Abstract
To explore whether CaMKII-dependent phosphorylation events mediate reperfusion arrhythmias, Langendorff perfused hearts were submitted to global ischemia/reperfusion. Epicardial monophasic or transmembrane action potentials and contractility were recorded. In rat hearts, reperfusion significantly increased the number of premature beats (PBs) relative to pre-ischemic values. This arrhythmic pattern was associated with a significant increase in CaMKII-dependent phosphorylation of Ser2814 on Ca2+-release channels (RyR2) and Thr17 on phospholamban (PLN) at the sarcoplasmic reticulum (SR). These phenomena could be prevented by the CaMKII-inhibitor KN-93. In transgenic mice with targeted inhibition of CaMKII at the SR membranes (SR-AIP), PBs were significantly decreased from 31 ± 6 to 5 ± 1 beats/3 min with a virtually complete disappearance of early-afterdepolarizations (EADs). In mice with genetic mutation of the CaMKII phosphorylation site on RyR2 (RyR2-S2814A), PBs decreased by 51.0 ± 14.7 %. In contrast, the number of PBs upon reperfusion did not change in transgenic mice with ablation of both PLN phosphorylation sites (PLN-DM). The experiments in SR-AIP mice, in which the CaMKII inhibitor peptide is anchored in the SR membrane but also inhibits CaMKII regulation of L-type Ca2+ channels, indicated a critical role of CaMKII-dependent phosphorylation of SR proteins and/or L-type Ca2+ channels in reperfusion arrhythmias. The experiments in RyR2-S2814A further indicate that up to 60% of PBs related to CaMKII are dependent on the phosphorylation of RyR2 Ser2814 site and could be ascribed to delayed-afterdepolarizations (DADs). Moreover, phosphorylation of PLN-Thr17 and L-type Ca2+ channels might contribute to reperfusion-induced PBs, by increasing SR Ca2+ content and Ca2+ influx.
Keywords: Ischemia, Reperfusion, heart, arrhythmias, CaMKII
INTRODUCTION
Reperfusion of the myocardium after a relatively brief period of ischemia may precipitate a pattern of arrhythmias with different levels of severity [1,2]. This is important in the clinical setting as revealed by the numerous reports indicating a significant role for these arrhythmias either during spontaneous reperfusion after coronary spasm or after thrombolysis subsequent to acute myocardial infarction [3–5]. Cardiac arrhythmias may arise from abnormalities in impulse propagation (due to reentry) or impulse initiation (due to focal or ectopic activity). In particular, there is clear evidence that a great number of reperfusion arrhythmias can be traced to ectopic sources [6–9]. Indeed, earlier experimental work has revealed that reperfusion arrhythmias may result from early-afterdepolarizations (EADs) [7]. Moreover recent findings described a close association between spontaneous Ca2+-oscillations, delayed-afterdepolarizations (DADs) and reperfusion arrhythmias [9,10]. These stimuli of non-reentry nature may give rise to episodes of ventricular tachycardia or fibrillation. However, the cellular and molecular bases of the focal arrhythmogenic event remain poorly understood.
Experiments from our laboratory have recently shown that the return to normal pH after a period of acidosis can evoke cardiac arrhythmias that are dependent on CaMKII [11]. This pattern of arrhythmias was initiated by a focal triggered mechanism, involving spontaneous diastolic Ca2+ release from cardiac ryanodine receptors (RyR2) on the sarcoplasmic reticulum (SR). The resulting elevation of cytosolic Ca2+ triggers the activation of the electrogenic Na+/Ca2+ exchanger (NCX), which in turn produces an arrhythmogenic depolarizing inward current (DAD) [11, 12]. Because of the facts that acidosis is a major component of myocardial ischemia and that CaMKII was found to be activated at the beginning of reperfusion [13–15], the present experiments were undertaken to explore whether CaMKII activity is involved in reperfusion arrhythmias. Our results indicated that reperfusion arrhythmias are tightly associated with CaMKII-dependent phosphorylation of SR proteins, in particular Ser2814 on RyR2 and Thr17 on phospholamban (PLN). Thus, these CaMKII targets would provide the molecular basis for triggering reperfusion arrhythmias, by contributing to increase SR Ca2+ load and to amplify RyR2-mediated SR Ca2+ leak.
METHODS
For a detailed description, see also the expanded materials and methods in Supplementary material online.
Animals
Experiments were performed on male Wistar rats (200–300 g body weight), transgenic mice (25–30 g) expressing four concatenated repeats of CaMKII-inhibitory peptide AIP targeted to the SR membrane (SR-AIP) [16], mice with genetic ablation of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) site on RyR2 (RyR2-S2814A) [17], and mice expressing a mutant PLN in which both phosphorylation residues (Ser16 and Thr17) were replaced by Ala (PLN-DM) (MMRRC, University of Missouri/Harlam, Mouse Regional Resource Center, NCRR, NIH) [18]. Age-matched wild type mice (WT) served as controls. Animals used in this study were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). The protocol was approved by the Ethics Committee of the Cardiovascular Research Center, National Research Council (CONICET, Argentina).
Ex vivo experiments: intact hearts
Animals were anaesthetized with an intraperitoneal injection of sodium pentobarbital (50mg/kg) and the heart was excised. Central thoracotomy and heart excision was performed immediately after phase III of anesthesia was reached, verified by the loss of pedal withdrawal reflex. Isolated hearts were perfused according to Langendorff technique at constant temperature (37°C) and flow (14 and 4 ml/min for rat and mouse hearts, respectively) as previously described [13,14].
