Abstract
Knowing threshold changes in brain lipids and lipid enzymes during dietary n-3 polyunsaturated fatty acid deprivation may elucidate dietary regulation of brain lipid metabolism. To determine thresholds, rats were fed for 15 weeks DHA-free diets having graded reductions of α-linolenic acid (α-LNA). Compared with control diet (4.6% α-LNA), plasma DHA fell significantly at 1.7% dietary α-LNA while brain DHA remained unchanged down to 0.8% α-LNA, when plasma and brain docosapentaenoic acid (DPAn-6) were increased and DHA-selective iPLA2 and COX-1 activities were downregulated. Brain AA was unchanged by deprivation, but AA selective-cPLA2, sPLA2 and COX-2 activities were increased at or below 0.8% dietary α-LNA, possibly in response to elevated brain DPAn-6. In summary, homeostatic mechanisms appear to maintain a control brain DHA concentration down to 0.8% dietary DHA despite reduced plasma DHA, when DPAn-6 replaces DHA. At extreme deprivation, decreased brain iPLA2 and COX-1 activities may reduce brain DHA loss.
Keywords: n-3 PUFA deprivation, rat brain, phospholipase A2, docosapentaenoic acid
INTRODUCTION
The brain is enriched in docosahexaenoic acid (DHA, 22:6n-3) and arachidonic acid (AA, 20:4n-6), which are critical for its normal structure and function [1–3]. In vertebrates, these polyunsaturated fatty acids (PUFAs) cannot be synthesized de novo from 2-carbon fragments, but can be elongated in liver (minimally in brain or heart) from their respective shorter-chain PUFA precursors, α-linolenic acid (α-LNA, 18:3n-3) and linoleic acid (LA, 18:2n-6) [4–7]. In humans, a low dietary n-3 PUFA intake or a low plasma DHA concentration has been correlated with increased risk for neuropsychiatric and/or neurodegenerative diseases [8, 9]. Dietary n-3 PUFA supplementation may be beneficial in these conditions [8, 10].
Multiple animal studies have been conducted to understand how dietary-derived n-3 PUFAs influence body integrity and metabolism. For example, in rats fed a DHA-free diet containing α-LNA at 4.6% total fatty acid, brain, heart and liver DHA concentrations are sufficient to maintain organ function, so this diet is considered n-3 PUFA "adequate" [2, 11]. In contrast, in rats fed a DHA-free diet containing 0.2% α-LNA, brain DHA concentrations are reduced, behavior is disturbed and brain derived neurotrophic factor (BDNF) is reduced compared with the 4.6% α-LNA diet, so this diet is considered n-3 PUFA “inadequate" or "deficient" [2, 12, 13]. Brain changes in rats fed this deficient diet include a prolonged DHA half-life; an increased concentration of docosapentaenoic acid (DPAn-6, 22:5n-6), an AA elongation product; reduced expression of enzymes that regulate DHA metabolism, Ca2+-independent phospholipase A2 (iPLA2 Type VI, iPLA2β) [14–17] and cyclooxygenase (COX)-1 [18, 19]; and increased expression of enzymes that regulate AA metabolism, cytosolic cPLA2 Type IV, secretory sPLA2 Type II and COX-2 [14, 20].
The brain lipid and enzyme changes in animals exposed to dietary n-3 PUFA deprivation, noted above and reported elsewhere [3, 21, 22], may not be clinically relevant because deprivation was too severe and prolonged, sometimes spanning several generations. This severity also limits the ability to identify causes and effects. To overcome these limitation, in the present study we exposed rats after weaning to 15 weeks of graded reductions in dietary n-3 PUFA content below the 4.6% α-LNA "adequate" level, and estimated when statistically significant changes in different lipid parameters first appeared (thresholds) in plasma, brain and liver.
MATERIALS AND METHODS
Materials
1-Palmitoyl-2-[1-14C] arachidonoyl-sn-glycerol-3-phosphorylcholine was purchased from PerkinElmer (Boston, MA, USA) and had a specific activity of 60 mCi/mmol. 1-Palmitoyl-2-[1-14C] palmitoyl-sn-glycerol-3-phosphorylcholine was purchased from GE Healthcare (Buckinghamshire, UK) and had specific activity of 53 mCi/mmol. The purity of each was > 95%, as determined by TLC, scintillation counting and GC. 1-Palmitoyl-2-arachidonoyl-sn-glycerol-3-phosphorylcholine, 1-palmitoyl-2-[1-14C] palmitoyl-sn-glycerol-3-phosphorylcholine and phosphatidylinositol 4, 5-bisphosphate were obtained from Avanti (Alabaster, AL, USA), protease inhibitor cocktail from Roche (Indianapolis, IN, USA). A high capacity cDNA reverse transcription kit, Taqman® gene expression master mix, and specific primers for real time RT-PCR were purchased from Applied Biosystems (Foster City, CA, USA).
Animals
Fischer-344 (CDF) male rat pups (19 days old) and their surrogate mothers, purchased from Charles River Laboratories (Portage, MI, USA), were housed in an animal facility with regulated temperature, humidity, and a 12 h light/12 h dark cycle. Lactating rats had free access to water and rodent chow formulation NIH-31 18-4 (Zeigler Bros, Gardners, PA, USA), which contained 4% (wt/wt) crude fat and (as percent total fatty acid) α-LNA (5.1%), eicosapentaenoic acid (20:5n-3) (2.0%), DHA (2.3%), LA (47.9%) and AA (0.02%) [23].
After nursing for 21 days, the pups were divided randomly into six groups and placed on a predetermined diet. They had free access to food and water, and their food was replaced every 2 or 3 days. After 15 weeks on a given diet, they were asphyxiated by CO2 inhalation and decapitated. The brains and liver were excised rapidly and frozen in 2-methylbutane cooled by dry ice to −50 °C, then stored at −80 °C until used. Blood was collected from the abdominal aorta with EDTA, and centrifuged at 1,500 rpm for 5 min. Plasma was removed and stored at 80 °C until assayed. The protocol was approved by the Animal Care and Use Committee of the Eunice Kennedy Schriver National Institute of Child Health and Human Development, and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23).
