Abstract
Background:
A global push to reduce the amount of saturated and trans-fatty acids, added salt and sugar in processed food, and to enhance fruit, vegetable and whole grain intake, while limiting energy intake, exists for most populations.
Objectives:
To redesign the International Choices Program (note: this is unrelated to the US Smart Choices Program), initially Netherlands focused, by an international board of scientists to create a generic, global front-of-pack nutrition logo system that helps consumers make healthier food choices and stimulates product reformulation.
Methods:
The Programme is a product-group-specific-nutrient-profiling approach with a distinction between basic and discretionary foods. The basic product groups are main contributors of essential and beneficial nutrients, and are based on food-based dietary guidelines from more than 20 countries across the globe. Generic criteria are derived from international nutrient recommendations for trans-fatty acids, saturated fatty acids, sodium, added sugar, fibre and energy, and evaluated against food composition data from 12 countries across Europe and market reality (actual foods on the market). Selected debates such as the source of fibre are also presented.
Results:
Generic criteria and a decision framework were developed to further define food categories, so as to meet the unique country- and region-specific dietary needs. The result is a complete set of criteria that is evaluated on a regular basis to ensure its alignment with international dietary patterns, new scientific insights and current developments within the food market.
Conclusions:
These guidelines are currently used in a number of countries across the globe, and are being evaluated for effectiveness. Completed studies have demonstrated an increase in consumer awareness, a positive effect on product innovation and a potential impact on nutrient intakes.
Keywords: food labelling, food/standards, nutrients, nutritive value, consumer health information, health promotion
Introduction
Excessive and unbalanced intake of energy, saturated fatty acids, trans-fatty acids, salt and sugar lead to increased risk for chronic non-communicable diseases (Joint WHO/FAO consultation, 2003). Reducing or minimising consumption of these nutrients in our diet, while increasing our consumption of fruits, vegetables and whole grains, has been the focus of extensive action by a number of governments and the food industry.
Front-of-pack (FOP) labelling and food reformulation have become subject to international stakeholder debate by regulators, scientists, the public health community and the food industry. The scientific community is concerned with aspects relating to science and credibility (Drewnowski and Fulgoni, 2008; Nestle and Ludwig, 2010; Scarborough et al., 2007, 2010), whereas the food industry would like to create a level playing field and global harmonisation (Trichterborn and Harzer, 2007; Lupton et al., 2010). Consumer organisations and the public health community want to protect consumers from being misled or confused, while also stimulating healthy eating. This is illustrated by initiatives in the United Kingdom on salt reduction and labelling (Food Standards Agency, 2005, 2009), and in Europe and Australia, where legislative bodies work on defining nutrient criteria for foods that are eligible to carry a health claim (Food Standards Australia New Zealand, 2007; EU Regulation (EC), 2007). In the United States, the Institute of Medicine and the Food and Drug Administration are evaluating existing FOP labelling systems following the failure of a multi-stakeholder initiative—the Smart Choices Program—led by the food industry. Unrelated to the International Choices Programme, it failed owing to criticism that its nutrient criteria allowed high sugar and high fat products to carry a healthy choice logo (Wartella et al., 2010). This highlights the importance of an independent scientific committee to define the actual criteria. In the fast changing food markets in developing countries, there is a growing interest in and need for tools to help consumers make healthier choices while their food supply is changing rapidly (Reardon et al., 2003; Popkin, 2008; Rivera et al., 2008).
Triggered by a governmental request to the food industry for one single healthy choice logo, the Choices Programme was first introduced in the Netherlands in 2006. The two main aims of the Programme are to help consumers make healthier food choices and to encourage food manufacturers to improve the composition of their products. Initially, criteria were developed and set for the Dutch market by an independent Dutch scientific committee advising the Choices Foundation Board (Dötsch-Klerk and Jansen, 2008). Since then, over 100 partners in food manufacturing, retail and food service (80% of the catering market) have joined the initiative in the Netherlands, and the Choices logo is being adopted in various other countries across the globe. This international roll-out has necessitated a complete re-evaluation of the criteria for further international applicability. In 2008, an international scientific committee was created, comprised of scientists from around the world. This paper describes the work of the international scientific committee, in terms of redefining product groups as well as the full set of criteria. The International Choices Programme has been subject to many complex and critical scientific debates, including on how to judge and monitor the food supply, and how to define what is healthy. Several critical issues that have been addressed are described within this paper.
Methods
A product-group-specific approach and selection of nutrients
The average population intake aims (Joint WHO/FAO consultation, 2003) have served as the starting point for developing criteria to evaluate food products according to their nutrient content. However, the range of fat, salt, added sugar, fibre and other nutrients between foods and beverages is far too great to create one set of criteria for all food products. Thus, product grouping is needed (Scarborough et al., 2007, 2010) to assure alignment with the aims of the International Choices Programme: to help consumers choose within a product group and to stimulate producers to improve the nutrient composition. This can only be achieved if criteria are separately set for different product groups—such as fats and beverages, rather than with a single set of criteria that compares foods across the food supply (Rayner et al., 2005; Drewnowski and Fulgoni, 2008; Katz et al., 2010).
Nutrient criteria have been developed for trans-fatty acids, saturated fatty acids, sodium and added sugars, because high intakes of these nutrients negatively affect health. The nutrient definitions and related health risks are provided in Table 1. The focus is not only on limiting the intake of nutrients with a negative impact on health, but also on ensuring the intake of essential and beneficial nutrients. To achieve this, a distinction has been made between basic foods and discretionary foods. Basic food product groups were based on product group classifications from food-based dietary guidelines used in more than 20 countries worldwide (see legend to Table 2), which significantly contribute to the intake of essential and beneficial nutrients (for example, vitamins, minerals) and water. Discretionary product groups do not significantly contribute to the intake of beneficial nutrients. They are included because they are eaten frequently, are important sources of trans-fatty acids, saturated fatty acids, sodium, added sugar and energy, and therefore targets for product innovation.
