Abstract
Marijuana is one of the most commonly used and abused drugs. Δ-9-tetrahydrocannabinol (Δ-9-THC), the primary psychoactive component in marijuana, is FDA-approved to ameliorate AIDS-associated wasting. Because cannabinoid receptors are expressed on cells of the immune system, it is possible that chronic Δ-9-THC use may impact HIV disease progression. Until recently, longitudinal, controlled, systems-approach studies on the effects of cannabinoids on disease progression were lacking. Data from our controlled studies in non-human primates show chronic Δ-9-THC administration prior to and during simian immunodeficiency virus (SIV) infection ameliorates disease progression, attenuates viral load and tissue inflammation, significantly reducing morbidity and mortality of SIV-infected macaques. Identification of possible mechanisms responsible for this modulation of disease progression is complicated due to the multiplicity of cannabinoid-mediated effects, tissue specific responses to the viral infection, multiple cellular mechanisms involved in inflammatory responses, coordinated neuroendocrine and localized responses to infection, and kinetics of viral replication. Emerging results from our studies reveal that the overall mechanisms mediating the protective effects of cannabinoids involve novel epigenomic regulatory mechanisms in need of systematic investigation. Here, we review the evidence supporting an immunomodulatory role for cannabinoids and its impact to disease progression with focus on HIV/SIV infection.
Keywords: cannabinoids, simian immunodeficiency virus, neuroimmune, epigenetics, HIV, DNA methylation
The cannabinoid system
Cannabis sativa (i.e., marijuana) contains over 60 different chemical constituents called cannabinoids (Dewey, 1986; Hollister, 1986). The cannabinoids include cannabidiol, cannabinol, and the major psychoactive constituent Δ-9-THC. An endogenous cannabinoid system consisting of anandamide and 2-arachidonylglycerol, derivatives of arachidonic acid, has also been described (Felder et al., 1996). These two endogenous cannabinoid ligands have short half-lives and likely function as neuromodulators at or near their site of synthesis. Their behavioral and physiological effects are similar to those of Δ-9-THC (Pertwee et al., 1993), and are mediated by binding to two major cannabinoid receptor subtypes, CB1 and CB2 (Felder and Glass, 1998; Pertwee, 1997). The CB1 receptor is preferentially expressed in the brain and has been identified as the mediator of cannabis-induced neurobehavioral effects (Ameri, 1999). The CB2 receptor is expressed primarily in peripheral tissues; it has 44% homology with the CB1 receptor (Munro et al., 1993), and is found on the surface of immune cells such as T- and B-cells, in the marginal zones of the spleen, and in tonsils. Both CB1 and CB2 receptors are coupled to Gi/o proteins and have been demonstrated to inhibit adenylate cyclase after short-term treatment (Howlett, 1995) and activate MAP kinase (Bouaboula et al., 1996). In addition to their neurobehavioral effects, cannabinoids affect the immune response. Several lines of evidence, derived mostly from in vitro studies, indicate that cannabinoids exert multiple effects on immune function that could modulate disease progression.
Cannabinoid Immunomodulation & HIV
Recreational and medicinal cannabinoid use in HIV-infected individuals
Antiretroviral therapy (ART) has substantially reduced morbidity and mortality in HIV-infected patients. HIV infection has become a more chronic disease during which co-morbid conditions such as drug abuse (Prentiss et al., 2004) have greater potential of altering the course of disease progression by affecting metabolic (Kino et al., 2003), immune, and neurobehavioral function (Lee et al., 2001). The greater likelihood of cannabinoid use, which is: a) frequent in the general population (Substance Abuse and Mental Health Services Administration, 2004); b) perceived by the lay public as having low health risk; and c) promoted as an appetite stimulant in AIDS patients, has the potential of affecting the progression of the disease (Bing et al., 2001; Nair et al., 2004; Bell et al., 1998; Khalsa and Royal, 2004; Galvan et al., 2003). Dronabinol (Marinol®), a synthetic form of Δ-9-THC, is approved by the Food and Drug Administration for treatment of HIV-associated anorexia (ElSohly et al., 2001). Although this approval has gained strong support from the lay public, little scientific evidence exists to support the efficacy of such an intervention (Watson et al., 2000). Moreover, few clinical studies have examined the impact of chronic Δ-9-THC use on the course and progression of HIV infection. Studies have looked at the association between marijuana use and rate of progression to AIDS in HIV+ males (Di Franco et al., 1996). Though not controlled, those studies did not show detrimental association between marijuana use and disease progression. Other prospective studies have examined the impact of short-term (21 day) Δ-9-THC administration (3 daily 0.9 g marijuana cigarettes; 3.95% Δ-9-THC) on HIV viral load. The results from that study did not show substantial elevation in viral load in HIV+ individuals receiving stable antiretroviral regimens containing nelfinavir or indinavir (Abrams et al., 2003), and thus, were considered to reflect the relative safety of short-term Δ-9-THC use in this patient population. Supporting evidence for this conclusion was provided by more recent studies that did not find short-term or long-term adverse effects of use of marijuana on CD4 cell count in over 400 HIV+ men followed for up to 11 years (Chao et al., 2008). However, controlled studies examining the impact of prolonged Δ-9-THC use and its impact on viral replication, immune phenotype markers, and overall disease progression remained lacking.
Relevance and contribution of our studies
The long-term effects of cannabinoid administration on the progression of HIV infection have not been previously examined. A controlled study examining the impact of chronic cannabinoid use on the immune, metabolic, and nutritional state of HIV+ individuals is complicated by the difficulty in selecting a homogenous patient population. In addition, the study of the host response from the time of initial infection to the development of the symptomatic phase is problematic. Furthermore, the confounding effect of prescription and non-prescription drugs frequently used by HIV+ individuals can alter the results obtained as well as their interpretation. The infection of rhesus macaques with simian immunodeficiency virus (SIV) circumvents these limitations, allowing for a controlled study design to identify alterations in biological systems at specific stages of the disease. Based on its genetic, antigenic, and biologic properties, SIV is the closest known relative of the human AIDS viruses (Gardner and Luciw, 1988). SIV infection results in a disease that is remarkably similar to HIV infection (Arthur et al., 1986). Intravenous inoculation with SIV results in peak viral load between days 14 and 21, and a well-established viral antigenemia after ~30 days. This temporal progression, which may vary by strain, is followed by an SIV-infected, yet asymptomatic period, that ultimately progresses to a clinical AIDS stage. Thus, SIV infection of rhesus macaques is recognized as one of the best animal models for studying the course of HIV infection.
We examined the impact of twice daily, intramuscular injection of Δ-9-THC (0.32 mg/kg) one month prior to intravenous infection with SIVmac251 in male rhesus macaques. Using this protocol of administration, we achieved consistent levels of drug across subjects, with average prevailing plasma Δ-9-THC levels of 22 ng/ml. Our studies show that chronic treatment with Δ-9-THC, decreases plasma and CSF viral load and tissue inflammation, significantly reducing morbidity and mortality of SIV-infected macaques (Molina et al., 2010). Interestingly, other than what appears to be better retention of body mass and attenuated inflammatory milieu, no significant alterations in immune phenotype were observed in animals administered Δ-9-THC for 28 days prior to and throughout the course of infection. In subsequent studies, we also demonstrated that chronic Δ-9-THC administration to uninfected macaques for up to 12 months did not alter lymphocyte subtypes, naïve or memory subsets, and proliferation and apoptosis of T lymphocytes when compared to time-matched vehicle-treated controls (LeCapitaine et al., 2011). However, those studies showed that chronic Δ-9-THC administration to naïve animals increased CD8+ and CD4+ lymphocyte expression of CXCR4. Whether this restricted modulation in lymphocyte co-receptor expression may play a role in host response to SIV infection is unclear. However, we speculate that these effects could potentially contribute to T cell homing to sites of infection. Similarly, clinical studies have failed to show significant alterations in immune cell phenotype in chronic cannabis users (Bredt et al., 2002).