Graded n-3 PUFA diets
The different n-3 PUFA diets, prepared by Dyets Inc. (Bethlehem, PA, USA), were based on the AIN-93G formulation [24, 25]. Each diet contained 10% crude fat, but a different amount of flaxseed oil. Fatty acid composition of each diet (µmol/g food, percent total fatty acid, or percent energy) is shown in Table 1. The n-3 PUFA “adequate” diet contained 7.8 µmol/g α-LNA (4.6 % of total fatty acid) [2]. The extreme “deficient” diet contained 0.25 µmol/g α-LNA (0.2% total fatty acid). The less deficient diets contained α-LNA at 3.8, 2.6, 1.7, or 0.8 % of total fatty acid. Other n-3 PUFAs were absent from all diets. Each diet contained 40 µmol/g LA (23–24% total fatty acid).
Table 1.
Composition of graded n-3 PUFA diets.
| Ingredient | 4.6 % ALA diet | 3.8 % ALA diet | 2.6 % ALA diet | 1.7% ALA diet | 0.8 % ALA diet | 0.2 % ALA diet | |
|---|---|---|---|---|---|---|---|
| g/100 g food | |||||||
| Carbohydrate | 60 | 60 | 60 | 60 | 60 | 60 | |
| Protein | 20 | 20 | 20 | 20 | 20 | 20 | |
| Fat | 10 | 10 | 10 | 10 | 10 | 10 | |
| Hydrogenated coconut oil | 6.00 | 6.12 | 6.25 | 6.37 | 6.50 | 6.62 | |
| Safflower oil | 3.23 | 3.26 | 3.29 | 3.32 | 3.35 | 33.8 | |
| Flaxseed oil | 0.77 | 0.62 | 0.46 | 0.31 | 0.15 | 0 | |
| Other ingredient | 10 | 10 | 10 | 10 | 10 | 10 | |
| Fatty acid concentration | % of total fatty acid concentration | ||||||
| 12:0 | 28.8 | 30.3 | 30.7 | 31.6 | 32.4 | 34.0 | |
| 14:0 | 12.5 | 12.9 | 13.1 | 13.3 | 14.0 | 14.6 | |
| 14:1n-5 | 0.04 | 0.04 | 0.03 | 0.04 | 0.05 | 0.05 | |
| 16:0 | 9.5 | 9.6 | 9.6 | 9.5 | 9.8 | 9.9 | |
| 16:1n-7 | 0.04 | 0.04 | 0.04 | 0.06 | 0.06 | 0.13 | |
| 18:0 | 8.0 | 7.9 | 8.1 | 8.3 | 9.3 | 8.0 | |
| 18:1n-9 | 8.4 | 7.7 | 8.0 | 7.6 | 7.1 | 5.9 | |
| 18:2n-6 | 27.9 | 27.7 | 27.9 | 27.9 | 27.5 | 27.2 | |
| 18:3n-3 | 4.6 | 3.8 | 2.6 | 1.7 | 0.8 | 0.2 | |
| Total saturated | 58.8 | 60.7 | 61.5 | 62.7 | 64.5 | 66.5 | |
| Total Monounsaturated | 8.5 | 7.8 | 8.0 | 7.7 | 7.2 | 6.1 | |
| n-6 PUFA | 27.9 | 27.7 | 27.9 | 27.9 | 27.5 | 27.2 | |
| n-3 PUFA | 4.6 | 3.8 | 2.6 | 1.7 | 0.8 | 0.2 | |
| n-6/n-3 | 4.2 | 5.1 | 7.0 | 11.8 | 23.4 | 132.9 | |
| Fatty acid content1 | µmol/g diet | ||||||
| 18:2n-6 | 40.5 ± 3.6 | 40.6 ± 1.8 | 44.1 ± 3.0 | 38.1 ± 0.8 | 41.1 ± 5.0 | 35.8 ± 2.7 | |
| 18:3n-3 | 6.7 ± 0.3 | 5.5 ± 0.6 | 4.0 ± 0.6 | 2.4 ± 0.1 | 1.2 ± 0.2 | 0.21 ± 0.03 | |
Data represent averages or means ± SD of 3 analyses
Lipid extraction and methylation
Methods of lipid extraction and methylation have been described [6, 19]. Total lipids from brain, liver and plasma were extracted by the Folch procedure [26], and fatty acids were transmethylated with 0.1% H2SO4-methanol for 3 h at 70 °C. Appropriate quantities of di-17:0 PC for total fatty acid analysis and of unesterified 17:0 for unesterified fatty acids were added as internal standards before transmethylation to fatty acid methyl esters.
Gas chromatography
Fatty acid methyl esters from brain and liver (nmol/g wet wt) and from plasma (nmol/ml plasma) were quantified with a gas chromatograph (6890N, Agilent Technologies, Palo Alto, CA, USA) equipped with an SP™-2330 fused silica capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness) (Supelco, Bellefonte, PA, USA) and a flame ionization detector [27]. Fatty acid concentrations were calculated by proportional comparison of peak areas to the area of the 17:0 internal standard.
Total RNA isolation and real time RT-PCR
Total RNA was isolated from brain using a commercial kit (RNeasy Lipid Tissue Kit; Qiagen, Valencia, CA, USA). cDNA was prepared from total RNA using a high-capacity cDNA Archive Kit (Applied Biosystems). mRNA levels of cPLA2 IV (Rn 00591916_m1), sPLA2 II (Rn 00580999_m1), iPLA2 VI (Rn 01504424_m1), COX-1 (Rn 00566881_m1), COX-2 (Rn 00568225_m1), 5-LOX (Rn 00563172_m1), 12-LOX (Rn 01461082_m1), 15-LOX (Rn 00696151_m1) and BDNF (Rn 01484928_m1) were measured by real time quantitative RT-PCR, using the ABI PRISM 7000 sequence detection system (Applied Biosystems). The fold change in gene expression was determined by the ΔΔCT method [28]. Data are expressed as relative level of the target gene in the n-3 PUFA deficient diet group normalized to the endogenous control (β-globulin, Rn_00560865_m1) and relative to the level with the n-3 PUFA “adequate” diet (calibrator). Each experiment was performed in triplicate with 6 independent samples per diet group.
Phospholipase A2 activities
A radioactivity method designed by the Dennis Group [29–31] was used to determine brain cPLA2 and iPLA2 activities. A commercial kit (Cayman, Ann Arbor, MI, USA) was used to measure sPLA2 activity.
Sample preparation
Brain tissue was homogenized with 3 vol homogenization buffer (10 mM HEPES, pH 7.5 containing 1 mM EDTA, 0.34 M sucrose and protease inhibitor cocktail (Roche)) using a glass homogenizer. The homogenized sample was centrifuged at 100,000 g for 1 h at 4 °C, and the supernatant was used for all PLA2 enzyme activity analyses. Supernatants were kept at −80 °C until use. Protein concentration was determined by the Bradford assay [32] (Bio-Rad, Hercules, CA, USA).
