Abstract
Dietary guidelines advise consumers to limit intake of red meat and choose lean protein sources, such as poultry and fish. Poultry consumption has been steadily increasing in the U.S., but the effect on cancer risk remains unclear. In a large U.S. cohort, we prospectively investigated poultry and fish intake and cancer risk across a range of malignancies in men and women. Diet was assessed at baseline (1995–1996) with a food frequency questionnaire in 492,186 participants of the National Institutes of Health-AARP Diet and Health Study. Over a mean follow-up of 9 years, we identified 74,418 incident cancer cases. In multivariate Cox proportional hazards regression models, we estimated the substitution and addition effects of white meat (poultry and fish) intake in relation to cancer risk. In substitution models with total meat intake held constant, a 10 gram (per 1,000 kilocalories) increase in white meat intake offset by an equal decrease in red meat intake was associated with a statistically significant reduced (3–20%) risk of cancers of the esophagus, liver, colon, rectum, anus, lung, and pleura. In addition models with red meat intake held constant, poultry intake remained inversely associated with esophageal squamous cell carcinoma, liver cancer, and lung cancer, but we observed mixed findings for fish intake. As the dietary recommendations intend, the inverse association observed between white meat intake and cancer risk may be largely due to the substitution of red meat. Simply increasing fish or poultry intake, without reducing red meat intake, may be less beneficial for cancer prevention.
Keywords: poultry, fish, canned tuna, cancer, cohort
Introduction
Meat is an integral component of the U.S. diet and national dietary guidelines emphasize lean protein sources, such as poultry and fish 1. For the prevention of cancer, the American Cancer Society specifically advises limiting intake of red and processed meats and choosing fish and poultry instead of beef, pork or lamb 2. However, over the last four decades total meat consumption in the U.S. has continued to rise, mainly due to an increase in poultry consumption, while red meat consumption is beginning to stabilize and fish consumption has remained low 3. Thus, understanding the role of poultry and fish intake in cancer risk within the U.S. population is of mounting importance.
While red and processed meat intake in relation to cancer risk has received considerable attention in recent years, intake of “white meat” (poultry and fish) has not been as extensively investigated in epidemiologic studies and for many cancer sites there is little or no prospective evidence available 4. The 2007 report from the World Cancer Research Fund and American Institute for Cancer Research concluded that the evidence for poultry intake and cancer risk was “too limited in amount, consistency, and quality to draw any conclusions,” while the evidence for fish intake was “limited to suggestive” of lower cancer risk and based primarily on studies of colorectal cancer 4. Poultry is generally considered a lean meat alternative to red meat, while the potential benefits of fish intake are linked to the anti-inflammatory and anti-carcinogenic effects of long-chain n-3 fatty acids 5. However, some species of seafood, including canned tuna, the most commonly consumed fish in the U.S., may contain mercury and other environmental contaminants 6–7.
We previously reported in the National Institutes of Health (NIH)-AARP Diet and Health Study, a U.S. diet and cancer cohort of half a million people, that red and processed meat intake was associated with an increased risk of cancers of the colorectum, lung, esophagus and liver 8, as well as all-cause, cardiovascular disease, and cancer mortality, while intake of white meat was inversely associated with cancer mortality 9. Given the current dietary recommendations for meat intake and cancer prevention, as well as the rise in poultry consumption within the U.S., we evaluated the substitution effect of white meat for red meat, as well as the addition effect of poultry and fish intake, in relation to cancer risk.
Materials and Methods
The NIH-AARP Diet and Health Study 10 is a large prospective cohort of U.S. men and women, aged 50–71 years, across six states (CA, FL, LA, NJ, NC, PA) and two metropolitan areas (Atlanta, GA and Detroit, MI). In 1995, AARP members were mailed a self-administered questionnaire with queries for demographics, dietary habits, and lifestyle characteristics. Of those who returned the baseline questionnaire (567,169 persons), 566,401 completed the survey satisfactorily and consented to be a part of the study. We further excluded proxy respondents (n = 15,760) and participants with prevalent cancer (as noted by cancer registry or self-report; n = 51,223) or end-stage renal disease (n = 997) at baseline, a death only report for any cancer (n = 1,804), zero person-years of follow-up (n = 36), or implausibly high (men: >6,141 kcal; women: >4,791 kcal) or low (men: <415 kcal; women: <318 kcal) total energy intake beyond twice the inter-quartile range of sex-specific Box-Cox transformed intake 11 (n = 4,395). Following exclusions, 293,466 men and 198,720 women (n = 492,186) were included in the present analysis. Analyses of ovarian, endometrial, and post-menopausal breast cancer, respectively, excluded women who reported an oophorectomy (n = 43,536), hysterectomy (n = 81,205), and pre-menopausal or uncertain menopausal status (n = 13,872) at baseline. The conduct of the NIH-AARP Diet and Health Study was reviewed and approved by the Special Studies Institutional Review Board of the U.S. National Cancer Institute and all participants gave informed consent by virtue of completing and returning the questionnaire. Follow-up for each subject began on the date of return of the baseline questionnaire (1995–1996) and continued until the date of cancer diagnosis, date of censoring due to loss to follow-up, death, or December 31, 2006, whichever came first.