Epicardial monophasic action potentials (MAPs)
MAPs were obtained by using a silver/silver chloride Ag/AgCl electrode apposed to the epicardial surface of the free left ventricular wall as previously described [11]. MAP recordings obtained satisfied previously documented criteria of a stable baseline and triangular MAP morphology, rapid upstroke phase, and consistent amplitude [11, 19]. Although MAP measurements are local, they were always associated with global changes in contractility. This makes it possible to correlate these electrical events with biochemical changes measured in the whole ventricle (see below).
Intracellular action potentials
Di-8-ANEPPS (Invitrogen, USA) was used to evaluate transmembrane action potentials (APs) in the epicardial layer of intact mouse hearts using a custom-made setup for Pulse Local-Field Fluorescence (PLFF) microscopy[20].
Experimental protocol
After stabilization, hearts were submitted to normothermic global ischemia (20 min for rat or 15 min for mice, respectively) followed by reperfusion (IR) [13,14]. Quantification of premature beats (PBs) was accomplished by counting the number of extra MAPs that do not follow the basal heart rhythm during the first 3 minutes of reperfusion. A group of hearts was freeze-clamped for biochemical assays at 1 min of reperfusion. Drugs were perfused 10 min before the onset of ischemia and during the reperfusion period. The concentration of DMSO used for dilution of drugs did not affect basal contractility and the pattern of ectopic activityin these experiments.
Biochemical assays
SR membrane vesicles were prepared from ventricular homogenates as previously described [21]. Protein concentrations were measured using the Bradford method with bovine serum albumin as the standard. The average yield was 1–2 mg membrane vesicles protein/g cardiac tissue.
Electrophoresis and Western Blot
For immunological detection of PLN and phosphorylated PLN, phospho-CaMKII and calsequestrin (CQS) 15–50 μg of membrane protein were electrophoresed per gel lane in 10% acrylamide gels according to Porzio and Pearson [13, 22]. For immunological detection of RyR2 and phosphorylated RyR2, 50 μg of membrane protein were electrophoresed per gel lane in 6% acrylamide gels according to Laemmli [21].
Statistics
Data are expressed as mean ± SEM. Statistical significance was determined by Student’s t-test for paired or unpaired observations as appropriate, and ANOVA when different groups were compared. The Newman-Keuls test was used to examine statistical differences observed with the ANOVA. A P value <0.05 was considered statistically significant.
RESULTS
Characterization of arrhythmias following reperfusion
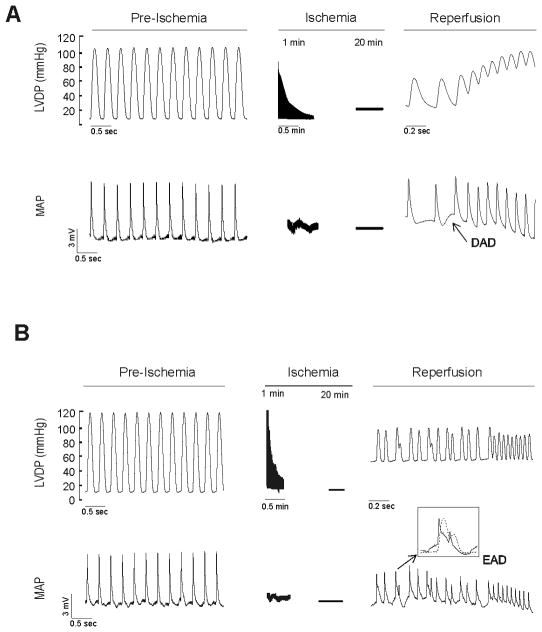
Previous experiments from different laboratories demonstrated that reperfusion after ischemia is associated with cardiac arrhythmias. Furthermore, it has been shown that the incidence of arrhythmias during reperfusion depends on the duration of the preceding ischemic period, the probability of obtaining arrhythmias being maximal after 20–30 min ischemia [2]. We therefore used a protocol of global ischemia of 20 min in rat or 15 min in mouse hearts to explore the occurrence of arrhythmias in the first 3 min of reperfusion. Typical patterns of arrhythmic activity observed at the onset of reperfusion are shown in Figure 1 by the simultaneous recording of left ventricular developed pressure (LVDP) and MAPs. Figure 1A shows an example of a premature beat (PB) that culminated into ventricular tachycardia. In some cases, as shown in Figure 1A, a membrane depolarization could be detected preceding the PB, suggesting the occurrence of a DAD. Figure 1B shows an arrhythmic pattern in which a depolarization starting before the completion of the action potential was observed, a typical feature of EADs. Thus, the arrhythmic pattern observed during the early reperfusion period seems to be triggered by both DADs and EADs, in agreement with previous findings [7, 9]. The number of beats triggered by EADs can be accurately determined from the MAP recordings. It was found that a small proportion of the total PBs (13.3 ± 5.6 %, i.e. 8 ± 3 of a total of 46 ± 5 beats/3 min) could be traced to EADs. In contrast, only some PBs triggered by DADs could be directly identified in MAP recordings. This does not necessarily mean that DADs-induced PBs were not occurring. Indeed, PBs that were not caused by EADs could be partially or totally attributed to DADs. In view of this possibility, we explored the underlying mechanisms of PBs upon reperfusion.
Figure 1. Left ventricular developed pressure (LVDP) and epicardial monophasic action potentials (MAPs) during ischemia/reperfusion protocol.
A: Representative recordings showing the appearance of reperfusion arrhythmias triggered bya delay after depolarization (DAD). B: Representative example in which the PBs are associated with an early afterdepolarization (EAD). The inset of the Figure indicates in an expanded time scale, the occurrence of EAD before the completion of the action potential.