Enzyme assay for radioisotope method
Concentrations were composed for a final incubation volume of 0.5 ml. For cPLA2 activity analysis, the cytosolic fraction (0.3 mg protein in one assay) was mixed with 100 mM HEPES, pH 7.5 containing 80 µM Ca2+, 2 mM dithiothreitol, 0.1 mg/ml fatty acid-free bovine serum albumin in 450 µl, then incubated with 50 µl substrate solution of 100 µM 1-palmitoyl-2-arachidonoyl-sn-glycerol-3-phosphorylcholine and phosphatidylinositol 4,5-bisphosphate (97:3) (containing approximately 100,000 dpm of 1-palmitoyl-2-[1-14C] arachidonoyl-sn-glycerol-3-phosphorylcholine in one assay) in 400 µM triton X-100 to start the enzyme reaction. For iPLA2 activity analysis, the cytosolic fraction (0.3 mg protein) was mixed with 100 mM HEPES, pH 7.5, 5 mM EDTA, 2 mM dithiothreitol, and 1 mM ATP in 450 µl, and 50 µl substrate mixture of 100 µM 1-palmitoyl-2-palmitoyl-sn-glycerol-3-phosphorylcholine (containing approximately 100,000 dpm of 1-palmitoyl-2-[1-14C] palmitoyl-sn-glycerol-3-phosphorylcholine) in 400 µM Triton X-100 was added to start the enzyme reaction.
Substrate preparation for radioisotope method
Substrates for iPLA2 and cPLA2 activity analyses were prepared daily. Appropriate amounts of cold and radiolabeled phospholipids were added to an appropriate amount of Triton X-100, and then the mixture was dried under nitrogen gas. Water was added to the residues to give a 10× lipid solution (1 mM phospholipid, 1,000,000 dpm, and 4 mM Triton X-100), which was mixed vigorously.
Enzyme assay
The cytosolic fraction (0.3 mg per assay) was mixed in a 450 µl assay mixture, and 50 µl substrate mixture was added to start the enzyme reaction. The reaction mixture was incubated for 30 min at 40 °C, and then 2.5 ml of Dole reagent (2-propanol, heptane: 0.5 M H2SO4, 400:100:20, vol/vol/vol) was added to stop the reaction. 1.5 ml of heptane and 1.5 ml H2O were added to the mixture, followed by vortexing and centrifugation. The upper phase (about 2 ml) was transferred to a tube, and 200 mg silicic acid (200–400 mesh) was added, followed by vortexing and centrifugation. The supernatant (1.5 ml) was transferred to a scintillation vial to which scintillation cocktail (Ready Safe™, Beckman Coulter, Fullerton, CA) plus 1% glacial acetic acid was added. Radioactivity of released unesterified fatty acid from substrate was counted as described above. Activities of iPLA2 and cPLA2 were expressed as the release rate of fatty acid from phospholipid. sPLA2 activity was measured using an assay kit (Cayman), according to the manufacturer's instructions.
Statistical analysis
Data are presented as mean ± SD (n = 6 for each group). Statistical significance was determined using a one-way ANOVA with Tukey’s multiple comparison. p < 0.05 was used as the cut off for statistical significance.
RESULTS
Effects of graded dietary α-LNA reductions on body weight
Body weight gain did not differ significantly over the 15-week feeding period between rats on any α-LNA deficient diet compared with the control 4.6% α-LNA diet. Average body weight (n = 6 per group) at 21 days equaled 25 ± 2 g (4.6% α-LNA), 25 ± 1 g (3.8%), 26 ± 2 g (2.6%), 26 ± 2 g (1.7%), 26 ± 2 g (0.8%), and 24 ± 3 g (0.2%), respectively (p > 0.05). Final body weight (after 15 weeks on a diet) was 338 ± 25 (4.6% α-LNA), 320 ± 11 g (3.8%), 338 ± 25 g (2.6%), 327 ± 19 g (1.7%), 346 ± 16 g (0.8%), and 325 ± 25 g (0.2%), respectively.
PUFA concentrations
Plasma
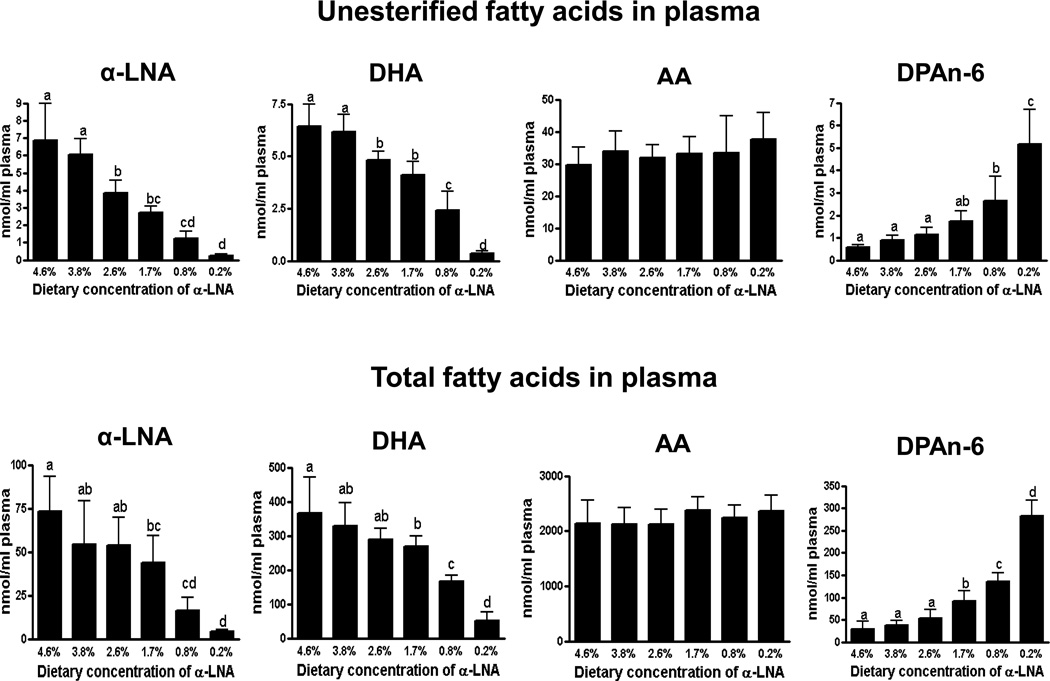
Compared with control values at 4.6% dietary α-LNA, statistically significant reductions in unesterified plasma α-LNA and DHA concentrations occurred at ≤ 2.6% dietary α-LNA. Total plasma α-LNA and DHA concentrations were reduced, and total plasma DPAn-6 was elevated at ≤ 1.7% dietary α-LNA (Figure 1). At ≤ 0.8% dietary α-LNA, unesterified plasma DPAn-6 was elevated. Neither total nor unesterified plasma AA was changed significantly compared with control by any deficient diet.
