Abstract
Smoking is the leading cause of preventable cancer deaths in the United States. Nicotine replacement therapies (NRT) have been developed to aid in smoking cessation, which decreases lung cancer incidence. However, the safety of NRT is controversial because numerous preclinical studies have shown that nicotine enhances tumor cell growth in vitro and in vivo. We modeled NRT in mice to determine the effects of physiological levels of nicotine on lung tumor formation, tumor growth or metastasis. Nicotine administered in drinking water did not enhance lung tumorigenesis after treatment with the tobacco carcinogen, NNK. Tumors that develop in this model have mutations in K-ras, which is a commonly observed in smoking-related, human lung adenocarcinomas. In a transgenic model of mutant K-ras-driven lung cancer, nicotine did not increase tumor number or size, and did not affect overall survival. Likewise, in a syngeneic model of lung cancer cell lines derived from NNK-treated mice, oral nicotine did not enhance tumor growth or metastasis. These data show that nicotine does not enhance lung tumorigenesis when given to achieve levels comparable to those of NRT, suggesting that nicotine has a dose threshold, below which it has no appreciable effect. These studies are consistent with epidemiological data showing that NRT does not enhance lung cancer risk in former smokers.
Keywords: Nicotine, NNK, K-ras, nicotine replacement therapy
Introduction
Lung cancer is the leading cause of cancer related deaths in the United States (1). Smoking accounts for over 90% of the lung cancer cases reported in men and 80% in women each year. K-Ras mutations are common in tobacco-related lung adenocarcinomas (2). K-Ras mutations enable Ras to remain in its active GTP-bound form, leading to activation of several signaling pathways including the PI3-kinase (PI3K), mitogen-activated protein kinase (MAPK) and Rac pathways (3). Patients with K-ras-mutant lung cancer have a particularly poor prognosis (4), thus emphasizing the importance of smoking cessation to decrease lung cancer incidence and mortality (5).
Nicotine is responsible for tobacco addiction, but can also be metabolized to form several lung carcinogens (6). Nicotine replacement therapy (NRT) is approved by the Food and Drug Administration (FDA) to help smokers overcome nicotine dependence, and is available in several forms such as gum, lozenges and patches. When used at the recommended dosage and duration, NRT is thought to be a safer option than continued smoking (7, 8). However, pre-clinical models have shown that nicotine can activate components of the PI3K, MAPK and Src pathways that increase lung cancer cell proliferation, survival and migration (9–12). Activation of these pathways by nicotine can also partially transform epithelial cells, induce an epithelial-mesenchymal transition (EMT), and increase chemotherapeutic resistance (9, 13, 14). When extrapolated to former smokers, these data have raised the possibility that NRT might contribute to adverse clinical outcomes. However, many of these preclinical studies have used nicotine at higher doses than those achieved in heavy smokers.
The aim of this study was to assess tumor promotion by nicotine in murine models of mutant K-ras-induced lung tumors when nicotine was administered to mimic NRT. Three models were used; a tobacco carcinogen-induced model, a transgenic model, and a syngeneic graft model. Nicotine was provided in the drinking water to achieve low steady state concentrations. In each model, there was no evidence of tumor promotion by nicotine when administered to achieve levels comparable to NRT users. These results suggest that NRT will not have an impact on the growth of human lung cancers bearing K-Ras mutations.
Materials and Methods
Mice
All animal studies were performed under a protocol approved by the Animal Care and Use Committee of the National Cancer Institute (NCI). A/J and C57BL/6 (Charles River; Fredrick, MD) mice were mated to generate F1 (AB6F1) mice that were used for all mouse experiments unless otherwise noted. For carcinogen studies, mice were divided into four groups, ten mice per group. Beginning at 6 weeks of age, two groups received three weekly intraperitoneal (IP) injections of 100mg/kg 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone(NNK)(Toronto Research Chemicals). One week after the last NNK injection, one NNK group and one control group were given 100 µg/mL nicotine (N0267, Sigma Aldrich) in their drinking water for 12 weeks. Water supplemented with nicotine was changed weekly and mice were sacrificed at 22 weeks of age.
KrasLA2/+ mice (15) in a C57Bl/6 background received nicotine in the drinking water or standard water starting at 3 weeks of age. Mice were sacrificed after a two-week treatment. For longer chronic studies, mice began treatment at six weeks of age and were sacrificed at 12 weeks of age. For survival studies, mice began nicotine treatment after six weeks of age and were sacrificed when they exhibited signs of morbidity. At sacrifice, livers, lungs, kidneys, spleen and obvious tumors were removed and preserved in NBF. For all studies, lungs were inflated using 10% neutral-buffered formalin (NBF) and peripheral lung tumors were counted and measured as previously described (16).
For syngeneic studies, AB6F1 mice between 6–10 weeks of age were injected subcutaneously in the flank with 1×105 CL13, IO33 or CL25 cells. One day after injection mice were randomized to receive nicotine at 100µg/ml in their drinking water or control water for the remainder of the study. Tumors were measured every other day and tumor volume was calculated using the formula (short2)(long)/2. Tissues were flash frozen for lysates or fixed in NBF for histology. Lungs were imbedded in paraffin and hematoxylin and eosin-stained slides were used for analysis of lung metastases.
For serum cotinine analysis, blood was collected in the early morning after 12 weeks of nicotine treatment. Samples were analyzed by LC/MS chromatography (SAIC, Fredrick, MD).