Pharmacologic inhibition of CaMKII prevents reperfusion arrhythmias in perfused rat hearts
Since CaMKII has been described as a pro-arrhythmogenic molecule and mediator of different type of arrhythmias [11,17,23,24], we investigated the hypothesis that CaMKII may be involved in the arrhythmic pattern observed in early reperfusion. We performed IR experiments in Langendorff-perfused rat hearts, similar to the ones described in Figure 1, in the presence and absence of the CaMKII inhibitor KN-93 or its inactive analogue, KN-92. Neither KN-93 nor KN-92 affected basal contractility and relaxation and MAP duration (MAPD) at the concentrations used (Table 1 of Supplementary Data).
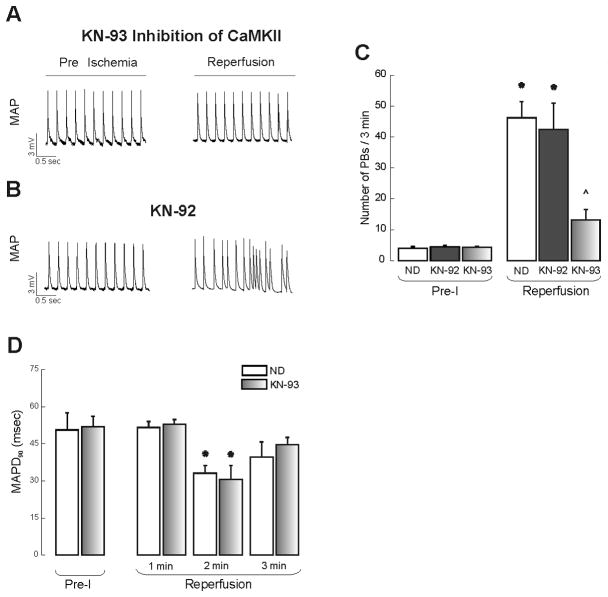
Figure 2A and B shows typical examples and overall results of this group of experiments. As shown in the summary data, the number of PBs observed upon reperfusion was decreased by 71.5 ± 7.4% in the presence of KN-93. Under these conditions EADs were not detected. Assuming that the PBs that were not associated to EADs could be triggered by DADs, KN-93 prevented 62.3 ± 6.8% of DADs. In contrast, KN-92 did not affect the occurrence of PBs upon reperfusion. These results suggest that CaMKII activity is responsible for most of early reperfusion arrhythmias. Upon reperfusion, MAPD was similarly and significantly decreased in the absence and presence of KN-93 with respect to pre-ischemic values (Figure 2D).
Figure 2. CaMKII-dependence of reperfusion arrhythmias.
Typical example (A) depicting that CaMKII-inhibition by 2.5 μM KN-93 significantly decreased the number of PBs. The inactive analogue KN-92 (B) failed to affect the reperfusion arrhythmic pattern. C: Overall results comparing the number of PBs in the pre-ischemic period (Pre-I) and in the first 3 min of reperfusion in the absence of drugs (ND) and in the presence of KN-92 and KN-93. D: MAP duration at 90 % repolarization (MAPD90) measured at Pre-I during the first 3 min of reperfusion in the absence and the presence of KN-93. n = 4–7. * indicates P < 0.05 with respect to Pre-I. ^ indicates P < 0.05 with respect to reperfusion with ND.
Reperfusion arrhythmias are strongly dependent on the SR in rat hearts
As shown in Figure 1, reperfusion arrhythmias could be attributed to both EADs and DADs. The occurrence of DADs was tightly associated with spontaneous Ca2+ release from cardiac RyR2. Indeed, when the SR function was disabled by the application of 1 μM ryanodine, a concentration known to diminish DADs [25,26], the number of PBs upon reperfusion were reduced from 46 ± 5 to 22 ± 6 beats/3min (See Figure 1 of Supplementary Data), implicating the SR as a main contributor to the generation of reperfusion arrhythmias.
Ca2+ release from cardiac RyR2 essentially depends on two factors: (1) The ability of the cell to increase SR Ca2+ content, which mainly relies on the activity of SERCA2a, and (2) the probability of spontaneous Ca2+ release through the RyR2, which primarily depends on the properties of these channels. Since CaMKII exerts a regulatory action on the SR Ca2+ uptake and release functions through phosphorylation of Thr17 on PLN (PLN-Thr17) and Ser2814 on RyR2 (RyR2-Ser2814), we investigated the possible role of these proteins in reperfusion arrhythmias. Therefore, the phosphorylation levels of PLN-Thr17 and RyR2-Ser2814 sites were examined at the onset of reperfusion.
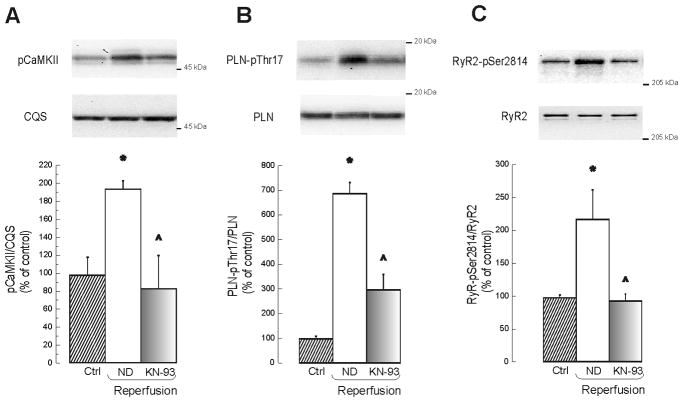
Figure 3A–C shows that CaMKII-autophosphorylation and CaMKII-dependent phosphorylation levels of PLN-Thr17 and that of RyR2-Ser2814, were significantly increased at the beginning of reperfusion. These phosphorylation events were reduced by KN-93 (P<0.05). In contrast, phosphorylation of the PLN-Ser16 site and that of RyR2-Ser2808, both dependent on PKA [21,22,27], did not change. In line with these findings, inhibition of PKA by 5 μM of H-89 failed to prevent reperfusion arrhythmias (See Figure 2C of Supplementary Data). Taken together, these results confirmed an increase in CaMKII activity levels at the beginning of reperfusion, as previously described [13,15]. These data also suggest that both RyR2-Ser2814 and PLN-Thr17 may be critical downstream targets of CaMKII involved in the genesis of reperfusion arrhythmias.