Figure 1.
Plasma unesterified and total fatty acid concentrations in rats fed different α-LNA containing diets for 15 weeks. Values are mean ± SD (n = 6 per group). Superscripts show significant differences at p < 0.05 from mean at 4.6% dietary α-LNA.
Liver
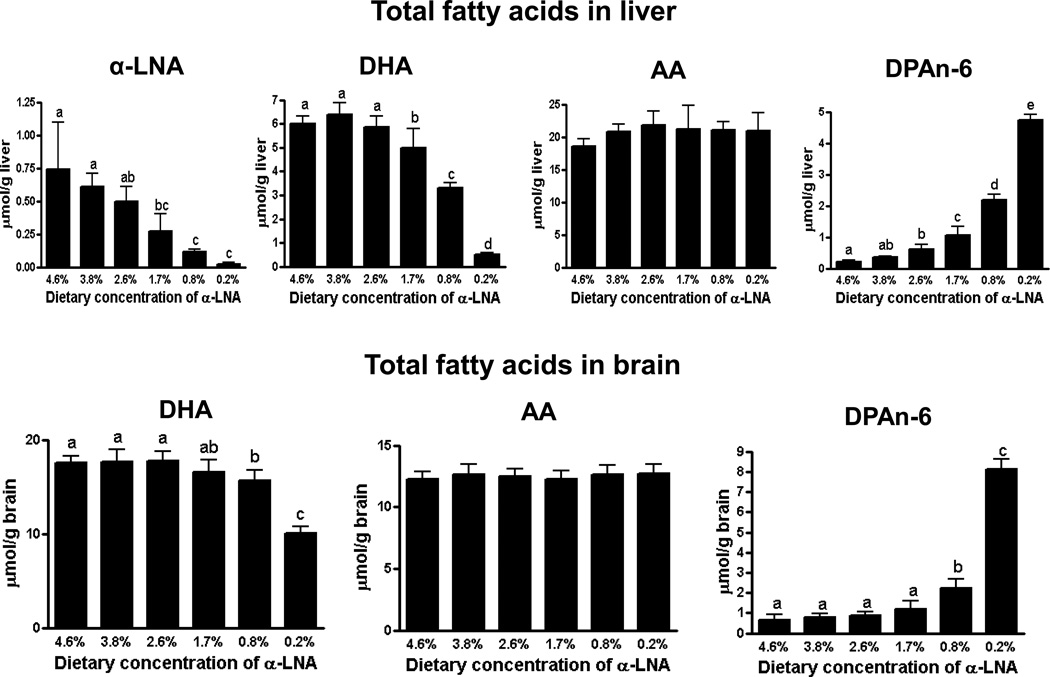
Compared with control, total liver DPAn-6 concentration was increased at ≤ 2.6% dietary α-LNA (Figure 2). Total liver α-LNA and DHA concentrations were reduced at ≤ 1.7% dietary α-LNA, but the total liver AA concentration was unchanged at all deprivations.
Figure 2.
Total fatty acid concentrations in liver and brain of rats fed different α-LNA containing diets for 15 weeks. Values are mean ± SD (n = 6 per group). Different superscripts show significant differences at p < 0.05. Superscripts show significant differences at p < 0.05 from mean at 4.6% dietary α-LNA.
Brain
Like plasma and liver AA concentrations, the brain AA concentration did not differ significantly from control at any level of dietary α-LNA deprivation (Figure 2). At 1.7% dietary α-LNA, when brain DHA did not differ from control, unesterified plasma DHA was 36% (4.11 nmol/ml vs. 6.45 nmol/ml) below control, and total plasma (98.3% esterified) DHA was 27% (269 nmol/ml vs. 367 nmol/ml) below control, demonstrating homeostatic regulation. Only at ≤ 0.8% dietary α-LNA was brain DHA reduced compared with control, while brain DPAn-6 was increased.
Brain enzyme mRNA and activity levels
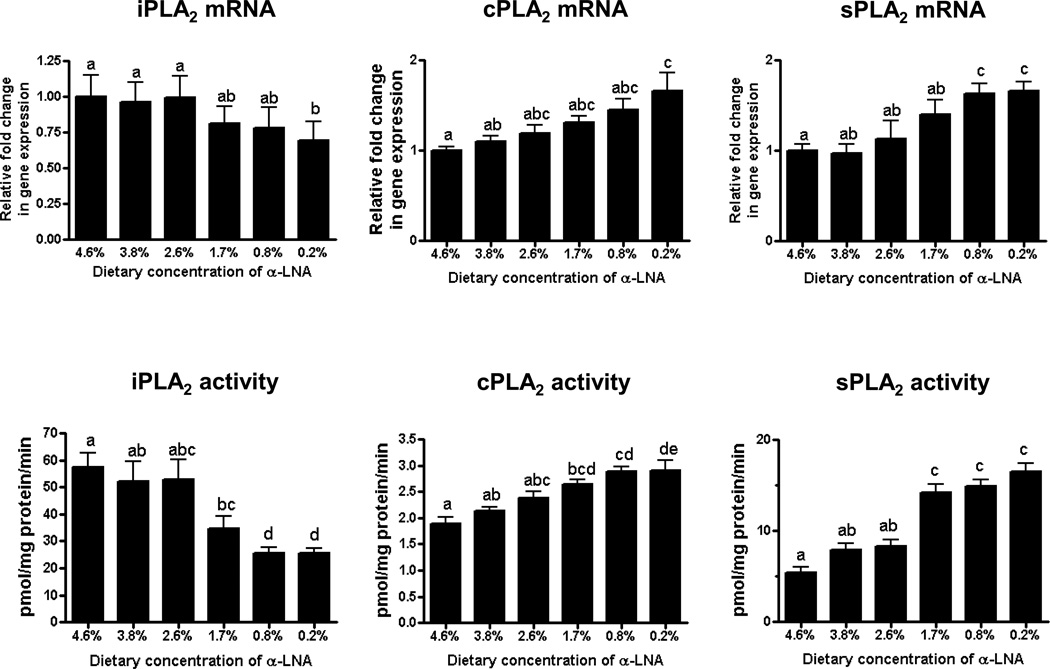