Cell Culture
IO33, CL13 and CL25 cells were derived from lung adenocarcinomas that developed in A/J mice after NNK administration, and were a generous gift from Dr. Steven Belinsky in 2005 (17). In cell lines, K-Ras mutation was verified by sequencing in 2007 and A/J background was verified by tumor growth in syngeneic mice. Cells used in experiments were within 8 passages of K-Ras mutation verification. Cells were maintained in RPMI 1640 supplemented with 5% FBS at 37°C and 5% carbon dioxide (CO2). For in vitro experiments, IO33, CL13 or CL25 cells were plated at 2.5 × 105 or 3 × 105 then serum starved in 0.1%FBS for 24 hours. Cells were then incubated with varying doses of nicotine (Sigma) for one hour.
For cell viability assays, IO33 and CL25 cells were seeded at 600–1200 cells/well in a 96 well plate. Nicotine was added to the media 24 hours after plating. For serum starvation, media was changed to RPMI with 0.1%FBS 24 hours before nicotine was added. Cells were fixed at 24, 48 and 72 hours after nicotine treatment by adding 10% TCA and incubating at 4C for 1 hour. All plates were stained with SRB as described previously (18). OD values were normalized to Day 0 reading for analysis.
Immunoblotting and Immunohistochemistry
Cell lysates were made in 4% SDS, 125mM Tris, pH 6.8, 20% glycerol and briefly sonicated. For tissue lysates, liver, lungs or tumors were flash frozen then ground in Ripa Buffer (Sigma) with added protease and phosphatase inhibitors. Immunoblotting was performed as previously described (19). Primary antibodies Akt (9272), pS473-Akt (4060), pT202/Y204-Erk1/2 (4376), pS235/236-ribosomal protein S6 (2211) and α-tubulin (2125) were obtained from Cell Signaling (Danvers, MA). All immunoblotting experiments were completed in triplicates.
For immunohistochemistry, lung tissues were imbedded in paraffin blocks (HistoServ), sectioned to slides and analyzed for protein expression with five mice per group. Briefly, slides were heated at 65°C then placed through xylene and ethanol washes. After inhibition of peroxidases (Invitrogen) and blocking (Vectastain), sections were incubated overnight at 4°C with primary antibody with the exception of the one hour incubation used with the CD3 (Dako, A0452) and ki67 (Novacastra) antibodies. Antibodies directed at pT202/Y204-Erk1/2 (4370), pS473-Akt (4060), pT308-Akt (9266), pS235/236-ribosomal protein S6 (2211) and Survivin (2808) were obtained from Cell Signaling and used according to manufacturer protocol. The antibody directed at Foxp3 was obtained from eBioscience (14-5773-82). Secondary Antibody (Vectastain) and DAB detection (Sigma) were used as previously described (20). Researchers were blinded before scoring (CM, ID) and followed the scoring for p-Akt, p-S6 and p-Erk protocol as previously published (21). Survivin and ki67 staining was quantified by counting the number of survivin positive cells in four 400× high powered fields (HPFs) per tumor. Numbers were averaged for all mice with at least five mice per group.
Unpaired student t-test by Prism software (GraphPad Software, La Jolla, CA, USA) was used for all analysis
Results
Levels of nicotine comparable to NRT do not enhance tobacco carcinogen-induced lung tumorigenesis in mice
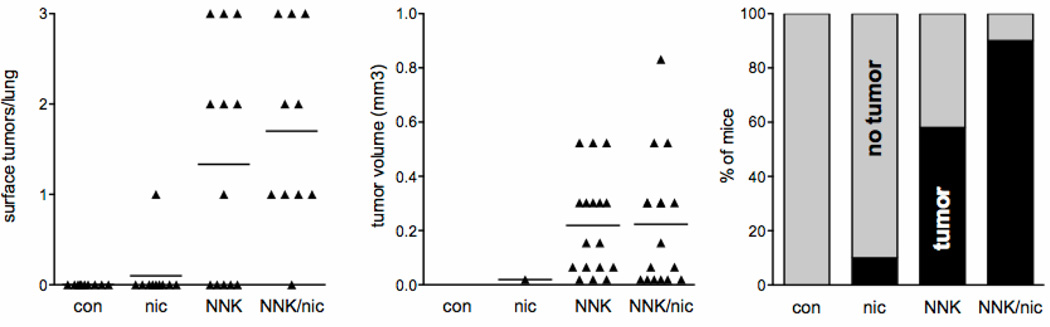
The A/J mouse has been widely used to study lung tumorigenesis and is susceptible to NNK-induced lung tumors. However, A/J mice are reported to show little preference for nicotine in drinking water, while C57BL/6 mice show a high preference (22). To generate mice that are both susceptible to NNK-induced lung tumors and have a preference for nicotine, A/J and C57BL/6 mice were crossed to generate AB6F1 mice. These mice were treated with NNK followed by nicotine administration in the drinking water to simulate a former smoker on NRT. 100 µg/ml of nicotine was chosen since concentrations above this did not result in increased daily nicotine dose to C57BL/6 mice (22). Nicotine treatment did not alter tumor multiplicity or size, as assessed by peripheral tumor number and tumor burden (Fig.1). As expected, tumor multiplicity was lower in these AB6F1 mice compared with pure A/J mice that develop approximately 25 lung tumors per mouse, and increased compared with pure C57BL/6 that do not develop lung tumors after NNK (23, 24). Nicotine had no effect on tumor incidence, even though 1/10 mice in the nicotine alone group developed one lung tumor. When this group was expanded to 30 mice, no additional mice developed lung tumors. Nicotine did appear to increase tumor incidence in NNK-treated mice but the difference was not statistically significant (Fig. 1). These studies were repeated after decreasing the dose of NNK to a single 100 mg/kg injection to highlight any effect of nicotine on incidence. The single dose of NNK led to 37% incidence of lung tumors. A single dose of NNK followed by nicotine at the same dosing schedule above led to a 31% incidence, which was not statistically different from the single dose NNK alone group (t-test p=0.78, n=13–19 per group, data not shown).
