated into 22:4n-6 (11 – 16% of preformed ARA) and 22:5n-6 (2 – 4% of preformed ARA) during early brain development in non-human primates (38). Some studies have indicated that ARA supplementation leads to increased neurogenesis and prepulse inhibition (39), alters brain catecholamines (40) and supports more normative values of brain N-arachidonylglycerol and ARA species of monoacylglycerols (41).
Studies with humans indicate that the rate of postnatal endogenous DHA synthesis is insufficient to support maximal DHA accretion in neural tissues (42,43). Furthermore, supplementation with ARA and DHA improves visual and cognitive performance compared with infants fed formula lacking preformed LCPUFA (3,4,6,44,45). In addition, positive correlations between LCPUFA status and visual maturation in term infants, and ARA and growth during the first year after preterm delivery, have been reported (46,47). Collectively, these observations serve as the basis for the addition of ARA and DHA to formula.
In the present study, the FA composition of the sow ration consisted of more than 50% linoleic acid with 3% alpha-linolenic acid. This composition led to a remarkably low DHA content in sow milk (2). The choice to include an MR reference group thus provided a unique opportunity to investigate the contribution of sow milk, essentially devoid of DHA but not ARA, to the LCPUFA status of the developing offspring. This treatment is representative of an extreme case of DHA-deficient breastmilk that is reflective of few human populations worldwide (2,48). Because of this, we advise caution in extending results observed with the MR group, in particular comparing MR pigs with the FR groups fed higher levels of DHA, to breastfeeding practices in humans.
In the present study, higher levels of dietary ARA were associated with increased heart and liver ARA, while brain ARA was constant and unaffected by ARA intake. Similarly, the neural tissue of the retina exhibited only a modest trend for an increase in ARA with increasing dietary ARA that was not supported by the linear regression analysis of the ARA dose-response data. The sensitivity of the heart to the dietary ARA level therefore suggests that the ARA content is influenced predominantly by preferential FA uptake and incorporation. Further, these data indicate that the rate of endogenous ARA biosynthesis may not be sufficient to match ARA accretion in the growing piglet heart. Several studies have noted a sensitivity of the nervous system and other tissues to ARA supplementation against a relatively high linoleic acid background. For example, ARA supplementation increased ARA in rat hippocampus (39) and increased in piglet striatum phosphatidylserine (40). Whelan et al. (49) noted ARA increases in many internal organ phospholipids in Syrian hamsters after an ARA supplementation relative to a background diet containing 30% linoleic acid. In humans, moderate consumption of ARA-rich foods (e.g. tropical fish, kangaroo meat) was shown to increase ARA, but not LA levels in plasma phospholipids (50,51).
The competition between n-3 and n-6 for tissue incorporation and the resulting effect on LCPUFA biosynthesis is well-studied. The relative availability of linoleic and alpha-linolenic acids, as well as their LCPUFA derivatives, drives the biosynthesis of n-3 and n-6 LCPUFA and modulates tissue levels of the two FA families in a reciprocal manner (19,52,53). Over the range of dietary ARA levels in the present study, tissue DHA was constant and unaffected by diet. Conversely, we observed a specific reduction in liver ARA with the highest DHA intakes, consistent with previous reports in pigs (18) and baboon neonates (22) consuming preformed DHA. These observations reinforce the concept that ARA and DHA in the liver are closely linked to diet because of the liver's central role in LCPUFA biosynthesis.
