Abstract
Overweight and obese individuals frequently restrict caloric intake to lose weight. The resultant weight loss, however, typically is followed by an equal or greater weight gain, a phenomenon called weight cycling. Most attention to weight cycling has focused on identifying its detrimental effects, but preclinical experiments indicating that intermittent caloric restriction or fasting can reduce cancer risk have raised interest in potential benefits of weight cycling. Although hypothesized adverse effects of weight cycling on energy metabolism remain largely unsubstantiated, there also is a lack of epidemiological evidence that intentional weight loss followed by regain of weight affects chronic-disease risk. In the limited studies of weight cycling and cancer, no independent effect on post-menopausal breast cancer but a modest enhancement of risk for renal cell carcinoma, endometrial cancer, and non-Hodgkin’s lymphoma have been reported. An effect of either intermittent caloric restriction or fasting in protecting against cancer is not supported by the majority of rodent carcinogenesis experiments. Collectively, the data argue against weight cycling and indicate that the objective of energy balance–based approaches to reduce cancer risk should be to strive to prevent adult weight gain and maintain body weight within the normal range defined by body mass index.
Keywords: cancer risk, energetics, intermittent caloric restriction, weight cycling
Introduction
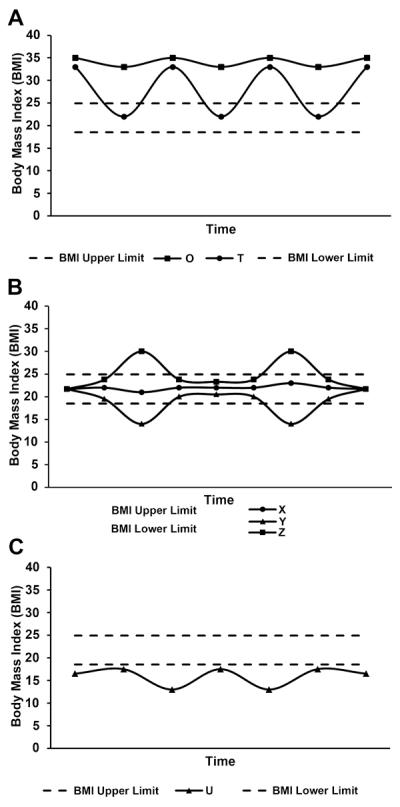
The prevalence of overweight and obesity, as defined by body mass index (BMI) > 24.9 (body weight in kg divided by height in m2), has increased at an epidemic rate over the last few decades (1), and an excess of adult body weight for height is associated with increased risk for a number of chronic diseases including certain types of cancer referred to as obesity-associated cancers (2-4). Rising obesity has stimulated increased efforts to lose weight (5, 6), and all approaches for inducing weight loss, in humans or animal models, involve reducing caloric intake relative to caloric need (7). Attempts to lose weight by any approach vary in their results, however, and the weight that is lost is frequently regained (8-10). This weight loss and regain in people indicates an intermittent dietary pattern that provides the link between intermittent caloric restriction in animal studies of energy balance and weight cycling in humans. Historically, repeated cycles of weight loss and regain have been called weight cycling, “yo-yo dieting,” or, more generally, weight fluctuation, weight variability, or weight instability (11). Weight cycling is a complex behavior and remains ill-defined, thus making it difficult to study in human populations or to simulate in animal models (12). The several reported patterns of weight cycling include the typical pattern in obese individuals, which is weight loss and then regain in a repetitive cycle (Fig. 1A). Less commonly considered patterns include weight cycling among normal-weight individuals (Fig. 1B) and weight cycling in individuals with a BMI below the normal range (Fig. 1C). These patterns and their many conceivable variants illustrate the complexity of investigating the effects of weight cycling in human populations. Cutter et al. proposed that the key elements to consider in characterizing weight cycling are the amplitude of the cycles (the amount of weight gained or lost), the frequency of cycling (the number of cycles experienced), and the duration of the cycles (the timeframe over which cycles occur: Days, weeks, months, years; ref. (12). Many other factors also can be considered, including the time during the lifecycle and stage during a disease process that weight cycling occurs (11).
Figure 1.
Patterns of weight cycling. BMI < 18.5 is underweight, 18.5 to 24.9 is considered normal weight, 25 to 29.9 is considered overweight, and ≥ 30 is considered obese. A, patterns of weight regulation involving obese individuals, either consistently in the obese range, individual O or transiently losing weight, regaining the weight, and repeating the cycle, individual T. B, individual X consistently maintains BMI in the normal range (18.5-24.9), with small weight fluctuations; individual Y engages in intermittent caloric restriction to induce weight loss, whereas individual Z periodically fails to regulate body weight and transiently attains a body weight above the normal range for BMI. C, individual U has a BMI that fluctuates entirely below the normal range. This type of underweight pattern can be associated with eating disorders, fad dietary practices, or natural disasters or wars leading to weight cycling that can reach down to starvation/famine levels of BMI.