Figure 1. Nicotine does not enhance lung tumorigenesis following NNK.
Tumor multiplicity (left, each point represents one mouse), tumor volume (middle, each point represents one tumor) and tumor incidence (right) after 12 weeks of oral nicotine treatment (n=10). Differences between tumor multiplicity and size ± nicotine were not significant within the control or NNK groups.
Serum cotinine levels measured at sacrifice averaged 137 ng/ml, which is comparable to 22 mg nicotine patch users (25). Females metabolize nicotine faster than their males due to the influence of estrogen on CYP2A6 (26). When cotinine levels were stratified based on gender, females achieved an average steady state level of 100 ng/mL while the males averaged 200 ng/mL (Supplementary Fig. S1). This concentration range of nicotine did not have observed toxicities.
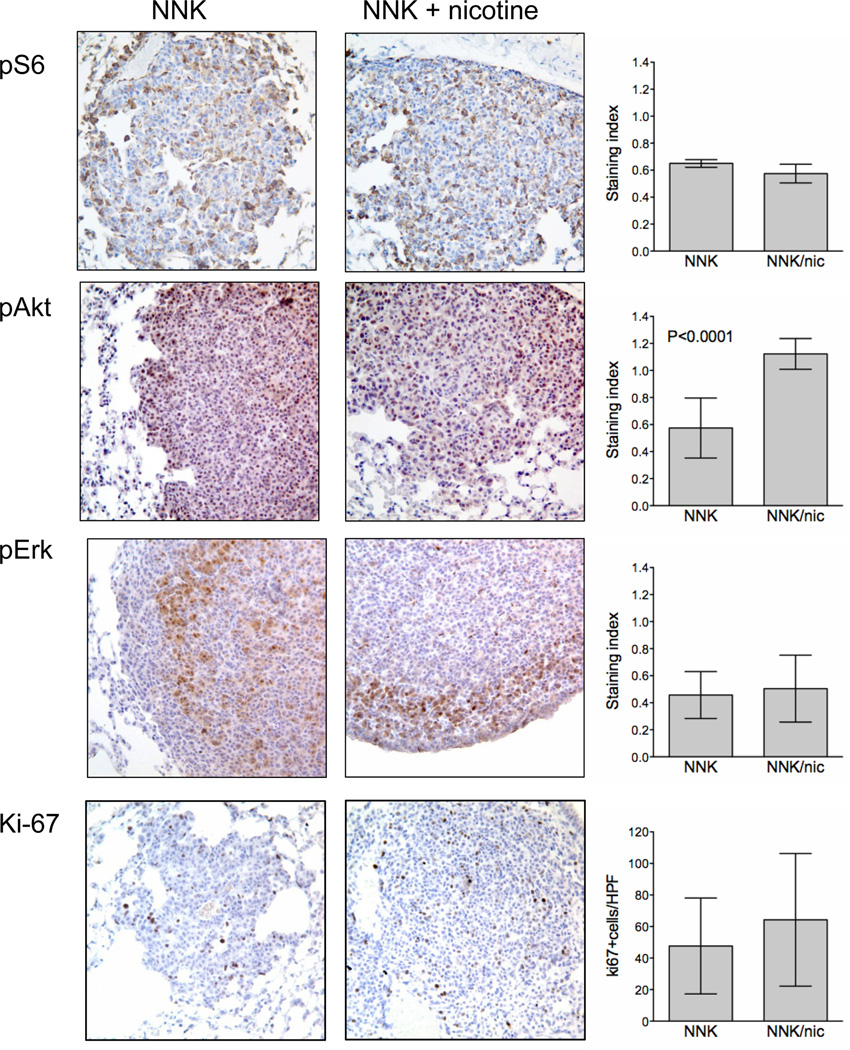
Nicotine can activate the MEK/ERK and PI3K/AKT pathways in vitro (9, 27, 28). In vivo, nicotine increased pS473-Akt expression in lung tumors but this did not correlate with increased Ki-67 or pT308-Akt staining (Fig. 2A and Supplementary Fig. S2). Because increased phosphorylation of Akt at both sites is necessary for full Akt kinase activation and increased proliferation, phosphorylation of only S473 is likely insufficient to promote tumor growth. No modulation of pS6 or pErk was observed. K-Ras mediated lung tumorigenesis requires the presence of Foxp3+-CD3+ regulatory T cells in the tumor microenvironment (29). Although nicotine alone has been reported to increase the suppressive effects of regulatory T cells (30), nicotine did not alter Foxp3+/CD3+ cell number in lung tumors or surrounding normal lung tissue (Supplementary Fig. S2)
Figure 2. Oral nicotine leads to Akt activation but not other growth promoting pathways in NNK-induced lung tumors.
