Abstract
Mesenchymal stem cell (MSC) therapy has demonstrated applications in vascular regenerative medicine. Although blood vessels exist in a mechanically dynamic environment, there has been no rigorous, systematic analysis of mechanical stimulation on stem cell differentiation. We hypothesize that mechanical stimuli, relevant to the vasculature, can differentiate MSCs toward smooth muscle (SMCs) and endothelial cells (ECs). This was tested using a unique experimental platform to differentially apply various mechanical stimuli in parallel. Three forces, cyclic stretch, cyclic pressure, and laminar shear stress, were applied independently to mimic several vascular physiologic conditions. Experiments were conducted using subconfluent MSCs for 5 days and demonstrated significant effects on morphology and proliferation depending upon the type, magnitude, frequency, and duration of applied stimulation. We have defined thresholds of cyclic stretch that potentiate SMC protein expression, but did not find EC protein expression under any condition tested. However, a second set of experiments performed at confluence and aimed to elicit the temporal gene expression response of a select magnitude of each stimulus revealed that EC gene expression can be increased with cyclic pressure and shear stress in a cell-contact-dependent manner. Further, these MSCs also appear to express genes from multiple lineages simultaneously which may warrant further investigation into post-transcriptional mechanisms for controlling protein expression. To our knowledge, this is the first systematic examination of the effects of mechanical stimulation on MSCs and has implications for the understanding of stem cell biology, as well as potential bioreactor designs for tissue engineering and cell therapy applications.
Keywords: Mechanobiology, Cyclic stretch, Cyclic pressure, Shear stress, Immunohistochemistry, PCR, Morphology, Proliferation, Mesenchymal stem cells, Vascular differentiation
1 Introduction
Although it is well known that the mechanical environment is important for maintaining the phenotype and functionality of terminally differentiated cells (Chien 2006; Resnick and Gimbrone 1995; Skalak et al. 1998), only recently has attention been given to the role that mechanical forces play in the differentiation of stem cells. Two studies (Hamilton et al. 2004; Park et al. 2004) were the first to demonstrate that cyclic uniaxial stretch was a driving factor in the differentiation of bone marrow-derived mesenchymal stem cells (MSCs) toward smooth muscle cells (SMCs). This work has been followed by a few studies, using 2-D and 3-D cultures, that have also demonstrated some degree of vascular cell (SMC and/or endothelial cell) differentiation from stem cells as a result of tension and cyclic stretch (Kurpinski et al. 2006b; Nieponice et al. 2006), shear stress and pressure (Kobayashi et al. 2004), or combined shear, stretch, and pressure (O’Cearbhaill et al. 2008). Other studies have demonstrated that osteogenic differentiation can result from mechanical stimuli (Li et al. 2004; Kreke et al. 2005; Scaglione et al. 2007).
While previous work indicates that mechanical stimulation in general guides MSC differentiation, these studies have predominantly been focused on SMCs, osteoblasts, and chondrocytes with little attention toward endothelial cells (ECs). In addition, there is a lack of studies utilizing cyclic pressure, even though this stimulus is physiologically relevant to the cardiovascular system and is an important stimulus for regulating the proliferation and functions both ECs and SMCs (Millgard and Lind 1998; Wolinsky 1970; Intengan and Schiffrin 2001). Moreover, the previous studies utilized a limited range of magnitudes and did not investigate the effects of frequency of dynamic mechanical stimuli, despite the wide range of both that are present from early development to maturity throughout the vasculature. These factors may be important to MSC differentiation, as they have been shown to influence other cell types (Nagatomi et al. 2001; Elder et al. 2001; Seliktar et al. 2000; Stegemann and Nerem 2003).
These previous studies have led us to the hypothesis that mechanical stimuli relevant to the vasculature will lead to MSC differentiation toward SMCs and ECs. We address this hypothesis here by utilizing a unique experimental design that allows evaluation of the differential effects of MSCs to cyclic stretching, cyclic pressure, and shear stress.
2 Materials and methods
2.1 Cell source
MSCs harvested from the bone marrow of Lewis rats (Javazon et al. 2001) were obtained from the Tulane Center for Gene Therapy (TCGT) under a material transfer agreement. Each lot of cells obtained from the TCGT was derived from a single rat, and two separate lots of cells were used to complete these studies. The MSCs were expanded in alpha modified Eagle’s media (α-MEM, Invitrogen, Carlsbad, CA) supplemented with pre-screened 20% fetal bovine serum (FBS, Atlanta Biologicals, Atlanta, GA), 1% antibiotic/an-timicotic (Invitrogen), and 10mM L-glutamine (Invitrogen). The multipotentiality of the MSCs obtained from the TCGT was confirmed with 2week culture in expansion media supplemented with osteogenic or adipogenic biochemical agents (Pittenger et al. 1999; Hamilton et al. 2004) and assayed with the histochemical stains Alizarin Red (Sigma, St. Louis, MS) and Oil Red O (Sigma), respectively.
2.2 Mechanical stimulation
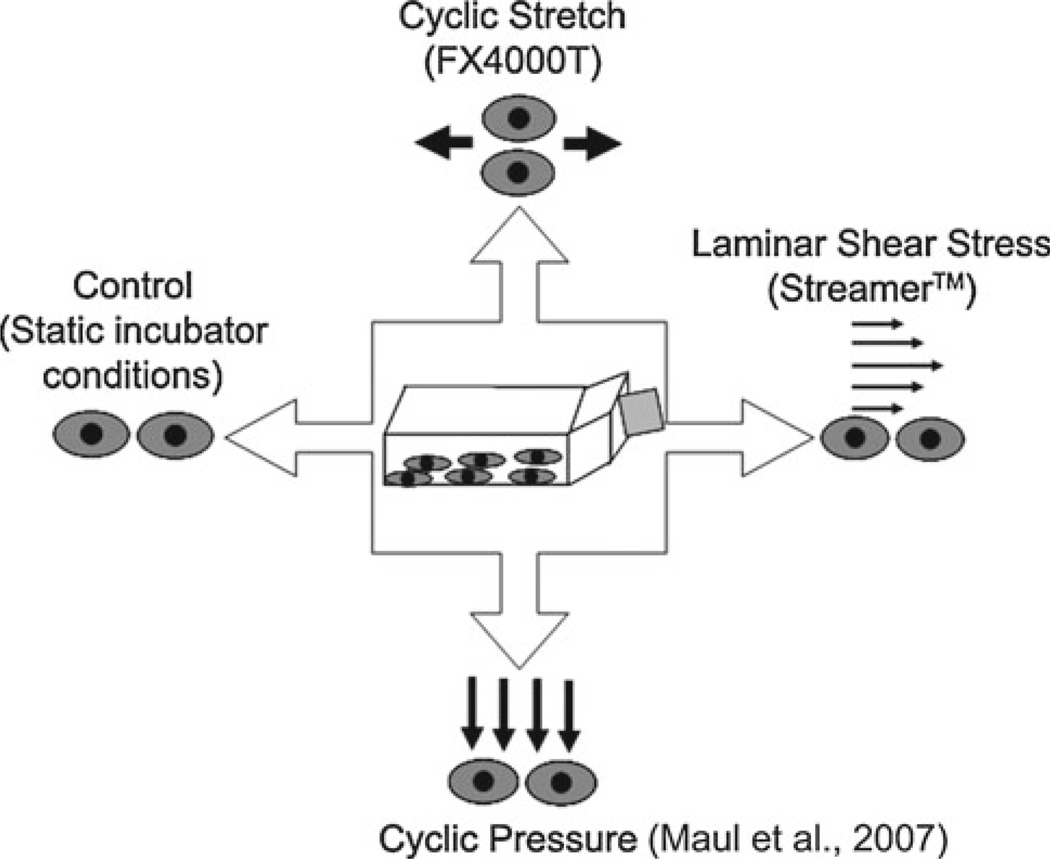
In order to rigorously and systematically study the differential effects of the three mechanical stimuli relevant to the cardiovascular system, a unique experimental protocol was developed (Fig. 1). This “Mechanical Panel” protocol permits the simultaneous exposure of a single population of cells (e.g., one T175 flask of MSCs) to cyclic stretch (CS), cyclic hydrostatic pressure (CP), and laminar shear stress (LSS) in an independent and parallel manner. This experimental design reduces the biological variability inherent in using a heterogeneous cell type by using the same population of cells in each of the mechanical systems and permits the paired comparison between each stimulus as well as the static control.
Fig. 1.
Mechanical stimulation is applied individually in parallel from a single population of cells to determine a differential response to each of the stimuli
In the first set of experiments designed to examine the effects of magnitude and frequency of applied stimuli, MSCs were seeded at 200 cells/cm2 on collagen type I-coated BioFlex™ (Flexcell International, Hillsborough, NC) deformable substrates for CS and control conditions or collagen type I-coated Culture Slips® (Flexcell) for LSS and CP conditions. The magnitudes and frequencies utilized for these experiments were chosen to be consistent with the magnitudes and frequencies found in the vasculature (Oluwole etal. 1997; Whaley and Wong 1999) and are listed in Table 1. A low seeding density was used to isolate the effects of the mechanical stimuli from other signaling mechanisms, such as cell-cell contacts, which can impact differentiation (Jaiswal et al. 1997). Forty-eight hours after seeding, the MSCs were loaded into either the FX-4000T cyclic tension device (Flexcell), Streamer shear stress device (Flexcell), a custom-built cyclic pressure system (Maul et al. 2007), or static incubator conditions and exposed to these environments for 5 days. For the Flexcell cyclic tension device, a sinusoidal waveform and arctangle loading posts were used to apply CS in a uniaxial fashion, while the solenoid valve driving the CP system generated a sawtooth waveform (Maul et al. 2007).
Table 1.