Figure 3. CaMKII is active at the onset of reperfusion.
Typical blots and overall results of experiments showing an increase in CaMKII autophosphorylation (A) and in the phosphorylation of two typical substrates of CaMKII: Thr17 site of PLN (B) and Ser2814 of RyR2 (C). Treatment with KN-93 prevented the CaMKII-dependent protein-phosphorylations detected during reperfusion. n = 3–6. * indicates P < 0.05 with respect to control (Ctrl). ^ indicates p < 0.05 with respect to reperfusion with ND.
Downstream targets of CaMKII during reperfusion
To gain further insight into the possible role of RyR2-Ser2814 and PLN-Thr17 phosphorylation and in an attempt to dissect the relative importance of these two phosphorylation events on reperfusion arrhythmias, we used two types of transgenic mice and a knock-in mouse model. Basal contractility, relaxation and MAPD of mice hearts used are shown in Table 2 of Supplementary Data. Wildtype (WT) mice showed a pattern of reperfusion arrhythmias similar to rats, although the total number of PBs was somewhat lower and the percentage of EADs somewhat higher (22.2 ± 6.8 % of total PBs). Moreover, a significant increase in CaMKII activity at the onset of reperfusion was also observed in mouse hearts (Figure 3 of Supplementary Data), in agreement with previous findings [14].
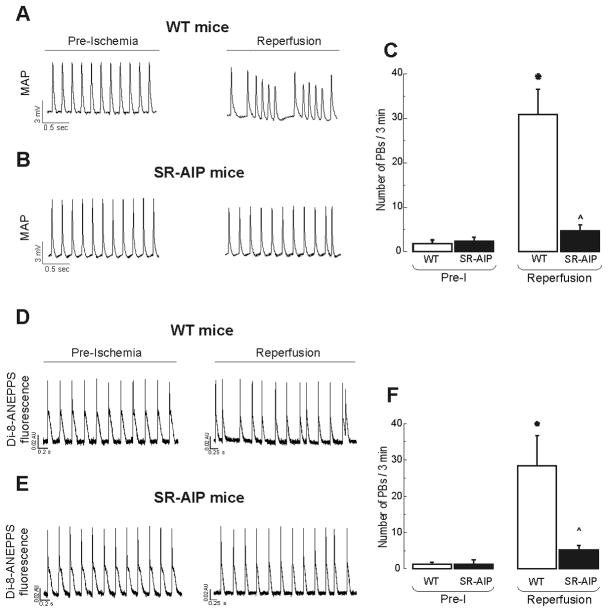
We first performed experiments in transgenic mice with targeted inhibition of CaMKII at the level of cardiac SR-membranes (SR-AIP). Figure 4A–C shows typical examples and overall results of these experiments. Arrhythmogenic afterdepolarizations were significantly decreased in SR-AIP mice. Figure 4D–F shows that similar results were obtained when transmembrane potentials were measured with the fluorescent dye DI-8-ANEPPS. No episodes of ventricular tachycardia during reperfusion were observed in SR-AIP mice. These experiments show that 83.9 ± 7.5 % of reperfusion PBs were prevented in SR-AIP mice and therefore dependent on CaMKII. Interestingly, virtually no EADs could be observed among the remaining PBs in SR-AIP mice. This is in consonance with the fact that, although CaMKII inhibition was specifically targeted to the SR, an inhibition of CaMKII-dependent regulation of L-type Ca2+ current also occurred in these mice [28]. Taken together the results indicate that reperfusion PBs are largely dependent on the effects of CaMKII at the level of SR and L-type Ca2+ channels.
Figure 4. CaMKII inhibition targeted to SR completely suppresses reperfusion arrhythmias.
Typical examples (A, B, D and E) and overall results (C and F) of experiments performed in WT mice and mice with targeted inhibition of CaMKII at the SR level (SR-AIP mice). A and B are MAP recordings, D and E are optical AP recordings obtained with the fluorescent dye Di-8-ANEPPS. Reperfusion arrhythmias were virtually completely inhibited in SR-AIP mice. n = 3–6. * P < 0.05 with respect to Pre-I. ^ indicates P < 0.05 with respect to reperfusion in WT mice.
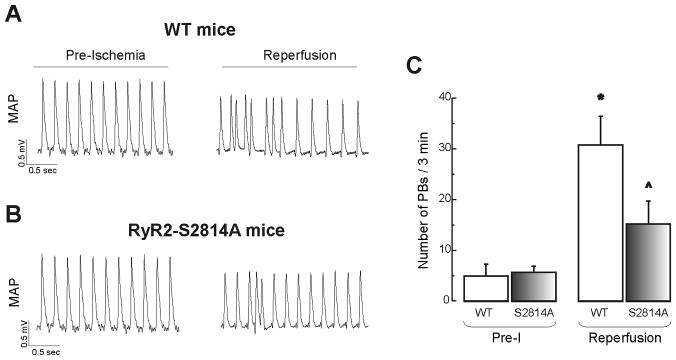
We then performed experiments in knock-in mice in which Ser2814 site of RyR2 was mutated to alanine (RyR2-S2814A) and transgenic mice expressing double-mutant PLN (PLN-DM) that lacks the regulatory PLN phosphorylation sites (Ser16A/Thr17A) to genetically inhibit CaMKII phosphorylation of RyR2 and PLN phosphorylation sites, respectively. In RyR2-S2814A mice the number of PBs detected in reperfusion was significantly reduced by 51.0 ± 14.7 % of total PBs (Figure 5A–C) or 60.8 ± 17.6 % when expressed relative to the CaMKII-dependent PBs according to the results in SR-AIP mice. The results point to CaMKII-dependent phosphorylation of RyR2 and DADs as key mechanisms involved in reperfusion arrhythmias.