Representative images of IHC for p-S6, p-Akt, p-Erk and Ki-67 in NNK-induced mouse lung tumors that developed in mice treated with or without 12 weeks of oral nicotine. Quantification (right) of IHC staining in tumors (n=5). Columns represent mean staining indices ± SD. For Ki-67, columns represent mean percentage of tumor cells with positive staining ± SD. Unless indicated, any differences are not significant.
Levels of nicotine comparable to NRT do not enhance mutant K-Ras-mediated lung tumorigenesis
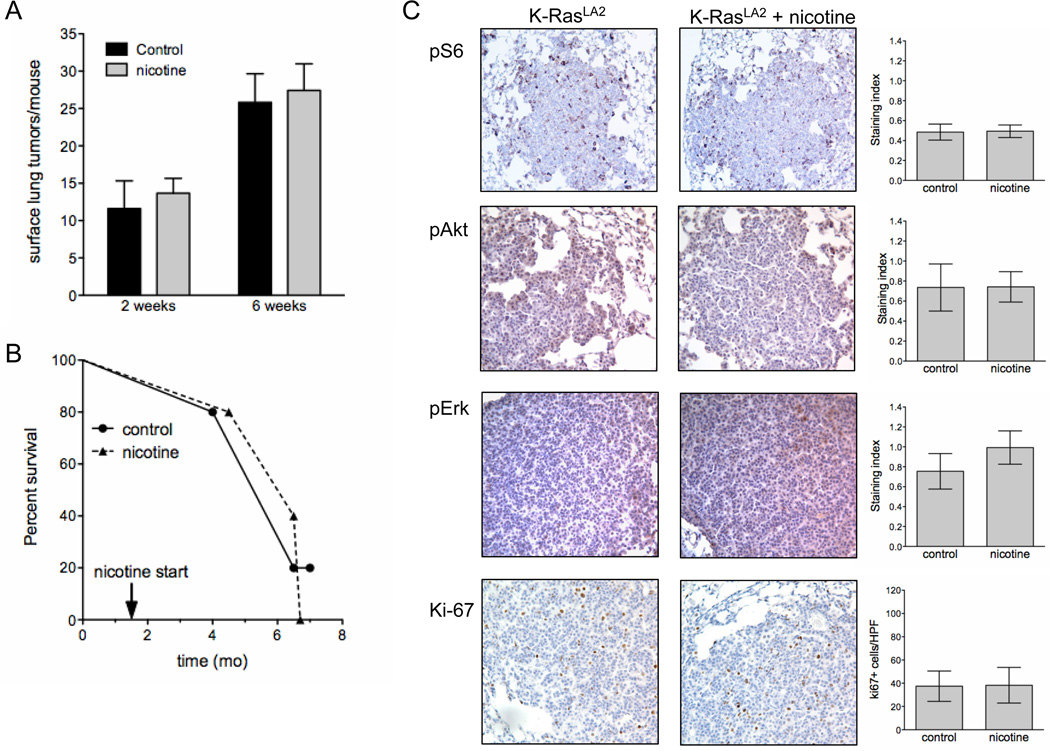
NNK-induced lung tumors in A/J mice exhibit K-ras mutations but are primarily adenomas that only progress to adenocarcinomas at later ages (31). A genetically engineered mouse model of mutant K-Ras (KRasLA2) was used to assess the effect of nicotine on tumor growth and progression. In this model, tumors are apparent as early as 2 weeks of age and progress to adenocarcinomas within several months, coincident with greatly increased tumor size (32). Two weeks of nicotine starting at 3 weeks of age did not change tumor multiplicity or tumor burden (Fig.3A and data not shown). To determine whether nicotine could increase tumor progression in older mice, six week-old mice were treated with nicotine for six weeks. There was no difference in peripheral tumor multiplicity, tumor size or tumor burden in the presence of nicotine, although tumor number and size were increased relative to younger mice (Fig. 3A). When initiated at six weeks of age and continued until death, nicotine also did not alter the overall life span of these mice (Fig. 3B). Cotinine levels were comparable to the AB6F1 mouse model (data not shown). In lung tumors from these mice, nicotine did not alter the activation of proteins associated with survival such as S6, Akt and Erk, and did not increase Ki-67 staining (Fig. 3C).
Figure 3. Nicotine does not enhance growth of lung tumors in KRasLA2 mice.
A, Average tumor multiplicity ± SD of peripheral lung tumors in KRasLA2 after two weeks and six weeks of oral nicotine treatment (n=5–7). B, Survival of KRasLA2 mice with or without oral nicotine treatment starting at 6 weeks of age (n=5). C, Representative images of IHC for p-S6, p-Akt, p-Erk and Ki-67 (right). Quantification of IHC (n=5). Columns represent mean staining indices ± SD. For Ki-67, columns represent mean percentage of tumor cells with positive staining ± SD. Unless indicated, any differences are not significant.