Applied stimuli for Mechanical Panel experiments to determine the dose–response of MSCs to different magnitudes and frequencies of mechanical stimulation
| Stimulus | Neonatal/sub-physiologic stimulus | Hypotensive stimulus | Normotensive stimulus | Hypertensive stimulus |
|---|---|---|---|---|
| Cyclic stretch | 1%, 2.75 Hz (CS-1HF) | 1%, 1 Hz (CS-1) | 5%, 1 Hz (CS-5) | 10%, 1 Hz (CS-10) |
| Cyclic pressure | 90/70mmHg, 2.75 Hz (CP-90HF) |
90/70mmHg, 1 Hz (CP-90) |
120/80mmHg, 1 Hz (CP-120) |
180/140 mmHg, 1 Hz (CP-180) |
| Shear stress | 1 dyne/cm2 (LSS-1) | 5 dynes/cm2 (LSS-5) | 10 dynes/cm2 (LSS-10) | 20 dynes/cm2 (LSS-20) |
| Control | Static incubator conditions (37°C, 5% CO2, 95% humidity) | |||
Neonatal frequency and blood pressure values were determined form normal newborn heart rate and blood pressure values found in Whaley and Wong 1999. Stretch and shear magnitudes were derived from an excellent review by Oluwole et al. 1997. Parenthetical terms denote abbreviations for each of the stimuli. For example, CS-1 denotes 1% 1 Hz cyclic stretch, and LSS-1 denotes 1 dyne/cm2 laminar shear stress. CS-1 HF denotes cyclic stretch at 1% at 2.75 Hz stimulation (high frequency)
In the second set of experiments, aimed at determining the temporal analysis of gene expression, MSCs were seeded at confluence (21,000 cells/cm2) on collagen type I-coated BioFlex™ (Flexcell) deformable substrates for stretch and control conditions or collagen type I-coated Culture Slips™ (Flexcell) for shear and pressure conditions. Forty-eight hours after seeding, the MSCs were loaded into each of the mechanical stimulation systems comprising the Mechanical Panel. The stimuli used were 10% cyclic stretch at 1Hz (CS-10), 120/80mmHg cyclic pressure at 1Hz (CP-120), and 20 dynes/cm2 laminar shear stress (LSS-20) and were chosen based upon the response of the MSCs during the 5-day experiments (see above) and from other related research on mechanobiology (Hamilton et al. 2004; Sumpio et al. 1994; Sato and Ohashi 2005; Wang et al. 2005). A confluent culture was used to generate enough genetic material for PCR analysis after 24 and 72 h of stimulation.
2.3 Histologic assessment of differentiation
The presence of bone-forming nodules was used to denote osteoblast differentiation and was assessed using Alizarin Red dye (Sigma, 40mM, pH 4.2), which stains calcium phosphate deposits. Accumulation of lipid droplets was used to denote adipocyte differentiation and was determined by incubating paraformaldehyde fixed MSCs with 60% isopropanol, followed by freshly prepared Oil Red O solution (Sigma, 0.3% in isopropanol mixed 3:2 with deionized water). The cells were counterstained with Harris-Hematoxylin (Sigma) for 1 minute followed by a tap water rinse. All samples were then digitally photographed (CoolPix, Nikon) at 100× on an inverted phase-contrast microscope (TS100, Nikon).
2.4 Assessment of morphology and cell density
At the termination of each 5-day experiment, samples were fixed with 4% paraformaldehyde, stained with Coomassie Brilliant Blue R-250 (Pierce, Rockford, IL) total protein stain (to enhance contrast) and digitally photographed (Cool-Pix, Nikon) under 100x magnification on an inverted phase-contrast microscope (Nikon). Cellular area, perimeter, major axis, minor axis, and angle of orientation were measured according to published techniques (Hamilton et al. 2004) using Scion Image (Scion Corp, Frederick, MD). Cell size is presented as the normalized cellular area, which was calculated by dividing the averaged cellular area for ten fields of view by the control values). In addition, the shape index (SI) was calculated for each cell in the field of view. The value of SI is 1 for cells that are perfect circles and approaches 0 for cells that are spindle shaped (Nerem et al. 1981; Kataoka et al. 1998). The shape index was averaged for all cells in ten random fields of view and normalized by subtraction of the average shape index for the control cells in each experiment. Negative values of the shape index relate to cells that are more spindle shaped than the controls, while positive values relate to cells that are more round than the controls. To quantify changes in cell density, the average number of nuclei (stained with Hoechst dye) from ten random fields of view was normalized by the average nuclei count from each control.
2.5 Immunohistochemistry (IHC)
To determine MSC differentiation towards vascular EC and SMC lineages, samples were fixed with 4% paraformalde-hyde for 5min and washed 3 times with 1X PBS. EC and SMC differentiation was assessed with IHC using standard techniques. Briefly, fixed samples were permeabilized with 0.1% Triton-X 100 and stained with primary and secondary antibodies (see Online Resource Table S1) diluted in PBG buffer (0.5% w/v BSA (Fraction V, Sigma), 0.15% w/v glycine (Sigma) in PBS). Non-specific binding of antibodies was blocked by incubation for 45min with 5% w/v BSA in PBS followed by 5 washes with PBG buffer. DAPI (Invitro-gen) was utilized for nuclear visualization. A negative control (absence of primary antibody) was prepared each time antibody staining occurred. All steps described for IHC staining occurred at room temperature.
2.6 Gene expression
Following stimulation in the Mechanical Panel, samples were processed for RNA isolation based upon the single-step RNA isolation method developed by Chomczynski et al. (Chomczynski and Sacchi 1987). For 5-day experiments, three of the six BioFlex wells and Culture Slips from each system were utilized for RNA isolation, whereas all BioFlex wells and Culture Slips were utilized in the temporal studies (24 and 72 h). Briefly, cell lysis and RNA isolation were performed with Trizol™ (Invitrogen) immediately following the withdrawal of each stimulus. The isolated RNA was purified using an RNeasy Kit (Qiagen, Valencia, CA) according to the manufacturer’s specifications. RNA quantity was assessed using a NanoDrop Spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington, DE), and RNA quality was assessed with a BioAnalyzer (Model 2100, Agilent Technologies). RNA samples attaining an RIN value of greater than 8 were selected for Real-Time PCR (RT-PCR).
Gene expression analysis was performed for our chosen end points using a validated TaqMan® low-density gene expression assay within a microfluidics card (Applied Biosystems, Foster City, CA) on a 7900 HT Fast Real-Time PCR System (Applied Biosystems). The gene names, phenotypic classifications, and Applied Biosystems catalog numbers for the custom-designed microfluidics card are listed in Online Resource Table S2. The data were analyzed by the ΔΔCT method with SDS Software (Version 2.0, Applied Biosystems) (Livak and Schmittgen 2001). Each sample was normalized to the most stable endogenous control gene (Gusb, systematically selected from 15 possible genes by analyzing samples from each of the possible magnitudes, frequencies, and stimuli types on an Endogenous Control array (Applied Biosystems) using GeNORM (Fernandes et al. 2008); data not shown), and a relative quantitation (RQ) analysis was then performed. The RQ values for each experiment were averaged to provide the mean change in gene expression compared to controls. Changes in gene expression greater than 30% of the control values were considered biologically relevant as previously described (Johnson et al. 2007; Hammond et al. 2005). Genes were grouped according to their classifications (e.g., osteoblast, SMC, EC) to help detect trends in related end points.
2.7 Statistical analysis
Morphologic measurements and cell densities were analyzed with SPSS (v.13, SPSS Inc., Chicago, IL) and are presented as the average ± standard error of the mean. The data were categorized according to each stimulus (control, CS, CP, or LSS). Paired t-tests were then used to make all comparisons between each stimulus with α = 0.05. Next one-way analysis of variance (ANOVA) was used to compare differences within each stimulus (CS, CP, or LSS) at each of the four different magnitudes and/or frequencies of stimulation. Forpost hoc testing, the Fisher’s least significant difference (LSD) was used to compare between groups with homogenous variance since all comparisons were proposed a priori. Data with unequal variance were compared using the Games-Howell test because of its utility with small sample sizes (n < 6) (Keppel and Wickens 2004). In addition to comparisons of means between magnitudes and frequencies of stimulation, the Spearman rank-correlation was used to determine possible relationships between the measured values (e.g., cell area, shape index, and cell density) and the frequency and magnitudes of stimulation.
Because the data from the measurement of cellular orientation were in degrees, circular statistics were used to analyze the distribution of the cellular orientation (Fisher 1993). For such data, a uniform distribution around the circumference of a circle was assumed to be the true population distribution and compared to each experimental condition using a modified Rayleigh statistic as described by Moore (1980). For graphical purposes, a linear histogram was used, with the measured angle for each cell being placed in one of eighteen bins between 0° and 180°, with a bin width of 10°.
For gene expression data, the threshold value for each gene was calculated, normalized against the endogenous control gene, and then normalized to the control, thus generating an RQ values for each mechanical stimulus (CP, CS, and LSS) and RQ= 1 for the control values. These RQ values were stored in a custom-built database (Microsoft Access 2003, Microsoft Corporation) and exported to SPSS software for statistical analysis. For comparisons to the control, a one-sample t-test was performed against μ = 1, with α = 0.05. For comparisons between stimuli and time points, one-way ANOVA with post hoc LSD testing at α = 0.05 was used to detect significant differences. To give some global sense to the data, a change index (CI) was calculated by averaging a score of 0 for no change, ±1 for a biologically relevant (>30% change) OR statistical (p < 0.1) trend, and ±2 for a biologically relevant (>30% change) AND statistically significant (p < 0.05) change for each of the genes in that category. Double arrows were used to indicate a majority increase or decrease (CI > 0.70) in the overall gene expression for that group from control values. Single arrows were used to denote a moderate change (0.25 ≤ CI ≤ 0.70) in the majority of the genes for a particular phenotype, and horizontal arrows to indicate very little change (CI < 0.25).