Possible protective mechanisms of cannabinoids
A potential mechanism for the overall protective effects seen with chronic Δ-9-THC is through suppression of the inflammatory response in SIV-infected macaques. Several studies have reported immunosuppressive and anti-inflammatory effects of cannabinoids (Klein et al., 2000; Faubert Kaplan and Kaminski, 2003; Ehrhart et al., 2005; Fischer-Stenger et al., 1993), and several lines of evidence indicate that cannabinoid immunomodulation results from cell-mediated events including attenuated cytokine production, decreased inflammatory cell recruitment, and protection from injury resulting from release of toxic mediators by infected cells. Cannabinoids, including Δ-9-THC, have been shown to have significant immunomodulatory effects (Friedman et al., 2003; Klein et al., 2003) on cytokine production and lymphocyte function and survival (Zhu et al., 1998; Friedman et al., 1995; Nahas et al., 1974) as well as cell-mediated immunity (Newton et al., 1994). Similar immunosuppressant effects on lymphocyte (Daul and Heath, 1975) and alveolar macrophage (Cabral et al., 1991) function have been reported in nonhuman primates. Furthermore, the potential of cannabinoids to regulate the activation and balance of human Th1/Th2 cells by a CB2 receptor-dependent pathway has been suggested by findings from several studies (Pross et al., 1990; Yuan et al., 2002). While the immunosuppressant effects of THC may be detrimental under certain conditions, emerging data suggest that this may not be the case in SIV/HIV infection (Reiss, 2010).
HIV thrives in a proinflammatory state
Uncontrolled HIV replication activates the immune system resulting in production of pro-inflammatory cytokines, a rise in lymphocyte proliferation and apoptosis (Meyaard et al., 1992), and an imbalance in Th1/Th2 responses (Romagnani et al., 1994). Moreover, viral replication is enhanced in an inflammatory state (Kotler et al., 1993; McGowan et al., 2004; Decrion et al., 2005), and chronic inflammatory states are correlated with increased levels of viremia (Groot et al., 2006) resulting from increased viral replication driven by pro-inflammatory cytokines (Connolly et al., 2005; Kfutwah et al., 2006). Studies have shown a modulatory effect of pro-inflammatory cytokines, particularly TNF-α, on viral replication (Decrion et al., 2005; Connolly et al., 2005; Kfutwah et al., 2006). In addition, compartmentalized viral replication in the genital tract increased in the presence of inflammation (Gumbi et al., 2008).
Alternative mechanisms that could specifically contribute to decreased pathogenesis of HIV/SIV infection
Recent studies have demonstrated that selective stimulation of CB2 receptors suppressed IFN-gamma-induced CD40 expression and JAK/STAT1 signaling and this resulted in decreased microglial TNF-alpha and nitric oxide production induced by IFN-gamma (Ehrhart et al., 2005). Others have also reported an ameliorating effect of cannabinoids on neuroinflammation (Cabral and Griffin-Thomas, 2008). Thus, a cannabinoid-mediated reduction in system-wide inflammation and CD4+ T cell activation may protect the host and lower viral replication as indicated by our data showing that SIV-infected macaques chronically treated with Δ-9-THC have decreased neuroinflammation and lower CNS viral load (Winsauer et al., 2011).
Cannabinoids protect from or decrease HIV viral replication
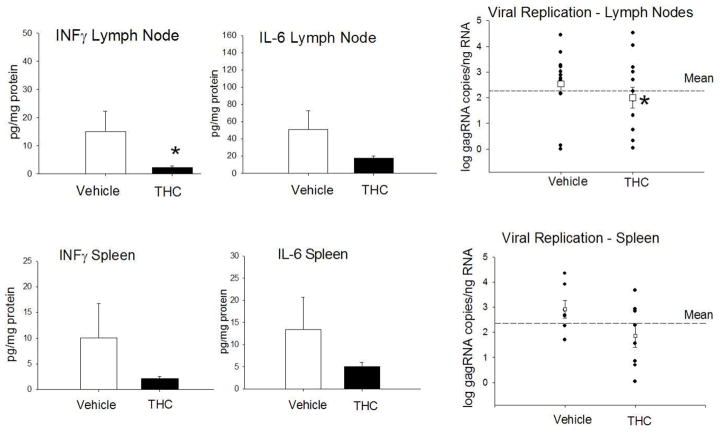
The reports in the literature describing effects of cannabinoids on HIV replication are conflicting. Studies have reported that 10 day Δ-9-THC administration (10 mg/kg) to immunodeficient mice (huPBL-SCID) implanted with human peripheral blood leukocytes (PBL), and infected with an HIV reporter construct, resulted in a transient increase in the percentage of PBL expressing CCR5 and CXCR4 (HIV co-receptors) as well as an increase in the number of HIV-infected cells (Roth et al., 2005). Moreover, in vitro studies have shown increased syncytia formation when MT-2 cells were cultured in the presence of cannabinoid agonists and HIV, suggesting increased infection and cytopathicity (Noe et al., 1998). In contrast, others have provided evidence that the synthetic cannabinoid WIN 55,212-2 potently inhibits HIV-1 expression in CD4+ lymphocytes and microglial cell cultures in a time- and concentration-dependent manner (Peterson et al., 2004). Moreover, Rock et al. demonstrated the involvement of CB2 receptors in cannabinoid antiviral activity in microglial cells suggesting that cannabinoid receptor agonists could directly suppress viral replication (Rock et al., 2007). This is strongly supported by the results from our studies showing a direct effect of cannabinoids on viral replication as seen by the reduced numbers of SIV-infected cells in cultures incubated with Δ-9-THC prior to infection (Molina et al., 2010). Our studies have also shown that Δ-9-THC-treated SIV-infected animals have lower viral loads in plasma as well as brain tissue when compared to control animals (Molina et al., 2010; Winsauer et al., 2011). Additional support for the possibility that chronic Δ-9-THC results in suppression of viral replication and that this is associated with suppressed inflammation is provided by findings from our recent studies in which animals received a prolonged period of Δ-9-THC treatment (15–18 months) prior to SIV infection. Our results show that the inflammatory milieu of lymph nodes and spleen at the time of sacrifice (approximately 5 months post-SIV infection) were lower in chronic Δ-9-THC-treated SIV-infected animals (Figure 1). In addition, this reduced inflammatory environment was associated with lower viral load in both tissues, particularly in lymph nodes, compared to vehicle-treated animals (Figure 1).
Figure 1.