Dietary Assessment
Dietary intake was assessed using a 124-item food frequency questionnaire (FFQ) developed and validated by the National Cancer Institute 11. Participants were asked to report their usual dietary intake of foods and beverages over the past year in both frequency of intake and portion size. The 1994–1996 USDA Continuing Survey of Food Intakes by Individuals was used to calculate nutrient intakes 12. Total white meat intake was the sum of all poultry and fish. Total poultry intake included chicken, turkey, ground poultry, as well as the processed poultry components of turkey or chicken cold cuts and low-fat versions of hot dogs and sausage. Poultry queries included line items for breaded/deep-fried chicken, other chicken (baked, broiled, roasted or stewed), chicken casseroles, sandwiches, and mixtures, as well as general habits of consuming skin and light or dark meat. Total fish intake included all types of finfish and shellfish, as well as canned tuna. Line items for fish differentiated breaded/deep-fried fish or fish sticks, other fish/seafood (not fried) and canned tuna. Total red meat intake included all types of fresh (beef, pork, hamburger, steak, and liver) and processed red meat (bacon, cold cuts, ham, hot dogs, and sausage, excluding low-fat versions made from poultry products).
Cancer Ascertainment
During follow-up, cancer cases were ascertained through linkage with the 8 original state cancer registries and an additional 2 states (AZ, TX). The cancer registries are certified by the North American Association of Central Cancer Registries as being at least 90% complete within 2 years of cancer incidence. The high quality of the NIH-AARP study case ascertainment methods are described in detail elsewhere 13. Vital status was ascertained though periodic linkage of the cohort to the Social Security Administration (SSA) Death Master File in the U.S., follow-up searches of the National Death Index Plus for participants matched to the SSA Death Master File, cancer registry linkage, questionnaire responses, and responses to other mailings. Invasive cancer diagnoses contributed to the incidence of the tumor site of the first diagnosis only during follow-up, excluding subsequent diagnoses at additional cancer sites. Cancer endpoints were defined by anatomic site and histology code using the third edition of the International Classification of Diseases for Oncology (ICD-0-3) 14.
Statistics
All dietary variables were adjusted for total energy intake using the nutrient density method and presented for ease of interpretability as grams per 1,000 kilocalories of total energy intake per day. Residual energy adjustment 15 produced similar results. For cancer sites with more than 100 cases, the association between poultry and fish intake and cancer risk was evaluated with Cox proportional hazards regression models with time since entry (person-years) as the underlying time metric. We confirmed that the Cox proportional hazards assumption was met through assessment of plots and interaction terms for the exposures with follow-up time. Hazard ratios (HR) and 95% confidence intervals (CI) are reported for 10 gram (per 1,000 kilocalories) increments of poultry and fish intake; and for sex-specific quintiles of intake lowest intake quintile represents the referent category). In categorical models, P-values for linear trend were calculated using the median value within quintiles.