Figure 5. CaMKII-dependent phosphorylation of RyR2-Ser2814 site is critical for generation of reperfusion arrhythmias.
Typical examples (A and B) and overall results (C) of reperfusion arrhythmias in WT mice and mice with genetic mutation of Ser2814 site of RyR2 (RyR2-S2814A mice). There was a significant decrease in PBs in RyR2-S2814A mice relative to WT. n = 3–6. * P < 0.05 with respect to Pre-I. ^ indicates P < 0.05 with respect to reperfusion in WT mice.
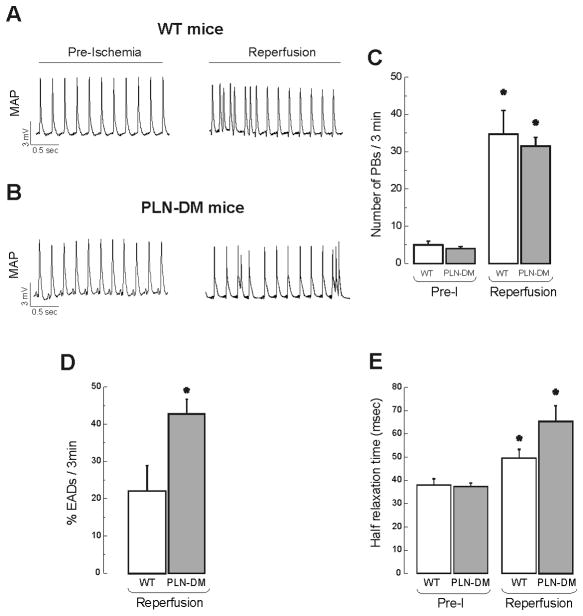
In contrast to the results in RyR2-S2814A mice, the number of reperfusion arrhythmias in non-phosphorytable PLN mice was not different from WT mice, as depicted in the typical examples and overall results in Figure 6A and B. However, CaMKII inhibition with KN-93, significantly reduced the number of PBs observed in PLN-DM mice (32 ± 2 PLN-DM vs. 10 ± 2 in PLN-DM + KN-93). A closer look to the arrhythmic pattern of these mice shows a significant increase in EADs with respect to WT animals: Whereas 42.8 ± 4.0 % of total PBs corresponded to EADs in PLN-DM mice, only 22.2 ± 6.8 % could be assigned to EADs in WT (Figure 6D). This finding is in line with the increase in L-type Ca2+ current described in PLN-DM mice [29]. Moreover, a significant increase in EADs in PLN-DM mice without changes in the total number of PBs, indicates a decrease in PBs other than EADs which might be related to the lack of CaMKII-dependent PLN phosphorylation. Additionally, the impairment in relaxation usually observed at the onset of reperfusion [13,14] was significantly more pronounced in PLN-DM mice than in WT-mice. Half relaxation time increased from 38.0 ± 2.7 msec in the preischemia to 65.4 ± 6.7 msec at 1 min of reperfusion in PLN-DM and from 37.3 ± 1.5 to 49.5 ± 3.8 msec in WT mice. These results reveal that Thr17 phosphorylation of PLN significantly influences the activity of SERCA2a and the enhancement of SR Ca2+ reuptake at the onset of reperfusion (Figure 6E). Taken together, these changes in PLN-DM mice indicate that a participation of CaMKII-dependent PLN phosphorylation in reperfusion arrhythmias cannot be dismissed.
Figure 6. Effect of genetic mutation of PLN-phosphorylation sites (PLN-DM mice) on reperfusion arrhythmias.
Typical examples of MAP recordings (A and B) and overall results (C), indicating that the number of PBs upon reperfusion was similar in transgenic mice with non-phosphorylatable PLN than in WT mice with intact PLN. The percentage of PBs triggered by EADs was increased in PLN-DM mice vs. WT mice (D). Half relaxation time at 1 min reperfusion was prolonged in PLN-DM mice with respect to WT mice (E). n = 4–7. * P < 0.05 with respect to Pre-I.
DISCUSSION
The multifunctional CaMKII has emerged as an important pro-arrhythmogenic signaling molecule in the setting of the long QT syndrome [30], cardiac hypertrophy [31] and cardiomyopathy [32]. Previous work pointed to the crucial role of CaMKII in generating EADs and triggered arrhythmias. Recent experiments from our laboratory showed that CaMKII is also responsible for DADs that trigger post-acidosis arrhythmias [11]. Since acidosis is an important component of ischemia, the present study was undertaken to investigate the possible participation of CaMKII in reperfusion arrhythmias. Reperfusion arrhythmias usually are of the ventricular tachycardia type and may degenerate in lethal ventricular fibrillation. Experimental evidence indicated that most of the ventricular tachycardia episodes leading to ventricular fibrillation at the onset of reperfusion, were initiated at the border of the reperfused zone by a non-reentrant mechanism and were maintained by both non-reentrant and reentrant mechanisms [6]. However, the nature of the inciting stimulus is still a matter of debate and different type of evidence points to EADs and DADs as the main trigger of the reperfusion arrhythmic pattern [6–9]. Some of these experiments have correlated the spontaneous Ca2+ oscillations typical of a Ca2+ overloaded SR, with reperfusion arrhythmias [9]. The present results indicated that the reperfusion arrhythmic pattern is largely dependent on CaMKII activation and that CaMKII-dependent phosphorylation of the RyR2-Ser2814 is the main mechanism involved in the generation of this type of arrhythmias. The results also point to DADs as main triggers of reperfusion arrhythmias.