Table 1. Nutrient definitions and health risks.
| Nutrient | Definition | Health risk with excessive intakes | Comments |
|---|---|---|---|
| SAFA | The sum of all types and sources of saturated fatty acids | Cardiovascular diseases, blood lipids (Joint WHO/FAO consultation, 2003; Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition, 2008; Elmadfa and Kornsteiner, 2009) | |
| TFA | All the geometrical isomers of monounsaturated and polyunsaturated fatty acids with non-conjugated, double carbon–carbon conjugations in the trans-configuration, and which are separated by at least one methylene group. Natural trans-fatty acids from meat and milk are excluded | Coronary heart disease, blood lipids (Joint WHO/FAO consultation, 2003; Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition, 2008; Elmadfa and Kornsteiner, 2009) | There are no limiting criteria for naturally occurring trans-fatty acids in dairy and meat, because these are difficult to influence through product reformulation. The saturated fat criteria defined for dairy and meat are considered sufficient to define the healthier options |
| Sodium | This includes both added sodium (for example, by salt or MSG) and sodium that is naturally present in one of the ingredients (for example, in yeast extract, protein hydrolysates) | Cardiovascular diseases, blood pressure (Joint WHO/FAO consultation, 2003; Strazullo et al., 2009) | |
| Added sugar | All monosaccharides, disaccharides and polyols with a caloric value of >3.5 kcal/g, from sources other than fruit, vegetables and milk products. This also includes natural sugars such as honey, syrups and (more than twice) concentrated fruit drinks It is assumed that ‘added sugar' is the same as ‘free sugar' | Dental diseases, obesity (Joint WHO/FAO consultation, 2003) | There are no physiological arguments to distinguish free or added sugar from total sugar. Added sugars, however, can be manipulated by manufacturers. In addition, fruit- and milk-based products should not be penalized for their intrinsic sugar content. Products high in free/added sugars are often nutrient-poor and energy-dense providing the so-called ‘empty calories' |
| Energy | The amount of energy from food products that is available for the metabolism of the body, expressed in kJ or kcal | Obesity, cardiovascular disease, diabetes, cancer (Joint WHO/FAO consultation, 2003) | |
| |
|
Health risks with too low intakes |
|
| Dietary fibre | The collective term for those substances that are not digested or taken up by the human small intestine and which have the chemical character of carbohydrates (suitable for human consumption) or compounds analogous to carbohydrates (see Codex) | Cardiovascular diseases, blood lipids, obesity (Joint WHO/FAO consultation, 2003) Insufficient micronutrient intakes with plant-based diets (Pascoe and Fulcher, 2008) | Codex fibre definition is currently under revision (Cummings et al., 2009) Source of fibre should be the main ingredients of the product |
Abbreviations: MSG, monosodium glutamate; SAFA, saturated fatty acids; TFA, trans-fatty acids.
Table 2. Overview of generic and product-group-specific criteriaa for basic and discretionary product groupsb.
|
Product group |
|
|
Nutrients to limit |
Fibre | ||||
|---|---|---|---|---|---|---|---|---|
| Comments | Energy | Saturated fat | Trans-fat | Sodium | Added sugars | |||
| Daily nutrient recommendations | From Joint WHO/FAO consultation (2001, 2003) | 2000 kcal per day | <10 en% | <1 en% | 2000 mg per day=1 mg/kcal (based on 2000 kcal per day) | <10 en% | 25 g per day=1.3 g/100 kcal (based on 2000 kcal per day) | |
| Generic criteria (energy based) | Generic energy-based criteria=daily nutrient recommendation+30% | NA | ⩽13 en% | ⩽1.3 en% | ⩽1.3 mg/kcal | ⩽13 en% | ⩾1.3 g/100 kcal | |
| Generic criteria, insignificancy levels (per 100 g) | Generic insignificancy levels=5% of daily nutrient recommendation per 100 g (based on 2000 kcal per day) | NA | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽100 mg/100 g | ⩽2.5 g/100 g | NA | |
| Basic product groups | ||||||||
| Fruit and vegetablesc | Fresh or fresh-frozen fruit, vegetables and legumes | All products without additives comply | NA | NA | NA | NA | NA | NA |
| Processed fruit and vegetables | Source of fibre can only be fruit and vegetables | NA | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽100 mg/100 g | Not added | ⩾1.3 g/100 kcal | |
| Fruit juices | Source of fibre can only be fruit | ⩽48 kcal/100 ml | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽100 mg/100 g | Not added | ⩾0.75 g/100 kcal | |
| Waterc | Water (plain) | NA | NA | NA | ⩽20 mg/100 ml | NA | NA | |
| Sources of carbohydratesc | Potatoes (unprocessed) | All products without additives comply | NA | NA | NA | NA | NA | NA |
| Potatoes (processed), pasta, noodles | Source of fibre can only be the main ingredient (for example, potatoes, grains) | NA | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽100 mg/100 g | Not added | ⩾1.3 g/100 kcal | |
| Rice | Source of fibre can only be rice | NA | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽100 mg/100 g | Not added | ⩾0.7 g/100 kcal | |
| Bread | Source of fibre can only be the main ingredient (for example, grains) | NA | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽500 mg/100 g | ⩽13 en% | ⩾1.3 g/100kcal | |
| Grains and cereal products | Source of fibre can only be grains | NA | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽100 mg/100 g | ⩽2.5 g/100 g | ⩾1.3 g/100 kcal | |
| Breakfast cereal products | Source of fibre can only be grains | NA | ⩽13 en% | ⩽0.1 g/100 g | ⩽500 mg/100 g | ⩽20 g/100 g | ⩾1.3 g/100 kcal | |