The sensitivity of the central nervous tissue DHA level has been previously investigated in numerous studies with term and preterm neonatal non-human primates (e.g. (21,22,54)). The brain consistently shows increased region-specific DHA accretion related to level and duration of preformed DHA feeding, and premature birth generally has a significant negative impact on DHA levels in this tissue type. In the present study, we observed higher levels of DHA in all tissues with increasing DHA content. Pigs in the high DHA group showed significantly higher levels of DHA in the cerebral cortex, retina and cerebellum compared with pigs in the low DHA group. Increases in mean DHA were 13%, 7% and 15%, respectively, for these neural tissues. Mean DHA levels in the hippocampus and globus pallidus were 17% and 15% higher in the high DHA group compared with the low DHA group; while these differences were not statistically significant, they may be of biological importance. The relative insensitivities of basal ganglia, limbic and midbrain regions to postnatal dietary DHA consumption, compared with the cerebral cortex, are consistent with data from baboons (22) and suggest that these deep brain regions may be influenced to a greater extent by the DHA status of the mother during gestation. Thus, these data support the recent dietary recommendations on total fat and fatty acids by the FAO/WHO for the provision of 200 mg/day DHA in the diets of pregnant and lactating women (55). Currently, there have only been a handful of investigations which have focused on the effects of ARA supplementation by women during pregnancy and lactation on neurodevelopment and other outcomes (56–58). Clearly, more work is needed in this area before it becomes possible to provide recommendations for ARA supplementation by expecting or nursing women.
In this study, pigs that exhibited the lowest neural tissue DHA also showed enriched tissue 22:4n-6, 22:5n-6 and 22:5n-3, with the greatest increases occurring in 22:5n-6. The increase in tissue 22:5n-6 in animals fed n-3-inadequate diets is well-characterized (59–61) and presumably occurs as an alternate substrate source for DHA-dependent functions in neural tissue. Recent examination of the frontal cortex from rat pups fed an n-3-deficient diet revealed a highly specific substitution of 22:5n-6, but not 22:4n-6 or ARA, for DHA in brain phosphatidylethanolamine and phosphatidylserine (62). Tight regulation of the “reciprocal replacement” of DHA and 22:5n-6 (59) among brain phospholipid molecular species illustrates the essential structure-function relationship between LCPUFA and outcomes such as vision or cognition. Functionally, low DHA levels in neural tissue cannot be fully compensated by increased 22:5n-6 and manifests as poorer electroretinogram performance (63) and spatial learning (64), and there is some evidence that high DHA enhances motor development (65). In the present study, the compositional difference in neural tissue 22-carbon LCPUFA between diets D1 and D3 is of particular interest. Pigs in the low DHA group had significantly lower DHA and higher 22:5n-6 in cerebral cortex, cerebellum and retina. DHA at 0.32% total FA is the level currently added to many infant formulas. Higher levels may provide further benefit for visual acuity and cognition, especially in target populations with endogenously low DHA status (5,9,11).
Changes in tissue FA composition may influence physiological function and alter safety outcomes and measures of overall development. In a separate report, we show that the dietary ARA level (0.09 – 1.0% total FA) had a negligible effect on safety-related outcomes, specifically clinical chemistries, hematology and immune function parameters in the subset of the present piglets fed 1.0% DHA (23). Likewise, there were no differences in growth among any of the dietary treatment groups and no differences in general behavior reported by daily caretakers.
In summary, results from the present study demonstrate a tissue-specific responsiveness to the dietary ARA level in rapidly growing neonatal pigs. Whereas brain ARA remained unaffected by diet ARA, heart and liver ARA levels were responsive. Dietary ARA level had no effect on DHA accretion in heart, liver or neural tissue. The magnification of heart ARA levels with increasing dietary ARA content suggests that the growing heart specifically incorporates and concentrates ARA at a rate that may exceed the biosynthetic capacity for ARA. Further investigations are warranted to determine the biological implication of heart ARA status in relation to an optimal balance of dietary n-6/n-3 for use in infant formulas.
Supplementary Material
Acknowledgements
The authors are grateful for the technical assistance of Lisa Furman, Peter Lawrence and Karl Roneker (Cornell University). This work was supported by DSM Food Specialties (Delft, The Netherlands) and Martek Biosciences Corp. (Columbia, MD). Mead Johnson Nutrition (Evansville, IN) provided oil for the experimental diets.