Nicotine does not augment tumor growth of syngeneic murine lung cancer cell lines
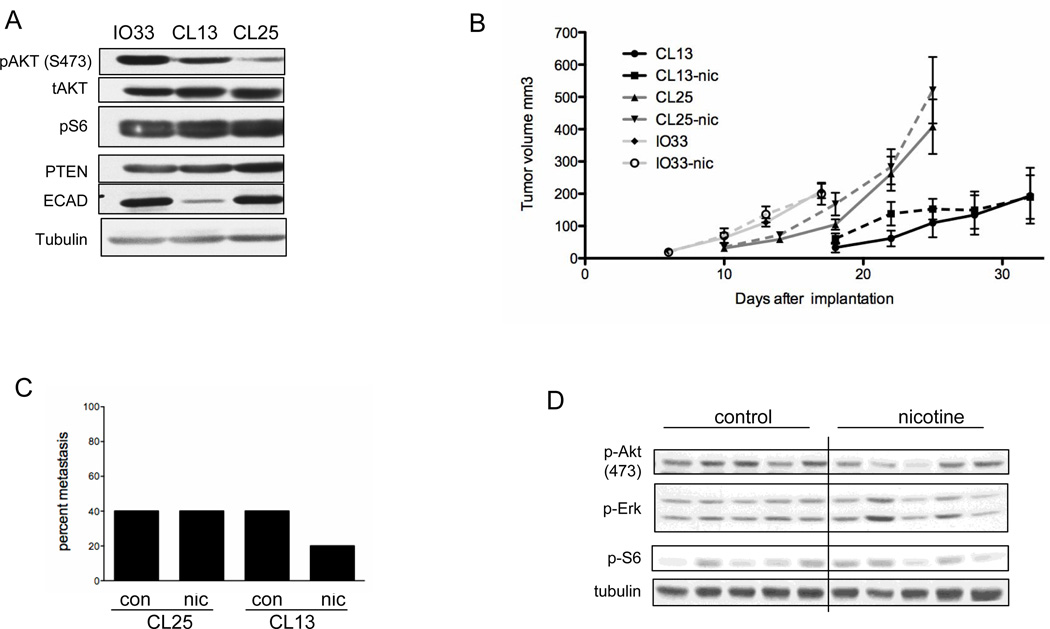
Because nicotine has been previously shown to enhance subcutaneous tumor growth of lung tumor cell lines in immunocompromised mice, murine lung tumor cell lines derived from NNK-induced lung adenocarcinomas were used for similar studies in immunocompetent, syngeneic mice. Three NNK-derived cell lines were used that exhibited differences in endogenous expression of survival pathways (Fig. 4A). IO33 and CL13 cells show relatively high expression of phosphorylated Akt compared with CL25. CL13 cells also have a mesenchymal phenotype as shown by a lack of E-cadherin expression and spindle shape in culture (Fig. 4A and data not shown). Oral nicotine did not enhance tumor growth of any of the three cell lines in AB6F1 mice (Fig. 4B). These same cell lines were subjected to an IP nicotine dosing regimen that had previously been shown to enhance xenograft tumor growth (10). IP dosing of nicotine did not enhance tumor growth of IO33 or CL25 cells (Fig. 5). Approximately 10% of AB6F1 mice died within 24 hours of 1 mg/kg IP nicotine injection (data not shown); therefore, subsequent experiments were done with lower doses. IP injections of nicotine at 0.6–1.0 mg/kg caused severe acute reactions in all mice that included tachypnea and immobility. Acute reactions to IP nicotine lasted 10–15 minutes, after which time the mice appeared to recover (data not shown). In addition to forming primary tumors, subcutaneously-injected CL25 and CL13 cells can metastasize to the lung. Similar to its lack of effect on primary tumors, nicotine did not substantially increase the incidence of metastasis in either cell line (Fig. 4C). The apparent decrease in metastasis of nicotine-treated CL13 cells was not statistically significant. Xenograft tumors from nicotine treated mice did not have activated Akt or Erk survival pathways (Fig. 4D and Supplementary Fig. S3).
Figure 4. Nicotine does not increase tumor growth in allograft models.
A, Immunoblot analysis of NNK-induced mouse lung tumor cell lines in the absence of nicotine. B, Tumor volume of IO33, CL13 and CL25 allograft tumors in AB6F1 mice with or without nicotine administration (n=5). C, Incidence of lung metastasis at completion of the allograft study. The percentage of mice with metastatic tumors were identified and quantified from H&E slides. D, Immunoblot analysis of components of the PI3K and MAPK pathways in allograft tumors. Tumors were harvested at sacrifice upon completion of the study.
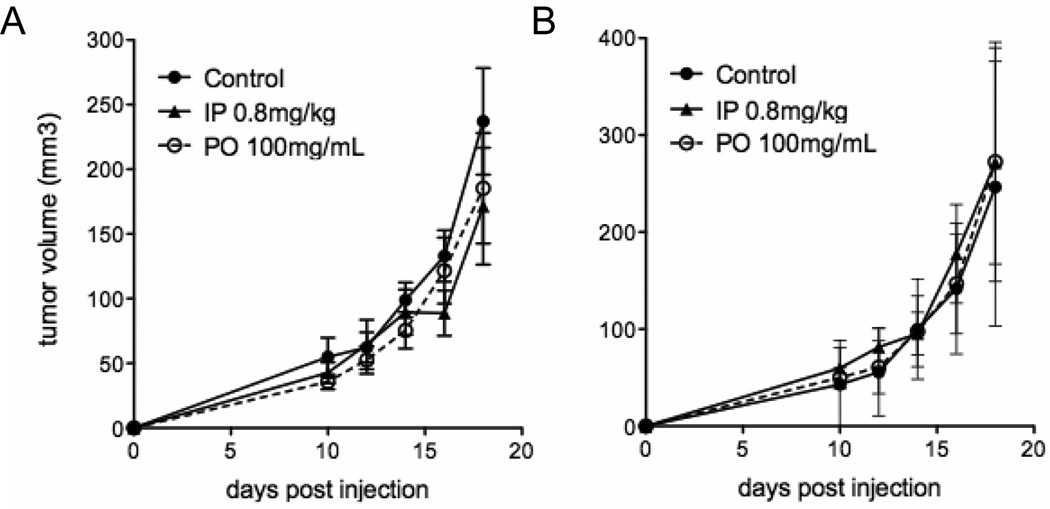
Figure 5. Intraperitoneal injection of nicotine does not increase tumor growth.