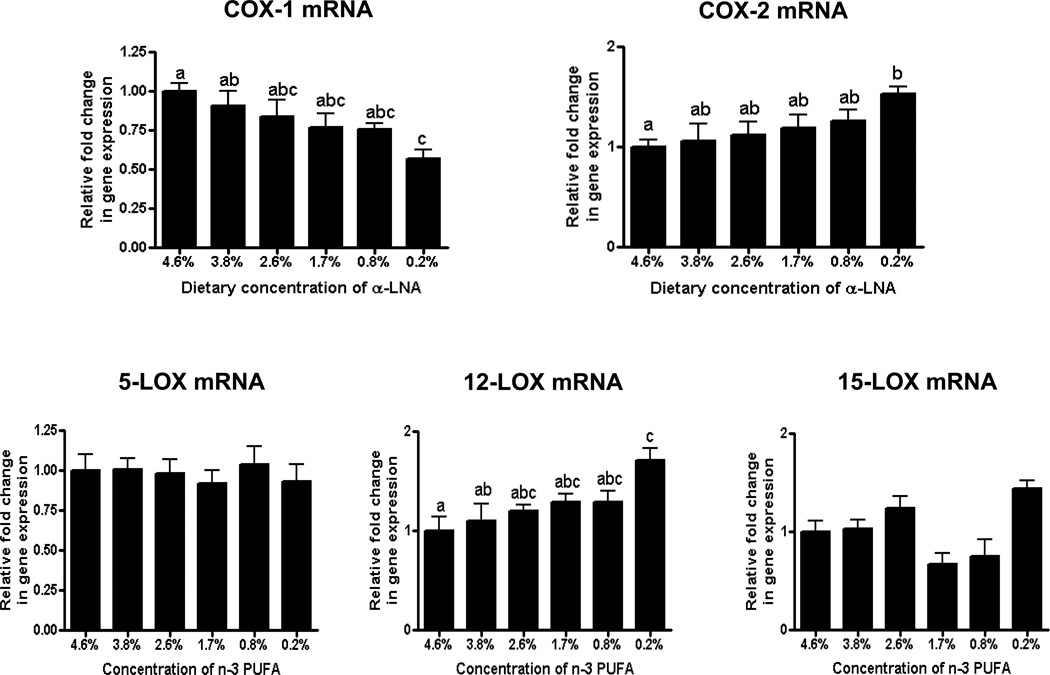
Brain iPLA2 activity was decreased and cPLA2 and sPLA2 activities were increased at ≤ 1.7% dietary α-LNA compared to control values (Figure 3). sPLA2 mRNA was increased at ≤ 0.8% α-LNA. Compared with control values, at 0.2% dietary α-LNA brain cPLA2, COX-2 and 12-LOX mRNA levels were increased and iPLA2 and COX-1 mRNA levels were decreased (Figure 4). No significant difference was found in 5-LOX or 15-LOX mRNA throughout deprivation (Figure 4).
Figure 3.
Brain mRNA and activity levels of iPLA2, cPLA2 and sPLA2 in rats fed different α-LNA containing diets for 15 weeks. Values are mean ± SD (n = 6 per group). Superscripts show significant differences at p < 0.05 from mean at 4.6% dietary α-LNA.
Figure 4.
Brain mRNA levels of COX and LOX enzymes sPLA2 mRNA in rats fed different α-LNA containing diets for 15 weeks. Values are mean ± SD (n = 6 per group). Superscripts show significant differences at p < 0.05 from mean at 4.6% dietary α-LNA.
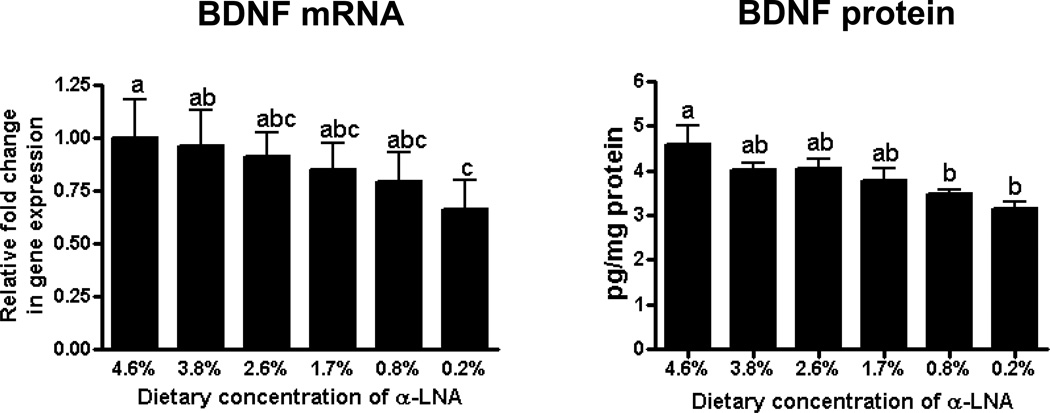
Brain BDNF mRNA and protein levels in brain
Compared with control, brain mRNA and protein levels of BDNF were decreased significantly at ≤ 0.8% and at 0.2% dietary α-LNA, respectively (Figure 5).
Figure 5.
Brain mRNA and protein of BDNF, in brains of rats fed different α-LNA containing diets for 15 weeks. Values are mean ± SD (n = 6 per group). Superscripts show significant differences at p < 0.05 from mean at 4.6% dietary α-LNA.