We constructed two types of models to adjust for intake of red meat and to test alternate hypotheses regarding white meat intake and cancer risk. In substitution models, total meat intake was held constant, such that an increase in white meat intake is offset by an equal decrease in red meat intake 16. Addition models evaluated the effect of an independent increase in white meat intake with red meat intake held constant 16. Multivariate models also included the following covariates: age (modeled as a continuous covariate), education (<8 year or unknown, 8–11 years, high school graduate, some college, college graduate), marital status, family history of cancer first degree relative), race (non-Hispanic white, non-Hispanic black, Hispanic/Asian/Pacific Islander/American Indian/Alaskan Native or unknown), body mass index (BMI; <18.5, 18.5 to <25, 25 to <30, 30 to <35, ≥35 kg/m2), smoking status (never, quit ≥ 10 years ago, quit 5 to 9 years ago, quit 1 to 4 years ago, quit <1 year ago or currently smoking and smoked ≤20 cigarettes/day, quit <1 year ago or currently smoking and smoked >20 cigarettes/day), frequency of vigorous physical activity (never/rarely, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5 or more times/week), menopausal hormone therapy in women (MHT; never, former, current use), total energy intake (continuous), alcohol intake (none, 0 to <5, 5 to <15, 15 to <30, ≥30 grams/day), fruit intake and vegetable intake [MyPyramid Equivalents Database (MPED) 17 servings per 1,000 kcal; modeled in quintiles]. Analyses for fish or poultry intake were mutually adjusted for the other type of white meat. We further evaluated potential confounding due to intake of fat, fiber, and calcium, as well as family history of specific cancers (first degree relative with colon, prostate, or breast cancer), and personal history of diabetes or hypertension; and parity, age at menarchy, and oral contraceptive use for female cancers. However, results did not meaningfully differ from those presented. Given that canned tuna was queried separately in the questionnaire, is commonly consumed in the U.S., and may be a key source of both long-chain n-3 fatty acids and mercury compared to other general types of fish queried 6–7, we evaluated associations with its intake separately. We also investigated associations differentiating processed poultry components and poultry or fish that was breaded/deep-fried; and conducted a lag analysis excluding the first 2-years of follow-up. We assessed whether any of the associations varied by sex and smoking status with statistical tests for interaction evaluating the significance of cross-product terms in multivariate-adjusted models. All statistical tests were two-sided and considered statistically significant at P<0.05. All statistical analyses were conducted using SAS 9.2 (SAS Institute, Inc.).
Results