3 Results
3.1 Osteogenic and adipogenic differentiation
Representative images for MSCs exposed to defined chemical media (see Online Resource Fig. S1) demonstrate that osteogenic and adipogenic differentiation occurred upon exposure to the defined chemical media and were multi-potent. No significant adipogenic or osteogenic differentiation occurred for any condition in the Mechanical Panel (see Online Resource Fig. S2). Although a few cells in the LSS-20 did show some adipogenic staining, comparison with the chemically induced cells demonstrates that this is well below what could be considered full differentiation. However, while this was the only condition where this phenomenon occurred, it may warrant future investigation.
3.2 Morphological changes as a result of mechanical stimulation
Qualitative assessment of Online Resource Fig. S3 clearly demonstrates that mechanical stimulation alters the MSC size, shape, and alignment. These observations were confirmed through a quantitative morphometric analysis.
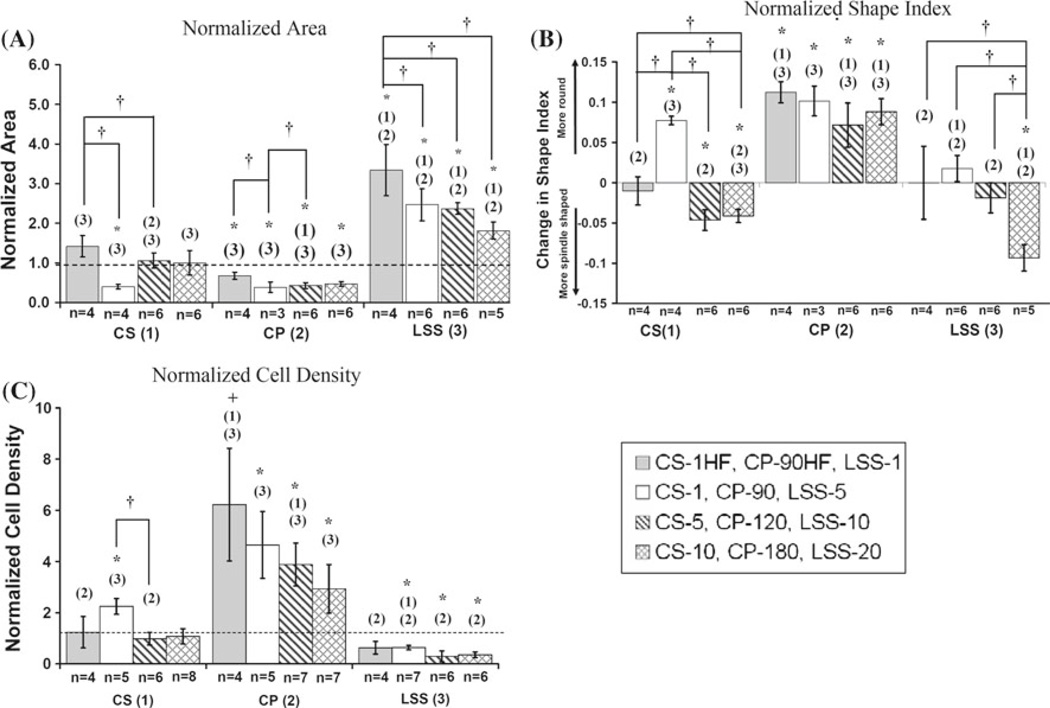
Cell size was significantly elevated for MSCs exposed to all magnitudes of LSS (Fig. 2a) and negatively correlated with LSS magnitude (ρ= −0.547, p < 0.05). There was a significant decrease in cellular area for MSCs exposed to all magnitudes and frequencies of CP when compared to controls. Within CP, the normalized area for CP-90HF was significantly larger than that for CP-90 and CP-120 and markedly larger (p = 0.06) than for CP-180. Within CS, the normalized area for CS-1 was significantly smaller than controls, CS-1HF, and CS-5. When comparing CS across the same frequency, there appeared to be a threshold effect but no significant correlation as a dose-response. Comparisons across stimuli indicate that MSCs exposed to CS or CP were significantly smaller in size than LSS.
Fig. 2.
a Average area measurements normalized by controls. b Changes in shape index relative to controls. c Cell density normalized by controls. The dashed line represents control values. All data are presented as mean +/− SEM. * denotes p < 0.05 compared to controls. + denotes p < 0.1 compared to controls. † denotes p < 0.05 for comparison of means within each stimulus. (1), (2), and (3) denote p < 0.05 for comparison of means between stimuli of the corresponding bar pattern where (1) = CS, (2) = CP, and (3) = LSS
The normalized shape index values for the various groups of MSCs are presented in Fig. 2b. The shape index values for LSS-1, LSS-5, and LSS-10 were not significantly different from controls. However, the shape index of LSS-20 was significantly smaller than the control, indicating that the MSCs were becoming more spindle shaped at this higher shear stress. Within the LSS stimulus, LSS-20 was significantly more spindle shaped than LSS10, LSS-5, and LSS-1. LSS demonstrated a strong dose-response (ρ= −0.792, p < 0.001), with increasing shear stress leading to amore spindle-shaped cell. The shape index values for all CP demonstrated a more rounded phenotype compared to controls, but within CP there was no differences between the various magnitudes or frequencies. The shape index values for CS-5 and CS-10 were significantly smaller compared to controls, indicating a more spindle shape. However, the shape index value for CS-1 was significantly higher than controls, indicating that the MSCs were more rounded. Within CS, all values were significantly different from each other, with the exception of CS-5 and CS-10. The shape index also showed a strong correlation to the magnitude (ρ = −0.639, p < 0.01) and frequency (ρ = −0.873, p < 0.01) of CS.
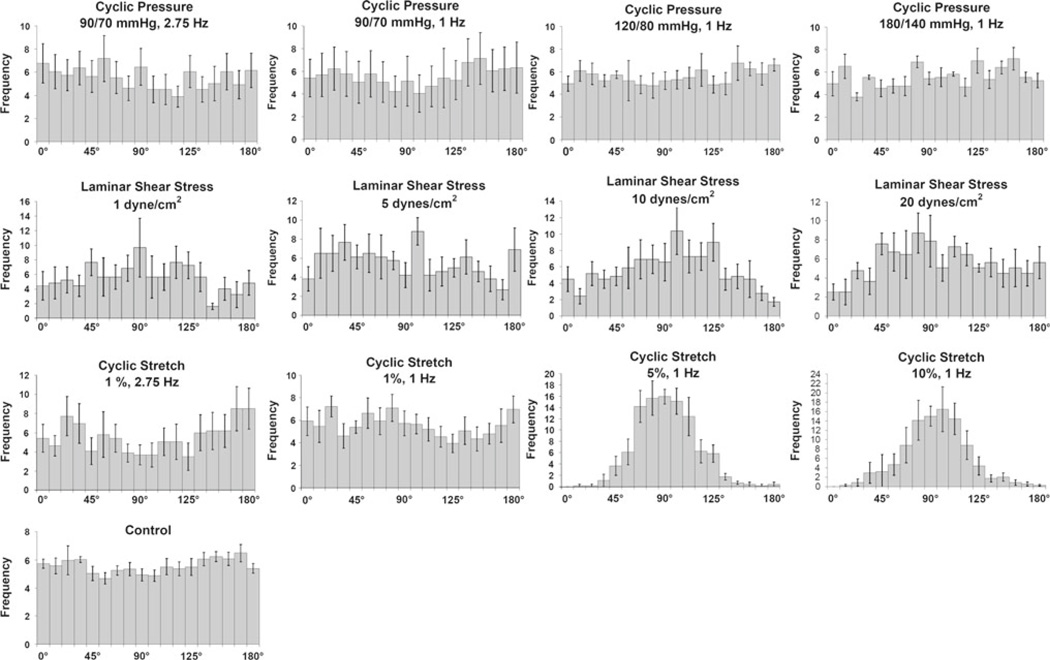
The orientation of MSCs was found to be random for all CP as well as for LSS-1 and LSS-5 (Fig. 3). However, LSS-10 and LSS-20 were markedly orientated parallel to flow. CS also showed a threshold to orientation, with random alignment at CS-1HF and CS-1, and alignment perpendicular to the stretch direction at CS-5 and CS-10.
Fig. 3.
Normalized histograms for cellular orientation in MSCs presented as the mean +/− SEM. Non-parametric analysis against a uniform distribution resulted in no statistical significant differences with any of the cyclic pressure regimens, which is in agreement with the normalized histogram for the control. No statistical difference was found for LSS at 1 dyne/cm2 and 5 dynes/cm2. However, LSS at 10 dynes/cm2 and 20 dynes/cm2, a preferred orientation begins to develop around 90°, which is in the direction of flow. CS at 1% 2.75 Hz and 1 % 1 Hz did not demonstrate any significant changes in alignment. However, higher magnitudes of CS (5% 1 Hz and 10% 1 Hz) demonstrated a significant change from a uniform distribution centered on 90°, which is perpendicular to the direction of stretch
Cell density
The quantitative results of these measurements are depicted in Fig. 2c and can qualitatively be seen in Online Resource Fig. S3. The cell density of MSCs exposed to CS-1HF, CS-5, and CS-10 was unchanged when compared to the controls. However, the cell density for CS-1 was significantly increased from controls. Although there appears to be possible dose- and frequency-responses for MSCs exposed to CS, the Spearman correlation coefficients for magnitude and frequency were non-significant for our data. MSCs exposed to CP-90, CP-120, and CP-180 had significantly increased cell densities compared to the controls. The cell density for CP90HF was elevated over controls, but this was not statistically significant (p = 0.07). There was also a weak, but statistically significant inverse correlation (ρ = −0.483, p < 0.05) between cell density and magnitudes of CP. Contrasted with CP, the cell density for MSCs exposed to LSS-5, LSS-10, and LSS-20 were significantly decreased when compared to controls. Only cell density for LSS-1 was not significantly different from the controls. A weak, but statistically significant decrease (ρ = −0.469, p < 0.05) in cell density was found for increasing magnitudes of LSS.