CaMKII-dependence of reperfusion arrhythmias
CaMKII can phosphorylate and modulate numerous proteins involved in Ca2+ handling, like voltage-gated Ca2+ channels, PLN and RyR2, all of which could contribute to arrhythmogenesis [21,27,33–35]. The present results showed that the arrhythmias upon reperfusion in the rat were significantly diminished by the CaMKII inhibitor KN-93 but not by its inactive analog KN-92, which makes unlikely that the inhibitory effect of KN-93 on reperfusion arrhythmias was due to non-specific effects of the drug [36]. Results in SR-AIP mice confirmed that most reperfusion arrhythmias could be attributed to CaMKII. Interpretation of the results in these mice should take into account that the expression of the CaMKII inhibitory peptide AIP in the SR membrane [16] produces not only the inhibition of CaMKII-dependent phosphorylation of the RyR2 and PLN but also of the L-type Ca2+ channels [28]. Therefore, this model suggests that CaMKII phosphorylation events occurring at these three levels: RyR2, PLN and L-type Ca2+ channels might play a role in reperfusion arrhythmogenesis.
CaMKII-dependent phosphorylation of RyR2 and PLN: their relative importance in reperfusion arrhythmias
PLN and RyR2 were both significantly phosphorylated at the onset of reperfusion on the CaMKII sites Thr17 and Ser2814 respectively, and inhibition of CaMKII blunted both phosphorylation events. These results point to the CaMKII phosphorylations of these two sites, as good candidates for playing a major role in reperfusion arrhythmias by regulating SR Ca2+-uptake and leak. This is in line with the concept that spontaneous Ca2+ release from the SR that may generate DADs depends on the interplay between the ability of SERCA2a to increase SR Ca2+ content and the properties of RyR2 [37]. In order to analyze the relative contribution of CaMKII-dependent phosphorylation of RyR2 to the generation of reperfusion arrhythmias, we used the novel knock-in mice, which express non-phosphorylatable Ser2814A on RyR2 [17]. We showed that reperfusion arrhythmias were decreased in these mice in a percentage that represents 60% of the CaMKII-dependent PBs. Assuming that SR Ca2+ content is not significantly different in Ser2814A mice with respect to WT mice, as it has been shown in isolated myocytes under basal conditions [24], it is possible to conclude that the reduction in PBs observed in these mice is attributable to the lack of CaMKII-dependent phosphorylation of RyR2 and could be ascribed to DADs. Moreover, the fact that in Ser2814A mice, reperfusion arrhythmias were significantly reduced but not fully blunted, indicates that a loss-of-function of RyR2 alone is not sufficient to completely prevent reperfusion-induced arrhythmogenesis.
In contrast to the situation in RyR2-S2814A mice, the number of reperfusion arrhythmias was not modified in PLN-DM mice. At first sight this could be interpreted as suggesting a negligible role of CaMKII-dependent phosphorylation of PLN in reperfusion arrhythmias. However, the lack of change in the number of PBs in these mice associated with a significant alteration in the arrhythmic pattern at the expense of a significant increase in EADs, might unmask a role of PLN phosphorylation. It is quite possible that the participation of PLN phosphorylation in reperfusion arrhythmias is being counterbalanced by the increase in L-type Ca2+ current typical of these mice [29]. This compensatory mechanism would explain both, the significant increase in EADs and the lack of alteration in the total number of PBs in PLN-DM mice when compared to WT animals.
Possible role of other CaMKII-targets on reperfusion arrhythmias
Previous findings convincingly showed the link between EADs initiation, L-type Ca2+ current facilitation, and CaMKII activation [33]. In the present experiments, the reperfusion arrhythmic pattern showed that 22% of PBs was triggered by EADs in WT mice. The lack of detectable EADs in SR-AIP mice would indicate that CaMKII-facilitation of L-type Ca2+ current is contributing to this type of triggered arrhythmias upon reperfusion. Moreover, the increase in L-type Ca2+ current could favor SR Ca2+ overload and the appearance of DADs [33]. Thus, an increase in CaMKII-dependent Ca2+ influx during AP may generate arrhythmias triggered by both EADs and DADs.
It has been shown that CaMKII may also affect Na+ and K+ channels [38,39]. By enhancing late INa, CaMKII-modulation of Na+ channels could prolong APD [38]. By modulating K+ currents, CaMKII has complex consequences in APD, prolonging or shortening the AP in mouse and rabbit hearts, respectively [39]. We did not observe changes in APD after KN-93 administration either under basal conditions or at the onset of reperfusion (Figure 2). These findings would preclude an effect of either the drug or of CaMKII on these channels under basal conditions and suggest that the contribution of CaMKII-dependent phosphorylations of these channels to the reperfusion arrhythmic pattern is not highly significant.
The results obtained in the presence of CaMKII inhibition in rat hearts and in SR-AIP mice indicate that most of reperfusion arrhythmias are triggered by CaMKII-dependent mechanisms. However, other signaling molecules might play a significant role. For instance, the increase in reactive oxygen species (ROS) generated at the onset of reperfusion [40] may be of critical importance in enhancing CaMKII activation by oxidation [41,42], thereby increasing reperfusion arrhythmias.
Taken together, our present results provide new insights into the mechanisms underlying the reperfusion arrhythmias. Our data suggest that CaMKII phosphorylation of RyR2-Ser2814 site is a key signaling event responsible for triggering PBs during the early reperfusion phase. CaMKII most likely amplifies SR Ca2+ leak, thus promoting reperfusion arrhythmias. Moreover, phosphorylation of other Ca2+ handling proteins including PLN-Thr17 and L-type Ca2+ channels might contribute to reperfusion-induced PBs at the onset of reflow, by increasing SR Ca2+ content and Ca2+ influx. These mechanisms, working in concert, provide the molecular bases for reperfusion arrhythmias.