Viral load in lymph nodes and spleen of Δ-9-THC-treated SIV-infected macaques show attenuated inflammation and viral replication. Chronic Δ-9-THC treatment resulted in lower INF-γ and IL-6 protein in lymph nodes and spleen. Viral RNA levels measured by quantitative real time RT-PCR as described previously (Molina et al., 2010) in lymph nodes and spleen of rhesus macaques treated chronically with Δ-9-THC (0.32 mg/kg; twice daily, i.m.) or vehicle for 15–18 months prior to inoculation with SIV. Tissues were collected at necropsy (113 to 503 days post-SIV infection). Chronic Δ-9-THC treatment resulted in lower levels of viral gagRNA in lymph nodes and spleen, as compared to vehicle-treated animals but reached statistical significance only in lymph nodes. *p≤0.05 determined by T-test.
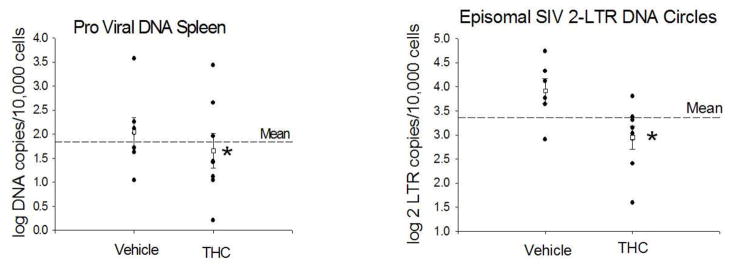
A more comprehensive analysis of SIV levels in spleen tissue reveals significantly lower numbers of spleen cells harboring SIV as reflected by the log DNA copies/cell (Figure 2). Moreover, levels of episomal SIV 2-long terminal repeat (2-LTR) DNA circles were also significantly lower in Δ-9-THC-treated SIV-infected animals (Figure 2). The 2-LTR circles are extra chromosomal products that remain in the nucleus after circularization and integration of the proviral DNA. These episomal products have been used as a marker of recent viral integration, and provide a discriminating measure of viral DNA entry into the nucleus after integration of the proviral DNA into the host cell chromosome (Mannioui et al., 2009). These results suggest that viral replication in immune tissues is reduced in Δ-9-THC-treated animals. Because SIV is known to persist in viral reservoirs, such as the spleen and lymph nodes, we speculate that control of this latent virus may contribute to improved course of infection in our macaque model.
Figure 2.
Proviral DNA, and episomal SIV 2-LTR circle DNA in spleen of vehicle and Δ-9-THC-treated SIV-infected animals obtained at necropsy (113 to 503 days post-SIV infection) of rhesus macaques treated chronically with Δ-9-THC or vehicle for 15–18 months prior to inoculation with SIV. The 2-LTR circles are extra chromosomal products that remain in the nucleus after circularization and integration of the proviral DNA. These episomal products have been used as a marker of recent viral integration, and provide a discriminating measure of viral DNA entry into the nucleus for comparison with proviral DNA copies integrated into the chromosome. *p<0.05 determined by T-test.
Additional protective effects of cannabinoids in HIV infection
In vitro studies, using a human macrophage-like cell line, have provided supporting evidence that cannabinoids attenuate HIV-1 Tat-induced migration, which in turn would be expected to contribute to decreased tissue inflammation (Raborn and Cabral, 2010). CB1 receptor agonists and elevation of endocannabinoid levels by the FAAH inhibitor URB597 prevented the downregulation of tight junction proteins ZO-1 and claudin-5, as well as inhibited Gp120-mediated damage of brain endothelium (Lu et al., 2008). While in vitro studies cannot recapitulate the in vivo state they do provide potential insight into the mechanisms occurring in vivo. Future studies are planned to examine the relevance of some of these findings, particularly as they pertain to barrier function. An additional modulator that has been proposed to affect progression of SIV infection has been the relative stress level of the host. This has been suggested as a mechanism contributing to the opiate modulation of SIV disease progression in studies by Donahoe et al.(Donahoe et al., 2009), which contrasted with that reported by Kumar et al. (Kumar et al., 2004). The neuroendocrine pathways involved in stress responses have been demonstrated to exert profound effects on immune function (Molina, 2006; Molina, 2005). However, no controlled studies have been conducted to examine the contribution of these mechanisms to SIV disease progression. Moreover, though invoked as a potential mechanism in studies by Donahoe et al., no measures of stress levels were obtained in those studies. Because cannabinoids may modulate hypothalamo-pituitary and sympathoadrenal activation (Brown and Dobs, 2002), we examined the potential role of attenuated stress levels, specifically the hypothalamo-pituitary-adrenal axis, as a mechanism for the cannabinoid-induced modulation of disease progression. Animals were housed in metabolic cages and 24 h urinary excretion of cortisol was measured for three consecutive days following an initial 24 h period of acclimation to the metabolic cage. As reflected in Table 1, no significant differences were noted in 24 h cortisol excretion assessed 3 months post-SIV infection in vehicle- or Δ-9-THC-treated animals. Whether cannabinoid administration can modulate stress levels during later periods of the infection remains to be explored. Moreover, whether Δ-9-THC altered sympathetic tone in immune tissues, such as the spleen, is yet to be investigated.
Table 1.
Average daily urinary cortisol excretion (ng/mg creatinine), plasma creatinine (mg/dl), and urinary creatinine excretion (mg/day) in vehicle-treated and Δ-9-THC-treated SIV-infected macaques measured at three months post-SIV infection. Animals were housed in metabolic cages and allowed 24 h to acclimate prior to performing 24 h urine collections on three consecutive days. Cortisol concentrations determined by radioimmunoassay have been normalized for urinary creatinine excretion. Plasma creatinine, urinary creatinine excretion, and glomerular filtration rate (GFR) are presented to reflect no difference in renal function at the time of urinary cortisol determinations. Values are average± SEM of N=4 in each group.
| Experimental Group | Urinary cortisol excretion (ng/mg creatinine/day) | Plasma creatinine (mg/dl) | Urinary creatinine excretion (mg/day) | GFR ml/min |
|---|---|---|---|---|
| Vehicle SIV-infected | 281±38 | 1.08±0.05 | 296±11 | 20±3 |
| Δ-9-THC SIV-infected | 250±72 | 1.05±0.03 | 283±7 | 19±2 |
GFR: glomerular filtration rate
Systems approach to study cannabinoid modulation of HIV disease
Taken together, the existing evidence suggests that cannabinoids may systematically modify host-pathogen interaction at various levels including the regulation of host immunity, inflammatory response, cell metabolism, as well as the ability of the virus to integrate into the host genome and replicate in host cells. Identification of possible mechanisms is complicated due to multiple aspects involved in the process, including tissue specific responses to the viral infection, multiple cellular mechanisms involved in inflammatory responses, coordinated neuroendocrine responses to infection, and kinetics of viral replication. Knowledge of specific cellular responses impacted by cannabinoids and the resulting modification in viral infectivity, integration, and replication can be obtained from in vitro studies. Transgenic models provide complementary information on the roles of specific proteins in the course of disease and the interaction with drugs of abuse. However, we speculate that shared pathways across organ systems may cooperatively affect viral kinetics and, as a result, disease progression. The multisystemic effects of cannabinoids could potentially impact several cell signaling and effector mechanisms involved in viral entry into host cells, integration into the host genome, viral replication, and damage to host cells, which likely contribute to the overall systemic response to the infection.