DISCUSSION
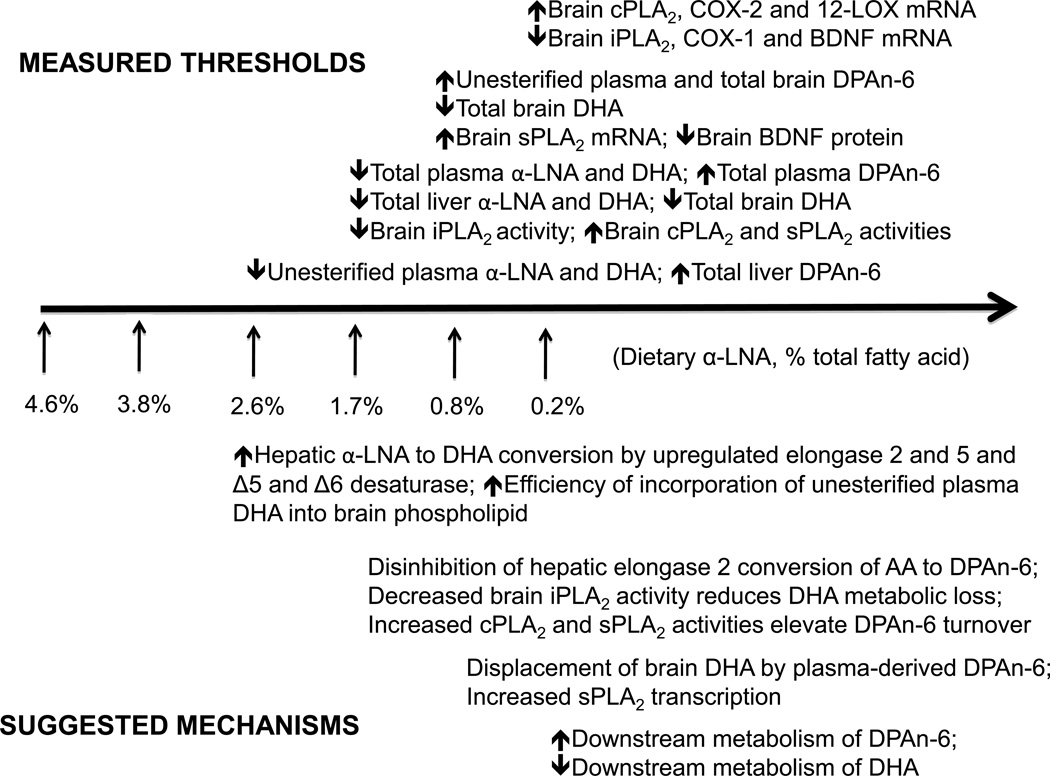
Figure 6 (top) relates the thresholds shown in Figures 1–5 to the degree of dietary α-LNA reduction, while Figure 6 (bottom) suggests mechanisms underlying the threshold relations (see below). Statistically significant reductions appeared first for unesterified plasma α-LNA and DHA, accompanied by increased total liver DPAn-6, at 2.6% dietary α-LNA. At ≤ 1.7% dietary α-LNA, total plasma and liver α-LNA and DHA were reduced, total plasma and liver DPAn-6 were elevated, brain iPLA2 activity was elevated, and brain cPLA2 and sPLA2 activities were reduced. At ≤ 0.8% dietary α-LNA, unesterified plasma and total brain DPAn-6 were elevated, total brain DHA was reduced, brain sPLA2 mRNA was elevated and BDNF protein was reduced. Finally, at 0.2% dietary α-LNA, brain iPLA2, COX-1 and BDNF mRNA were reduced, while brain cPLA2, COX-2 and 12-LOX mRNA were increased. Brain, liver and plasma AA concentrations and brain 5-LOX and 15-LOX mRNA levels were not changed significantly by any deprivation regimen, although changes in AA concentration in specific brain phospholipids may have occurred [12].
Figure 6.
Summary (top) and interpretation (bottom) of correlated threshold changes in plasma, brain and liver lipid measurements in rats fed diets containing declining concentrations of α-LNA for 15 weeks, in comparison to values with dietary n-3 PUFA adequate 4.6% α-LNA (see text).
A major finding of this study is that the brain DHA concentration was maintained at its control level at 4.6% dietary α-LNA down to 1.7% dietary α-LNA, despite reductions in unesterified plasma DHA of 36% (4.11 vs. 6.45 nmol/ml) and of total plasma DHA (98.3% esterified) of 27% (269 vs. 367 nmol/ml) below their respective control levels. Even when brain DHA was reduced significantly at 0.8% dietary α-LNA, the reduction was only 11% (15.7 vs. 17.6 µmol/g wet wt), much less than reductions of 64% (2.42 vs. 6.45 nmol/ml) in unesterified plasma DHA and of 54% (168 vs. 367 nmol/ml) in total plasma DHA below control levels. Because unesterified plasma DHA is the replacement source of brain DHA lost by metabolism [33, 34], there must be homeostatic brain mechanisms that resist a decline in brain DHA concentration despite markedly reduced plasma unesterified DHA.
Brain expression of DHA-selective iPLA2 IV [15, 16, 35]and of COX-1 (which can be functionally coupled to iPLA2 [18, 36]) did not differ from control at 2.6% dietary α-LNA. To maintain the brain DHA concentration with this diet, other brain DHA-releasing enzymes may have been downregulated, such as plasmalogen-selective PLA2, cPLA2γ, sPLA2, phospholipase C, and iPLA2γ [14, 37, 38], but this remains to be tested. Reduced β-oxidation of DHA within mitochondria DHA also could have limited DHA loss from brain [39, 40]. Such effects would prolong brain DHA half-life, which was demonstrated in rats fed the 0.2% α-LNA diet [6]. At 1.7% dietary α-LNA, the reduced activity of DHA-selective iPLA2 IV [15–17] would have helped to limit DHA loss from brain.
Dietary studies by Stark et al. suggest that DPAn-6 does not compete with DHA at for esterification into brain phospholipid, at a normal DHA concentration [41, 42]. At 0.8% dietary α-LNA, however, their data indicate that replacement would occur, because of the reduced plasma unesterified DHA and increased plasma unesterified DPAn-6 available for brain incorporation [40, 41, 43]. The invariance of the total brain AA concentration with all diets, and the absence of a change in AA turnover with 3-generational n-3 PUFA deprivation in rats [44], support this interpretation. As the threshold elevation in total plasma DPAn-6 at 1.7% dietary α-LNA followed the threshold elevation in liver DPAn-6 at 2.6% dietary α-LNA, it is likely that plasma DPAn-6 was increased by its increased synthesis and secretion by the liver, secondary to upregulation of elongases 2 and 5 and Δ6 desaturase. Activities of these enzymes in liver are much higher than in brain and, unlike brain activities, can be upregulated by dietary n-3 PUFA deprivation [19, 45–47].
Reduced brain activities of iPLA2 IV and COX-1 at ≤ 1.7% dietary α-LNA suggest reduced DHA release from phospholipid and downstream metabolism, tending to preserve DHA [3, 6, 18], and agree with the reported COX-1 protein increase at 0.2% dietary α-LNA [18]. These changes were accompanied by elevated activities of enzymes of the AA cascade, cPLA2, sPLA2, COX-2, and 12-LOX, which have been shown to be functionally coupled in various studies [18, 36, 48–50]. However, since the brain AA concentration was unchanged throughout α-LNA deprivation, we propose that the elevated expression of these enzymes might be related to the elevated brain esterified DPAn-6. Supporting this interpretation, DPAn-6 can be metabolized through the LOX pathway [51], and 12-LOX can convert DPAn-6 to anti-inflammatory oxylipins [52]. Further, the elevated brain DPAn-6 concentration in rats fed the 0.2% α-LNA diet is accompanied by increased DPAn-6 turnover in brain phospholipids (Igarashi et al., unpublished observations).