Clinical Implications
Reperfusion and revascularization therapies (thrombolysis and percutaneous coronary intervention) have the primary aim of salvaging viable tissue. However, they may also induce undesirable effects, among which arrhythmias is one of the most detrimental. Our findings have potentially important clinical implications with respect to reperfusion arrhythmias, since they identified CaMKII as a main determinant of early reperfusion arrhythmias. Furthermore, our data indicate that phosphorylation of Ser2814 on RyR2, Thr17 on PLN and L-type Ca2+ channels are downstream targets of CaMKII linked to the onset of reperfusion arrhythmias. Previous work suggests a promising role for future therapies targeting CaMKII function in the treatment of heart disease and cardiac arrhythmias [17,39,43,44]. Our new findings expand these earlier studies by showing that CaMKII inhibition, particularly at the SR level, provides potential new avenues for treating reperfusion arrhythmias. The extension of this knowledge to larger mammals and patients is necessary and critical in devising strategies that could modify the levels of CaMKII-dependent phosphorylation, protecting against ventricular arrhythmias and death.
Supplementary Material
Acknowledgments
We wish to thank Dr. Ariel Escobar for mounting the set up to measure optical transmembrane potential in the intact heart.
Funding
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina (PIP # 2139 to A.M.) and National Institutes of Health (FOGARTY Grant # 1 R03 TW007713-01 to A.M.) and by Agencia Nacional de Promoción Científica y Técnica, Argentina (PICT # 1321 to C.M-W). R.B. is a fellow from CONICET. M.S., C.A.V., C.M-W, L.V. and A.M. are established Investigators of CONICET. X.H.T.W. is a ‘W.M. Keck Foundation Distinguished Young Scholar in Medical Research’, and is supported by National Institutes of Health (NIH/NHLBI grants R01-HL089598 and R01-HL091947) and Fondation Leducq Award to the ‘Alliance for Calmodulin Kinase Signaling in Heart Disease’
Footnotes
Disclosures
None.
References
- 1.Manning AS, Coltart DJ, Hearse DJ. Ischemia and reperfusion-induced arrhythmias in the rat. Effects of xanthine oxidase inhibition with allopurinol. Circ Res. 1984;55:545–548. doi: 10.1161/01.res.55.4.545. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- 3.Salerno JA, Previtali M, Chimienti M, Klersy C, Bobba P. Vasospasm and ventricular arrhythmias. NY Acad Sci. 1984;427:222–233. doi: 10.1111/j.1749-6632.1984.tb20786.x. [DOI] [PubMed] [Google Scholar]
- 4.Terkelsen CJ, Sørensen JT, Kaltoft AK, Nielsen SS, Thuesen L, Bøtker HE, et al. Prevalence and significance of accelerated idioventricular rhythm in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2009;104:1641–1646. doi: 10.1016/j.amjcard.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Pride YB, Appelbaum E, Lord EE, Sloan S, Cannon CP, Sabatine MS, et al. TIMI Study Group. Relation between myocardial infarct size and ventricular tachyarrhythmia among patients with preserved left ventricular ejection fraction following fibrinolytic therapy for ST-segment elevation myocardial infarction. Am J Cardiol. 2009;104:475–479. doi: 10.1016/j.amjcard.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Pogwizd SM, Corr PB. Electrophysiologic mechanisms underlying arrhythmias due to reperfusion of ischemic myocardium. Circulation. 1987;72:404–426. doi: 10.1161/01.cir.76.2.404. [DOI] [PubMed] [Google Scholar]
- 7.Priori SG, Mantica M, Napolitano C, Schwartz PJ. Early afterdepolarizations induced in vivo by reperfusion of ischemic myocardium. A possible mechanism for reperfusion arrhythmias. Circulation. 1990;81:1911–1920. doi: 10.1161/01.cir.81.6.1911. [DOI] [PubMed] [Google Scholar]
- 8.Pogwizd SM, Corr PB. The contribution of nonreentrant mechanisms to malignant ventricular arrhythmias. Basic Res Cardiol. 1992;87 (Suppl 2):115–129. doi: 10.1007/978-3-642-72477-0_11. [DOI] [PubMed] [Google Scholar]
- 9.Lakireddy V, Bub G, Baweja P, Syed A, Boutjdir M, El-Sherif N. The kinetics of spontaneous calcium oscillations and arrhythmogenesis in the in vivo heart during ischemia/reperfusion. Heart Rhythm. 2006;3:58–66. doi: 10.1016/j.hrthm.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Brooks WW, Conrad CH, Morgan JP. Reperfusion induced arrhythmias following ischaemia in intact rat heart: role of intracellular calcium. Cardiovasc Res. 1995;29:536–542. [PubMed] [Google Scholar]
- 11.Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, et al. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Heart Circ Physiol. 2008;295:H1669–H1683. doi: 10.1152/ajpheart.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci USA. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vittone L, Mundiña-Weilemann C, Said M, Ferrero P, Mattiazzi A. Time course and mechanisms of phosphorylation of phospholamban residues in ischemia-reperfused rat hearts. Dissociation of phospholamban phosphorylation pathways. J Mol Cell Cardiol. 2002;34:39–50. doi: 10.1006/jmcc.2001.1488. [DOI] [PubMed] [Google Scholar]
- 14.Said M, Vittone L, Mundiña-Weilenmann C, Ferrero P, Kranias EG, Mattiazzi A. Role of dual-site phospholamban phosphorylation in the stunned heart: insights from phospholamban site-specific mutants. Am J Physiol Heart Circ Physiol. 2003;285:H1198–H1205. doi: 10.1152/ajpheart.00209.2003. [DOI] [PubMed] [Google Scholar]
- 15.Mattiazzi A, Mundiña-Weilenmann C, Vittone L, Said M. Phosphorylation of phospholamban in ischemia-reperfusion injury: functional role of Thr17 residue. Mol Cell Biochem. 2004;263:131–136. [PubMed] [Google Scholar]