| Meatc | Meat, poultry, eggs (unprocessed) | Naturally occurring trans-fat from meat is excluded | NA | ⩽1.1 g/100 g or ⩽13 en%d | ⩽0.1 g/100 g | ⩽100 mg/100 g | Not added | NA |
| Processed meat, meat products and meat substitutes | Naturally occurring trans-fat from meat is excluded | NA | ⩽1.1 g/100 g or ⩽13 en% | ⩽0.1 g/100 g | ⩽900 mg/100 g | ⩽2.5 g/100 g | NA | |
| Fishc | Fresh or fresh–frozen fish, shellfish and crustaceans | NA | ⩽1.1 g/100 g or ⩽30% of total fat | ⩽0.1 g/100 g | ⩽100 mg/100 g | Not added | NA | |
| Processed fish or fish products | NA | ⩽1.1 g/100 g or ⩽30% of total fat | ⩽0.1 g/100 g | ⩽450 mg/100 g | Not added | NA | ||
| Dairyc | Milk (products) | Naturally occurring trans-fat from milk is excluded | NA | ⩽1.4 g/100 g | ⩽0.1 g/100 g | ⩽100 mg/100 g | ⩽5 g/100 g | NA |
| Cheese (products) | Naturally occurring trans-fat from milk is excluded | NA | ⩽15 g/100 g | ⩽0.1 g/100 g | ⩽900 mg/100 g | Not added | NA | |
| Oils and fatsc | Oils, fats and fat containing spreads | Naturally occurring trans-fat from milk is excluded | NA | ⩽30% of total fat | ⩽1.3 en% | ⩽1.3 mg/kcal | Not added | NA |
| Composite dishes | Main course | Naturally occurring trans-fat from meat or milk is excluded Source of fibre can only be one of the main ingredient (for example, potatoes, vegetables, grains) | 400–700 kcal per serving | ⩽1.1 g/100 g or ⩽13 en% | ⩽0.1 g/100 g or ⩽1.3 en% | ⩽2.2 mg/kcal | ⩽2.5 g/100 g or ⩽13 en% | 1.25 g/100 kcal |
| Filled sandwiches/rolls | Naturally occurring trans-fat from meat or milk is excluded Source of fibre can only be the main ingredients (for example, vegetables, grains) | ⩽350 kcal per serving | ⩽1.1 g/100 g or ⩽13 en% | ⩽0.1 g/100 g or ⩽1.3 en% | ⩽1.9 mg/kcal | ⩽2.5 g/100 g or ⩽13 en% | ⩾0.8 g/100 kcal | |
| Discretionary product groups | ||||||||
| Soups | ⩽100 kcal/100 g | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽300 mg/100 g | ⩽2.5 g/100 g | NA | ||
| Meal sauces | ⩽100 kcal/100 g | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽450 mg/100 g | ⩽2.5 g/100 g | NA | ||
| Other sauces (on water basis) | ⩽100 kcal/100 g | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽750 mg/100 g | NA | NA | ||
| Other sauces (emulsions) | ⩽350 kcal/100 g | ⩽1.1 g/100 g or 30% total fat | ⩽0.1 g/100 g or 1.3 en% | ⩽750 mg/100 g | ⩽2.5 g/100 g or ⩽13 en% | NA | ||
| Snacks (pastry, edible ice, sweet and savoury snacks) | ⩽110 kcal per serving | ⩽1.1 g/100 g or ⩽13 en% | ⩽0.1 g/100 g or ⩽1.3 en% | ⩽400 mg/100 g | ⩽20 g/100 g | NA | ||
| Beverages | ⩽20 kcal/100 ml | ⩽1.1 g/100 g | ⩽0.1 g/100 g | ⩽20 mg/100 g | NA | NA | ||
| Bread toppings incl. hummus-like products | NA | ⩽13 en% | ⩽1.3 en% | ⩽400 mg/100 g | ⩽30 g/100 g | NA | ||
| All other products | ⩽1.1 g/100 g or ⩽13 en% | ⩽0.1 g/100 g or ⩽1.3 en% | ⩽100 mg/100 g or ⩽1.3 mg/kcal | ⩽2.5 g/100 g or ⩽13 en% | NA | |||
| National deviation example | ||||||||
| Soft white cheese (Israel) | Naturally occurring trans-fat from milk is excluded | NA | ⩽2 g/100 g | ⩽0.1 g/100 g | ⩽400 mg/100 g | Not added | NA | |
Abbreviation: NA, not applicable.
1 kcal=4.186 kJ; en%=for a specific food: energy delivered by this nutrient (kcal), divided by the total energy content (kcal) of the food, multiplied by 100.
Criteria are applicable to all foods and beverages, except infant and follow-up formulas; products containing >0.5% alcohol.
For a detailed description of product groups, see Supplementary Appendix 4 ‘Overview of product group descriptions, criteria and rationales'.
Basic product groups are based on the product group classifications as used in food-based dietary guidelines in more than 20 countries: Australia, Belgium, Canada, China, Denmark, Finland, France, Germany, Greece, Hungary, Malaysia, Mexico, Namibia, The Netherlands, Philippines, Portugal, Singapore, Spain, Sweden, Turkey, UK and Europe. See Supplementary Appendix 4 for references.
If possible, the generic criteria are fixed per product group: either as en% or g/100 g. However, if this leads to the needless exclusion of low-energy foods, both generic criteria (en% or as g/100 g) can be used.
An emphasis on healthy choices in basic product groups is encouraged by setting the criteria for discretionary foods at a more restrictive level than for basic foods. This is explained below. Table 2 (second column) provides an overview of all product groups.
Fibre was the subject of much debate. Indeed, manufacturers often add artificial or isolated fibres such as inulin as a ‘beneficial nutrient' to many foods. However, the effects on health of these isolated fibres are inconclusive (Cummings et al., 2009), and these purified fibres do not provide the micronutrients and phytochemicals that are present in sources of naturally occurring fibre, such as whole grains (Pascoe and Fulcher, 2008). The significance of this in terms of public health is great for countries such as Mexico, where tortillas represent around a quarter of the calories consumed (Popkin, 2008). Therefore, to promote fibre intake, a fibre criterion was added for relevant product groups. In line with the evidence, and to ensure sufficient micronutrient intake, the source of fibre must originate from the actual ingredients of the product group (for example, whole grain, vegetables).
Furthermore, as the Choices Programme aims to promote appropriate energy intake, an energy criterion has been defined for product groups that either substantially contribute to energy intake (for example, main courses and filled sandwiches) or for which a limited consumption is recommended (discretionary product groups): for example, sugar-sweetened beverages (Popkin et al., 2006).