The multiplicity of factors involved in HIV disease progession, their intricate relevance, connectivity, and interaction cannot be effectively reconciled using isolated organ, cellular, or molecular approaches and requires alternative approaches to their investigation. Systems biology analysis is a powerful approach to identifying salient cellular and molecular signatures prevailing during the infection in the presence of cannabinoid use. In ongoing investigations, we are beginning to examine the contribution of genome-wide transcriptional modulation to the cannabinoid-associated disease phenotype. Emerging data from our laboratory indicate that Δ-9-THC produces marked alterations in gene expression in the duodenum of SIV-infected macaques. When subjected to pathway analysis, differential gene expression patterns reveal important and functionally relevant systems involved in host response to infection, including those involved in the immune/stress response, cell adhesion and tight junction signaling pathways. The gastrointestinal tract contains a significant fraction of the body’s lymphoid tissue, the gut-associated lymphoid tissue (GALT). The GALT, comprised of specialized lymphoid tissue, Peyer’s patches and activated lymphocytes dispersed throughout the epithelium and lamina propria of the intestines, has been identified as a primary site of SIV/HIV replication (Kotler et al., 1993), and is the site of primary interaction with antigens. Many of the lymphoid cells found in the gut are the effector memory T cell subtype (CD4+CD95+CD28−) and express CCR5, the co-receptor used by SIV/HIV for cell entry during the early stage of infection. This makes them primary targets for viral infection and proliferation (Mohri et al., 1998) leading to a rapid and persistent decrease in the CD4+ effector cells in GALT that is not paralleled in the periphery (Verhoeven et al., 2008). CD4+ T cell depletion is associated with an increase in both apoptosis (Pandrea et al., 2007; Li et al., 2005) and proliferation of GALT T lymphocytes and with increased tissue inflammation, immune activation, and decreased tissue repair (McGowan et al., 2004; Decrion et al., 2005). This enhanced inflammatory response has been proposed to impair the replenishment of CD4+ T cells in the GALT (Li et al., 2005; Sankaran et al., 2008), and to contribute to maintenance of viral load. We speculate that cannabinoids modulate intestinal inflammatory responses through CB2 receptors expressed in cells of the GALT (Thuru et al., 2007), rendering protection from the localized activation of inflammation in SIV-infected animals, potentially reducing tissue injury associated with enhanced viral replication in GALT.
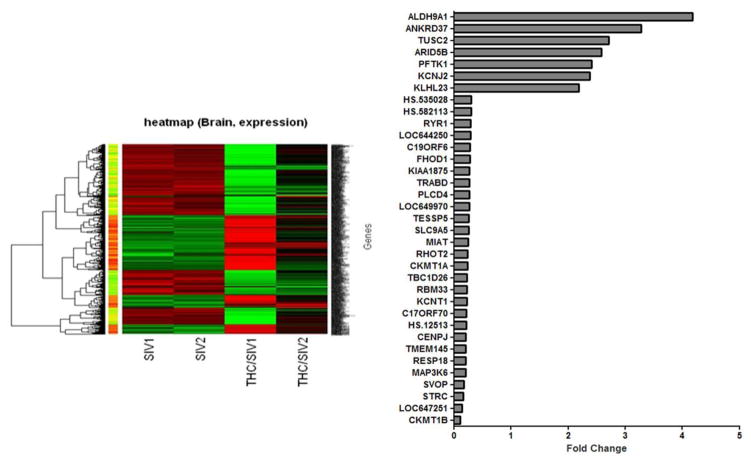
The brain is an early target in HIV pathology (Hurtrel et al., 1991) and plays a central role in modulating the neuroendocrine signals that regulate immune responses. The central nervous system is a possible site for Δ-9-THC-induced immunomodulation which could directly affect SIV/HIV neuropathogenesis. Focused analysis of gene microarray data should provide insight into the network of pathways that potentially mediate the modulatory effects of cannabinoids. Hence, we have performed microarray analysis on the brains (lateral cerebellum) of vehicle-treated SIV-infected and Δ-9-THC-treated SIV-infected macaques (Figure 3). These animals were treated with vehicle or Δ-9-THC for 15–18 months and SIV-infected for approximately 5 months. Our results show that chronic Δ-9-THC resulted in significant changes in expression of 153 genes (7 upregulated and 146 downregulated genes) compared to the vehicle-treated SIV-infected animals. Several genes that were differentially regulated could potentially modulate inflammation and HIV infectivity. For example, in the Δ-9-THC-treated SIV-infected animals, mRNA for I-kappa-B kinase subunit gamma (IKBKG), coactivator-associated arginine methyltransferase (CARM1), and GLE1 were decreased by 45% or less as compared to the vehicle-treated SIV-infected animals. IKBKG encodes the regulatory subunit of the inhibitor of κB kinase complex, which activates NF-κB pathway. Additionally, CARM1, which has been shown to be a novel transcriptional coactivator of NF-κB (Covic et al., 2005), was downregulated in the Δ-9-THC animals. NF-κB signaling affects many cellular processes including the transcription of proinflammatory genes. Additionally, viruses including HIV have evolved mechanisms to modulate the NF-κB pathway for enhanced viral replication, host cell survival and immune response and evasion. Because chronic Δ-9-THC decreases the mRNA for IKBG and CARM1, which would be expected to result in decreased activation of the NF-κB pathway, these findings suggest a potential mechanism for the decreased inflammatory milieu and viral load in the brains of the Δ-9-THC-treated SIV-infected macaques. The translocation of viral mRNA from the nucleus to the cytoplasm is mediated via HIV Rev proteins. A nuclear reporter signal (NES) is required for REV to move through the nuclear pore complex. It has been reported that GLE1 contains a NES and is localized at nuclear pore complexes, indicating that GLE1 is an RNA-export factor (Murphy and Wente, 1996). Thus, one could speculate that the downregulation of GLE1 by chronic Δ-9-THC treatment may decrease the export of HIV from the nucleus resulting in decreased viral production. This prediction is in agreement with our results from viral kinetics in spleen and lymph nodes (Figures 1 and 2) as well as with our observations of suppressed viral replication in MT4-R5 cells (Molina et al., 2010). The regional and cellular specificity of these changes remains to be determined.
Figure 3.
Microarray analysis of brains (lateral cerebellum) of 2 vehicle-treated SIV-infected and 2 Δ-9-THC-treated SIV-infected macaques. Animals were treated with vehicle or Δ-9-THC for 15–18 months and SIV-infected for approximately 5 months. Results show that chronic Δ-9-THC resulted in significant changes in expression of 153 genes (7 upregulated and 146 downregulated genes) compared to the vehicle-treated SIV-infected animals. The histogram identifies all genes that were significantly up- (>2-fold) and downregulated (>70%). The Illumina Custom algorithm, which assumes that target signal intensity is normally distributed among replicates of the same condition, was used to compare the level of gene expression. The signals were normalized by cubic spline normalization method and then transformed to log2 scale. Rank Product, a non-parametric statistical method based on the ranks of fold changes (Breitling et al., 2004), was employed to detect differentially expressed genes. Rank Product detects genes that are consistently found among the most upregulated/downregulated genes in a number of replicate experiments. With the selection of 200 permutations and 0.01 as the cutoff for p-value, two tables, one for upregulated genes, another for downregulated genes, were obtained.