The focus of this review is on the pattern of weight cycling illustrated in Figure 1A and, further, on the animal-model correlative of this pattern of human weight cycling. Over the last decade, a series of reports using genetically engineered or transplantable tumor models have indicated that intermittent caloric restriction or intermittent fasting, which represents an extreme form of caloric restriction, may exert beneficial effects against cancer, generating scientific interest in this approach (13-21). We will assess the evidence on the use of intermittent caloric restriction, which results in weight cycling, as an intervention tool for the prevention and control of cancer.
Searching for Detrimental Effects of Weight Cycling
Investigations of weight cycling have primarily been based on the assumption that it has negative health consequences. The majority of work has centered on the concern that weight cycling promotes the development of excessive weight gain. Specific hypotheses that have been evaluated include that weight cycling 1) impairs future weight loss and promotes future weight gain, 2) increases food/caloric efficiency, 3) increases relative, total, and/or central adiposity, 4) increases preference for dietary fat, 5) decreases caloric expenditure, 6) increases lipogenic enzyme activity, and 7) promotes insulin resistance (22, 23). However, while stimulating considerable investigation, none of these effects has been substantiated in rodent experiments or clinical studies (9, 11, 23, 24).
The perception that weight cycling has negative consequences on human health has also been disseminated by reports that it increases morbidity and mortality (25-28). These early reports of adverse effects, however, have been shown to be due to a failure to account for intentionality of weight loss. For example, intentional weight loss was associated with a nearly 25% reduced all-cause mortality [hazard rate ratio (HRR) = 0.76; 95% confidence interval (CI), 0.60–0.97] compared with a one-third higher such risk for unintentional weight loss (HRR = 1.31; 95% CI, 1.01–1.70; ref. (29). As summarized in ref. (30), when unintentional weight loss studies are excluded, the majority of evidence fails to support an adverse effect of weight cycling on health, with the exception of gallbladder stones, which have a higher frequency in people who weight cycle (31, 32).
Cancer and Weight Cycling
Cancers of the breast (postmenopausal), colon, endometrium, esophagus, kidney (renal cell), and pancreas have been reported to be associated with obesity based on exhaustive reviews of the effects of body weight, adiposity, weight gain, and weight loss on the prevalence of cancer (2, 33), and recent evidence indicates that prostate cancer may be added to this list (3). Despite extensive investigations of factors related to energetics and cancer, the effects of weight cycling have been reported only for breast, endometrium, kidney, and lymphopoetic cancers (Table 1). In a large prospective study of weight change and breast cancer, no evidence was found to support an independent effect of weight cycling, defined as losing 20 pounds or more and gaining at least half of them back within a year [odds ratio (OR) = 1.0; 95% CI, 0.9–1.1; ref. (34)]. Similarly, a case-control study of the effects of body size in relation to postmenopausal breast cancer found a non-significant increase in risk (OR = 2.11; 95% CI, 1.00–4.44) among women who exhibited a fluctuating pattern of body size, defined as body weight varying between ≥ the median of the control group and < than the median, throughout adulthood (35). On the other hand, two studies in the Women’s Health Initiative do indicate an association of weight cycling with increased risk. First, the incidence of renal cell carcinoma was increased in postmenopausal women who experienced intentional weight cycling (10 or more pounds) 10 or more times relative to stable-weight women [relative risk (RR) = 2.6; 95% CI, 1.6–4.2; P-trend = 0.0005; ref. (36)]. Second, women had an increased risk of developing non-Hodgkin’s lymphoma if they intentionally lost at least 50 pounds 3 or more times (hazard ratio (HR) = 1.97; 95% CI, 0.93–4.16; P-trend by frequency = 0.09) or 20–49 pounds ≥ 3 times (HR = 1.55; 95% CI, 1.00–2.40; P-trend = 0.05), but there was a reduced risk of non-Hodgkin’s lymphoma associated with smaller amounts of weight loss (10–19 pounds ≥ 3 times; HR = 0.78; 95% CI, 0.46–1.33; P-trend = 0.40, ref. (37). Similar non-statistically significant, trends of altered risk were seen in association with multiple myeloma and leukemia.
Table 1.