Development of criteria: the scientific approach
Generic criteria and reference amounts
A first step in the development of the nutrient criteria relates to the energy-based translation of international nutrient recommendations (Joint WHO/FAO consultation, 2003) into food-specific generic criteria. The generic criteria are defined as ‘nutrient recommendation +30%'. This additional 30% has been added to the criteria, as the average diet consist of many foods—not all of which contain nutrients that should be limited (saturated fatty acids, trans-fatty acids, sodium, added sugar). An additional 30% allows more room for foods that do contribute to the intake of these nutrients on top of recommendations (Table 2). This 30% is an arbitrary starting point, which can be lowered when the nutritional quality of the diet has improved. Intake modelling was used to substantiate that adding 30% does not counter the objectives of the programme (Van Raaij et al., 2008; Roodenburg et al., 2009).
In developing the criteria, it was important to look at products with low absolute levels of nutrients or energy. This is because the generic criteria expressed as a percentage of energy might lead to unrealistic scores (for example, saturated fatty acids in soups). Therefore, to ensure that low-energy-dense products would not be needlessly excluded, specific criteria were developed for these ‘insignificant levels' of nutrients. A ‘level of insignificance' was defined as ‘<5% of the daily nutrient recommendation in grams or milligrams per 100 g of a food product', according to an energy intake of 2000 kcal per day (Table 2).
Product-group-specific criteria
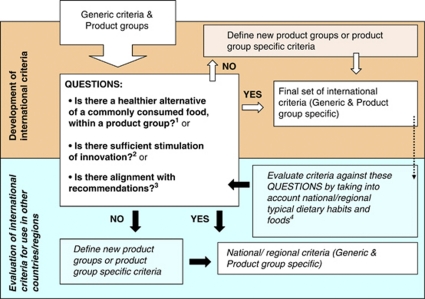
Generic criteria were applied where possible. In addition typical food habits need to be taken into account. In principle, the number of product groups should be limited and are determined by a decision framework: a new product group or product-group-specific criteria can only be defined under the conditions outlined in Figure 1.
Figure 1.
Decision framework for defining new product groups or new product-group-specific criteria. 1In this question, we assume that people choose within a product group. Example: a new product group ‘Rice' was derived from ‘Grains and cereal products' by defining a product-group-specific criterion for fibre, so that some high fibre rice can comply. 2Examples: product-group-specific criterion for sodium was defined for soups, because of taste reasons (as a generic sodium criterion would lead to soups without a salty taste), whereas for sodium in cheese and bread it was defined according to technological constrains (that is, salt is needed for the preparation of bread and cheese). 3Example: a product-group-specific criterion for SAFA was defined for the product group ‘Fish products' to allow more products to comply, which are important for unsaturated fatty acid intake. 4Within the Israeli diet, soft white cheese is an important source of calcium and protein. As previous criteria for ‘Cheese' were too lenient and not encouraging innovation, it was therefore adapted (see Supplementary Appendix 1).
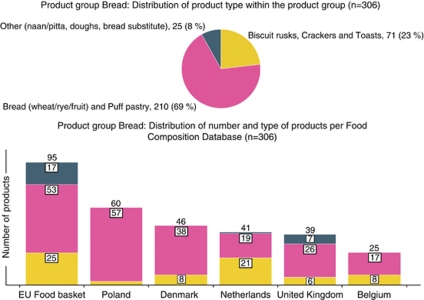
To encourage the intake of essential and beneficial nutrients, consumers should have access to more healthy choices within basic product groups—as these provide essential nutrients—than within the discretionary product groups. For basic product groups, the aim was to have at least 20% of products comply with the criteria within a given product group, and approximately 10% for the discretionary product groups. These percentages (10 and 20) were used as a starting point for deciding when a product-group-specific criterion is needed and what the criterion should be. To determine the actual percentages and the existing variations in product composition on the international market, a food composition test database was created. It consists of food composition data for 7000 foods from 12 European countries (Belgium, Denmark, France, Germany, Ireland, Italy, the Netherlands, Norway, Poland, Spain, Sweden and the United Kingdom; for details see legend of Figure 2). This selection was based on geographic representation, data completeness, costs and availability—both electronically and in the English language. For more details, see Supplementary Appendix 2 ‘Development of a test database on food composition'.
Figure 2.
Description of the test database on food composition for the product group bread: number and type of products. This database consists of 7000 foods from the following sources: 21% of the foods come from the limited EU database (EFSA, 2008), in the following quantities: France (29%), Spain (17%), Germany (12%), Italy, Sweden, UK, Denmark (each 8%), Ireland (7%) and Norway (6%). Foods from other databases are as follows: the Netherlands (20%) (Stichting Nederlands Voedingstoffenbesluit, 2006), the United Kingdom (17%) (Food Standards Agency, 2002), Belgium (Rijksadministratief Centrum, 2004) and Denmark (Danish Institute for Food and Veterinary Research, 2005) (both 14%), Poland (13%) (National Food and Nutrition Institute (IZZ, 2005).
During the scientific debates, factors such as public health nutrition, food market reality (actual foods on the market) and the typical examples of indicator foods were used to further define the criteria. Some examples are addressed in the next section.
Most of the work was carried out between February 2008 and June 2009, during which time the International Scientific Committee of the Choices Programme met four times. Final changes were made in May 2010, and decisions were made with the consensus of all committee members.
Results
Definition of the actual criteria
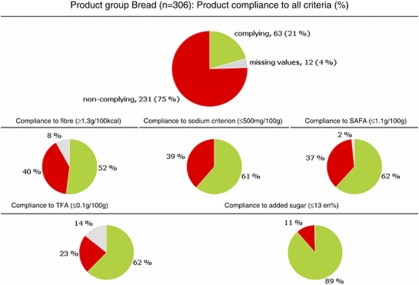
For the product group bread, Figure 2 shows the data description and Figure 3 shows the percentage of products that comply (overall and per key-nutrient). The legend in Figure 3 shows examples of indicator foods that do or do not comply with the criteria. Statistics, distributions and plots of the key-nutrient content were used when necessary (data not shown). For all product groups, all of this information is available in Supplementary Appendix 3 ‘Detailed characteristics per product group'. Table 2 shows the overall criteria for all product groups.