Comparisons made within each stimulus showed a single statistically significant difference for an increase in cell density for CS-1 compared to CS-5. Comparisons between stimuli revealed increases in cell density for CP compared to both CS and LSS at nearly all levels. The only other significant difference was for CS-1 compared to LSS-5.
3.3 Immunohistochemistry
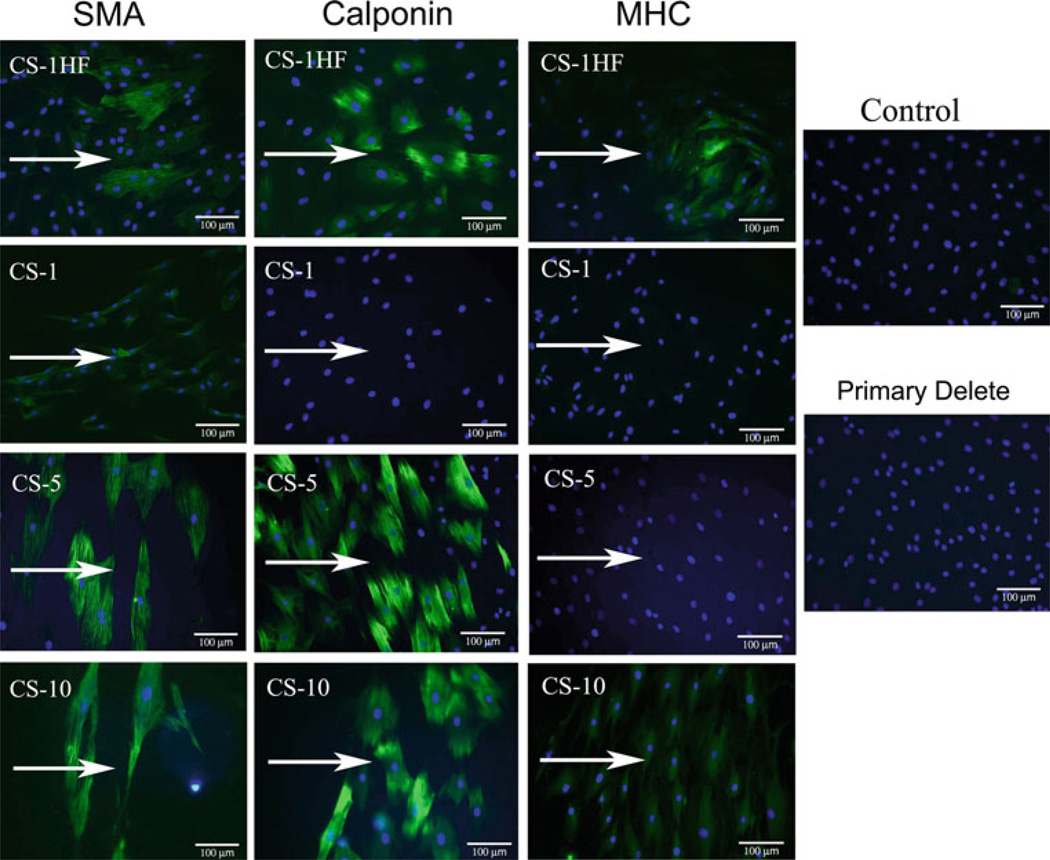
One of the main objectives of this work was to determine what role each of the three mechanical stimuli in the Mechanical Panel have on the differentiation of stem cells toward cardiovascular lineages. Table 2 summarizes the findings and representative images from the entire study (n ≥ 4) for each condition are found in Online Resource Fig. S4–S9. The significant finding of our study was the apparent dose-dependent change in SMC protein expression with exposure to CS (Fig. 4). We found that CS displayed a dose-dependent expression of SMC protein markers at 1 Hz, as well as at higher frequency stimulation, which also resulted in expression of all three markers in a more heterogeneous manner. These increases SMC protein expression coincide with the change in cell shape (see Fig. 2b) toward a more spindle-shaped morphology. Conversely CP, LSS, or control conditions did not induce the expression of any of these SMC-related proteins, despite similar changes in morphology toward a more spindle shape after exposure to LSS-10 or LSS-20. In addition to the SMC protein expression following CS, we also observed that these cells constitutively express flk-1 (see Online Resource Fig. S7), which was maintained under all levels of CS, as well as in CP-180, LSS-10, and LSS-20. Lower magnitudes of CP and LSS resulted in a loss of flk-1 expression. No expression of the EC-specific proteins platelet endothelial cell adhesion molecule (PECAM, see Online Resource Fig. S8) or von Willebrand Factor (vWF, see Online Resource Fig. S9) was found under any condition.
Table 2.
Summary of IHC results for Mechanical Panel experiments
| Stimulus | SMA | Calponin | MHC | Flk-1 | PECAM | vWF |
|---|---|---|---|---|---|---|
| CS-1HF | +/− | +/− | +/− | ++ | – | – |
| CS-1 | + | – | – | ++ | – | – |
| CS-5 | ++ | ++ | – | ++ | – | – |
| CS-10 | ++ | ++ | + | ++ | – | – |
| CP-90HF | – | – | – | – | – | – |
| CP-90 | – | – | – | – | – | – |
| CP-120 | – | – | – | – | – | – |
| CP&-180 | – | – | – | ++ | – | – |
| LSS-1 | – | – | – | – | – | – |
| LSS-5 | – | – | – | – | – | |
| LSS-10 | – | – | – | + | – | – |
| LSS-20 | – | – | – | + | – | – |
| Control | – | – | – | ++ | – | – |
++ indicates strong, homogenous staining intensity. + indicates a moderate to weak homogeneous (>80% positive cells) staining intensity.
+/− indicates heterogeneous staining (approximately 50% of the cells were positive in a given field of view). – indicates no significant (<3% positive cells) staining was evident
Fig. 4.
MSCs exposed to CS expressed SMC proteins with increasing levels of differentiation with increasing magnitudes of stretch at 1 Hz. Increasing the frequency of stimulation also caused local expression of all SMC proteins assayed and may indicate a population-specific frequency response. Arrows indicate the direction of applied stimulation. Note qualitatively the effect of CS on cellular alignment
3.4 Temporal analysis of gene expression
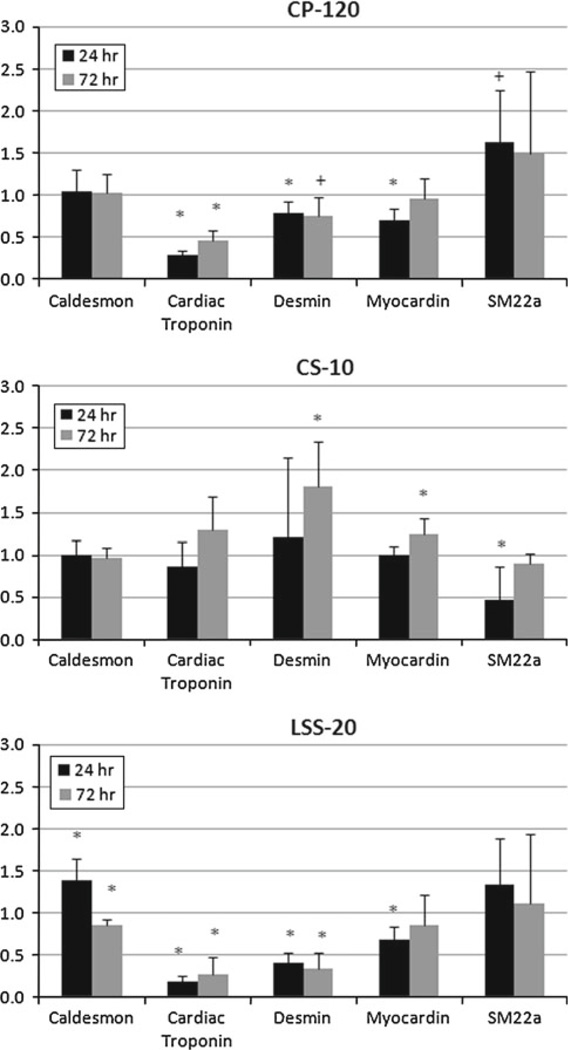
The effect of the Mechanical Panel on the temporal expression of genes from a variety of phenotypes appeared highly promiscuous, with gene expression being detected from a number of lineage-specific markers. The results of the averaged RQ values are presented in Online Resource Tables S3–S8. CS showed more trends toward upregulation than downregulation in the muscle-related genes by 72h (Fig. 5 and Online Resource Table S3). Specific among these were the significant upregulation of desmin and myocardin by 72h. The most notable exception was the expression of SM22α at 24 h, which was significantly downregulated. This contrasted with the response of both CP and LSS, which demonstrated higher expression of SM22α. Further, CP and LSS also significantly inhibited cardiac troponin expression at both time points, whereas CS remained unchanged at the 24 h time point, but was tending toward upregulation by 72 h.
Fig. 5.