A, IO33 and B, CL25 allograft tumor growth curves after intraperitoneal injection of nicotine (IP) or water (control) or oral nicotine (PO). Any differences are not significant.
Nicotine does not promote growth of NNK-induced mouse lung tumor cell lines in vitro
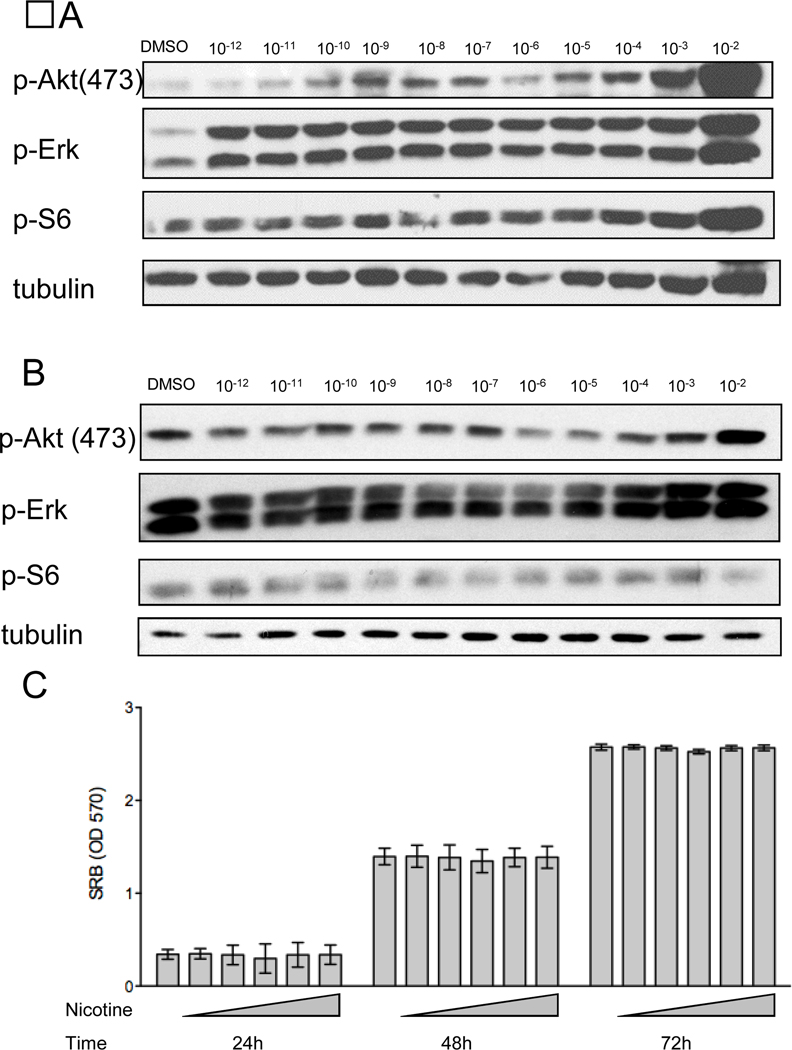
Since nicotine can rapidly stimulate survival pathways in vitro, the NNK-derived cell lines were treated with varying doses of nicotine to determine whether they might be refractory to these stimulatory effects of nicotine. Nicotine activated the Akt and Erk pathways at low physiological nicotine doses in CL25 cells when they were serum deprived (Fig. 6A). However, activation was not observed when nicotine was added to cells grown in 5% FBS (Fig. 6B). The dependence of serum was confirmed when proliferation was analyzed with or without serum starvation. Nicotine did not promote cellular proliferation in low serum (data not shown), and there was no effect when nicotine was added in the presence of 5% FBS (Fig. 6D). These results show that physiologically relevant concentrations of nicotine do not promote cell proliferation in vitro under normal growth conditions (5% FBS).
Figure 6. Nicotine only activates components of PI3K and MAPK under serum starvation conditions.
Immunoblotting analysis of Cl25 cells after one-hour nicotine treatment at indicated doses in 0.5% serum (A) or 5% serum (B). C, SRB assay of CL25 cells in 5% serum. Prior to nicotine treatment, OD570 values were approximately 0.1. The first bar for each time point is untreated cells. Nicotine doses (from left to right for each time point) were 1 nM, 10 nM, 100 nM, 1 µM and 10 µM. Any differences are not significant.