Weight cycling and cancer risk: Epidemiological studies
| Cancer site | Risk assessment | Amplitudea | Frequency | Duration | Ref. |
|---|---|---|---|---|---|
| Breast (postmenopausal, prospective) |
OR = 1.0; 95% CI, 0.9–1.1 |
≥ 20 lbs. lost; ≥ 10 lbs. regain |
≥ 1cycle | 1 year | (34) |
| Breast (postmenopausal, case-control) |
OR = 2.11; 95% CI, 1.00–4.44 |
≥ Control median weight; < Control median |
Not indicated |
Adult life | (35) |
| Renal cell carcinoma (postmenopausal women, prospective) |
RR = 2.6; 95% CI, 1.6–4.2, P-trend = 0.0005 |
≥ 10 lbs. | ≥ 10 cycles | Adult life | (36) |
| Renal cell carcinoma (case-control) |
OR = 2.31; 95% CI, 1.04–5.12 |
≥ 10 lbs. | ≥ 3 cycles | Adult life | (38) |
| Endometrial (population-based case-control) |
OR = 1.27; 95% CI, 1.00–1.61, P-trend = 0.05 |
≥ 20 lbs. lost; ≥ 10 lbs. regained |
1 cycle | 1 year | (39) |
| Non-Hodgkin’s lymphoma (postmenopausal women, prospective) |
HR = 1.97; 95% CI, 0.93–4.16, P-trend = 0.09 |
≥ 50 lbs. | ≥ 3 cycles | 20 years | (37) |
| HR = 1.55; 95% CI, 1.00–2.40, P-trend = 0.05 |
20 lbs.–49 lbs. | ≥ 3 cycles | 20 years | ||
| HR = 0.78; 95% CI, 0.46–1.33, P-trend = 0.40 |
10 lbs.–19 lbs. | ≥ 3 cycles | 20 years |
Classification of weight cycling as defined in ref. (12).
In a case-control study, the risk for renal cell carcinoma also was elevated in female weight cyclers (OR = 2.31; 95% CI, 1.04–5.12, for ≥ 3 weight cycles of ≥ 10 pounds; P-trend = 0.05), but not in men (38). A population-based case-control study found that a history of weight cycling (greater than 20-pound weight loss with at least half regained within a year) was associated with a modest increase in the risk of endometrial cancer after adjustment for BMI and other factors (OR = 1.27; 95% CI, 1.00–1.61; P-trend = 0.05; ref. (39).
Rodent Carcinogenesis Studies
Chemically induced models
Kritchevsky et al. (40) investigated effects of weight cycling (induced by alternating periods of caloric restriction and ad libitum feeding) on the promotion phase of 7,12 dimethyl[α]benzanthrancene (DMBA)-induced mammary cancer, modeling common patterns of weight regulation via dieting (Table 2). They evaluated effects of 25% caloric restriction relative to ad libitum feeding at different times during the promotion/progression phase of mammary carcinogenesis and for cycles of 4 or 8 weeks. Body weight and tumor latency graphs from that study showed that retardation of weight gain suppressed mammary tumor development. When rats had free access to food and accelerated weight gain, however, the rate of tumor occurrence increased, particularly when short-term (4 week) caloric restriction was followed by an extended (12 week) period of ad libitum feeding and weight regain (50% tumor incidence, ad libitum–alone, versus 60% tumor incidence in caloric restriction followed by ad libitum feeding). Findings of Sylvester et al. (41) on caloric restriction during tumor promotion parallel those of Kritchevsky et al., despite employing 50% caloric restriction relative to ad libitum feeding and imposing a restriction on all components of the diet instead of just on calories, a distinction described in detail in ref. (42). In another study, an effort to control the magnitude of weight cycling involved subjecting DMBA-treated obese adult Wistar rats to 4 cycles of 50% caloric restriction relative to ad libitum fed rats to achieve a 20% weight loss followed by weight regain (43). A reduced tumor incidence occurred in weight-cycled animals, but the difference was not statistically significant (mammary tumor incidence of 18% ad libitum versus 9% weight-cycled).
Table 2.