This project was also supported in part by Award T32DK007158 from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ARA
arachidonic acid
- DHA
docosahexaenoic acid
- FA
fatty acid
- FAME
FA methyl ester
- FR
formula-reared
- LCPUFA
long chain polyunsaturated fatty acid
- MR
maternal-reared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data associated with this article can be found in the online version at http://www.sciencedirect.com/science/journal/09523278.
Conflict of interest statement: C. Tyburczy, K. S. D. Kothapalli, W. J. Park, B. S. Blank, K. L. Bradford, J. P. Zimmer and J. T. Brenna report no conflicts of interest. C. M. Butt and N. Salem, Jr. are employees of Martek Biosciences Corp.
References
- 1.Rulis AM. Agency Response Letter GRAS Notice No. GRN 000080. CFSAN/Office of Premarket Approval. 2001. [Google Scholar]
- 2.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 3.Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical studies. Crit Rev Food Sci Nutr. 2005;45:205–229. doi: 10.1080/10408690590956378. [DOI] [PubMed] [Google Scholar]
- 4.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl A):S70–75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Carlson SE. Early determinants of development: a lipid perspective. Am J Clin Nutr. 2009;89:1523S–1529S. doi: 10.3945/ajcn.2009.27113G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan AS, Astwood JD, Gautier S, Kuratko CN, Nelson EB, Salem N., Jr. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids. 2010;82:305–314. doi: 10.1016/j.plefa.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Koletzko B, Cetin I, Brenna JT. Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98:873–877. doi: 10.1017/S0007114507764747. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2009;81:151–158. doi: 10.1016/j.plefa.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6:437–449. doi: 10.1053/siny.2001.0093. [DOI] [PubMed] [Google Scholar]
- 10.Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, Simmer K, Colditz PB, Morris S, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. Jama. 2009;301:175–182. doi: 10.1001/jama.2008.945. [DOI] [PubMed] [Google Scholar]
- 11.Birch EE, Carlson SE, Hoffman DR, Fitzgerald-Gustafson KM, Fu VL, Drover JR, Castaneda YS, Minns L, Wheaton DK, et al. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am J Clin Nutr. 2010;91:848–859. doi: 10.3945/ajcn.2009.28557. [DOI] [PubMed] [Google Scholar]
- 12.Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr. 1994;60:189–194. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- 13.Huang MC, Brenna JT, Chao AC, Tschanz C, Diersen-Schade DA, Hung HC. Differential tissue dose responses of (n-3) and (n-6) PUFA in neonatal piglets fed docosahexaenoate and arachidonoate. J Nutr. 2007;137:2049–2055. doi: 10.1093/jn/137.9.2049. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh AT, Brenna JT. Dietary docosahexaenoic acid but not arachidonic acid influences central nervous system fatty acid status in baboon neonates. Prostaglandins Leukot Essent Fatty Acids. 2009;81:105–110. doi: 10.1016/j.plefa.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Farquharson J, Jamieson EC, Logan RW, Patrick WJ, Howatson AG, Cockburn F. Age- and dietary-related distributions of hepatic arachidonic and docosahexaenoic acid in early infancy. Pediatr Res. 1995;38:361–365. doi: 10.1203/00006450-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Boswell K, Koskelo EK, Carl L, Glaza S, Hensen DJ, Williams KD, Kyle DJ. Preclinical evaluation of single-cell oils that are highly enriched with arachidonic acid and docosahexaenoic acid. Food Chem Toxicol. 1996;34:585–593. doi: 10.1016/0278-6915(96)00019-1. [DOI] [PubMed] [Google Scholar]