Figure 3.
Product group bread: the percentage of complying and non-complying products are given: overall product score and a score per nutrient. Examples of complying foods (eligible to use the logo): rye bread, averageb; wholemeal bread averagec, crisp bread rye 18% fibre Ryvita Morkt (dark)d; examples of foods that do not comply (including non-complying nutrients): white bread, premiumc (sodium, fibre), crackerse (TFA, SAFA, sodium, fibre); crisp bread, knackerbrot, wheat, finef (SAFA, fibre). Sources: bStichting Nederlands Voedingstoffenbesluit (2006); cFood Standards Agency (2002); dEFSA (2008); eRijksadministratief Centrum (2004); fDanish Institute for Food and Veterinary Research (2005). SAFA, saturated fatty acids; TFA, trans-fatty acids; en%, for a specific food: energy delivered by added sugar (kcal), divided by the total energy content (kcal) of the food and multiplied by 100.
To illustrate the scientific debate, a few examples are addressed below. Supplementary Appendix 4 ‘Overview of product group descriptions, criteria and rationales' contains all of the debated rationales for the product-group-specific criteria, including an overview of the changes made to the original Netherlands-based criteria.
For example, the rationale for encouraging innovation (Figure 1) was applied to the energy criterion in beverages. Owing to concerns that the consumption of sugar-rich beverages can adversely affect energy intake, weight gain and increase the risk of health problems (Vartanian et al., 2007), beverages should not contribute by more than 10% to daily energy intake (Popkin et al., 2006). However, people will continue to consume these sugar-sweetened drinks. Therefore, reformulation should be encouraged. Currently, standard energy containing drinks contain 10–12 g of sugar/100 g (40–48 kcal/100 g). As a first step without forcing manufacturers to use artificial sweeteners, the criterion has been set at 20 kcal/100 g or 100 ml, which is the equivalent of 5 g of sugar. This will be progressively lowered over time. It is also important to note that on the one hand a considerable number of consumers do not consume sweeteners (Euromonitor International, 2009; Mattes and Popkin, 2009), and on the other, there are concerns about the possible effects of excessive sweetness on long-term habituation to sugar and sweet foods (Avena et al., 2009). By relying on a gradual sugar reduction approach, it is expected that once consumers have gotten used to a less sweet taste, this will also facilitate the lowering of sugar levels in other product groups such as milk products and breakfast cereals.
Another example is pure (100%) fruit juice consumption, which contributes to the intake of fruit, but also to a high-energy intake and the possibility of weight gain and diabetes (Sanigorski et al., 2007; Odegaard et al., 2010). As sugar levels are determined by legislation (Codex, 2005), there is little room for innovation. Therefore, an energy criterion has been set at 48 kcal/100 ml to exclude fruit juices that are too high in energy. Thus, the rationale applied here is alignment with the recommendations (Figure 1) to stimulate fruit intake, but also to limit energy intake from fruit juices.
The decision framework can be used to evaluate the criteria globally against other dietary habits and food compositions. For example: for white soft cheese—a major contributor of protein and calcium in Israel—the existing criteria did not discriminate the healthier option. Therefore, new criteria were defined (Table 2). The International Scientific Committee used the feedback from the Israeli Scientific Committee (Appendix 1 ‘Israeli case study') as a basis for the rationales to decide on needs to add product groups and criteria (Figure 1).
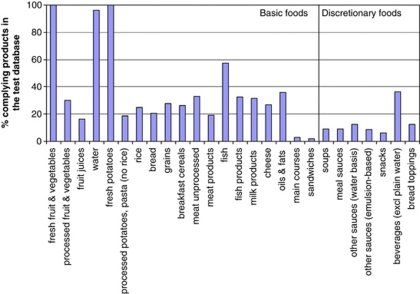
Figure 4 shows the percentage of products that comply with the test database, and illustrates that there are more products that comply within the basic product groups than within the discretionary product groups. In some cases, the percentage of complying products are far below (for example, sandwiches and main courses) or above (for example, fresh fruit and vegetables, fresh potatoes, water and beverages) the targeted 20% for basic foods or 10% for discretionary foods. For sandwiches and main courses, data from the test database are limited. Real examples from the (Dutch) market illustrate that criteria did stimulate food manufactures to innovate. Consumption of fresh fruit and vegetables, fresh potatoes and water needs to be encouraged, as these provide essential nutrients. These foods are therefore all eligible to carry the Choices logo. Regarding the discretionary product groups, there is a relatively high percentage (36%) of beverages that comply. These are however exclusively limited to coffee, tea, flavoured water and light beverages.
Figure 4.
Percentages of products that comply based on the test database. Results are shown per product group, for basic foods and discretionary foods.
Discussion
This paper provides an overview of the development of nutrient profiles used for determining international Choices logo eligibility. It involves a product-group-specific approach, with a distinction between basic and discretionary foods. It is assumed that consumers should have a healthier choice within a given product group. The nutrient criteria are evaluated on a regular basis (every 3 years) by an independent scientific committee. The evaluations take into account the latest developments in nutrition science and food technology, as well as within the market place, with the ultimate aim of achieving the population dietary intake aims (Joint WHO/FAO consultation, 2003). The unique feature of this nutrient-profiling system is its decision framework that enables international applicability and translation to other dietary habits.
Many different organisations have developed nutrient-profiling systems for various purposes (Stockley et al., 2007). Some—such as the Swedish Keyhole (Livsmedelsverket, 2009) and the New Zealand Pick the Tick (The National Heart Foundation, 2002)—have been around for more than 20 years. Others such as the traffic light system in the United Kingdom (Food Standards Agency, 2009) have been promoted by government agencies. Systems that rely on one set of criteria for the entire food supply (Rayner et al., 2005; Drewnowski and Fulgoni, 2008; Katz et al., 2010) are less aimed at stimulating product reformulation within a product category, and may actually reinforce current high animal source food diets (Drewnowski and Fulgoni, 2008). In addition, within these systems, there is little scientific reasoning behind the applied weighing of ‘nutrients to limit' against ‘nutrients to encourage'.