Intermittent caloric restriction and cancer incidence: Rodent experiments
| Cancer sitea | Risk assessmentb |
Amplitudec | Frequency | Duration | Ref.d |
|---|---|---|---|---|---|
| Mammary, DMBA- induced, rat |
RR = 1.20; 95% CI, 0.68-2.11, NS |
25% CR | 1 cycle | 4 wk CR; 12 wk AL |
(40) |
| RR = 0.90; 95% CI, 0.47-1.73, NS |
25% CR | 1 cycle | 4 wk AL, 8 wk CR, 4 wk AL |
||
| Mammary, DMBA- induced, rat |
RR = 0.74; 95% CI, 0.49-1.12, NS |
50% CR | 1 cycle | 5 wk AL, 4 wk CR, 12 wk AL |
(41) |
| Mammary, DMBA- induced, rat |
RR = 0.50; 95% CI, 0.14-1.84, NS |
50% CR | 4 cycles | Loss of 20% body weight and then re- gained |
(43) |
| Mammary, DMBA- induced, rat |
RR = 0.89; 95% CI, 0.59-1.35, NS |
40% CR | 15 cycles | 48 hr AL, 48 hr CR for 9 wk |
(44) |
| Mammary, DMBA- induced, rat |
RR = 0.90; 95% CI, 0.63-1.30, NS |
40% CR | 16 cycles | 48 hr AL, 48 hr CR for 10wk |
(45) |
| Mammary, MNU- induced, rat |
RR=1.22; 95% CI, 0.89-1.68, NS |
33% CR | 4 cycles | 1 wk CR, 3 wk AL |
(46) |
| Mammary, MMTV- TGF-α (lepob/+) transgenic mouse |
RR = 0.04; 95 % CI, 0.01-0.30, P < 0.05 |
50% CR | 12 cycles | 3 wk CR, 3 wk, AL | (13) |
| Mammary, MMTV- TGF-α/Lepr+Leprdb transgenic mouse |
RR = 0.18; 95% CI, 0.09-0.40, P < 0.001 |
50% CR | 12 cycles | 3 wk CR, 3 wk, AL | (14) |
| Mammary, MMTV- TGF-α transgenic mouse |
RR = 0.13; 95% CI, 0.06-0.28, P < 0.05 |
50% CR | 12 cycles | 3 wk CR, 3 wk, AL | (15) |
| Mammary, MMTV- Neu transgenic mouse |
RR = 0.60; 95% CI, 0.27- 1.33, NS |
50% CR | 12 cycles | 3 wk CR, 3 wk, AL | (18) |
| Multiple tumor types, p53 deficient (p53+/−) mouse |
RR = 0.64; 95% CI, 0.31-1.32, NS |
24-hr fast | 28 - 40 cycles |
1 day/wk for 7-10 mo |
(20) |
| Mammary, spontaneous in DBA inbred mice |
RR = 1.12; 95% CI, 0.94-1.34, NS |
24-hr fast | 220 cycles | 24-hr fast, 2 times/wk for 110 wk |
(54) |
Abbreviations: CR, caloric restriction; AL, ad libitum; NS, not significant; MNU, 1-methyl-1-nitrosourea. DBA refers to a specific inbred mouse strain.
Information is provided on the cancer site, agent used to induce cancer, and the rodent species used.
The experimental approaches and number of rodents per treatment group varied markedly among the reported experiments. In order to provide a more uniform format in which to view and interpret experimental findings, risk assessment was limited to cancer incidence, and RR estimates were calculated using the cancer incidence and N for each group. Ad libitum– fed rodents were used as the referent group, and intermittently calorically restricted rodents were used as the experimental group. RR and CIs for the RR were calculated using the method described in ref. (56).
Classification of weight cycling as defined in ref. (12).
Two studies discussed in the text are not included in the Table (refs. (16, 17) because final aggregated cancer incidence data could not be accurately ascertained from the reported data. Similarly, refs (21, 55) are not included because the primary endpoint of these prostate (LAPC-4 cells) xenograft studies was survival; the effect of intermittent caloric restriction was not statistically significant in either study.
Mehta et al. investigated cycles of 48-hour 40% caloric restriction followed by 48 hours during which rats were fed the same diet as age-matched, ad libitum–fed animals in order to prevent over-eating (also termed rebound eating) relative to the ad libitum–fed control rats (44). This pattern of feeding and weight cycling virtually eliminated the protective effect on carcinogenesis associated with chronic caloric restriction (tumor incidence of 63% ad libitum versus 23% chronic caloric restriction versus 57% intermittent caloric restriction), a finding that was duplicated (45). Tagliaferro et al. had similar findings with intermittent caloric restriction during the promotion/progression phase of 1-methyl-1-nitrosourea–induced rat mammary carcinogenesis; 1 week of 33% caloric restriction followed by 3 weeks of paired re-feeding (to prevent rebound eating) for 16 weeks (4 cycles) did not protect against mammary carcinogenesis (tumor incidence of 54% ad libitum versus 66% intermittent caloric restriction; ref. (46).