Some of the brain mRNA changes in this study may have reflected the reduced brain DHA concentration [18]. For example, COX-2 and cPLA2 gene transcription are regulated by nuclear factor (NF)-κB [53, 54], and DHA can inhibit NF-κB activity via a peroxisome proliferator activated receptor-dependent mechanism [55]. DHA and its 15-LOX product, 10,17S-docosatriene, decreased hippocampal NF-κB and COX-2 gene expression in other models [56].
The reduced brain BDNF protein and mRNA levels between 0.8% and 0.2% dietary α-LNA agree with evidence that DHA can be neuroprotective via a BDNF mechanism [13, 21]. At 0.2% dietary α-LNA, BDNF downregulation is accompanied by reduced cAMP response element binding protein transcription factor activity and reduced p38 mitogen-activated protein kinase activity [13]. Reduced BDNF has been reported in human brain disease [57].
Our results with the extreme 0.2% dietary α-LNA deprivation are consistent with other rodent studies on effects of n-3 PUFA deprivation [2, 11, 12, 22]. Such studies could not identify threshold changes because they were too severe, limiting their clinical extrapolation. The present study involving graded reductions in dietary α-LNA in the absence of dietary DHA, with a constant duration (15 weeks) of dietary exposure, leads us to propose the cause and effect relations discussed above and outlined in Figure 6 (bottom).
Our results may clarify whether dietary n-3 PUFA supplementation is necessary in human subjects [58]. That the brain DHA concentration did not fall below control level down to 1.7% dietary α-LNA, despite the 36% reduction in unesterified plasma DHA, suggests that comparable reductions in humans would not necessarily have pathophysiological consequences. Supporting this suggestion, a 33% lesser blood DHA concentration in vegetarians than in omnivores [59] was not associated with a significant difference in mood, or in general mortality or mortality from any cause [60, 61]. Additionally, 4-month dietary DHA supplementation in preschool children, which increased blood DHA from 1.0% to 3.2% of total fatty acid, did not enhance scores on any of four cognitive tests [62].
In summary, by quantifying effects of 15-week-long graded dietary α-LNA reductions in rats, we have shown that the brain DHA concentration, which is considered critical for brain function and metabolism, was maintained at its control level despite a profound reduction in plasma DHA. Brain DHA declined after plasma DPAn-6, likely derived by liver synthesis, was elevated in the presence of reduced plasma unesterified DHA, and this change might be related to increased expression of cPLA2, sPLA2 and COX-2. Because human studies involving n-3 PUFA deprivation have not reported increased plasma DPAn-6 levels, the results imply that brain DHA in such studies was not reduced to an extent that caused pathophysiological changes. This conclusion agrees with the absence of reported neuropathological effects in vegetarians compared with omnivores. An adequate brain DHA content with initially falling dietary α-LNA must be maintained by homeostatic mechanisms that remain to be elucidated. With extreme experimental deprivation, additional mechanisms include reduced expression of DHA-metabolizing iPLA2 and COX-1, and changes are accompanied by reduced brain BDNF expression.
ACKNOWLEDGEMENTS
This work was supported by Intramural Program of the National Institute on Aging, National Institutes of Health. We thank Dr. Ameer Taha and the NIH Fellows Editorial Board for editing the manuscript.
Abbreviations
- AA
arachidonic acid
- BDNF
brain derived neurotrophic factor
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- DPAn-6
docosapentaenoic acid
- LA
linoleic acid
- α-LNA
α-linolenic acid
- LOX
lipoxygenase
- cPLA2
cytosolic phospholipase A2
- sPLA2
secretory PLA2
- iPLA2
Ca2+-independent PLA2
- PUFA
polyunsaturated fatty acid
- sn
stereospecifically numbered
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 2.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 3.Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr Opin Lipidol. 2002;13:267–272. doi: 10.1097/00041433-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Holman RT. Nutritional and functional requirements for essential fatty acids. Prog Clin Biol Res. 1986;222:211–228. [PubMed] [Google Scholar]
- 5.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Rat heart cannot synthesize docosahexaenoic acid from circulating alpha-linolenic acid because it lacks elongase-2. J Lipid Res. 2008;49:1735–1745. doi: 10.1194/jlr.M800093-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 7.Demar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem. 2005;94:1063–1076. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- 8.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci. 2000;22:474–480. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- 9.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 10.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Upregulated liver conversion of alpha-linolenic acid to docosahexaenoic acid in rats on a 15 week n-3 PUFA-deficient diet. J Lipid Res. 2007;48:152–164. doi: 10.1194/jlr.M600396-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 14.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 15.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramadan E, Rosa AO, Chang L, Chen M, Rapoport SI, Basselin M. Extracellular-derived calcium does not initiate in vivo neurotransmission involving docosahexaenoic acid. J Lipid Res. 51:2334–2340. doi: 10.1194/jlr.M006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basselin M, Rosa AO, Ramadan E, Cheon Y, Chang L, Chen M, Greenstein D, Wohltmann M, Turk J, Rapoport SI. Imaging decreased brain docosahexaenoic acid metabolism and signaling in iPLA2{beta} (VIA)-deficient mice. J Lipid Res. doi: 10.1194/jlr.M008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, Reed GA, McCarson KE. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: Interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33:1279–1292. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriguchi T, Lim SY, Greiner R, Lefkowitz W, Loewke J, Hoshiba J, Salem N., Jr Effects of an n-3-deficient diet on brain, retina, and liver fatty acyl composition in artificially reared rats. J Lipid Res. 2004;45:1437–1445. doi: 10.1194/jlr.M400087-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta. 2009;1791:132–139. doi: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 25.Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr. 1993;123:1923–1931. doi: 10.1093/jn/123.11.1923. [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Washizaki K, Smith QR, Rapoport SI, Purdon AD. Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in the anesthetized rat. J Neurochem. 1994;63:727–736. doi: 10.1046/j.1471-4159.1994.63020727.x. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Cole GM, Lim GP, Yang F, Teter B, Begum A, Ma Q, Harris-White ME, Frautschy SA. Prevention of Alzheimer's disease: Omega-3 fatty acid and phenolic antioxidant interventions. Neurobiol Aging. 2005;26 Suppl 1:133–136. doi: 10.1016/j.neurobiolaging.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Lucas KK, Dennis EA. Distinguishing phospholipase A2 types in biological samples by employing group-specific assays in the presence of inhibitors. Prostaglandins Other Lipid Mediat. 2005;77:235–248. doi: 10.1016/j.prostaglandins.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Yang HC, Mosior M, Ni B, Dennis EA. Regional distribution, ontogeny, purification, and characterization of the Ca2+-independent phospholipase A2 from rat brain. J Neurochem. 1999;73:1278–1287. doi: 10.1046/j.1471-4159.1999.0731278.x. [DOI] [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Purdon D, Arai T, Rapoport S. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. J Lipid Res. 1997;38:526–530. [PubMed] [Google Scholar]
- 34.DeMar JC, Jr, Lee HJ, Ma K, Chang L, Bell JM, Rapoport SI, Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Strokin M, Sergeeva M, Reiser G. Role of Ca(2+)-independent phospholipase A(2) and n-3 polyunsaturated fatty acid docosahexaenoic acid in prostanoid production in brain: perspectives for protection in neuroinflammation. Int J Dev Neurosci. 2004;22:551–557. doi: 10.1016/j.ijdevneu.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Murakami M, Kambe T, Shimbara S, Kudo I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J Biol Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- 37.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strokin M, Sergeeva M, Reiser G. Prostaglandin synthesis in rat brain astrocytes is under the control of the n-3 docosahexaenoic acid, released by group VIB calcium-independent phospholipase A2. J Neurochem. 2007;102:1771–1782. doi: 10.1111/j.1471-4159.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- 39.Gavino GR, Gavino VC. Rat liver outer mitochondrial carnitine palmitoyltransferase activity towards long-chain polyunsaturated fatty acids and their CoA esters. Lipids. 1991;26:266–270. doi: 10.1007/BF02537135. [DOI] [PubMed] [Google Scholar]
- 40.Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: Review and critical analysis. Brain Res. Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- 41.Stark KD, Lim SY, Salem N., Jr Artificial rearing with docosahexaenoic acid and n-6 docosapentaenoic acid alters rat tissue fatty acid composition. J Lipid Res. 2007;48:2471–2477. doi: 10.1194/jlr.M700317-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Stark KD, Lim SY, Salem N., Jr Docosahexaenoic acid and n-6 docosapentaenoic acid supplementation alter rat skeletal muscle fatty acid composition. Lipids Health Dis. 2007;6:13. doi: 10.1186/1476-511X-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavino VC, Cordeau S, Gavino G. Kinetic analysis of the selectivity of acylcarnitine synthesis in rat mitochondria. Lipids. 2003;38:485–490. doi: 10.1007/s11745-003-1088-7. [DOI] [PubMed] [Google Scholar]
- 44.Contreras MA, Chang MC, Rosenberger TA, Greiner RS, Myers CS, Salem N, Jr, Rapoport SI. Chronic nutritional deprivation of n-3 alpha-linolenic acid does not affect n-6 arachidonic acid recycling within brain phospholipids of awake rats. J Neurochem. 2001;79:1090–1099. doi: 10.1046/j.1471-4159.2001.00658.x. [DOI] [PubMed] [Google Scholar]
- 45.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 47.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J. Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 49.Kuwata H, Nonaka T, Murakami M, Kudo I. Search of factors that intermediate cytokine-induced group IIA phospholipase A2 expression through the cytosolic phospholipase A2- and 12/15-lipoxygenase-dependent pathway. J Biol Chem. 2005;280:25830–25839. doi: 10.1074/jbc.M500168200. [DOI] [PubMed] [Google Scholar]
- 50.Bosetti F, Rintala J, Seemann R, Rosenberger TA, Contreras MA, Rapoport SI, Chang MC. Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E(2) concentration in rat brain. Mol Psychiatry. 2002;7:845–850. doi: 10.1038/sj.mp.4001111. [DOI] [PubMed] [Google Scholar]
- 51.Sprecher H, Careaga MM. Metabolism of (n-6) and (n-3) polyunsaturated fatty acids by human platelets. Prostaglandins Leukot Med. 1986;23:129–134. doi: 10.1016/0262-1746(86)90175-7. [DOI] [PubMed] [Google Scholar]
- 52.Dangi B, Obeng M, Nauroth JM, Teymourlouei M, Needham M, Raman K, Arterburn LM. Biogenic synthesis, purification, and chemical characterization of anti-inflammatory resolvins derived from docosapentaenoic acid (DPAn-6) J Biol Chem. 2009;284:14744–14759. doi: 10.1074/jbc.M809014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morri H, Ozaki M, Watanabe Y. 5'-flanking region surrounding a human cytosolic phospholipase A2 gene. Biochem Biophys Res Commun. 1994;205:6–11. doi: 10.1006/bbrc.1994.2621. [DOI] [PubMed] [Google Scholar]
- 54.Rivest S. Activation of the nuclear factor kappa B (NF-kappaB) and cyclooxygenase-2 (COX-2) genes in cerebral blood vessels in response to systemic inflammation. Mol Psychiatry. 1999;4:500. [PubMed] [Google Scholar]
- 55.Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2005;92:187–195. doi: 10.1007/s10549-005-2415-z. [DOI] [PubMed] [Google Scholar]
- 56.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 57.Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 37:596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kris-Etherton PM, Hill AM. N-3 fatty acids: food or supplements? J Am Diet Assoc. 2008;108:1125–1130. doi: 10.1016/j.jada.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 59.Rosell MS, Lloyd-Wright Z, Appleby PN, Sanders TA, Allen NE, Key TJ. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am J Clin Nutr. 2005;82:327–334. doi: 10.1093/ajcn.82.2.327. [DOI] [PubMed] [Google Scholar]
- 60.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford) Am J Clin Nutr. 2009;89:1613S–1619S. doi: 10.3945/ajcn.2009.26736L. [DOI] [PubMed] [Google Scholar]
- 61.Beezhold BL, Johnston CS, Daigle DR. Vegetarian diets are associated with healthy mood states: a cross-sectional study in seventh day adventist adults. Nutr J. 9:26. doi: 10.1186/1475-2891-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryan AS, Nelson EB. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: a randomized, placebo-controlled, double-blind study. Clin Pediatr (Phila) 2008;47:355–362. doi: 10.1177/0009922807311730. [DOI] [PubMed] [Google Scholar]