Cannabinoid modulation of SIV infection: role for epigenetics
Recently, regulation of gene expression by epigenetic mechanisms has gained much attention. Two primary modes of epigenetic modification occur in eukaryotic cells, DNA methylation and histone modification. DNA methylation is the most characterized epigenetic modification. Small changes in DNA methylation density can have profound biological effects through modulation of gene expression. A prerequisite for understanding the function of DNA modification in disease progression is knowledge of the distribution of these modifications in the genome. While the impact of cannabinoids on DNA methylation has not yet been examined, recent studies have shown CB1 receptor-mediated increases in DNA methyltransferase (DNMT) activity in cells via activation of the p38 and p442/44 mitogen-activated protein kinase (MAPK) pathways. This enhancement of DNMT activity is associated with a dramatic increase in DNA methylation and inhibition of gene expression related to several cell functions in differentiating cells (Paradisi et al., 2008). Results from our ongoing studies have provided evidence that this mechanism may be involved in the immunomodulation observed in cannabinoid-treated animals.
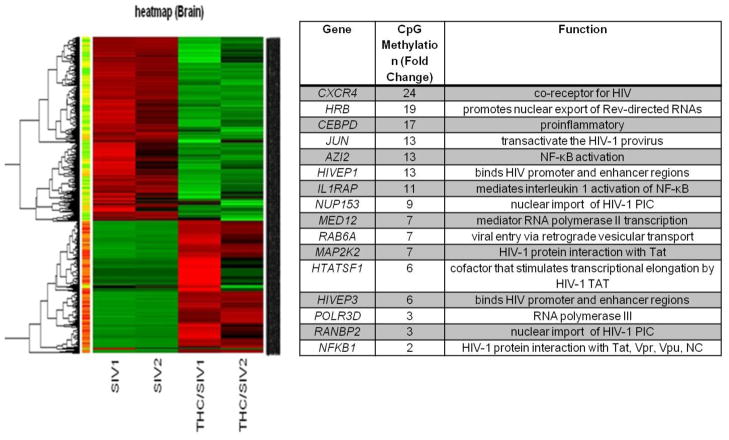
Ongoing studies from our laboratory have begun to examine the cannabinoid effects on DNA methylation in tissues we consider to be critical checkpoints for HIV/SIV replication and pathogenesis. CpG methylation arrays were performed on the same brain tissue as the microarray study (Figure 4). Gene expression and DNA methylation pattern analyses reveal that at least 50% of the genes that are differentially expressed in Δ-9-THC-treated SIV-infected vs. vehicle-treated SIV-infected animals can be linked to differential DNA methylation patterns. Of interest, is the finding of relevant hypermethylated genes that can impact viral entry, integration, and production as well as inflammation, shown in the insert table of Figure 4. Among the most relevant genes CXCR4, nucleoporin 153kDa (NUP153), RAN binding protein 2 (RANBP2), Human immunodeficiency virus type 1 enhancer binding protein (HIVEP1), HIV-1 Rev binding protein (HRB), human immunodeficiency virus type I enhancer binding protein 3 (HIVEP3), HIV TAT specific factor 1 (HTATSF1), mediator subunit complex 12 (MED12), CCAAT-enhancer binding protein delta (C/EBPD), and interleukin 1 receptor accessory protein (IL1RAP) are all hypermethylated (ranging from 3- to 24-fold greater) in the Δ-9-THC-treated SIV-infected macaques compared to the vehicle-treated SIV-infected macaques. CXCR4, a chemokine receptor, in addition to contributing to inflammatory cell recruitment can be used as a coreceptor for HIV entry into cells, particularly during later stages of the infection. The HIV preintegration complex (PIC) enters the nucleus using nuclear pore complexes. It has been shown that RANBP2 and NUP153 are cofactors for HIV-1 PIC nuclear entry (Sabri et al., 2007; Zhang et al., 2010). HIVEP1 binds specific DNA sequences in the promoter and enhancer regions of several viruses, including HIV. The HRB gene product binds the HIV Rev activation domain and promotes nuclear export of Rev-directed RNAs. HIVEP3 binds both promoter and enhancer regions of HIV. HTATSF1 codes for a cofactor that stimulates transcriptional elongation by HIV-1 TAT, which binds the HIV-1 promoter. The hypermethylation and predicted subsequent suppression of gene expression, suggests a novel hypothesis that Δ-9-THC may hijack the expression of key proteins required for viral cell and nuclear entry, HIV promoter and enhancer binding, and transcriptional elongation of HIV. Future studies are planned to determine whether expression of these factors is indeed affected in the brain of Δ-9-THC-treated animals and to identify cellular specificity of the changes observed. Nevertheless, our results strongly suggest that Δ-9-THC, through epigenetic mechanisms, is altering viral kinetics in the brain. These mechanisms agree with the viral kinetics we observed in the spleen (Figure 3). Similar viral kinetic analysis of brain tissues are in progress. In addition, our results suggest that the hypermethylation of C/EBPD, a regulator of several inflammatory mediators including IL-1, IL-6, TNF-α (Li et al., 2005), and IL1RAP which mediates IL-1 activation of NF-κB, would be important epigenetic mechanisms of neuroimmunomodulation relevant to HIV/SIV neuropathology. This speculation is supported by our findings that among SIV-infected animals treated chronically with Δ-9-THC, none to date has demonstrated substantial CNS findings on routine histological analysis at necropsy (Winsauer et al., 2011).
Figure 4.
DNA methylation patterns in brain (lateral cerebellum) of Δ-9-THC- and vehicle-treated SIV-infected macaques obtained at necropsy (113 to 503 days post-SIV infection). DNA methylation profiles were established using the Infinium Methylation27, which interrogates more than 27,000 CpG islands in more than 14,000 genes. Data was normalized and the distribution of the fraction (methylation fraction) was assumed to be normally distributed among replicates. Similar to the analysis of microarray data, we adopted the non-parametric procedure, Rank Product, to identify CpG islands whose methylation patterns show increasing or decreasing trends between control and SIV, and between VEH/SIV and THC/SIV. The methylation ratios were extracted after average normalization and then were used for detecting differential methylation patterns. With the selection of 200 permutations and 0.01 as the cutoff for p-value, two tables, one for upregulated islands, another for downregulated islands, were obtained. Meanwhile, genes spanned by these differential CpG islands were obtained via the annotation in Illumina studio. The table insert identifies some of the hypermethylated genes that are potentially delaying/decreasing SIV disease progression.
Alternatively, cannabinoids could also be modulating the expression of miRNAs, a novel class of endogenous, small, noncoding RNAs that negatively regulate gene expression via degradation or translational inhibition of their target mRNAs. MicroRNAs are important regulators of cell differentiation, proliferation/growth, mobility, and apoptosis (Zhang, 2008). Although the functional significance of specific miRNA expression is largely unclear, studies indicate that a broad range of inflammatory conditions is associated with dysregulation of miRNA expression. In addition, they are required for mounting a potent innate immune response (miR-155) and suppressing premature innate immune cell (neutrophil) activation (miR-223). While HIV/SIV infection results in CD4+ T cell depletion, widespread tissue inflammation, and localized/generalized immune cell activation, data from our ongoing studies indicate these changes are considerably suppressed in cannabinoid-treated SIV-infected animals. Although interesting, several questions including the molecular mechanisms underlying the protective effects of cannabinoids remain unaddressed. Does cannabinoid administration induce the expression of specific miRNAs? Are cannabinoid-induced miRNAs suppressing inflammation by selectively targeting the mRNA of proinflammatory genes? Are cannabinoid-induced miRNAs regulating the activity of the mRNA for genes associated with immune cell activation? Results from our ongoing studies indicate that chronic Δ-9-THC alters expression of miRNA in SIV-infected macaques (Table 2). Several miRNAs were found to be differentially expressed in CD4+ cells, intestinal mucosa, and brain when Δ-9-THC-treated SIV-infected macaques were compared to vehicle-treated SIV-infected macaques. Moreover, specific miRNAs were shown to be differentially expressed in CD4+ T cells, intestinal mucosa (colon), and brain of Δ-9-THC-treated SIV-infected macaques.