- 17.Suarez A, Faus MJ, Gil A. Dietary long-chain polyunsaturated fatty acids modify heart, kidney, and lung fatty acid composition in weanling rats. Lipids. 1996;31:345–348. doi: 10.1007/BF02529883. [DOI] [PubMed] [Google Scholar]
- 18.de la Presa-Owens S, Innis SM, Rioux FM. Addition of triglycerides with arachidonic acid or docosahexaenoic acid to infant formula has tissue- and lipid class-specific effects on fatty acids and hepatic desaturase activities in formula-fed piglets. J Nutr. 1998;128:1376–1384. doi: 10.1093/jn/128.8.1376. [DOI] [PubMed] [Google Scholar]
- 19.Blank C, Neumann MA, Makrides M, Gibson RA. Optimizing DHA levels in piglets by lowering the linoleic acid to alpha-linolenic acid ratio. J Lipid Res. 2002;43:1537–1543. doi: 10.1194/jlr.m200152-jlr200. [DOI] [PubMed] [Google Scholar]
- 20.Huang MC, Chao A, Kirwan R, Tschanz C, Peralta JM, Diersen-Schade DA, Cha S, Brenna JT. Negligible changes in piglet serum clinical indicators or organ weights due to dietary single-cell long-chain polyunsaturated oils. Food Chem Toxicol. 2002;40:453–460. doi: 10.1016/s0278-6915(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 21.Sarkadi-Nagy E, Wijendran V, Diau GY, Chao AC, Hsieh AT, Turpeinen A, Nathanielsz PW, Brenna JT. The influence of prematurity and long chain polyunsaturate supplementation in 4-week adjusted age baboon neonate brain and related tissues. Pediatr Res. 2003;54:244–252. doi: 10.1203/01.PDR.0000072795.38990.F2. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh AT, Anthony JC, Diersen-Schade DA, Rumsey SC, Lawrence P, Li C, Nathanielsz PW, Brenna JT. The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids. Pediatr Res. 2007;61:537–545. doi: 10.1203/pdr.0b013e318045bec9. [DOI] [PubMed] [Google Scholar]
- 23.Tyburczy C, Kothapalli KSD, Park WJ, Blank BS, Liu Y-C, Nauroth JM, Zimmer JP, Salem J, N., Brenna JT. Negligible effect of dietary arachidonic acid (ARA) levels on growth, clinical chemistry and immune function in domestic piglets. Br J Nutr. 2011 doi: 10.1017/S000711451100359X. In press. [DOI] [PubMed] [Google Scholar]
- 24.Cabrera RA, Boyd RD, Jungst SB, Wilson ER, Johnston ME, Vignes JL, Odle J. Impact of lactation length and piglet weaning weight on long-term growth and viability of progeny. J Anim Sci. 2010;88:2265–2276. doi: 10.2527/jas.2009-2121. [DOI] [PubMed] [Google Scholar]
- 25.Garces R, Mancha M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal Biochem. 1993;211:139–143. doi: 10.1006/abio.1993.1244. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Nijland M, Miller M, Ford S, Nathanielsz PW, Brenna JT. The influence of maternal early to mid-gestation nutrient restriction on long chain polyunsaturated fatty acids in fetal sheep. Lipids. 2008;43:525–531. doi: 10.1007/s11745-008-3186-1. [DOI] [PubMed] [Google Scholar]
- 27.Brenna JT, Tyburczy C. Identification of FAME double bond location by covalent adduct chemical ionization (CACI) tandem mass spectrometry. AOCS Lipid Library. 2010 http://lipidlibrary.aocs.org/topics/caci_ms/index.htm. [Google Scholar]
- 28.Innis SM. The colostrum-deprived piglet as a model for study of infant lipid nutrition. J Nutr. 1993;123:386–390. doi: 10.1093/jn/123.suppl_2.386. [DOI] [PubMed] [Google Scholar]