Temporal changes in muscle-related gene expression for each component of the Mechanical Panel. CS-10 showed the more conisten trend toward upregulation by 72 h, despite several genes being down-regulated at 24 h. * p < 0.05; + p < 0.10
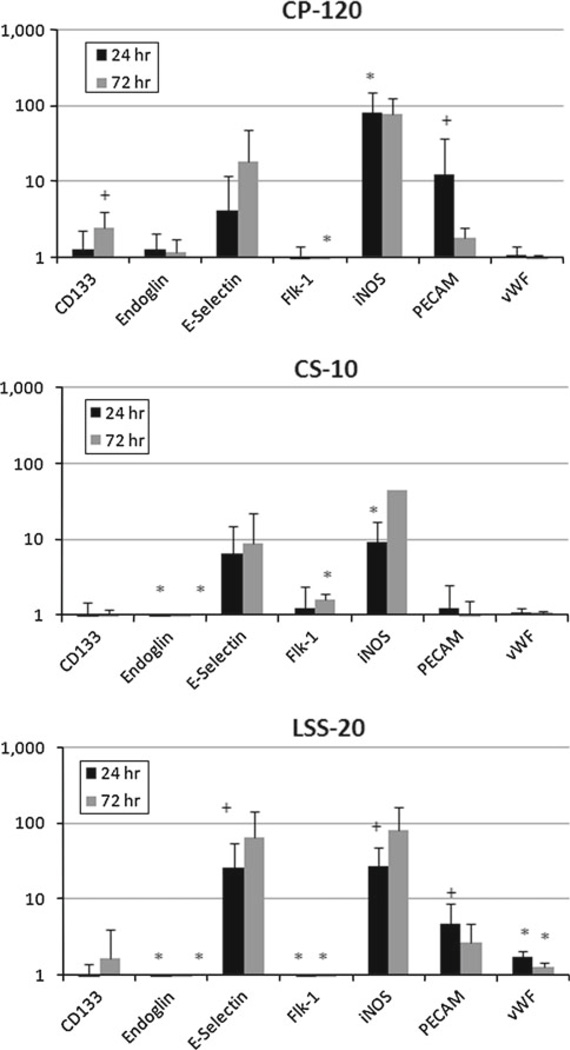
The expression of endothelial-related genes (Fig. 6 and Online resource Table S4) was more significantly affected by CP and LSS. Genes including CD133, von Willebrand Factor, E-selectin, and PECAM exhibited significant increases for at least one time point for CP and LSS. As in the muscle-related gene expression, there was a lone exception—Flk-1—which was significantly downregulated in both CP and LSS, but was significantly upregulated by CS by the 72h time point. Both E-selectin and iNOS were highly variable due to low copy numbers in both the controls and the individual Mechanical Panel components.
Fig. 6.
Temporal changes in endothelial-related gene expression were significantly higher than the muscle-related genes. LSS-20 and CP-120 demonstrated more significant and sustained endothelial-specific genes than CS-10. * p < 0.05; + p < 0.10
Gene expression for all soluble factor-related genes investigated (Online Resource Table S5) was significantly upregulated by LSS at both times. CP was the only other component of the Mechanical Panel which demonstrated transient upregulation of two soluble factor-related genes— FGF-2 and VEGF-A. CS appeared to have minimal impact on the expression of these genes with expression remaining near control levels for both time points.
Similar to the soluble factor-related genes, LSS significantly increased expression for all ECM genes with the exception of collagen IV, which was significantly downregulated at both time points (Online Resource Table S6). CS showed significant upregulation of collagen III and downregulation of both collagen IV and elastin at both time points. In fact, collagen IV was predominantly downregulated for each Mechanical Panel condition at each time point. Twenty-four hours of CP was the only Mechanical Panel component that demonstrated a trend toward upregulation but with high variability. CP also had a stronger effect on elastin gene expression, which was trending toward upregulation at 24h and was significantly upregulated by 72 h.
Similar to both endothelial-related and the soluble factor-related genes, LSS showed a significant increase in osteoblast-related gene expression early on at 24 h for all tested genes except osteocalcin, which was significantly downregulated at 24 and 72h (Online Resource Table S7). The osteo-inductor BMP2 as well as an early matrix protein osteopontin was significantly upregulated for CS at 24 and 72h, while the more mature marker of bone formation, oste-ocalcin, was unchanged. Osteocalcin remained unchanged relative to controls for both CP and CS and was significantly downregulated by LSS at both 24 and 72 h. Alkaline phosphatase expression tended to be increased by mechanical stimulation, with the exception of CS at 24 h, which was significantly downregulated.
Gene expression for chondrocyte- and adipocyte-related markers (Online Resource Table S8) was relatively unremarkable following mechanical stimulation. PPAR-γ and aggrecan expression were similar to control levels for both time points. Only LSS at 72 h showed a significant downregulation of aggrecan. The expression of collagen II, which is used as a marker for mature chondrocyte differentiation, was highly variable due to low copy number and can most likely be thought to be non-expressed under control conditions. Mechanical Panel stimulation appeared to cause some expression, but at extremely low copy numbers, and only CP and CS at 24 h showed statistically significant increases. Finally, cyclin B1 (Online Resource Table S8), which is part of the control mechanism for the G2/M transition in the cell cycle (Ito 2000), was used to assess cell cycle regulation changes that may have been occurring under mechanical stimulation. At 24h, both CP and LSS demonstrated a statistically significant downregulation of cyclin B1. The drop was most severe under LSS and persisted through 72 h, while expression under CP returned toward control values. CS showed no change at 24 h and a significant increase in cyclin B1 expression at 72h.
4 Discussion
The use of our Mechanical Panel experimental design allowed for the differential analysis of the effects of the three dominant mechanical stimuli found in the vasculature on stem cell morphology, growth, and differentiation. We have found that MSCs are sensitive to different types of forces and will respond in a dose-dependent manner to both the magnitude and/or frequency of CS and LSS, but display a relatively constant effect as a result of other forces such as CP. A more detailed discussion of our findings in light of previous work follows.
4.1 Differential morphology and cell density changes
Our results demonstrate that cyclic pressure increased proliferation and resulted in a significantly smaller and more rounded cell. Pressure-induced proliferation has been demonstrated in other cell types including SMCs (Watase et al.1997; Cappadona et al. 1999; Stover and Nagatomi 2007) and ECs (Schwartz et al. 1999; Sumpio et al. 1994) and may therefore represent a potential tool for rapid expansion of cells for tissue engineering or cell therapy.
Cyclic stretch had varying effects on MSCs depending upon the magnitude and frequency of stimulation. The morphologic changes we have reported at higher magnitudes of cyclic stretch (CS-5 and CS-10) are in agreement with previously published data in other stem cells (Hamilton et al. 2004; Lee et al. 2006; Park et al. 2004). The changes in alignment are characteristic of stress-shielding and occur in any cell type that is free to move in two dimensions (Park et al. 2004; Lee et al. 2006; Mills et al. 1997). Alignment was perpendicular to the direction of strain because the Flexcell cyclic tension system employs a fixed boundary for the membrane, resulting a in a true uniaxial stretch. The effect of the higher frequency cyclic stretch eliminated this orientation, which has been explored in more detail elsewhere (De et al. 2007). Unlike previously published studies (Hamilton et al. 2004), CS did not have a negative impact on proliferation, which may be due to the high serum content (20% FBS) used for the culture of these cells and the duration of the Mechanical Panel experiments. Although higher growth factor levels may overwhelm some mechanisms by which the cells respond to mechanical stimuli, we maintained the same serum content during the experiments across the stimuli which allows us to conclude that any changes in cell behavior would be a direct result of the mechanical stimulation. Whether the threshold values of morphological changes or protein and gene expression will be maintained at different serum levels, for the same mechanical stimuli, is a potential area for future exploration and may shed light onto the interplay between growth factor and mechanical stimulation mechanisms.
Like cyclic stretch, laminar shear stress induced dose-dependent changes in size, shape, alignment, and cell density. We are uncertain whether this decrease in cell number was related to an inhibition of proliferation, or positive selection of non-proliferating cells caused by washing away cells attempting to divide. However, there is evidence in the literature showing a decrease in proliferation by shear stresses greater than 5 dynes/cm2 in mature ECs (Riha et al. 2005; Akimoto et al. 2000; Wasserman et al. 2002), potentially pointing to this as a conserved mechanism.
4.2 Differential protein expression
We found that SMC protein expression was restricted to MSCs exposed to cyclic stretch and was strongly dependent on the magnitude and frequency of applied cyclic stretch. Our findings are consistent with previously published data on stem cells only exposed to a single loading regimen (Riha et al. 2007; Hamilton et al. 2004; Park et al. 2004). However, our study has determined there are important thresholds for the expression of specific SMC proteins under 1 Hz stimulation. In addition, within the CS samples expressing SMA, lower magnitudes of stimulation displayed a weaker fluorescent signal than higher magnitudes of CS. CS-1HF demonstrated what appeared to be a stronger intensity signal for SMA, but the expression was restricted to smaller pockets within the field of view, as opposed to the general staining found for the other CS samples at 1 Hz. We believe these effects may be related to a specific subpopulation of cells that may be more sensitive to the frequency of stimulation. In fact, this effect of frequency with magnitude may be better characterized a single metric; namely, strain rate. Future work specifically evaluating a wide range of strain rate may be able to shed more light on the role of these factors on cell behavior. In addition, these studies also provide corroborating evidence for the relationship between cell shape and differentiation, as the types of SMC proteins expressed byMSCs exposed to cyclic stretch increased with changes in cell shape toward a more spindle morphology (see Figs. 2 and 4). The expression of SMC proteins in other cells has been linked to RhoA expressionand the reorganization of the actin filaments (Lu et al. 2001), and RhoA and ROCK have been implicated in other lineage commitments by MSCs dependent upon cellular shape (McBeath et al. 2004). Future experiments should be directed at determining the relationship between these GTP-binding proteins and MSC differentiation in light of mechanical stimulation.
While we did not see any other markers of EC differentiation apart from flk-1, the presence of flk-1 suggests the potential for these cells to differentiate to ECs since it is associated with EC differentiation during development (Yamamoto et al. 2005; Yamashita 2004). Our findings of constitutive expression of flk-1 in addition to SMC-related proteins following exposure to CS may be a similar phenomenon reported in SMC precursors derived from the blood (Simper et al. 2002). It is also worth noting that, despite not being able to visualize protein expression for vWF, there was evidence that the gene itself is expressed in all the conditions, with some changes due CP or low LSS (see Online Resource Table S9). This dual expression of SMC and EC markers has also figured prominently during development of the vascular plexus and may be related to angiogenesis/vasculogenesis (Hirschi and Majesky 2004). Furthermore, lack of staining for osteoblasts or adipocytes suggests that mechanical stimuli relevant to the vasculature may be inducing vasculature-specific differentiation.