- 16.Ji Y, Li B, Reed TD, Lorenz JN, Kaetzel MA, Dedman JR. Targeted inhibition of Ca2+/Calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17. J Biol Chem. 2003;278:25063–25071. doi: 10.1074/jbc.M302193200. [DOI] [PubMed] [Google Scholar]
- 17.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brittsan AG, Carr AN, Schmidt AG, Kranias EG. Maximal inhibition of SERCA2 Ca2+ affinity by phospholamban in transgenic hearts overexpressing a non-phosphorylatable form of phospholamban. J Biol Chem. 2000;275:12129–12135. doi: 10.1074/jbc.275.16.12129. [DOI] [PubMed] [Google Scholar]
- 19.Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol. 2001;12:1286–1294. doi: 10.1046/j.1540-8167.2001.01286.x. [DOI] [PubMed] [Google Scholar]
- 20.Valverde CA, Kornyeyev D, Ferreiro M, Petrosky AD, Mattiazzi A, Escobar AL. Transient Ca2+ depletion of the sarcoplasmic reticulum at the onset of reperfusion. Cardiovasc Res. 2010;85:671–680. doi: 10.1093/cvr/cvp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrero P, Said M, Sánchez G, Vittone L, Valverde C, Donoso P, et al. Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during beta-adrenergic stimulation. J Mol Cell Cardiol. 2007;43:281–291. doi: 10.1016/j.yjmcc.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundiña-Weilenmann C, Ferrero P, Said M, Vittone L, Kranias EG, Mattiazzi A. Role of phosphorylation of Thr17 residue of phospholamban in mechanical recovery during hypercapnic acidosis. Cardiovasc Res. 2005;66:114–122. doi: 10.1016/j.cardiores.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Erickson JR, Anderson ME. CaMKII and its role in cardiac arrhythmia. J Cardiovasc Electrophysiol. 2008;19:1332–1336. doi: 10.1111/j.1540-8167.2008.01295.x. [DOI] [PubMed] [Google Scholar]
- 24.vanOort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutjdir M, el-Sherif N, Gough WB. Effects of caffeine and ryanodine on delayed afterdepolarizations and sustained rhythmic activity in 1-day-old myocardial infarction in the dog. Circulation. 1990;81:1393–1400. doi: 10.1161/01.cir.81.4.1393. [DOI] [PubMed] [Google Scholar]
- 26.Zucchi R, Ronca F, Ronca-Testoni S. Modulation of sarcoplasmic reticulum function: a new strategy in cardioprotection? Pharmacology & Therapeutics. 2001;89:47–65. doi: 10.1016/s0163-7258(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 27.Wehrens XHT, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: A critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol. 2007;42:196–205. doi: 10.1016/j.yjmcc.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brittsan AG, Ginsburg KS, Chu G, Yatani A, Wolska BM, Schmidt AG, et al. Chronic SR Ca2+-ATPase inhibition causes adaptive changes in cellular Ca2+ transport. Circ Res. 2003;92:769–776. doi: 10.1161/01.RES.0000066661.49920.59. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaMkinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol Heart Circ Physiol. 1999;276:H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 32.Xiao H, Wang M, Du Y, Yuan J, Cheng X, Chen Z, et al. Arrhythmogenic autoantibodies against calcium channel lead to sudden death in idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2011;13:264–270. doi: 10.1093/eurjhf/hfq198. [DOI] [PubMed] [Google Scholar]
- 33.Anderson ME. Multiple downstream proarrhythmic targets for calmodulin kinase II: Moving beyond an ion channel-centric focus. Cardiovasc Res. 2007;73:657–666. doi: 10.1016/j.cardiores.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Lee TS, Karl R, Moosmang S, Lenhardt P, Klugbauer N, Hofmann F, et al. Calmodulin kinase II is involved in voltage dependent facilitation of the L-type Cav1.2 calcium channel: Identification of the phosphorylation sites. J Biol Chem. 2006;281:25560–25567. doi: 10.1074/jbc.M508661200. [DOI] [PubMed] [Google Scholar]
- 35.Mundiña-Weilenmann C, Vittone L, Ortale M, de Cingolani GC, Mattiazzi A. Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J Biol Chem. 1996;271:33561–33567. doi: 10.1074/jbc.271.52.33561. [DOI] [PubMed] [Google Scholar]
- 36.Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun. 2006 Jul 14;345(4):1606–10. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 37.O’Neill SC, Miller L, Hinch R, Eisner DA. Interplay between SERCA and sarcolemmal Ca2+ efflux pathways controls spontaneous release of Ca2+ from the sarcoplasmic reticulum in rat ventricular myocytes. J Physiol. 2004;559:121–128. doi: 10.1113/jphysiol.2003.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner S, Hacker E, Grandi E, Weber SL, Dybkova N, Sossalla S, et al. Ca2+/Calmodulin kinase II differentially modulates potassium currents. Circ Arrhythm Electrophysiol. 2009;3:285–294. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolli R, Marbán E. Molecular and Cellular Mechanisms of Myocardial Stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 41.Erickson JR, Joiner MA, Guan X, Kutschke W, Yang J, Oddis CV, et al. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palomeque J, Velez Rueda O, Sapia L, Valverde CA, Salas M, Vila-Petroff M, et al. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+ Calmodulin Protein Kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 43.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, et al. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 44.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nature Med. 2005;1:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.