Collectively, these reports indicate that short-term bouts of reduced caloric intake do not offer sustained protection against mammary cancer; whereas, maintenance of a lower body weight or weight loss to a maintained lower body weight is protective despite how the lower body weight was achieved. These findings parallel the epidemiological evidence that adult weight gain is associated with an increased risk for postmenopausal breast cancer and that loss of excess weight for height is accompanied by a reduction in this risk (35, 47-49). The data from chemically induced cancer models are also consistent with the epidemiologic observation that weight cycling does not exert an independent effect on breast-cancer risk (34).
Genetically engineered mouse models of mammary and prostate cancer and lymphoma
Intermittent and chronic caloric restriction
Weight cycling has been studied in various genetically engineered mouse models of both non-obesity– and obesity-associated cancer. A series of papers by Cleary and coworkers reported results of intermittent caloric restriction involving 3 weeks of 50% caloric restriction followed by 3 weeks of feeding matched (to prevent overeating) to the intake of ad libitum–fed mice in a mouse model of breast cancer induced by mouse mammary tumor virus (MMTV)-driven overexpression of transforming growth factor alpha (TGF-α). They performed weight-cycling experiments in mice that were heterozygous for a defect in leptin (lepob/+), creating a predisposition towards obesity (13, 14), and in mice that were not (15). Intermittent caloric restriction protected against mammary cancer in both models (average incidence across studies: 75.4% For ad libitum–fed mice versus 9.6% for mice on intermittent caloric-restriction). At first glance it appears that these results are at odds with the work in chemically induced models and with the epidemiologic findings summarized earlier. Although it is difficult to reconcile the results of one of these genetically engineered–model studies (13) with the other published work, the other two studies in this series (14, 15) indicated that growth curves and final body weights were significantly lower in intermittently caloric-restricted mice than in ad libitum–fed mice. When viewed in this light, the results are consistent with the findings of Kritchevsky et al. (40) and Sylvester et al. (41).
The experiments in the MMTV-TGF-α model also found that the effect of 50% intermittent caloric restriction, the type of weight cycling illustrated in Figure 1B, was statistically significant and greater than that of 25% chronic caloric restriction in reducing cancer incidence, despite a similar overall intake of calories in both restricted groups. It is not clear, however, if this difference in effect on carcinogenic response was due to direct effects of the magnitude of caloric restriction (50% intermittent versus 25% chronic) on host systemic factors such as insulin-like growth factor-1, leptin, or adiponectin and/or to direct effects on cell autonomous factors, such as the activity of the signaling network involving mammalian target of rapamycin, that modulate the carcinogenic process (19, 50). Alternatively, the differential effects of 25% chronically imposed and 50% intermittently imposed caloric restriction could have been a consequence of indirect effects on MMTV-driven transgene expression since activity of the MMTV promoter has been reported to be inhibited by dietary restriction (51-53). Other studies suggest that the observations from the MMTV-TGF-α model may not be generalizable. Neither intermittent nor chronic caloric restriction statistically significantly reduced tumor response in an MMTV-driven Her-2/Neu-overexpression model of breast cancer (mammary tumor incidences of 37.5% ad libitum, 33% chronic caloric restriction, and 22.5% intermittent caloric restriction; ref. (18), and intermittent caloric restriction caused only a modest and transient prolongation of tumor latency in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model of prostate cancer (16, 17).
Intermittent fasting
The effect of fasting one day per week and feeding matched to the diet of ad libitum–fed mice the other 6-days (in order to prevent rebound eating) versus the effect of chronic caloric restriction (i.e., 40% restriction relative to ad libitum–fed mice each day of the week) was studied in genetically engineered p53-deficient mice, where cancer development is considered inevitable (20). Although multiple tumor burden was reduced by either fasting or chronic caloric restriction, none of the differences were statistically significant (tumor incidence 40% ad libitum, 23% chronic caloric restriction, and 26% fasting). Although not in genetically engineered models, other studies support the genetically engineered findings. Tannenbaum and Silverstone found that intermittent fasting (24-hour fast two times per week) failed to inhibit spontaneous mammary cancer in the inbred DBA-mouse strain (tumor incidences of 80% ad libitum and 89% intermittent fasting; ref. (54). The effects of intermittent fasting were also investigated in a xenograft model of prostate cancer. An initial report indicated that intermittent fasting produced a non-significant trend toward improved survival following transplantation of LAPC-4 human prostate cancer cells into severe combined immunodeficiency (SCID) mice (21), but a larger follow-up study failed to detect a protective effect (55). Given that fasting is generally not recommended for weight control, these negative findings provide no support for considering the extreme method of caloric control by fasting for reducing cancer risk.