4.3 Temporal changes in gene expression
A summary of our results, broken down by gene group, is found in Table 3. It should be noted that the relative quan-titation of the data for certain genes has a high variability. This variability comes primarily from two sources: complete lack of expression in one or more control groups and a heterogeneous starting cell population. Since many of the genes analyzed in this study should not be expressed by any cell type other than the terminally differentiated cell type (e.g., E-selectin, osteocalcin, cardiac troponin), it is expected that the copy number in the control population may be rather low or undetectable. Therefore, expression in the other mechanically stimulated group may be relatively quan-titated at 10× or higher; but in certain instances when the control cells do express the gene in sufficient quantities to be detected, the relative quantitation is much lower. This hints at a second primary source of variability. MSCs by nature are a heterogeneous population of cells, even if they have been clonally isolated. This is because they have a plastic phenotype and may begin to differentiate within a given culture based on the local microenvironment (Quesenberry and Aliotta 2008; Kuhn and Tuan 2010). A heterogeneous population of cells may therefore lead to the self-selection of a particular population based on the mechanical stimulus employed. We attempted to control for this phenomenon by designing our experiments to be conducted in parallel to apply all mechanical stimuli to a well-mixed population of these heterogeneous cells. Therefore, expression of certain genes may be a function of the starting subphenotype of the population for that experiment set or a subset of the population being more responsive to a given stimulus. However, because all experiments were done identically, any relative change in gene expression is a true representation of the potential for that stimulus to invoke such a change in MSCs. While we may be able to speak in general terms about the role of these stimuli in initiating gene expression, further experimentation may be able to dissect out these phenomena.
Table 3.
Summary of the changes in gene expression for phenotypic groups by our Mechanical Panel of stimulation
| Gene group | Cyclic pressure (h) |
Shear stress (h) |
Cyclic stretch (h) |
|||
|---|---|---|---|---|---|---|
| 24 | 72 | 24 | 72 | 24 | 72 | |
| Muscle | ↓ | ↓ | ↓ | ↓↓ | ↓ | ↑↑ |
| Endothelial | ↑ | ↑ | ↑ | ↑ | ↓ | ↔ |
| Bone | ↔ | ↑ | ↑↑ | ↔ | ↑ | ↑↑ |
| Cartilage | ↑↑ | ↑ | ↔ | ↓ | ↑ | ↑ |
| Fat | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ |
| Growth Factor | ↑↑ | ↔ | ↑↑ | ↑↑ | ↔ | ↔ |
| ECM | ↑↑ | ↔ | ↑↑ | ↑ | ↓ | ↑ |
| Proliferation | ↓↓ | ↔ | ↓↓ | ↓↓ | ↔ | ↑↑ |
To give some global sense to the data, a change index (CI) was calculated by averaging a score of 0 for no change, ±1 for a biological or statistical (p < 0.1) trend, and ±2 for a biologically relevant and statistically significant (p < 0.05) change for each of the genes in that category. ↑↑ and ↓↓ indicate a majority increase or decrease (CI > 0.70)in the overall gene expression for that group from control values. Single arrows denote a moderate change (0.25 ≤ CI ≤ 0.70) in the majority of the genes for a particular phenotype, and ↔ indicates very little change (CI < 0.25)
In general, LSS and CP tended to downregulate muscle-related genes, which is in contrast to other results where shear stress and pressure-dominated shear stress increased the protein expression of SMA and SM-MHC in marrow stromal cells expressing Stro-1 (Kobayashi et al. 2004). However, the studies by Kobayashi et al. utilized a pre-incubation period of 7–21 days before exposure to mechanical stimulation and found that the longer pre-incubation period increased SMC protein expression. This may be related to the phenomenon describe in one of the earliest MSC papers by Galmiche and Charbord, where they found that extensive culture of bone marrow inevitably increased SMC protein expression (Galmiche et al. 1993). Based upon the decreased expression of myocardin in our system, which is necessary for nearly all SMC proteins, including SM22α, SMA, caldesmon, calponin, and SM-MHC (Owens et al. 2004; Du et al. 2003; Chen et al. 2002; Yoshida et al. 2004), we believe that that these genes would also be diminished by LSS and CP in our Mechanical Panel, which is consistent with our findings on the subconfluent 5-day Mechanical Panel experiments. Conversely, CS stimulation started with a trend toward downregulation at 24 h and then increased to control levels or higher at 72 h. We believe this is due to the morphological changes that occur in the first 24 h to shield the cells from the imposed stresses, followed by increases in these genes to produce the proteins determined in the 5-day subconfluent studies. Similarly, O’Cearbhaill et al. found increases in SMC gene expression (α-SMA and calponin) within 24h of application of all three forces simultaneously. In their pseudovessel system, it would appear that stretch either dominated the changes in gene expression or an unexpected synergy occurred with all three forces. Another important factor may also be the substrate, which was fibronectin in their work compared with collagen I in ours. Simultaneous signaling from multiple forces, and/or forces and biochemical/integrin signaling may in fact create as-yet unknown synergies that drive cellular behavior. Such phenomenon have been discovered in cancer biology and may eventually be found in stem cell differentiation as well (Janes et al. 2008).
Endothelial-related gene expression tended to be increased by both CP and LSS at 24 and 72h, leading to the conclusion that the MSCs are becoming more endothelial-like under these mechanical stimuli. E-selectin and iNOS had little or no detectable expression under control and CS conditions, but were found under CP and LSS. Our endothelial-related gene expression results are in agreement with other work with embryonic stem cells (Yamamoto et al. 2005; Wang et al. 2005). However, we hypothesize that the increase in endothelial-related genes under these conditions made be dependent upon cell-cell contact. Some preliminary gene expression data from the subconfluent 5-day Mechanical Panel portion of this study showed that gene expression for vWF was on average upregulated under CP (but not statistically significant) and strongly downregulated under low LSS (Online Resource Table S9). The 5-day Mechanical Panel experiments were initiated with the MSCs in a highly subconfluent state (200 cells/cm2), and only the MSCs exposed to CP reached confluence by the end of the experimental period, which was the only condition to demonstrate an increased vWF gene expression for those studies. In the experiments examining temporal gene expression, all experiments were begun at confluence (20,000 cells/cm2), and both LSS and CP show upregulated endothelial-related gene expression.
The expression of growth factors, including VEGF-A, was consistently upregulated by LSS at 24 and 72h. Similar results for the regulation of VEGF have been shown for embryonic stem cells (Wang et al. 2005) and vascular endothelial cells (Conklin et al. 2002) exposed to shear stresses similar in magnitude to those used in our study. Despite the increases in both TGF-β and VEGF-A gene expression under LSS, their receptor (endoglin and flk-1) gene expression was downregulated under LSS. Given that positive-feedback loops have been shown to exist between TGFβ and endoglin (Conley et al. 2000; Rodriguez-Barbero et al. 2001), the downregulation of endoglin in conjunction with the upreg-ulation of TGF-β in our LSS studies may be indicating a post-transcriptional or post-translational modification of these factors that results in no net, active protein production
Although previous reports on gene expression in MSC have indicated that no transcripts for osteopontin should be detected in non-osteoblast differentiated MSC (Kim et al. 2005), osteopontin mRNA has been detected in a variety of other cells including vascular SMCs and ECs, with implications for atherosclerosis (Isoda et al. 2002; Xie et al. 2001). The presence of osteopontin in other cell types has also been attributed to its potential role in cell adhesion because it contains the Arg-Gly-Asp-Ser adhesion domain which is capable of binding fibronectin (Butler 1989; Ruoslahti 1988). In our experiments, we find that osteopontin expression can be variable, but is consistently expressed. However, osteocalcin gene expression was significantly downregulated under LSS and unchanged for the remainder of the conditions, indicating that a more comprehensive panel of genes should be required to determine osteoblast differentiation. Likewise, the expression of aggrecan and collagen II have previously been used for definitive markers of chondrocyte differentiation. Yet, these genes were readily expressed in almost all conditions and in some instances were upregulated. Longer time points may be necessary to allow for the accumulation of proteins associated with these genes to be visible histologically. Pressure has previously been implicated in driving chondrocyte differentiation, but at much higher pressures (>5 MPa) than those presented here (< 1 MPa) (Angele et al. 2003; Elder et al. 2005, 2001). Other important differences between this study and previous studies focused on chondrocyte differentiation are the use of pellet cultures and of constant or low frequency pressure. Similar to our observation that endothelial gene expression is dependent on cell-cell contacts, the local microenvironment may play a significant role in the activation of the chondrocyte lineage under hydrostatic pressure.
Cyclin B1 was used to detect changes in the cell cycle progression as a result of mechanical stimulation (Ito 2000; Liao et al. 2004). Our data indicate a significant increase in cyclin B1 expression following exposure to CS at 72h, consistent with a similar study (Kurpinski et al. 2006a). In agreement with our cell densities after 5 days of LSS on subconfluent MSCs (Fig. 2B), LSS was a strong downregulator of cyclin B1 expression, effectively arresting the cell in the G2/M phase of the cell cycle. Similar results have been shown for ECs under laminar shear stress (Garcia-Cardena et al. 2001; Wasserman and Topper 2004). Cyclin B1 was also transiently decreased at 24 h by CP before returning to baseline levels at 72 h. There is other evidence in the literature that CP increases proliferation in bone marrow-derived cells as well as ECs, SMCs, and chondrocytes (Maul et al. 2007; Sumpio et al. 1994; Shin et al. 2002; Stover and Nagatomi 2007) (see also Fig. 2B). More recently, mechanisms involving VEGF (Shin et al. 2002) and the PI3k/Akt pathway (Stover and Nagatomi 2007) have been proposed for CP-increased proliferation. Our results suggest that contact inhibition of the MSCs played a more dominant role in their cell cycle progression, which is in contrast to the effect of pressure on aortic ECs that lose their contact inhibition in the presence of pressure (Ohashi et al. 2007). It is possible that the arrest at the G2/M phase of the cell cycle may be a result of other paracrine signals previously unavailable to the MSCs in our earlier experiments, which were only just reaching confluence in the subconfluent Mechanical Panel experiments. Further studies utilizing other markers of proliferation should be undertaken to obtain a more accurate proliferation profile of these cells.