The scientific basis behind the nutrient profiles that are presented here are the international nutrient recommendations (Joint WHO/FAO consultation, 2003) and food composition data. Others have based their nutrient profiles on expert judgment, and dietary requirements (Rayner et al., 2005; Drewnowski and Fulgoni, 2008), on actual intakes (Food Standards Agency, 2005) or on existing food standards and technical and taste arguments in addition to the dietary intake aims (Nijman et al., 2007). Ultimately, it is difficult to judge which nutrient-profiling system is better than the other. Indeed, although various validation methods for nutrient profiles have been published (Azais-Braesco et al., 2006; Arambepola et al., 2008; Scarborough et al., 2010), there is no gold standard (Towsend, 2010). The Choices criteria have been evaluated by an independent scientific committee, and by estimating their potential improvements on habitual nutrient intakes (Van Raaij et al., 2008; Roodenburg et al., 2009).
However, the scientific approach behind the Choices Programme has some limitations. Indeed, the food composition databases that are used represent—at best—average nutrient composition values. In most cases, they are fairly outdated and cannot keep pace with thousands of new foods continuously introduced onto the food market. Nevertheless, the major advantage of the current approach is its transparency—all the data are publically available. Furthermore, evaluation of the nutrient criteria according to European food composition data is a first step in developing nutrient criteria for international use. International applicability requires the evaluation of criteria against data from other regions on dietary habits and food composition (if available) based on the above-mentioned decision framework. This has been carried out for Israel. An additional advantage of this process is the coinciding incentive of collecting food composition data in regions where these data are limited.
Consumer research in four European countries demonstrates that a simple front-of-pack label—such as a health logo—is most appropriate within a shopping environment where quick decisions have to be made (Feunekes et al., 2008). This has served as the basis of the Choices logo: since the launch of the Choices Programme in the Netherlands, studies are being carried out on its effectiveness. These studies reveal that there is increasing recognition and appreciation of the logo by consumers, especially from those interested in health (Vyth et al., 2009). A study on current consumer behaviour in supermarkets reveals that people who are health conscious purchase more products bearing the Choices logo (Vyth et al., 2010a). Effects of the Choices logo on sales in Dutch workplace canteens are limited (Vyth et al., 2011). Others have shown mixed effects on sales of front-of-pack labelling in the supermarket environment (Sacks et al., 2009; Sutherland et al., 2010). Additional studies demonstrate positive effects on product reformulation (Vyth et al., 2010b); thus, by improving the composition of products, consumers with less interest in health can also be reached. Currently, effectiveness research is not limited to the Netherlands, as it has also been initiated in other countries, including Poland.
In summary, the nutrient criteria for logo eligibility developed by the international Choices Programme's global panel of scientists are a transparent, science-based tool designed to encourage both consumers and producers towards a healthier food supply. International applicability makes these nutrient profiles a useful tool to stimulate product innovation and consumer choice globally. This is also true for the fast-changing food markets of developing and transitional countries, including India, Mexico and Brazil.
Acknowledgments
The committee would like to extend its thanks to Leon Jansen (LJ) from the Choices International Foundation and Petra Dekker (PD) from Frieslandcampina for their help in writing this manuscript and for their technical assistance. The committee also wishes to thank Mariska Dötsch (MD), Olivia Howell-Davies (OH), Michiel Meeuse (MM), Andrea Sekulovic (AS), Renske Visser (RV) from Unilever Research and Development Vlaardingen, Anika de Mul from the VU University Amsterdam, Amalia Waxman and Yaara Tirosh from Israel for their technical assistance. AR, AS, MD, MM, OH, RV and PD have signed an agreement with either Unilever or Frieslandcampina, thereby agreeing not to share any content of the discussion on the criteria, so as to ensure a sound scientific process (Supplementary text agreement 2). The work is supported by the Choices International Foundation. Members of the International Scientific Committee (ISC) of the Choices Programme receive no payment, except for reimbursement of travel-related expenses. Members of the ISC have signed a ‘Terms of reference' form, which discloses their relevant relations (Supplementary text agreement 1).
NS, GP, GV, MDA, MR, MG, RE, VS and JCS have no financial conflict of interest. BP receives his salary funding from NIH (70%), the University of North Carolina (UNC) and the Robert Wood Johnson foundation (25%). He is involved in random-controlled trials at UNC and the Mexican National Institute of Public Health funded by Nestlé's Water and Danone Water research groups, respectively. AA receives compensation as a consultant for Danone and the California Almond Board, and is a member of the the Global Dairy Platform's executive board. He also receives speaker fees from various food producers as well as research grants and food products for research purposes from more than 100 different food companies. AR is employed by Unilever and seconded at the VU University Amsterdam.
Footnotes
Supplementary Information accompanies the paper on European Journal of Clinical Nutrition website (http://www.nature.com/ejcn)
Contributors: The manuscript was drafted by AJCR, BMP and JCS on behalf of the International Scientific Committee of the Choices Programme: BM Popkin, Carolina Population Center, University of North Carolina, Chapel Hill, NC, USA; N Steyn, Human Sciences Research Council, Cape Town, South Africa; G Pekcan, Department of Nutrition and Dietetics, Hacettepe University, Ankara, Turkey; G Vansant, Faculty of Medicine, Catholic University Leuven, Leuven, Belgium; MD Vaz de Almeida, Faculty of Nutrition and Food Sciences, University of Porto, Porto, Portugal; M Raats, Faculty of Arts and Human Sciences, University of Surrey, Surrey, UK; M Gurinovic, Institute for Medical Research, University of Belgrade, Belgrade, Serbia; R Endevelt, Maccabi Health Services, Tel Aviv, Israel; A Astrup, Department of Human Nutrition, Faculty of Life Sciences, University of Copenhagen, Frederiksberg, Denmark; V Somoza, Research Platform for Molecular Food Science, University of Vienna, Vienna, Austria; JC Seidell, Department of Health Sciences, VU University Amsterdam, Amsterdam, The Netherlands.