During a mean follow-up of 9.1 years, we identified 51,419 cancer cases in men and 22,999 cancer cases in women. The 10th and 90th percentiles of dietary intakes of total poultry, total fish, and canned tuna (per 1,000 kilocalories per day) respectively, were: 4.2 g to 43.8 g, 2.1 g to 22.9 g, and 0 g to 6.9 g in men; and 4.8 g to 51.6 g, 2.0 to 23.5, and 0.2 g to 8.6 g in women. Poultry intake was only modestly correlated (r = 0.17) with fish intake. Less than 3% of total poultry intake was processed and approximately one-third of the total fish intake came from canned tuna (data presented in the text only). As presented in Table 1, participants in the highest quintile of total white meat (poultry and fish) intake, compared with those in the lowest, were more likely to be college graduates and less likely to be current smokers and physically inactive. Red meat intake was similar across quintiles of white meat intake, while participants in the highest quintile of white meat intake consumed more vegetables and less alcohol than those in lowest quintile. Trends in characteristics of participants were similar across quintiles of poultry and fish intake, as well as by sex (data not shown).
Table 1.
Means and proportions for selected baseline characteristics of the NIH-AARP Diet and Health Study cohort (n = 492,186) by quintiles of total white meat intake
| Total White Meat | |||||
|---|---|---|---|---|---|
| Characteristic | Q1 | Q2 | Q3 | Q4 | Q5 |
| White meat, g/1000 kcal), mean | 8.9 | 18.5 | 27.6 | 40.0 | 72.6 |
| Poultry (g/1000 kcal), mean | 5.3 | 11.5 | 17.7 | 26.6 | 51.2 |
| Fish (g/1000 kcal), mean | 3.6 | 7.0 | 9.9 | 13.4 | 21.4 |
| Canned tuna (g/1000 kcal), mean | 1.2 | 2.2 | 3.0 | 3.9 | 5.9 |
| Red meat (g/1000 kcal), mean | 31.8 | 35.8 | 36.8 | 36.3 | 32.1 |
| Age, mean | 63 | 62 | 62 | 62 | 62 |
| White, Non-Hispanic, % | 91 | 92 | 92 | 91 | 90 |
| College and post-college, % | 31 | 35 | 39 | 42 | 45 |
| Currently married, % | 65 | 69 | 70 | 70 | 69 |
| Positive family history of cancer, % | 48 | 49 | 49 | 49 | 49 |
| Never smoker, % | 33 | 34 | 36 | 37 | 36 |
| Current smoker or quit <1 year ago, % | 19 | 16 | 14 | 12 | 10 |
| Alcohol intake (g/day), mean | 18.4 | 13.0 | 11.6 | 10.2 | 8.4 |
| BMI (kg/m2), mean | 26.7 | 27.1 | 27.2 | 27.2 | 27.4 |
| Obese (BMI≥ 30), % | 19 | 21 | 22 | 22 | 23 |
| Physical Activity (Vigorous ≥ 20 mins), % | |||||
| Never | 24 | 20 | 18 | 17 | 16 |
| ≥ 5 times / week | 19 | 18 | 19 | 19 | 21 |
| Current MHT use, % of women | 41 | 44 | 44 | 46 | 45 |
| Dietary intakes per day, mean | |||||
| Fruit (MPED servings/1000 kcal) | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Vegetables (MPED servings/1000 kcal) | 2.0 | 2.1 | 2.2 | 2.3 | 2.5 |
| Total energy intake (kcal) | 1,941 | 1,844 | 1,826 | 1,810 | 1,757 |
N= 293,466 men; 198,720 women
Abbreviations: Q, Quintile; MHT, BMI, Body Mass Index, Menopausal Hormone Therapy, MPED, MyPyramid Equivalents Database
Substitution of white meat for red meat
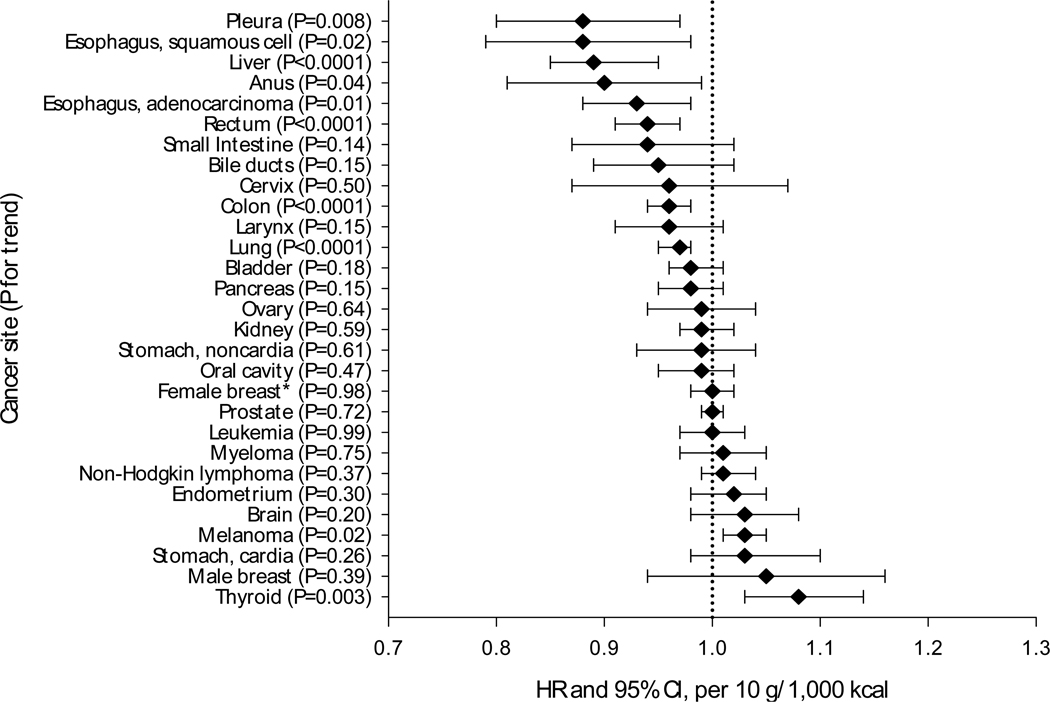
Figure 1 represents the risk associated with a 10 gram increase in white meat intake offset by a 10 gram decrease in red meat intake (total meat intake held constant). We observed 10% or greater reductions in risk for esophageal squamous cell carcinomas and cancers of the pleura, liver, and anus; and 3% to 7% reductions in risk for esophageal adenocarcinomas, and cancers of the colon, rectum, and lung. Conversely, we observed an elevated risk (3–8%) of melanoma and thyroid cancer. Intake of either poultry or fish, in substitution models for red meat, was similarly associated with lower risk of cancers of the esophagus, colon, rectum, liver, and lung, while only fish intake was associated with higher risk of melanoma (data not shown).