Table 2.
Differentially expressed (up and downregulated) miRNAs in CD4+ T cells isolated from a Δ-9-THC-treated SIV-infected macaque showing at least 2-fold change in expression when compared to a vehicle-treated SIV-infected macaque. Log2 ratios were converted to fold changes. The miRNAs miR-142-3p, -142-5p and -150 have been previously shown to be expressed abundantly in naïve T cells. However, upon cellular activation (differentiation into activated and memory T cells) the expression of all three miRNAs is significantly downregulated. The increased expression of these three miRNAs observed in the Δ-9-THC-treated SIV-infected macaque may indirectly suggest reduced or significantly lower levels of immune activation, a hallmark of HIV disease progression.
| Upregulated miRNAs (n=58) | Downregulated miRNAs (n=13) | ||
|---|---|---|---|
| miRNA ID | Fold Change | miRNA ID | Fold Change |
| hsa-miR-125b-1*/mmu-miR-125b-3p/rno-miR-125b-3p | 2.54 | hsa-miR-1237 | −2.4 |
| hsa-miR-1264 | 3.14 | hsa-miR-1249 | −2.93 |
| hsa-miR-134/mmu-miR-134/rno-miR-134 | 2.66 | hsa-miR-126/mmu-miR-126-3p/rno-miR-126 | −2.36 |
| hsa-miR-142-3p/mmu-miR-142-3p/rno-miR-142-3p | 2.81 | hsa-miR-139-3p/mmu-miR-139-3p/rno-miR-139-3p | −2.41 |
| hsa-miR-142-5p/mmu-miR-142-5p/rno-miR-142-5p | 2.39 | hsa-miR-223/mmu-miR-223/rno-miR-223 | −2.75 |
| hsa-miR-148b/mmu-miR-148b/rno-miR-148b-3p | 2.5 | hsa-miR-370/mmu-miR-370/rno-miR-370 | −61.9 |
| hsa-miR-150/mmu-miR- 150/rno-miR-150 | 2.05 | hsa-miR-372 | −2.4 |
| hsa-miR-181a/mmu-miR-181a/rno-miR-181a | 2.19 | hsa-miR-373 | −2.69 |
| hsa-miR-181c/mmu-miR-181c/rno-miR-181c | 2.1 | hsa-miR-532-5p/mmu-miR-532-5p/rno-miR-532-5p | −3.44 |
Of interest, our results indicate that three miRNAs (mir-142-3p, -142-5p and -150) previously shown to be highly enriched in naïve T cells (Wu et al., 2007), and significantly downregulated upon cellular activation (differentiation into activated and memory T cells), are expressed at high levels in the CD4+ T cells of the Δ-9-THC-treated SIV-infected animals (Table 2). Additionally, results from similar analysis of intestinal mucosa reflect increased expression of several miRNAs including miR-492, a miRNA predicted to target the IL-22 receptor. Moreover, significant downregulation of miR-21 was observed in the brain of Δ-9-THC-treated SIV-infected macaques. This miRNA was previously shown to be upregulated in several inflammatory conditions (Moschos et al., 2007; Lu et al., 2009; Wu et al., 2008). These findings suggest that Δ-9-THC changes miRNA expression resulting in lower levels of immune activation in Δ-9-THC-treated SIV-infected macaques than in vehicle-treated SIV-infected macaques. Furthermore, they concur with our observations of lower pro-inflammatory cytokine expression in brains of those animals and are the focus of current investigations.
Summary and Perspectives
Taken together, various lines of evidence demonstrate that chronic Δ-9-THC initiated prior to, and continued throughout the course of SIV infection does not impair, and may enhance, the host’s ability to control viral load, and prolongs survival. While the small group sizes and natural variation in SIV disease is a limitation of primate studies, the consistent findings of immunosuppression, decreased viral loads, and reduction in neuropathologies in different cohorts of animals as well as in tissues central to control of disease progression strongly supports an important immunomodulatory role for cannabinoids. Whether similar responses would have been observed if Δ-9-THC administration was initiated after the acute infection period, and whether chronic cannabinoid administration would interact with antiretroviral therapy effectiveness, pharmacokinetics, and/or toxicity, remains to be determined and warrants further studies. Furthermore, how the concentrations of Δ-9-THC achieved in our studies compare with those in patient populations consuming either oral or smoked Δ-9-THC remains to be examined. Moreover, whether similar protective effects would be observed with higher circulating levels of Δ-9-THC remains to be established. What is clear is that a systems approach is necessary to integrate understanding of the biomedical consequences of chronic cannabinoid administration on disease progression. Novel directions embracing the identification of genomic mechanisms promise to elucidate previously unidentified mechanisms important in disease progression and control of infection. Furthermore, these new mechanisms may lead to the development of therapeutic targets directed at improved morbidity and mortality.
Acknowledgments
Source of support: DA020419 and DA030053
The authors would like to thank all of the individuals that have provided technical and analytical support (Jaime Hubbell, Tesslyn Land, Edith Walker, Blake Lewis, Nedra Lacour), as well as critical scientific discussions with Drs. Joe Moerschbaecher, Robert Siggins, Leslie Birke, Robin McGoey, and Lisa Harrison-Bernard. The authors are indebted to Dr. Marilyn Huestis from NIDA Intramural Research Program for THC analysis.