Supplementary Material
References
- Arambepola C, Scarborough P, Rayner M. Validating a nutrient profile model. Public Health Nutr. 2008;11:371–378. doi: 10.1017/S1368980007000377. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azais-Braesco V, Goffi C, Labouze E. Nutrient profiling: comparison and critical analysis of existing systems. Public Health Nutr. 2006;9:613–622. doi: 10.1079/phn2006966. [DOI] [PubMed] [Google Scholar]
- Codex General Standard for Fruit Juices and Nectars 2005Codex Stan247-2005.
- Cummings JH, Mann JI, Nishida C, Voster HH. Dietary fibre: an agreed definition. Lancet. 2009;373:365–366. doi: 10.1016/S0140-6736(09)60117-3. [DOI] [PubMed] [Google Scholar]
- Danish Institute for Food and Veterinary Research, Food Informatics, Department of Nutrition 2005. Danish Food Composition Databank 2005, revision 6.0.
- Dötsch-Klerk M, Jansen L. The Choices programme: a simple front-of-pack stamp making healthy choices easy. Asia Pac J Clin Nutr. 2008;17:383–386. [PubMed] [Google Scholar]
- Drewnowski A, Fulgoni V., III Nutrient profiling of foods: creating a nutrient-rich food index. Nutr Rev. 2008;66:23–39. doi: 10.1111/j.1753-4887.2007.00003.x. [DOI] [PubMed] [Google Scholar]
- Elmadfa I, Kornsteiner M. Fats and fatty acid requirements for adults. Ann Nutr Metab. 2009;55:56–75. doi: 10.1159/000228996. [DOI] [PubMed] [Google Scholar]
- EU Regulation (EC) No. 1924/2006 of the European Parliament and of the council of 20 December 2006 on nutrition and health claims made on foods. Off J Eur Union. 2007;12:3–15. [Google Scholar]
- Euromonitor International 2009Trade sources/national statisticsAvailable at: http://www.euromonitor.com/ research_methodology.aspx (last accessed October 2009).
- European Food Safety Agency (EFSA) EU Limited Food Basket of Representative Food Items for the Evaluation of the Impact of Nutrient Profiles on the Eligibility of Food to Claims. EFSA: Parma, Italy; 2008. [Google Scholar]
- Feunekes GIJ, Gortemaker IA, Willems AA, Lion R. Front-of-pack nutrition labelling: testing effectiveness of different nutrition labelling formats front-of-pack in four European countries. Appetite. 2008;50:57–70. doi: 10.1016/j.appet.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Food Standards Australia New Zealand (FSANZ) 2007Consultation Paper proposal P293 nutrition, health and related claims (2007)Available at: http://www.foodstandards.gov.au/_srcfiles/ P293_Consultation_Paper.pdf (last accessed March 2010).
- Foods Standards Agency 2002McCance and Widdowson's the Composition of Foods: Summary Edition6th edn.Food Standards Agency: London [Google Scholar]
- Food Standards Agency 2005Effects of reducing salt in processed food on the population's salt intake—the salt modelAvailable at:http://www.food.gov.uk/healthiereating/salt/ saltmodel (last accessed October 2009).
- Food Standards Agency 2009Front-of-pack (FOP) nutrition labelling policy reviewAvailable at: http://www.food.gov.uk/foodlabelling/signposting/ policyreview/(last accessed March 2009).
- Joint WHO/FAO consultation 2003Diet, nutrition, and the prevention of chronic diseasesJoint WHO/FAO consultation: Geneva. Technical Report Series No. 916. [PubMed]
- Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition 2008. Interim Summary of Conclusions and Dietary Recommendations on Total Fat & Fatty Acids, Geneva.
- Joint FAO/WHO/UNU Expert Consultation 2001Human energy requirementsFAO: Rome. Food and Nutrition Technical Report Series.
- Katz DL, Njike VY, Rhee LO, Reinhold A, Ayoop KT. Performance characteristics of NuVal and the Overall Nutritional Quality Index (ONQI) Am J Clin Nutr. 2010;91:1102S–1108S. doi: 10.3945/ajcn.2010.28450E. [DOI] [PubMed] [Google Scholar]
- Livsmedelsverket. National Food Administration 2009The Keyhole SymbolAvailable at: http://www.slv.se/en-gb/Group1/Food-and-Nutrition/ Keyhole-symbol/(last accessed 4 January 2011).
- Lupton JR, Balentine DA, Black RM, Hildwine R, Ivens BJ, Kennedy ET, et al. The Smart Choices front-of package nutrition labelling program: rationale and development of the nutrition criteria. Am J Clin Nutr. 2010;91:1078S–1089S. doi: 10.3945/ajcn.2010.28450B. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Food and Nutrition Institute (IZZ) 2005Polish Food Composition Data Base Tables of Nutritional Value of Food Products and Dishes3rd edition extended and updated.IZZ: Warsaw [Google Scholar]
- Nestle M, Ludwig DS. Front-of-package food labels. JAMA. 2010;303:771–772. doi: 10.1001/jama.2010.179. [DOI] [PubMed] [Google Scholar]
- Nijman CAJ, Zijp IM, Sierksma A, Roodenburg AJC, Leenen R, Van den Kerkhoff C, et al. A method to improve the nutritional quality of foods and beverages based on dietary recommendations. Eur J Clin Nutr. 2007;61:461–471. doi: 10.1038/sj.ejcn.1602548. [DOI] [PubMed] [Google Scholar]
- Odegaard AO, Koh WP, Arakawa K, Yu MC, Pereira MA. Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes: the Singapore Chinese Health Study. Am J Epidemiol. 2010;171:701–708. doi: 10.1093/aje/kwp452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe DA, Fulcher RG.2008Biochemistry and compartmentalization of cereal grain components and their functional relationship to mammalian healthIn: Marquant L, Jacobs DR (eds).Whole Grains and Health Blackwell Publishing: Ames; 89–165. [Google Scholar]
- Popkin BM, Armstrong LE, Bray GM, Caballero B, Frei B, Willett WC. A new proposed guidance system for beverage consumption in the United States. Am J Clin Nutr. 2006;83:529–542. doi: 10.1093/ajcn.83.3.529. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The World is Fat: The Fads, Trends, Policies, and Products that are Fattening the Human Race. Avery-Penguin Group: New York; 2008. [Google Scholar]
- Rivera JA, Rosas-Peralta M, Aguilar-Salinas CA, Popkin BM, Willett WC. Consumo de bebidas y prevención de la obesidad. Salud Publ Mex. 2008;50:173–195. doi: 10.1590/s0036-36342008000200011. [DOI] [PubMed] [Google Scholar]
- Rayner M, Scarborough P, Stockley L.2005Nutrient profiles: applicability of currently proposed model for uses in relation to promotion of food to children aged 5–10 and adults(Monograph online). Available at: http://www.food.gov.uk/multimedia/pdfs/ nutprofmodelforadults.pdf(last accessed October 2009).