Figure 1. Multivariate Hazard Ratiosa and 95% Confidence Intervals for substitution effect of a 10 gram increase in total white meat intake and cancer risk, NIH-AARP Diet and Health Study (n = 492,186).
a Substitution (for red meat) model: adjusted for total meat intake (10g/1000 kcal), age, sex, education, marital status, family history of cancer, race, body mass index, smoking status, frequency of vigorous physical activity, menopausal hormone therapy in women, and intake of alcohol, fruit, vegetables, and total energy
* Post-menopausal cases only
Addition of white meat
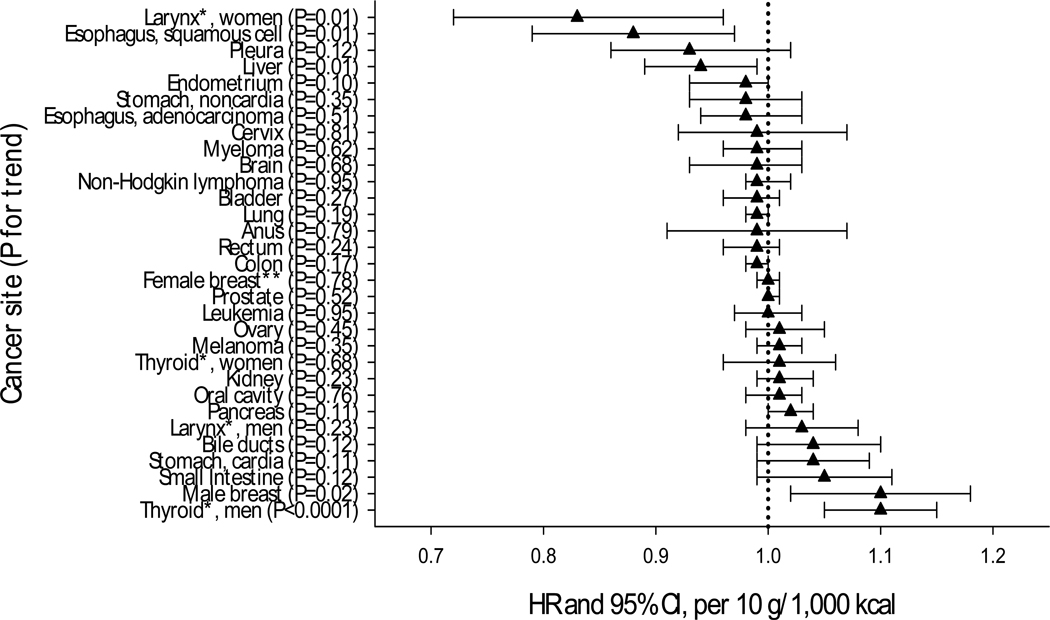
The results of the additive analysis represent the risk associated with an independent increase in white meat intake, while holding red meat intake constant. Total white meat intake was inversely associated with cancers of the esophagus, liver, and lung, but positively associated with melanoma (data not shown). A 10 gram increase in poultry intake (Figure 2) was associated with lower risk of liver cancer and esophageal squamous cell carcinoma, as well as laryngeal cancer in women only (Pinteraction sex= 0.01). In men, poultry intake was positively associated with thyroid (Pinteraction sex= 0.03) and male breast cancer. Those in the highest, compared with lowest quintile of poultry intake had a lower risk of lung, bladder, and rectal cancer (Table 2). Among never smokers, the inverse association between poultry intake and lung cancer risk remained consistent [HR and 95% CI for fifth versus first quintile: 0.78 (0.58–1.03); Ptrend=0.04, and 10 g/1000 kcal: 0.95 (0.91–0.99); Ptrend=0.03; data in text only].
Figure 2. Multivariate Hazard Ratiosa and 95% Confidence Intervals for addition effect of a 10 gram increase in poultry intake and cancer risk, NIH-AARP Diet and Health Study (n = 492,186).
a Addition model: adjusted for red meat intake and fish intake, age, sex, education, marital status, family history of cancer, race, body mass index, smoking status, frequency of vigorous physical activity, menopausal hormone therapy in women, and intake of alcohol, fruit, vegetables, and total energy
* Significant interaction with sex
** Post-menopausal cases only
Table 2.