Conclusions
An individual’s body weight depends on the balance between caloric intake and caloric expenditure. In adults, small body-weight fluctuations occur throughout the day and during the course of a week. Over time, net trends in energy balance (positive, negative, or equilibrium) result in healthy or unhealthy weight for height. Available data fail to make a compelling case that weight cycling exerts either beneficial or detrimental effects on health independent of effects associated with BMI. Rather, the weight of evidence reinforces the current public health recommendations regarding weight management: 1) Maintain adult BMI in the target range of 18.5 to 24.9 (this range may differ depending on race) by preventing weight gain (the major cause of departing the range), and 2) monitor and correct BMI above 24.9 by initiating weight loss to return to the target range. It is clear that weight cycling is an undesirable public health goal since health benefits of maintaining adult weight in the desirable range for height are well-documented. The substantial evidence reviewed here shows that the cycle of weight regain following intentional weight loss generally does not reduce cancer risk, and the focus of weight-cycling research should be on ways to break this cycle.
Acknowledgements
The authors thank Mary C. Playdon, Shawna B. Matthews, and John N. McGinley for their technical assistance in the preparation of this manuscript.
Grant Support Supported in part by U.S. Public Health Service Grant R01 CA126704 from the National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund, American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington, DC: 2007. [Google Scholar]
- 3.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbaramaiah K, Howe LR, Bhardwaj P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4(3):329–46. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Weiss EC, Galuska DA, Khan LK, Serdula MK. Weight-control practices among U.S. adults, 2001-2002. Am J Prev Med. 2006;31(1):18–24. doi: 10.1016/j.amepre.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Baradel LA, Gillespie C, Kicklighter JR, et al. Temporal changes in trying to lose weight and recommended weight-loss strategies among overweight and obese Americans, 1996-2003. Prev Med. 2009;49(2-3):158–64. doi: 10.1016/j.ypmed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of Diet and Exercise, Alone or Combined, on Weight and Body Composition in Overweight-to-Obese Postmenopausal Women. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss EC, Galuska DA, Kettel KL, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999-2002. Am J Prev Med. 2007;33(1):34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1 Suppl):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 10.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29(10):1153–67. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 11.Weight cycling. National Task Force on the Prevention and Treatment of Obesity. JAMA. 1994;272(15):1196–202. [PubMed] [Google Scholar]
- 12.Cutter G, St JS, Brunner R, et al. Methodological issues in weight cycling. Ann Behav Med. 1996;18(4):280–9. doi: 10.1007/BF02895290. [DOI] [PubMed] [Google Scholar]
- 13.Cleary MP, Jacobson MK, Phillips FC, et al. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-alpha female mice. Cancer Epidemiol Biomarkers Prev. 2002;11(9):836–43. [PubMed] [Google Scholar]
- 14.Cleary MP, Hu X, Grossmann ME, et al. Prevention of mammary tumorigenesis by intermittent caloric restriction: does caloric intake during refeeding modulate the response? Exp Biol Med (Maywood ) 2007;232(1):70–80. [PubMed] [Google Scholar]
- 15.Rogozina OP, Bonorden MJL, Grande JP, Cleary MP. Serum Insulin-like Growth Factor-I and Mammary Tumor Development in Ad libitumΓÇôFed, Chronic CalorieΓÇôRestricted, and Intermittent CalorieΓÇôRestricted MMTV-TGF-+¦ Mice. Cancer Prevention Research. 2009;2(8):712–9. doi: 10.1158/1940-6207.CAPR-09-0028. [DOI] [PubMed] [Google Scholar]
- 16.Bonorden MJ, Rogozina OP, Kluczny CM, et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr Cancer. 2009;61(2):265–75. doi: 10.1080/01635580802419798. [DOI] [PubMed] [Google Scholar]