5 Conclusion
We have demonstrated that mechanical stimulation has significant effects on the morphology, cell density, and differentiation in rat MSCs and that these effects are dependent on the type, magnitude, and frequency of the applied stimulation. While there are differences between the responses of rat and human MSCs (Javazon et al. 2001), the use of human physiologic conditions in these experiments resulting in the effects described in the paper may in fact point to a fundamental response of cells to these stimuli. The use of our parallel stimulation may also point to fundamental changes based upon the applied mechanical stimulus despite the additional biochemical cues given by the high serum content used in the culture media. Our results have extended previous observations that cyclic stretch can drive stem cell differentiation toward an SMC phenotype and have improved our understanding of the role cyclic stretch plays in differentiation by defining the threshold magnitudes and frequencies at which these changes occur. As with all studies, there are some limitations in our study that should be noted. First, we did not create a pulsatile shear condition in our system for direct comparison to the cyclic nature of the pressure and stretch components of the study. Adding a frequency component to the shear stress studies may impact the results since the other two forces did indeed display a frequency response in morphology and/or protein expression. Also, we were unable to control for the surface rigidity in these experiments. Several other researchers have demonstrated the effects of surface rigidity on cellular behavior showing influence on cell growth and differentiation (Fu et al. 2010; Discher et al. 2005; Engler et al. 2004). However, Engler et al. have demonstrated that there is a limit to the ability of cells to sense rigidity, which decreases when the stiffness of the surface approaches 10 kPa (Engler et al. 2004). We have previously examined the mechanical properties of the BioFlex membranes and found them to be roughly 6kPa (Vande Geest et al. 2004), which may lessen the potential impact of comparisons between the Culture Slips and BioFlex membranes. Finally, our requirement for enough genetic material for analysis at early time points forced us to change our seeding density. We initially believed that this would not impact the outcome of the experiments, but based on the observation that change in gene expression appears to be somewhat linked to starting density, we cannot extrapolate from the protein data generated in the first set of 5-day experiments to the gene expression data in the second set of temporal experiments. However, despite limited EC protein expression, our preliminary gene expression data do suggest that EC-related genes are expressed as a result of CP. We have demonstrated that cyclic pressure increases cell number and that a correlation exists between cell size, shape, and proliferation. To our knowledge, this work is the first systematic study examining multiple types of mechanical stimulation in parallel across a broad range of magnitudes and multiple frequencies and has future applications in bioreactor design and regenerative medicine research.
Utilizing our Mechanical Panel approach, we were able to determine the temporal changes in gene expression by MSCs exposed to three mechanical stimuli. The use of confluent cultures may have created a more physiologic signaling environment, resulting in EC-related gene expression under CP and LSS, which was not seen in previous work. This leads to further questions about whether the cells were pre-primed for EC gene expression because they were confluent or whether additional paracrine or cell– cell junction signaling is required for the expression of EC-related genes under these two mechanical stimuli. Our data for CS at 24h indicated a decrease in muscle-related gene expression, but by 72 h, several muscle-related genes including cardiac troponin, desmin, and myocardin were increasing suggesting that the cells are potentially entering a myofibroblast phenotype. Perhaps with additional cues, including longer exposure time or biochemical stimulation such as TGF-β (Riha et al. 2007; Lee et al. 2006), MSCs under mechanical stimulation may continue down the path toward functional SMCs and ECs, which would provide important steps forward for vascular regenerative medicine.
Supplementary Material
Acknowledgments
Funding for this research was provided by the National Institute of Health R01 HL069368 and T32 EB001026, the Ruth L. Kirschstein National Research Service Award, andanAmerican Heart Association post-doctoral fellowship. Portions of this work were also supported by the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center (P30 CA43703).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10237-010-0285-8) contains supplementary material, which is available to authorized users.
Contributor Information
Timothy M. Maul, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA 15219, USA The McGowan Institute for Regenerative Medicine, University of Pittsburgh, 450 Technology Drive. Suite 300, Pittsburgh, PA 15219, USA; The Center for Vascular Remodeling and Regeneration, University of Pittsburgh, Pittsburgh, PA 15219, USA.
Douglas W. Chew, Department of Surgery, University of Pittsburgh, Pittsburgh, PA 15219, USA The McGowan Institute for Regenerative Medicine, University of Pittsburgh, 450 Technology Drive. Suite 300, Pittsburgh, PA 15219, USA; The Center for Vascular Remodeling and Regeneration, University of Pittsburgh, Pittsburgh, PA 15219, USA.
Alejandro Nieponice, Department of Surgery, University of Pittsburgh, Pittsburgh, PA 15219, USA; The McGowan Institute for Regenerative Medicine, University of Pittsburgh, 450 Technology Drive. Suite 300, Pittsburgh, PA 15219, USA; The Center for Vascular Remodeling and Regeneration, University of Pittsburgh, Pittsburgh, PA 15219, USA.
David A. Vorp, Email: vorpda@upmc.edu, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA 15219, USA; Department of Surgery, University of Pittsburgh, Pittsburgh, PA 15219, USA; The McGowan Institute for Regenerative Medicine, University of Pittsburgh, 450 Technology Drive. Suite 300, Pittsburgh, PA 15219, USA; The Center for Vascular Remodeling and Regeneration, University of Pittsburgh, Pittsburgh, PA 15219, USA.
References
- Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(sdi1/cip1/waf1) Circ Res. 2000;86(2):185–190. doi: 10.1161/01.res.86.2.185. [DOI] [PubMed] [Google Scholar]
- Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21(3):451–457. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2–3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Cappadona C, Redmond EM, Theodorakis NG, McKillop IH, Hendrickson R, Chhabra A, Sitzmann JV, Cahill PA. Phe-notype dictates the growth response of vascular smooth muscle cells to pulse pressure in vitro. Exp Cell Res. 1999;250(1):174–186. doi: 10.1006/excr.1999.4502. [DOI] [PubMed] [Google Scholar]
- Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34(10):1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- Chien S. Molecular basis of rheological modulation of endo-thelial functions: Importance of stress direction. Biorheology. 2006;43(2):95–116. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of rna isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and vegf expression in porcine arterial endothe-lial cells. J Surg Res. 2002;102(1):13–21. doi: 10.1006/jsre.2001.6295. [DOI] [PubMed] [Google Scholar]
- Conley BA, Smith JD, Guerrero-Esteo M, Bernabeu C, Vary CP. Endoglin, a tgf-beta receptor-associated protein, is expressed by smooth muscle cells in human atherosclerotic plaques. Atherosclerosis. 2000;153(2):323–335. doi: 10.1016/s0021-9150(00)00422-6. [DOI] [PubMed] [Google Scholar]
- De R, Zemel A, Safran SA. Dynamics of cell orientation. Nat Phys. 2007;3(9):655–659. [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23(7):2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder SH, Fulzele KS, McCulley WR. Cyclic hydrostatic compression stimulates chondroinductionofc3h/10t1/2 cells. Biomech Model Mechanobiol. 2005;3(3):141–146. doi: 10.1007/s10237-004-0058-3. [DOI] [PubMed] [Google Scholar]
- Elder SH, Goldstein SA, Kimura JH, Soslowsky LJ, Spengler DM. Chondrocyte differentiation is modulated by frequency and duration of cyclic compressive loading. Ann Biomed Eng. 2001;29(6):476–482. doi: 10.1114/1.1376696. [DOI] [PubMed] [Google Scholar]
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86(1):617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JMO, Mommens M, Hagen O, Babiak I, Solberg C. Selection of suitable reference genes for real-time pcr studies of atlantic halibut development. Comp Biochem Physiol Part B: Biochem Mol Biol. 2008;150(1):23–32. doi: 10.1016/j.cbpb.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical analysis of circular data. Cambridge: Melbourne; 1993. [Google Scholar]
- Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7(9):733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82(1):66–76. [PubMed] [Google Scholar]
- Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA. Biomechanical activation of vascular endo-thelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA. 2001;98(8):4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DW, Maul TM, Vorp DA. Characterization of the response of bone marrow derived progenitor cells to cyclic strain: Implications for vascular tissue engineering applications. Tissue Eng. 2004;10(3/4):361–370. doi: 10.1089/107632704323061726. [DOI] [PubMed] [Google Scholar]
- Hammond JP, Broadley MR, Craigon DJ, Higgins J, Emmerson ZF, Townsend HJ, White PJ, May ST. Using genomic DNA-based probe-selection to improve the sensitivity of high-density oligonucleotide arrays when applied to heterologous species. Plant Methods. 2005;1(1):10. doi: 10.1186/1746-4811-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Majesky MW. Smooth muscle stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276(1):22–33. doi: 10.1002/ar.a.10128. [DOI] [PubMed] [Google Scholar]
- Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 Pt 2):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- Isoda K, Nishikawa K, Kamezawa Y, Yoshida M, Kusuhara M, Moroi M, Tada N, Ohsuzu F. Osteopontin plays an important role in the development of medial thickening and neointimal formation. Circ Res. 2002;91(1):77–82. doi: 10.1161/01.res.0000025268.10302.0c. [DOI] [PubMed] [Google Scholar]
- Ito M. Factors controlling cyclin b expression. Plant Mol Biol. 2000;43(5–6):677–690. doi: 10.1023/a:1006336005587. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64(2):295–312. [PubMed] [Google Scholar]