Footnotes
Disclaimers: none
References
- 1.Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003;139:258–266. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 3.Arthur LO, Gilden RV, Marx PA, Gardner MB. Simian acquired immunodeficiency syndrome. Prog Allergy. 1986;37:332–352. doi: 10.1159/000318452. [DOI] [PubMed] [Google Scholar]
- 4.Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121 (Pt 11):2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 5.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 6.Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 7.Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol. 2002;42:82S–89S. doi: 10.1002/j.1552-4604.2002.tb06007.x. [DOI] [PubMed] [Google Scholar]
- 8.Breitling R, Armengaud P, Amtmann A, Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Brown TT, Dobs AS. Endocrine effects of marijuana. J Clin Pharmacol. 2002;42:90S–96S. doi: 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 10.Cabral GA, Griffin-Thomas L. Cannabinoids as therapeutic agents for ablating neuroinflammatory disease. Endocr Metab Immune Disord Drug Targets. 2008;8:159–172. doi: 10.2174/187153008785700118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabral GA, Stinnett AL, Bailey J, Ali SF, Paule MG, Scallet AC, Slikker W., Jr Chronic marijuana smoke alters alveolar macrophage morphology and protein expression. Pharmacol Biochem Behav. 1991;40:643–649. doi: 10.1016/0091-3057(91)90376-d. [DOI] [PubMed] [Google Scholar]
- 12.Chao C, Jacobson LP, Tashkin D, Martinez-Maza O, Roth MD, Margolick JB, Chmiel JS, Rinaldo C, Zhang ZF, Detels R. Recreational drug use and T lymphocyte subpopulations in HIV-uninfected and HIV-infected men. Drug Alcohol Depend. 2008;94:165–171. doi: 10.1016/j.drugalcdep.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HIV disease-a review and rationale for new therapeutic approaches. AIDS Rev. 2005;7:168–180. [PubMed] [Google Scholar]
- 14.Covic M, Hassa PO, Saccani S, Buerki C, Meier NI, Lombardi C, Imhof R, Bedford MT, Natoli G, Hottiger MO. Arginine methyltransferase CARM1 is a promoter-specific regulator of NF-kappaB-dependent gene expression. EMBO J. 2005;24:85–96. doi: 10.1038/sj.emboj.7600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daul CB, Heath RG. The effect of chronic marihuana usage on the immunological status of rheusus monkeys. Life Sci. 1975;17:875–881. doi: 10.1016/0024-3205(75)90438-5. [DOI] [PubMed] [Google Scholar]
- 16.Decrion AZ, Dichamp I, Varin A, Herbein G. HIV and inflammation. Curr HIV Res. 2005;3:243–259. doi: 10.2174/1570162054368057. [DOI] [PubMed] [Google Scholar]
- 17.Dewey WL. Cannabinoid pharmacology. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- 18.Di Franco MJ, Sheppard HW, Hunter DJ, Tosteson TD, Ascher MS. The lack of association of marijuana and other recreational drugs with progression to AIDS in the San Francisco Men’s Health Study. Ann Epidemiol. 1996;6:283–289. doi: 10.1016/s1047-2797(96)00022-1. [DOI] [PubMed] [Google Scholar]
- 19.Donahoe RM, O’neil SP, Marsteller FA, Novembre FJ, Anderson DC, Lankford-Turner P, McClure HH. Probable deceleration of progression of Simian AIDS affected by opiate dependency: studies with a rhesus macaque/SIVsmm9 model. J Acquir Immune Defic Syndr. 2009;50:241–249. doi: 10.1097/QAI.0b013e3181967354. [DOI] [PubMed] [Google Scholar]
- 20.Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ElSohly MA, deWit H, Wachtel SR, Feng S, Murphy TP. Delta9-tetrahydrocannabivarin as a marker for the ingestion of marijuana versus Marinol: results of a clinical study. J Anal Toxicol. 2001;25:565–571. doi: 10.1093/jat/25.7.565. [DOI] [PubMed] [Google Scholar]
- 22.Faubert Kaplan BL, Kaminski NE. Cannabinoids inhibit the activation of ERK MAPK in PMA/Io-stimulated mouse splenocytes. Int Immunopharmacol. 2003;3:1503–1510. doi: 10.1016/S1567-5769(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 23.Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- 24.Felder CC, Nielsen A, Briley EM, Palkovits M, Priller J, Axelrod J, Nguyen DN, Richardson JM, Riggin RM, Koppel GA, Paul SM, Becker GW. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett. 1996;393:231–235. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- 25.Fischer-Stenger K, Dove Pettit DA, Cabral GA. Delta 9-tetrahydrocannabinol inhibition of tumor necrosis factor-alpha: suppression of post-translational events. J Pharmacol Exp Ther. 1993;267:1558–1565. [PubMed] [Google Scholar]
- 26.Friedman H, Klein TW, Newton C, Daaka Y. Marijuana, receptors and immunomodulation. Adv Exp Med Biol. 1995;373:103–113. doi: 10.1007/978-1-4615-1951-5_15. [DOI] [PubMed] [Google Scholar]
- 27.Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003;16:209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galvan FH, Burnam MA, Bing EG. Co-occurring psychiatric symptoms and drug dependence or heavy drinking among HIV-positive people. J Psychoactive Drugs. 2003;35(Suppl 1):153–160. doi: 10.1080/02791072.2003.10400510. [DOI] [PubMed] [Google Scholar]
- 29.Gardner MB, Luciw PA. Simian immunodeficiency viruses and their relationship to the human immunodeficiency viruses. AIDS. 1988;2(Suppl 1):S3–10. doi: 10.1097/00002030-198800001-00002. [DOI] [PubMed] [Google Scholar]
- 30.Groot F, van Capel TM, Schuitemaker J, Berkhout B, de Jong EC. Differential susceptibility of naive, central memory and effector memory T cells to dendritic cell-mediated HIV-1 transmission. Retrovirology. 2006;3:52. doi: 10.1186/1742-4690-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumbi PP, Nkwanyana NN, Bere A, Burgers WA, Gray CM, Williamson AL, Hoffman M, Coetzee D, Denny L, Passmore JA. Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T-cell responses in the female genital tract during chronic HIV infection. J Virol. 2008;82:8529–8536. doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- 33.Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- 34.Hurtrel B, Chakrabarti L, Hurtrel M, Maire MA, Dormont D, Montagnier L. Early SIV encephalopathy. J Med Primatol. 1991;20:159–166. [PubMed] [Google Scholar]
- 35.Kfutwah AK, Mary JY, Nicola MA, Blaise-Boisseau S, Barre-Sinoussi F, Ayouba A, Menu E. Tumour necrosis factor-alpha stimulates HIV-1 replication in single-cycle infection of human term placental villi fragments in a time, viral dose and envelope dependent manner. Retrovirology. 2006;3:36. doi: 10.1186/1742-4690-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalsa JH, Royal W. Do drugs of abuse impact on HIV disease? J Neuroimmunol. 2004;147:6–8. doi: 10.1016/j.jneuroim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Kino T, Mirani M, Alesci S, Chrousos GP. AIDS-related lipodystrophy/insulin resistance syndrome. Horm Metab Res. 2003;35:129–136. doi: 10.1055/s-2003-39072. [DOI] [PubMed] [Google Scholar]
- 38.Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- 39.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 40.Kotler DP, Reka S, Clayton F. Intestinal mucosal inflammation associated with human immunodeficiency virus infection. Dig Dis Sci. 1993;38:1119–1127. doi: 10.1007/BF01295730. [DOI] [PubMed] [Google Scholar]
- 41.Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78:11425–11428. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeCapitaine NJ, Zhang P, Winsauer P, Walker E, Vande Stouwe C, Porretta C, Molina PE. Chronic Delta-9-tetrahydrocannabinol Administration Increases Lymphocyte CXCR4 Expression in Rhesus Macaques. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984–1997. JAMA. 2001;285:1308–1315. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 45.Lu TS, Avraham HK, Seng S, Tachado SD, Koziel H, Makriyannis A, Avraham S. Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells. J Immunol. 2008;181:6406–6416. doi: 10.4049/jimmunol.181.9.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182:4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mannioui A, Bourry O, Sellier P, Delache B, Brochard P, Andrieu T, Vaslin B, Karlsson I, Roques P, Le Grand R. Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques. Retrovirology. 2009;6:106. doi: 10.1186/1742-4690-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGowan I, Elliott J, Fuerst M, Taing P, Boscardin J, Poles M, Anton P. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37:1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 49.Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RP, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 50.Mohri H, Bonhoeffer S, Monard S, Perelson AS, Ho DD. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science. 1998;279:1223–1227. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