- 17.Bonorden MJ, Rogozina OP, Kluczny CM, et al. Cross-sectional analysis of intermittent versus chronic caloric restriction in the TRAMP mouse. Prostate. 2009;69(3):317–26. doi: 10.1002/pros.20878. [DOI] [PubMed] [Google Scholar]
- 18.Pape-Ansorge KA, Grande JP, Christensen TA, Maihle NJ, Cleary MP. Effect of moderate caloric restriction and/or weight cycling on mammary tumor incidence and latency in MMTV-Neu female mice. Nutr Cancer. 2002;44(2):162–8. doi: 10.1207/S15327914NC4402_07. [DOI] [PubMed] [Google Scholar]
- 19.Rogozina OP, Bonorden MJ, Seppanen CM, Grande JP, Cleary MP. Effect of Chronic and Intermittent Calorie Restriction on Serum Adiponectin and Leptin and Mammary Tumorigenesis. Cancer Prev Res (Phila) 2011 doi: 10.1158/1940-6207.CAPR-10-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23(5):817–22. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 21.Buschemeyer WC, III, Klink JC, Mavropoulos JC, et al. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate. 2010;70(10):1037–43. doi: 10.1002/pros.21136. [DOI] [PubMed] [Google Scholar]
- 22.Brownell KD, Greenwood MRC, Stellar E, Shrager EE. The effects of repeated cycles of weight loss and regain in rats. Physiology & Behavior. 1986;38(4):459–64. doi: 10.1016/0031-9384(86)90411-7. [DOI] [PubMed] [Google Scholar]
- 23.Reed GW, Hill JO. Weight cycling: a review of the animal literature. Obes Res. 1993;1(5):392–402. doi: 10.1002/j.1550-8528.1993.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeffery RW. Does weight cycling present a health risk? Am J Clin Nutr. 1996;63(3 Suppl):452S–5S. doi: 10.1093/ajcn/63.3.452. [DOI] [PubMed] [Google Scholar]
- 25.Brownell KD, Rodin J. Medical, metabolic, and psychological effects of weight cycling. Arch Intern Med. 1994;154(12):1325–30. [PubMed] [Google Scholar]
- 26.Blair SN, Shaten J, Brownell K, Collins G, Lissner L. Body weight change, all-cause mortality, and cause-specific mortality in the Multiple Risk Factor Intervention Trial. Ann Intern Med. 1993;119(7 Pt 2):749–57. doi: 10.7326/0003-4819-119-7_part_2-199310011-00024. [DOI] [PubMed] [Google Scholar]
- 27.Lissner L, Odell PM, D’Agostino RB, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324(26):1839–44. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
- 28.Lee IM, Paffenbarger RS., Jr Change in body weight and longevity. JAMA. 1992;268(15):2045–9. [PubMed] [Google Scholar]
- 29.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional Weight Loss and Death in Overweight and Obese U.S. Adults 35 Years of Age and Older. Annals of Internal Medicine. 2003;138(5):383–9. doi: 10.7326/0003-4819-138-5-200303040-00007. [DOI] [PubMed] [Google Scholar]
- 30.Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169(9):881–6. doi: 10.1001/archinternmed.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syngal S, Coakley EH, Willett WC, et al. Long-term weight patterns and risk for cholecystectomy in women. Ann Intern Med. 1999;130(6):471–7. doi: 10.7326/0003-4819-130-6-199903160-00003. [DOI] [PubMed] [Google Scholar]
- 32.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Weight cycling and risk of gallstone disease in men. Arch Intern Med. 2006;166(21):2369–74. doi: 10.1001/archinte.166.21.2369. [DOI] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer . Physical Activity, Weight Control, and Cancer. IARC Press; Lyon: 2002. [Google Scholar]
- 34.Trentham-Dietz A, Newcomb PA, Egan KM, et al. Weight change and risk of postmenopausal breast cancer (United States) Cancer Causes Control. 2000;11(6):533–42. doi: 10.1023/a:1008961931534. [DOI] [PubMed] [Google Scholar]
- 35.Eng SM, Gammon MD, Terry MB, et al. Body size changes in relation to postmenopausal breast cancer among women on Long Island, New York. Am J Epidemiol. 2005;162(3):229–37. doi: 10.1093/aje/kwi195. [DOI] [PubMed] [Google Scholar]
- 36.Luo J, Margolis KL, Adami HO, et al. Body size, weight cycling, and risk of renal cell carcinoma among postmenopausal women: the Women’s Health Initiative (United States) Am J Epidemiol. 2007;166(7):752–9. doi: 10.1093/aje/kwm137. [DOI] [PubMed] [Google Scholar]
- 37.De Roos AJ, Ulrich CM, Ray RM, et al. Intentional weight loss and risk of lymphohematopoietic cancers. Cancer Causes Control. 2010;21(2):223–36. doi: 10.1007/s10552-009-9453-5. [DOI] [PubMed] [Google Scholar]