- Reardon T, Timmer CP, Barrett CB, Berdegue JA. The rise of supermarkets in Africa, Asia, and Latin America. Am J Agric Econ. 2003;85:1140–1146. [Google Scholar]
- Rijksadministratief Centrum 2004. Belgische Voedingsmiddelentabel Nubel: Brussel.
- Roodenburg AJC, Temme EHM, Howell Davies O, Seidell JC. Potential impact of the Choices Programme on nutrient intakes of the Dutch population. Nutr Bull. 2009;34:318–323. [Google Scholar]
- Sacks G, Rayner M., Swinburn BM. Impact of front-of-pack ‘traffic-light' nutrition labelling on consumer food purchases in the UK. Health Promot Int. 2009;24:344–352. doi: 10.1093/heapro/dap032. [DOI] [PubMed] [Google Scholar]
- Sanigorski AM, Bell AC, Swinburn BM. Association of key foods and beverages with obesity in Australian schoolchildren. Public Health Nutr. 2007;10:152–157. doi: 10.1017/S1368980007246634. [DOI] [PubMed] [Google Scholar]
- Scarborough P, Arambepola C, Kaur A, Bhatnagar P, Rayner M. Should nutrient profile models be ‘category specific' or ‘across-the board'? A comparison of the two systems using diets of British adults. EurJ Clin Nutr. 2010;64:553–560. doi: 10.1038/ejcn.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough P, Boxer A, Rayner M, Stockley L. Developing nutrient profile models: a systematic approach. Public Health Nutr. 2007;10:330–336. doi: 10.1017/S1368980007223870. [DOI] [PubMed] [Google Scholar]
- Strazullo P, Kandala NB, Cappuccio FP. Salt intake, stroke and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichting Nederlands Voedingsstoffenbestand . NEVO Food Composition Database. Voedingscentrum: Den Haag; 2006. [Google Scholar]
- Stockley L, Rayner M, Kaur A.2007Nutrient profiles for use in relation to food promotion and children's diet: update of 2004 reviewAvailable at: http://www.food.gov.uk/healthiereating/advertisingtochildren /nutlab/nutprofilereview/ nutprofilelitupdatedec07 (last accessed October 2009).
- Sutherland LA, Kaley LA, Fischer L. Guiding stars: the effect of a nutrition navigation program on consumer purchases at the supermarket. Am J Clin Nutr. 2010;91:1090S–1094S. doi: 10.3945/ajcn.2010.28450C. [DOI] [PubMed] [Google Scholar]
- The National Heart Foundation of New Zealand 2002Pick the Tick The Food Information Program. Guidelines for Tick Approval. The National Heart Foundation of New ZealandAvailable at: http://www.heartfoundation.org.nz (last accessed 15 October 2009).
- Towsend M. Where is the science? What will it take to show that nutrient profiling systems work. Am J Clin Nutr. 2010;91:1109S–1115S. doi: 10.3945/ajcn.2010.28450F. [DOI] [PubMed] [Google Scholar]
- Trichterborn J, Harzer G. An industry perspective on nutrition profiling in the European environment of public health and nutrition. Nutr Bull. 2007;32:295–302. [Google Scholar]
- Van Raaij J, Hendriksen M, Verhagen H. Potential for improvement of population diet through reformulation of commonly eaten foods. Public Health Nutr. 2008;12:1–6. doi: 10.1017/S1368980008003376. [DOI] [PubMed] [Google Scholar]
- Vartanian LM, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97:667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyth EL, Steenhuis IHM, Heymans MW, Roodenburg AJC, Brug J, Seidell JC. Influence of placement of a nutrition logo on cafeteria menu items on lunchtime food choices at Dutch work sites. J Am Diet Assoc. 2011;111:131–136. doi: 10.1016/j.jada.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Vyth EL, Steenhuis IHM, Mallant SF, Mol ZL, Brug J, Temminghof M, et al. A front-of-pack nutrition logo: a quantitative and qualitative process evaluation in the Netherlands. J Health Commun. 2009;14:631–645. doi: 10.1080/10810730903204247. [DOI] [PubMed] [Google Scholar]
- Vyth EL, Steenhuis IHM, Roodenburg AJC, Brug J, Seidell JC.2010bFront-of-pack nutrition label stimulates healthier product development: a quantitative analysis Int J Behav Nutr Phys Act 765 http://www.ijbnpa.org/content/7/1/65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyth EL, Steenhuis IHM, Vlot JA, Wulp A, Hogenes MG, et al. Actual use of a front-of-pack nutrition logo in the supermarket: consumers' motives in food choice. Public Health Nutr. 2010a;13:1882–1889. doi: 10.1017/S1368980010000637. [DOI] [PubMed] [Google Scholar]
- Wartella EAL, Lichtenstein AH, Boon CS.2010Examination of Front-of-Package Nutrition Rating Systems and Symbols: Phase 1 ReportCommittee on Examination of Front-of-Package Nutrition Ratings Systems and Symbols; Institute of Medicine.National Academy Press: Washington, DC; [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.