- Janes KA, Reinhardt HC, Yaffe MB. Cytokine-induced signaling networks prioritize dynamic range over signal strength. Cell. 2008;135(2):343–354. doi: 10.1016/j.cell.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19(3):219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Oph-thalmol Vis Sci. 2007;48(7):3161–3177. doi: 10.1167/iovs.06-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N, Ujita S, Sato M. Effect of flow direction on the morphological responses of cultured bovine aortic endothelial cells. Med Biol Eng Comput. 1998;36(1):122–128. doi: 10.1007/BF02522869. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: a researchers handbook. 4th edn. Upper Saddle River: Pearson; 2004. [Google Scholar]
- Kim DH, Yoo KH, Choi KS, Choi J, Choi SY, Yang SE, Yang YS, Im HJ, Kim KH, Jung HL, Sung KW, Koo HH. Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine. 2005;31(2):119–126. doi: 10.1016/j.cyto.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Yasu T, Ueba H, Sata M, Hashimoto S, Kuroki M, Saito M, Kawakami M. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol. 2004;32(12):1238–1245. doi: 10.1016/j.exphem.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36(6):1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: Implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222(2):268–277. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechano-sensing by mesenchymal stem cells. Proc Natl Acad Sci USA. 2006;103(44):16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpinski K, Park J, Thakar RG, Li S. Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain. Mol Cell Biomech. 2006;3(1):21–34. [PubMed] [Google Scholar]
- Lee WC, Rubin JP, Marra KG. Regulation of alpha-smooth muscle actin protein expression in adipose-derived stem cells. Cells Tissues Organs. 2006;183(2):80–86. doi: 10.1159/000095512. [DOI] [PubMed] [Google Scholar]
- Li YJ, Batra NN, You L, Meier SC, Coe IA, Yellowley CE, Jacobs CR. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22(6):1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Liao XD, Wang XH, Jin HJ, Chen LY, Chen Q. Mechanical stretch induces mitochondria-dependent apoptosis in neonatal rat cardiomyocytes and g2/m accumulation in cardiac fibroblasts. Cell Res. 2004;14(1):16–26. doi: 10.1038/sj.cr.7290198. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2-[delta][delta]ct method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J, Landerholm TE, Wei JS, Dong X-R, Wu S-P, Liu X, Nagata K-i, Inagaki M, Majesky MW. Coronary smooth muscle differentiation from proepicardial cells requires rhoa-med-iated actin reorganization and p160 rho-kinase activity. Dev Biol. 2001;240(2):404–418. doi: 10.1006/dbio.2001.0403. [DOI] [PubMed] [Google Scholar]
- Maul TM, Hamilton DW, Nieponice A, Soletti L, Vorp DA. A new experimental system for the extended application of cyclic hydrostatic pressure to cell culture. J Biomech Eng. 2007;129(1):110–116. doi: 10.1115/1.2401190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Millgard J, Lind L. Acute hypertension impairs endothelium-dependent vasodilation. Clin Sci (Colch) 1998;94(6):601–607. doi: 10.1042/cs0940601. [DOI] [PubMed] [Google Scholar]
- Mills I, Cohen CR, Kamal K, Li G, Shin T, Du W, Sumpio BE. Strain activation of bovine aortic smooth muscle cell proliferation and alignment: Study of strain dependency and the role of protein kinase a and c signaling pathways. J Cell Physiol. 1997;170(3):228–234. doi: 10.1002/(SICI)1097-4652(199703)170:3<228::AID-JCP2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Moore BR. A modification of the rayleigh test for vector data. Biometrika. 1980;67(1):175–180. [Google Scholar]
- Nagatomi J, Arulanandam BP, Metzger DW, Meunier A, Bizios R. Frequency- and duration-dependent effects of cyclic pressure on select bone cell functions. Tissue Eng. 2001;7(6):717–728. doi: 10.1089/107632701753337672. [DOI] [PubMed] [Google Scholar]
- Nerem RM, Levesque MJ, Cornhill JF. Vascular endothelial morphology as an indicator of the pattern of blood flow. J Bio-mech Eng. 1981;103(3):172–176. doi: 10.1115/1.3138275. [DOI] [PubMed] [Google Scholar]
- Nieponice A, Maul TM, Cumer JM, Soletti L, Vorp DA. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J Biomed Mater Res A. 2006;81A(3):523–530. doi: 10.1002/jbm.a.31041. [DOI] [PubMed] [Google Scholar]
- O’Cearbhaill ED, Punchard MA, Murphy M, Barry FP, McHugh PE, Barron V. Response of mesenchymal stem cells to the bio-mechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials. 2008;29(11):1610–1619. doi: 10.1016/j.biomaterials.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Sugaya Y, Sakamoto N, Sato M. Hydrostatic pressure influences morphology and expression of ve-cadherin of vascular endothelial cells. J Biomech. 2007;40(11):2399–2405. doi: 10.1016/j.jbiomech.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Oluwole BO, Du W, Mills I, Sumpio BE. Gene regulation by mechanical forces. Endothel: J Endothel Cell Res. 1997;5(2):85–93. doi: 10.3109/10623329709079866. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88(3):359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Quesenberry PJ, Aliotta JM. The paradoxical dynamism of marrow stem cells: Considerations of stem cells, niches, and microve-sicles. Stem Cell Rev. 2008;4(3):137–147. doi: 10.1007/s12015-008-9036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick N, Gimbrone MA. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9(10):874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Roles of hemo-dynamic forces in vascular cell differentiation. Ann Biomed Eng. 2005;33(6):772–779. doi: 10.1007/s10439-005-3310-9. [DOI] [PubMed] [Google Scholar]
- Riha GM, Wang X, Wang H, Chai H, Mu H, Lin PH, Lumsden AB, Yao Q, Chen C. Cyclic strain induces vascular smooth muscle cell differentiation from murine embryonic mesenchymal progenitor cells. Surgery. 2007;141(3):394–402. doi: 10.1016/j.surg.2006.07.043. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Barbero A, Obreo J, Eleno N, Rodriguez-Pena A, Duwel A, Jerkic M, Sanchez-Rodriguez A, Bernabeu C, Lopez-Novoa JM. Endoglin expression in human and rat mesangial cells and its upregulation by tgf-beta1. Biochem Biophys Res Commun. 2001;282(1):142–147. doi: 10.1006/bbrc.2001.4526. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Sato M, Ohashi T. Biorheological views of endothelial cell responses to mechanical stimuli. Biorheology. 2005;42(6):421–441. [PubMed] [Google Scholar]
- Scaglione S, Wendt D, Miggino S, Papadimitropoulos A, Fato M, Quarto R, Martin I. Effects of fluid flow and calcium phosphate coating on human bone marrow stromal cells cultured in a defined 2d model system. J Biomed Mater Res A. 2007;86(2):411–419. doi: 10.1002/jbm.a.31607. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Bizios R, Medow MS, Gerritsen ME. Exposure of human vascular endothelial cells to sustained hydrostatic pressure stimulates proliferation. Involvement of the alphaV integrins. Circ Res. 1999;84(3):315–322. doi: 10.1161/01.res.84.3.315. [DOI] [PubMed] [Google Scholar]
- Seliktar D, Black RA, Vito RP, Nerem RM. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28(4):351–362. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- Shin HY, Smith ML, Toy KJ, Williams PM, Bizios R, Gerritsen ME. Vegf-c mediates cyclic pressure-induced endothelial cell proliferation. Physiol Genomics. 2002;11(3):245–251. doi: 10.1152/physiolgenomics.00068.2002. [DOI] [PubMed] [Google Scholar]
- Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002;106(10):1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- Skalak TC, Price RJ, Zeller PJ. Where do new arterioles come from? Mechanical forces and microvessel adaptation. Microcircu-lation. 1998;5(2–3):91–94. [PubMed] [Google Scholar]
- Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31(4):391–402. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- Stover J, Nagatomi J. Cyclic pressure stimulates DNA synthesis through the pi3k/akt signaling pathway in rat bladder smooth muscle cells. Ann Biomed Eng. 2007;35(9):1585–1594. doi: 10.1007/s10439-007-9331-9. [DOI] [PubMed] [Google Scholar]
- Sumpio BE, Widmann MD, Ricotta J, Awolesi MA, Watase M. Increased ambient pressure stimulates proliferation and morphologic changes in cultured endothelial cells. J Cell Phys-iol. 1994;158(1):133–139. doi: 10.1002/jcp.1041580117. [DOI] [PubMed] [Google Scholar]
- Vande Geest JP, Di Martino ES, Vorp DA. An analysis of the complete strain field within flexercell membranes. J Biomech. 2004;37(12):1923–1928. doi: 10.1016/j.jbiomech.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25(9):1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- Wasserman SM, Mehraban F, Komuves LG, Yang RB, Tomlinson JE, Zhang Y, Spriggs F, Topper JN. Gene expression profile of human endothelial cells exposed to sustained fluid shear stress. Physiol Genomics. 2002;12(1):13–23. doi: 10.1152/physiolgenomics.00102.2002. [DOI] [PubMed] [Google Scholar]
- Wasserman SM, Topper JN. Adaptation of the endothelium to fluid flow: in vitro analyses of gene expression and in vivo implications. Vasc Med. 2004;9(1):35–45. doi: 10.1191/1358863x04vm521ra. [DOI] [PubMed] [Google Scholar]
- Watase M, Awolesi MA, Ricotta J, Sumpio BE. Effect of pressure on cultured smooth muscle cells. Life Sci. 1997;61(10):987–996. doi: 10.1016/s0024-3205(97)00603-6. [DOI] [PubMed] [Google Scholar]
- Whaley L, Wong D. Nursing care of infants and children. 5th edn. St. Louis: Mosby; 1999. [Google Scholar]
- Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ Res. 1970;26(4):507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]
- Xie Z, Pimental DR, Lohan S, Vasertriger A, Pligavko C, Colucci WS, Singh K. Regulation of angiotensin ii-stimulated osteopon-tin expression in cardiac microvascular endothelial cells: role of p42/44 mitogen-activated protein kinase and reactive oxygen species. J Cell Physiol. 2001;188(1):132–138. doi: 10.1002/jcp.1104. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288(4):H1915–1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- Yamashita JK. Differentiation and diversification of vascular cells from embryonic stem cells. Int J Hematol. 2004;80(1):1–6. doi: 10.1532/ijh97.04043. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kawai-Kowase K, Owens GK. Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential embryonic cells. Arterioscler Thromb Vasc Biol. 2004;24(9):1596–1601. doi: 10.1161/01.ATV.0000137190.63214.c5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.