- 51.Molina PE. Opioids and opiates: analgesia with cardiovascular, haemodynamic and immune implications in critical illness. J Intern Med. 2006;259:138–154. doi: 10.1111/j.1365-2796.2005.01569.x. [DOI] [PubMed] [Google Scholar]
- 52.Molina PE. Neurobiology of the stress response: contribution of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock. 2005;24:3–10. doi: 10.1097/01.shk.0000167112.18871.5c. [DOI] [PubMed] [Google Scholar]
- 53.Molina PE, Winsauer P, Zhang P, Walker E, Birke L, Amedee A, Stouwe CV, Troxclair D, McGoey R, Varner K, Byerley L, Lamotte L. Cannabinoid Administration Attenuates the Progression of Simian Immunodeficiency Virus. AIDS Res Hum Retroviruses. 2010;27(6):585–592. doi: 10.1089/aid.2010.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG, Lindsay MA. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics. 2007;8:240. doi: 10.1186/1471-2164-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 56.Murphy R, Wente SR. An RNA-export mediator with an essential nuclear export signal. Nature. 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- 57.Nahas GG, Suciu-Foca N, Armand JP, Morishima A. Inhibition of cellular mediated immunity in marihuana smokers. Science. 1974;183:419–420. doi: 10.1126/science.183.4123.419. [DOI] [PubMed] [Google Scholar]
- 58.Nair MP, Mahajan S, Hewitt R, Whitney ZR, Schwartz SA. Association of drug abuse with inhibition of HIV-1 immune responses: studies with long-term of HIV-1 non-progressors. J Neuroimmunol. 2004;147:21–25. doi: 10.1016/j.jneuroim.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 59.Newton CA, Klein TW, Friedman H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect Immun. 1994;62:4015–4020. doi: 10.1128/iai.62.9.4015-4020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noe SN, Nyland SB, Ugen K, Friedman H, Klein TW. Cannabinoid receptor agonists enhance syncytia formation in MT-2 cells infected with cell free HIV-1MN. Adv Exp Med Biol. 1998;437:223–229. doi: 10.1007/978-1-4615-5347-2_25. [DOI] [PubMed] [Google Scholar]
- 61.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paradisi A, Pasquariello N, Barcaroli D, Maccarrone M. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. J Biol Chem. 2008;283:6005–6012. doi: 10.1074/jbc.M707964200. [DOI] [PubMed] [Google Scholar]
- 63.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 64.Pertwee RG, Stevenson LA, Griffin G. Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. Br J Pharmacol. 1993;110:1483–1490. doi: 10.1111/j.1476-5381.1993.tb13989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peterson PK, Gekker G, Hu S, Cabral G, Lokensgard JR. Cannabinoids and morphine differentially affect HIV-1 expression in CD4(+) lymphocyte and microglial cell cultures. J Neuroimmunol. 2004;147:123–126. doi: 10.1016/j.jneuroim.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 66.Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. J Acquir Immune Defic Syndr. 2004;35:38–45. doi: 10.1097/00126334-200401010-00005. [DOI] [PubMed] [Google Scholar]
- 67.Pross SH, Klein TW, Newton CA, Smith J, Widen R, Friedman H. Differential suppression of T-cell subpopulations by thc (delta-9-tetrahydrocannabinol) Int J Immunopharmacol. 1990;12:539–544. doi: 10.1016/0192-0561(90)90118-7. [DOI] [PubMed] [Google Scholar]
- 68.Raborn ES, Cabral GA. Cannabinoid inhibition of macrophage migration to the trans-activating (Tat) protein of HIV-1 is linked to the CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 2010;333:319–327. doi: 10.1124/jpet.109.163055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reiss CS. Cannabinoids and Viral Infections. Pharmaceuticals (Basel) 2010;3:1873–1886. doi: 10.3390/ph3061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rock RB, Gekker G, Hu S, Sheng WS, Cabral GA, Martin BR, Peterson PK. WIN55,212-2-mediated inhibition of HIV-1 expression in microglial cells: involvement of cannabinoid receptors. J Neuroimmune Pharmacol. 2007;2:178–183. doi: 10.1007/s11481-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 71.Romagnani S, Del Prete G, Manetti R, Ravina A, Annunziato F, De Carli M, Mazzetti M, Piccinni MP, D’Elios MM, Parronchi P. Role of TH1/TH2 cytokines in HIV infection. Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 72.Roth MD, Tashkin DP, Whittaker KM, Choi R, Baldwin GC. Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci. 2005;77:1711–1722. doi: 10.1016/j.lfs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 73.Sabri N, Roth P, Xylourgidis N, Sadeghifar F, Adler J, Samakovlis C. Distinct functions of the Drosophila Nup153 and Nup214 FG domains in nuclear protein transport. J Cell Biol. 2007;178:557–565. doi: 10.1083/jcb.200612135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, Dandekar S. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Substance Abuse and Mental Health Services Administration. NSDUH Series H-24 DHHS Publication No. SMA 04–3963. 2004. Overview of Findings from the 2003 National Survey on Drug Use and Health Office of Applied Studies. [Google Scholar]
- 76.Thuru X, Chamaillard M, Karsak M, Gantier E, Erdual E, Dubuquoy C, Phillipe D, Rousseaux C, Wang YC, Dubus P, Stalewski J, Menzaghi F, Schteingart C, Zimmer A, Riviere P, Desreumaus P. Cannabinoid receptor 2 is required for homeostatic control of intestinal inflammation. Gastroenterology. 2007;132:A228. [Google Scholar]
- 77.Verhoeven D, Sankaran S, Silvey M, Dandekar S. Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J Virol. 2008;82:4016–4027. doi: 10.1128/JVI.02164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57:547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- 79.Winsauer PJ, Molina PE, Amedee AM, Filipeanu CM, McGoey RR, Troxclair DA, Walker EM, Birke LL, Stouwe CV, Howard JM, Leonard ST, Moerschbaecher JM, Lewis PB. Tolerance to chronic delta-9-tetrahydrocannabinol (Delta-THC) in rhesus macaques infected with simian immunodeficiency virus. Exp Clin Psychopharmacol. 2011;19:154–172. doi: 10.1037/a0023000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635.e24. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 81.Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA, Manjunath N. miRNA profiling of naive, effector and memory CD8 T cells. PLoS One. 2007;2:e1020. doi: 10.1371/journal.pone.0001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol. 2002;133:124–131. doi: 10.1016/s0165-5728(02)00370-3. [DOI] [PubMed] [Google Scholar]
- 83.Zhang C. MicroRNomics: a newly emerging approach for disease biology. Physiol Genomics. 2008;33:139–147. doi: 10.1152/physiolgenomics.00034.2008. [DOI] [PubMed] [Google Scholar]
- 84.Zhang R, Mehla R, Chauhan A. Perturbation of host nuclear membrane component RanBP2 impairs the nuclear import of human immunodeficiency virus -1 preintegration complex (DNA) PLoS One. 2010;5:e15620. doi: 10.1371/journal.pone.0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Wang JF, Kunos G, Groopman JE. Cannabinoid modulation of Kaposi’s sarcoma-associated herpesvirus infection and transformation. Cancer Res. 2007;67:7230–7237. doi: 10.1158/0008-5472.CAN-07-0960. [DOI] [PubMed] [Google Scholar]
- 86.Zhu W, Friedman H, Klein TW. Delta9-tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of Bcl-2 and caspase-1. J Pharmacol Exp Ther. 1998;286:1103–1109. [PubMed] [Google Scholar]