- 29.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 30.Boland LM, Drzewiecki MM. Polyunsaturated fatty acid modulation of voltage-gated ion channels. Cell Biochem Biophys. 2008;52:59–84. doi: 10.1007/s12013-008-9027-2. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 32.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 33.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Rat heart cannot synthesize docosahexaenoic acid from circulating alpha-linolenic acid because it lacks elongase-2. J Lipid Res. 2008;49:1735–1745. doi: 10.1194/jlr.M800093-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darios F, Ruiperez V, Lopez I, Villanueva J, Gutierrez LM, Davletov B. Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010;11:528–533. doi: 10.1038/embor.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- 36.Connell E, Darios F, Broersen K, Gatsby N, Peak-Chew SY, Rickman C, Davletov B. Mechanism of arachidonic acid action on syntaxin-Munc18. EMBO Rep. 2007;8:414–419. doi: 10.1038/sj.embor.7400935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odutuga AA. Reversal of brain essential fatty-acid deficiency in the rat by dietary linoleate, linolenate and arachidonate. Int J Biochem. 1981;13:1035–1038. doi: 10.1016/0020-711x(81)90010-0. [DOI] [PubMed] [Google Scholar]
- 38.Wijendran V, Lawrence P, Diau GY, Boehm G, Nathanielsz PW, Brenna JT. Significant utilization of dietary arachidonic acid is for brain adrenic acid in baboon neonates. J Lipid Res. 2002;43:762–767. [PubMed] [Google Scholar]
- 39.Maekawa M, Takashima N, Matsumata M, Ikegami S, Kontani M, Hara Y, Kawashima H, Owada Y, Kiso Y, et al. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS One. 2009;4:e5085. doi: 10.1371/journal.pone.0005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Presa Owens S, Innis SM. Diverse, region-specific effects of addition of arachidonic and docosahexanoic acids to formula with low or adequate linoleic and alpha-linolenic acids on piglet brain monoaminergic neurotransmitters. Pediatr Res. 2000;48:125–130. doi: 10.1203/00006450-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc Natl Acad Sci U S A. 2001;98:6402–6406. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salem N, Jr., Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci U S A. 1996;93:49–54. doi: 10.1073/pnas.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunnane SC, Francescutti V, Brenna JT, Crawford MA. Breast-fed infants achieve a higher rate of brain and whole body docosahexaenoate accumulation than formula-fed infants not consuming dietary docosahexaenoate. Lipids. 2000;35:105–111. doi: 10.1007/s11745-000-0501-6. [DOI] [PubMed] [Google Scholar]
- 44.Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res. 1998;44:201–209. doi: 10.1203/00006450-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Carlson SE, Ford AJ, Werkman SH, Peeples JM, Koo WW. Visual acuity and fatty acid status of term infants fed human milk and formulas with and without docosahexaenoate and arachidonate from egg yolk lecithin. Pediatr Res. 1996;39:882–888. doi: 10.1203/00006450-199605000-00024. [DOI] [PubMed] [Google Scholar]
- 46.Birch EE, Castaneda YS, Wheaton DH, Birch DG, Uauy RD, Hoffman DR. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am J Clin Nutr. 2005;81:871–879. doi: 10.1093/ajcn/81.4.871. [DOI] [PubMed] [Google Scholar]
- 47.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci U S A. 1993;90:1073–1077. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyuar KB, Min Y, Ghebremeskel K, Khalil AK, Elbashir MI, Cawford MA. Milk of northern Sudanese mothers whose traditional diet is high in carbohydrate contains low docosahexaenoic acid. Acta Paediatr. 2010 doi: 10.1111/j.1651-2227.2010.01940.x. [DOI] [PubMed] [Google Scholar]
- 49.Whelan J, Surette ME, Hardardottir I, Lu G, Golemboski KA, Larsen E, Kinsella JE. Dietary arachidonate enhances tissue arachidonate levels and eicosanoid production in Syrian hamsters. J Nutr. 1993;123:2174–2185. doi: 10.1093/jn/123.12.2174. [DOI] [PubMed] [Google Scholar]