Discussion
We used three mouse models to demonstrate that oral nicotine, at steady state levels relevant to NRT, does not enhance lung tumorigenesis after NNK exposure, and does not enhance growth or metastasis of nascent lung tumors bearing K-Ras mutations. The NNK-induced lung tumor model is particularly relevant since it models a former smoker using NRT. The main clinical implication of these studies is that doses of nicotine that mirror use of NRT may not be harmful in the setting of prior tobacco use, but they shed no insight into possible effects of NRT in current smokers. Similar results have been reported by Murphy et al., who show that oral administration of low doses of nicotine has no effect on NNK-induced lung tumorigenesis (33). These investigators varied the timing of nicotine in relation to NNK exposure as well as length of treatment, and observed no differences in tumor multiplicity, size, or histology. Our study and that of Murphy et al. are in conflict with prior studies that showed that nicotine augmented NNK-induced lung tumors and/or lung tumor xenograft growth and metastasis (10). We believe the principal difference in the outcomes of these studies is that studies that show a tumor promoting effect of nicotine used high intraperitoneal dosing that may not be reflective of sustained, lower nicotine doses typical of NRT.
For NNK, there is a threshold effect for lung carcinogenesis in mice, where doses of 2 µmol or below do not have appreciable tumorigenic activity, while doses above 2 µmol show a linear correlation with lung tumor multiplicity (34). A similar phenomenon may occur for nicotine where low doses or low dose rates do not have appreciable tumor promotion activity while larger doses do. In support of this, one study found no effect on growth of three small cell lung tumor xenografts using Alzet minipumps to deliver 20 or 200 µg of nicotine per day (35). In contrast, high dose IP nicotine three times per week for 28 weeks in NNK-treated mice increased both lung tumor number and tumor size (10). In the studies presented here, NRT-level nicotine did not augment either lung tumor number or size following NNK exposure. The previous study used IP dosing of nicotine, which delivered approximately 8 times the daily dose used here in a single bolus dose; this study also used 40% higher NNK dosing and over twice the duration of nicotine treatment. In an attempt to replicate these studies and compare IP dosing schedule with oral dosing, all injected mice developed severe acute respiratory distress and/or seizures, and several mice died within 24 hours (data not shown). Lower doses of IP nicotine did not enhance syngeneic tumor growth, similar to what was observed for oral dosing.
In vitro, nicotine has been shown to stimulate growth-promoting pathways that are frequently activated in lung tumors, particularly the mutant K-Ras-associated PI3K/AKT and MEK/ERK pathways (27, 28, 36). In normal lung and lung tumors, nicotine can bind to nicotinic acid receptors potentially stimulating these growth-promoting pathways (28, 37). Nicotine increased Akt, Erk and mTor pathway activation in vitro but these effects were largely dependent on the absence of serum (Fig. 6), where basal levels of pathway activation are lower. In NNK-treated mice, nicotine partially enhanced Akt activation but this was not sufficient to propagate to the mTor pathway (pS6 in Fig. 2). In addition, these pathways were not elevated in tumors from the syngeneic model (Fig. 4D and supplementary Fig. 3). This suggests that activation of these pathways by nicotine in vitro in low serum may not be relevant to what occurs with physiological levels of nicotine in vivo.
Another consideration for these types of studies is strain background. Previous studies have shown stimulation of tumor growth in Balb/c, C57BL/6 and nude mice using IP dosing of nicotine, or in Balb/c using a nicotine patch (10, 38, 39). Recessive traits relating to nicotine metabolism or response may be present in inbred strains. Here, AB6F1 mice were used in most studies. Because of the mixed background, there may be fewer strain-dependent variables than when using pure backgrounds. Likewise, there may be certain populations that are at higher risk for nicotine-stimulated tumor growth as there are for risk for nicotine dependence. However, epidemiological studies have indicated that large populations are not at increased risk of lung cancer due to NRT (8). The studies presented here support those findings using multiple mouse models and physiological administration of nicotine.
Supplementary Material
References
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Husgafvel-Pursiainen K, Hackman P, Ridanpaa M, Anttila S, Karjalainen A, Partanen T, et al. K-ras mutations in human adenocarcinoma of the lung: association with smoking and occupational exposure to asbestos. Int J Cancer. 1993;53:250–256. doi: 10.1002/ijc.2910530213. [DOI] [PubMed] [Google Scholar]
- 3.Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascaux C, Iannino N, Martin B, Paesmans M, Berghmans T, Dusart M, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi N, Mochizuki-Kobayashi Y, Utsunomiya O. Quantitative relationship between cumulative cigarette consumption and lung cancer mortality in Japan. Int J Epidemiol. 2000;29:963–968. doi: 10.1093/ije/29.6.963. [DOI] [PubMed] [Google Scholar]
- 6.Hecht SS, Chen CB, Ornaf RM, Hoffmann D, Tso TC. Chemical studies on tobacco smoke LVI. Tobacco specific nitrosamines: origins, carcinogenicity and metabolism. IARC Sci Publ. 1978:395–413. [PubMed] [Google Scholar]