- 38.Lindblad P, Wolk A, Bergstrom R, Persson I, Adami HO. The role of obesity and weight fluctuations in the etiology of renal cell cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 1994;3(8):631–9. [PubMed] [Google Scholar]
- 39.Trentham-Dietz A, Nichols HB, Hampton JM, Newcomb PA. Weight change and risk of endometrial cancer. Int J Epidemiol. 2006;35(1):151–8. doi: 10.1093/ije/dyi226. [DOI] [PubMed] [Google Scholar]
- 40.Kritchevsky D, Welch CB, Klurfeld DM. Response of mammary tumors to caloric restriction for different time periods during the promotion phase. Nutr Cancer. 1989;12(3):259–69. doi: 10.1080/01635588909514025. [DOI] [PubMed] [Google Scholar]
- 41.Sylvester PW, Aylsworth CF, Van Vugt DA, Meites J. Influence of underfeeding during the “critical period” or thereafter on carcinogen-induced mammary tumors in rats. Cancer Res. 1982;42(12):4943–7. [PubMed] [Google Scholar]
- 42.Thompson HJ, Zhu Z, Jiang W. Dietary energy restriction in breast cancer prevention. J Mammary Gland Biol Neoplasia. 2003;8(1):133–42. doi: 10.1023/a:1025743607445. [DOI] [PubMed] [Google Scholar]
- 43.Buison AM, Pellizzon MA, Brogan KE, Barnes MJ, Jen KL. Weight cycling did not increase tumor incidence in high fat-fed rats treated with a low dose of 7,12-dimethylbenz(1)anthracene. Nutr Res. 2005;25:1097–108. [Google Scholar]
- 44.Mehta RS, Harris SR, Gunnett CA, Bunce OR, Hartle DK. The effects of patterned calorie-restricted diets on mammary tumor incidence and plasma endothelin levels in DMBA-treated rats. Carcinogenesis. 1993;14(8):1693–6. doi: 10.1093/carcin/14.8.1693. [DOI] [PubMed] [Google Scholar]
- 45.Harris SR, Brix AE, Broderson JR, Bunce OR. Chronic energy restriction versus energy cycling and mammary tumor promotion. Proc Soc Exp Biol Med. 1995;209(3):231–6. doi: 10.3181/00379727-209-43897. [DOI] [PubMed] [Google Scholar]
- 46.Tagliaferro AR, Ronan AM, Meeker LD, et al. Cyclic food restriction alters substrate utilization and abolishes protection from mammary carcinogenesis female rats. J Nutr. 1996;126(5):1398–405. doi: 10.1093/jn/126.5.1398. [DOI] [PubMed] [Google Scholar]
- 47.Harvie M, Howell A, Vierkant RA, et al. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2005;14(3):656–61. doi: 10.1158/1055-9965.EPI-04-0001. [DOI] [PubMed] [Google Scholar]
- 48.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord. 2003;27(12):1447–52. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- 49.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 50.Dogan S, Johannsen AC, Grande JP, Cleary MP. Effects of intermittent and chronic calorie restriction on mammalian target of rapamycin (mTOR) and IGF-I signaling pathways in mammary fat pad tissues and mammary tumors. Nutr Cancer. 2011;63(3):389–401. doi: 10.1080/01635581.2011.535968. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar NH, Fernandes G, Telang NT, Kourides IA, Good RA. Low-calorie diet prevents the development of mammary tumors in C3H mice and reduces circulating prolactin level, murine mammary tumor virus expression, and proliferation of mammary alveolar cells. Proc Natl Acad Sci U S A. 1982;79(24):7758–62. doi: 10.1073/pnas.79.24.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engelman RW, Fukaura Y, Hamada N, Good RA, Day NK. Dietary restriction permits normal parturition and lactation but suppresses mouse mammary tumor virus proviral transcription even after mammary involution. Cancer Res. 1991;51(19):5123–8. [PubMed] [Google Scholar]
- 53.Haraguchi S, Good RA, Engelman RW, Greene S, Day NK. Prolactin, epidermal growth factor or transforming growth factor-alpha activate a mammary cell-specific enhancer in mouse mammary tumor virus-long terminal repeat. Mol Cell Endocrinol. 1997;129(2):145–55. doi: 10.1016/s0303-7207(97)04053-7. [DOI] [PubMed] [Google Scholar]
- 54.Tannenbaum A, SILVERSTONE H. Failure to inhibit the formation of mammary carcinoma in mice by intermittent fasting. Cancer Res. 1950;10(9):577–9. [PubMed] [Google Scholar]
- 55.Thomas JA, Antonelli JA, Lloyd JC, et al. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis. 2010;13(4):350–5. doi: 10.1038/pcan.2010.24. [DOI] [PubMed] [Google Scholar]
- 56.Armitage P, Berry G. Statistical Methods in Medical Research. 3rd ed Blackwell; London: 1994. [Google Scholar]