- 50.Sinclair AJ, Johnson L, O'Dea K, Holman RT. Diets rich in lean beef increase arachidonic acid and long-chain omega 3 polyunsaturated fatty acid levels in plasma phospholipids. Lipids. 1994;29:337–343. doi: 10.1007/BF02537187. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair AJ, O'Dea K, Dunstan G, Ireland PD, Niall M. Effects on plasma lipids and fatty acid composition of very low fat diets enriched with fish or kangaroo meat. Lipids. 1987;22:523–529. doi: 10.1007/BF02540369. [DOI] [PubMed] [Google Scholar]
- 52.Mohrhauer H, Holman RT. Alteration of the Fatty Acid Composition of Brain Lipids by Varying Levels of Dietary Essential Fatty Acids. J Neurochem. 1963;10:523–530. doi: 10.1111/j.1471-4159.1963.tb09855.x. [DOI] [PubMed] [Google Scholar]
- 53.Su HM, Keswick LA, Brenna JT. Increasing dietary linoleic acid in young rats increases and then decreases docosahexaenoic acid in retina but not in brain. Lipids. 1996;31:1289–1298. doi: 10.1007/BF02587915. [DOI] [PubMed] [Google Scholar]
- 54.Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.FAO/WHO . Interim summary of conclusions and dietary recommendations on total fat and fatty acids. WHO HQ; Geneva: Nov 10–14, 2010. 2008. [Google Scholar]
- 56.van Goor SA, Dijck-Brouwer DA, Doornbos B, Erwich JJ, Schaafsma A, Muskiet FA, Hadders-Algra M. Supplementation of DHA but not DHA with arachidonic acid during pregnancy and lactation influences general movement quality in 12-week-old term infants. Br J Nutr. 2010;103:235–242. doi: 10.1017/S0007114509991528. [DOI] [PubMed] [Google Scholar]
- 57.van Goor SA, Dijck-Brouwer DA, Hadders-Algra M, Doornbos B, Erwich JJ, Schaafsma A, Muskiet FA. Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins Leukot Essent Fatty Acids. 2009;80:65–69. doi: 10.1016/j.plefa.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 58.van Goor SA, Schaafsma A, Erwich JJ, Dijck-Brouwer DA, Muskiet FA. Mildly abnormal general movement quality in infants is associated with higher Mead acid and lower arachidonic acid and shows a U-shaped relation with the DHA/AA ratio. Prostaglandins Leukot Essent Fatty Acids. 2009;82:15–20. doi: 10.1016/j.plefa.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Galli C, White HB, Jr., Paoletti R. Lipid alterations and their reversion in the central nervous system of growing rats deficient in essential fatty acids. Lipids. 1971;6:378–387. doi: 10.1007/BF02531374. [DOI] [PubMed] [Google Scholar]
- 60.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340:810–813. doi: 10.1016/0140-6736(92)92684-8. [DOI] [PubMed] [Google Scholar]
- 61.Greiner RS, Catalan JN, Moriguchi T, Salem N., Jr. Docosapentaenoic acid does not completely replace DHA in n-3 FA-deficient rats during early development. Lipids. 2003;38:431–435. doi: 10.1007/s11745-003-1080-2. [DOI] [PubMed] [Google Scholar]
- 62.Brand A, Crawford MA, Yavin E. Retailoring docosahexaenoic acid-containing phospholipid species during impaired neurogenesis following omega-3 alpha-linolenic acid deprivation. J Neurochem. 2010;114:1393–1404. doi: 10.1111/j.1471-4159.2010.06866.x. [DOI] [PubMed] [Google Scholar]
- 63.Diau GY, Loew ER, Wijendran V, Sarkadi-Nagy E, Nathanielsz PW, Brenna JT. Docosahexaenoic and arachidonic acid influence on preterm baboon retinal composition and function. Invest Ophthalmol Vis Sci. 2003;44:4559–4566. doi: 10.1167/iovs.03-0478. [DOI] [PubMed] [Google Scholar]
- 64.Lim SY, Hoshiba J, Moriguchi T, Salem N., Jr. N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr Res. 2005;58:741–748. doi: 10.1203/01.PDR.0000180547.46725.CC. [DOI] [PubMed] [Google Scholar]
- 65.Champoux M, Hibbeln JR, Shannon C, Majchrzak S, Suomi SJ, Salem N, Jr., Higley JD. Fatty acid formula supplementation and neuromotor development in rhesus monkey neonates. Pediatr Res. 2002;51:273–281. doi: 10.1203/00006450-200203000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.