- 7.Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High-dose nicotine patch therapy. Percentage of replacement and smoking cessation. JAMA. 1995;274:1353–1358. [PubMed] [Google Scholar]
- 8.Murray RP, Connett JE, Zapawa LM. Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob Res. 2009;11:1076–1082. doi: 10.1093/ntr/ntp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catassi A, Servent D, Paleari L, Cesario A, Russo P. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: implications on lung carcinogenesis. Mutat Res. 2008;659:221–231. doi: 10.1016/j.mrrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Shen T, Le W, Yee A, Kamdar O, Hwang PH, Upadhyay D. Nicotine induces resistance to chemotherapy in nasal epithelial cancer. Am J Rhinol Allergy. 2010;24:e73–e77. doi: 10.2500/ajra.2010.24.3456. [DOI] [PubMed] [Google Scholar]
- 14.Chen RJ, Ho YS, Guo HR, Wang YJ. Long-term nicotine exposure-induced chemoresistance is mediated by activation of Stat3 and downregulation of ERK1/2 via nAChR and beta-adrenoceptors in human bladder cancer cells. Toxicol Sci. 2010;115:118–130. doi: 10.1093/toxsci/kfq028. [DOI] [PubMed] [Google Scholar]
- 15.Johnson L, Mercer K, Greenbaum D, Bronson R, Crowley D, Tuveson D, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 16.Hollander MC, Maier CR, Hobbs EA, Ashmore AR, Linnoila RI, Dennis PA. Akt1 deletion prevents lung tumorigenesis by mutant K-ras. Oncogene. 2011 doi: 10.1038/onc.2010.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones-Bolin SE, Johansson E, Palmisano WA, Anderson MW, Wiest JS, Belinsky SA. Effect of promoter and intron 2 polymorphisms on murine lung K-ras gene expression. Carcinogenesis. 1998;19:1503–1508. doi: 10.1093/carcin/19.8.1503. [DOI] [PubMed] [Google Scholar]
- 18.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 19.Brognard J, Clark A, Ni Y, Dennis P. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 20.Granville CA, Warfel N, Tsurutani J, Hollander MC, Robertson M, Fox SD, et al. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin Cancer Res. 2007;13:2281–2289. doi: 10.1158/1078-0432.CCR-06-2570. [DOI] [PubMed] [Google Scholar]
- 21.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson SF, Marks MJ, Collins AC. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 1996;124:332–339. doi: 10.1007/BF02247438. [DOI] [PubMed] [Google Scholar]
- 23.Upadhyaya P, Kenney PM, Hochalter JB, Wang M, Hecht SS. Tumorigenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol enantiomers and metabolites in the A/J mouse. Carcinogenesis. 1999;20:1577–1582. doi: 10.1093/carcin/20.8.1577. [DOI] [PubMed] [Google Scholar]
- 24.Anderson LM, Hecht SS, Dixon DE, Dove LF, Kovatch RM, Amin S, et al. Evaluation of the transplacental tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in mice. Cancer Res. 1989;49:3770–3775. [PubMed] [Google Scholar]
- 25.Hurt RD, Dale LC, Offord KP, Lauger GG, Baskin LB, Lawson GM, et al. Serum nicotine and cotinine levels during nicotine-patch therapy. Clin Pharmacol Ther. 1993;54:98–106. doi: 10.1038/clpt.1993.117. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai JR, Chong IW, Chen CC, Lin SR, Sheu CC, Hwang JJ. Mitogen-activated protein kinase pathway was significantly activated in human bronchial epithelial cells by nicotine. DNA Cell Biol. 2006;25:312–322. doi: 10.1089/dna.2006.25.312. [DOI] [PubMed] [Google Scholar]
- 28.West K, Brognard J, Clark A, Linnoila I, Yang X, Swain S, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granville CA, Memmott RM, Balogh A, Mariotti J, Kawabata S, Han W, et al. A central role for Foxp3+ regulatory T cells in K-Ras-driven lung tumorigenesis. PLoS One. 2009;4:e5061. doi: 10.1371/journal.pone.0005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang DW, Zhou RB, Yao YM, Zhu XM, Yin YM, Zhao GJ, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther. 335:553–561. doi: 10.1124/jpet.110.169961. [DOI] [PubMed] [Google Scholar]
- 31.Belinsky SA, Devereux TR, Foley JF, Maronpot RR, Anderson MW. Role of the alveolar type II cell in the development and progression of pulmonary tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the A/J mouse. Cancer Res. 1992;52:3164–3173. [PubMed] [Google Scholar]
- 32.Jackson E, Willis N, Mercer K, Bronson R, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy SE, von Weymarn LB, Schutten MM, Kassie F, Modiano JF. Chronic nicotine consumption does not influence 4-(methynitrosamino)-1-(3-pyridyl)-1-butanone induced lung tumorigenesis. Cancer Prev Res (Phila Pa) 2011 doi: 10.1158/1940-6207.CAPR-11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson LA, Hecht SS. O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991;51:5557–5564. [PubMed] [Google Scholar]
- 35.Pratesi G, Cervi S, Balsari A, Bondiolotti G, Vicentini LM. Effect of serotonin and nicotine on the growth of a human small cell lung cancer xenograft. Anticancer Res. 1996;16:3615–3619. [PubMed] [Google Scholar]
- 36.Chu M, Guo J, Chen CY. Long-term exposure to nicotine, via ras pathway, induces cyclin D1 to stimulate G1 cell cycle transition. J Biol Chem. 2005;280:6369–6379. doi: 10.1074/jbc.M408947200. [DOI] [PubMed] [Google Scholar]
- 37.Nishioka T, Guo J, Yamamoto D, Chen L, Huppi P, Chen CY. Nicotine, through upregulating pro-survival signaling, cooperates with NNK to promote transformation. J Cell Biochem. 2010;109:152–161. doi: 10.1002/jcb.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarzynka MJ, Guo P, Bar-Joseph I, Hu B, Cheng SY. Estradiol and nicotine exposure enhances A549 bronchioloalveolar carcinoma xenograft growth in mice through the stimulation of angiogenesis. Int J Oncol. 2006;28:337–344. [PMC free article] [PubMed] [Google Scholar]
- 39.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.