Multivariate Hazard Ratiosa and 95% Confidence Intervals for additive effect of an increase in poultry or fish intake and cancer risk, NIH-AARP Diet and Health Study (n = 492,186)
| Q1 | Q2 | Q3 | Q4 | Q5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer site | Dietary intake | ref | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | Ptrend |
| Oral cavity | |||||||||||
| n=1,305 | Poultry | 1.00 | 1.07 | 0.90–1.26 | 0.97 | 0.81–1.16 | 1.11 | 0.93–1.32 | 1.05 | 0.87–1.26 | 0.62 |
| Fish | 1.00 | 1.03 | 0.87–1.22 | 1.00 | 0.84–1.18 | 0.97 | 0.81–1.16 | 0.90 | 0.75–1.09 | 0.17 | |
| Larynx | |||||||||||
| n=472 (M) | Poultryb | 1.00 | 0.89 | 0.68–1.17 | 1.00 | 0.76–1.32 | 0.96 | 0.72–1.29 | 1.04 | 0.77–1.41 | 0.61 |
| n=95 (F) | Poultryb | 1.00 | 1.04 | 0.61–1.77 | 0.72 | 0.39–1.34 | 0.79 | 0.42–1.50 | 0.27 | 0.10–0.71 | 0.004 |
| Fish | 1.00 | 1.01 | 0.78–1.29 | 0.88 | 0.68–1.15 | 1.00 | 0.77–1.31 | 1.03 | 0.79–1.36 | 0.68 | |
| Esophagus, squamous cell | |||||||||||
| n=185 | Poultry | 1.00 | 0.97 | 0.66–1.42 | 0.67 | 0.43–1.05 | 0.44 | 0.26–0.76 | 0.69 | 0.42–1.13 | 0.04 |
| Fish | 1.00 | 0.95 | 0.62–1.47 | 0.99 | 0.63–1.55 | 0.93 | 0.58–1.48 | 0.98 | 0.61–1.59 | 0.84 | |
| Esophagus, adenocarcinoma | |||||||||||
| n=553 | Poultry | 1.00 | 0.92 | 0.71–1.19 | 0.85 | 0.65–1.11 | 1.03 | 0.79–1.35 | 0.95 | 0.72–1.26 | 0.92 |
| Fish | 1.00 | 0.94 | 0.73–1.21 | 0.88 | 0.68–1.14 | 0.83 | 0.63–1.09 | 0.78 | 0.59–1.03 | 0.06 | |
| Stomach, cardia | |||||||||||
| n=418 | Poultry | 1.00 | 0.77 | 0.56–1.04 | 0.90 | 0.66–1.22 | 0.99 | 0.73–1.34 | 1.00 | 0.73–1.36 | 0.37 |
| Fish | 1.00 | 1.03 | 0.76–1.39 | 0.96 | 0.70–1.31 | 1.05 | 0.77–1.43 | 0.98 | 0.71–1.35 | 0.85 | |
| Stomach, noncardia | |||||||||||
| n=510 | Poultry | 1.00 | 1.20 | 0.79–1.33 | 0.92 | 0.70–1.21 | 0.91 | 0.69–1.21 | 0.80 | 0.59–1.07 | 0.10 |
| Fish | 1.00 | 1.08 | 0.82–1.41 | 0.81 | 0.60–1.09 | 1.22 | 0.93–1.61 | 1.11 | 0.84–1.48 | 0.27 | |
| Small Intestine | |||||||||||
| n=379 | Poultry | 1.00 | 0.98 | 0.63–1.53 | 1.21 | 0.78–1.87 | 1.44 | 0.94–2.21 | 1.41 | 0.91–2.17 | 0.07 |
| Fish | 1.00 | 1.00 | 0.66–1.50 | 1.28 | 0.86–1.88 | 0.70 | 0.44–1.10 | 0.84 | 0.54–1.30 | 0.99 | |
| Colon | |||||||||||
| n=5,095 | Poultry | 1.00 | 1.04 | 0.96–1.13 | 0.96 | 0.88–1.05 | 1.03 | 0.95–1.13 | 0.97 | 0.89–1.07 | 0.43 |
| Fish | 1.00 | 0.98 | 0.90–1.07 | 0.98 | 0.90–1.07 | 0.96 | 0.88–1.05 | 0.95 | 0.87–1.04 | 0.30 | |
| Rectum | |||||||||||
| n=1,884 | Poultry | 1.00 | 0.87 | 0.76–1.00 | 0.99 | 0.86–1.14 | 0.95 | 0.82–1.10 | 0.84 | 0.72–0.98 | 0.08 |
| Fish | 1.00 | 0.95 | 0.82–1.09 | 0.81 | 0.70–0.94 | 0.87 | 0.75–1.01 | 0.96 | 0.83–1.11 | 0.88 | |
| Anus | |||||||||||
| n=164 | Poultry | 1.00 | 1.01 | 0.63–1.62 | 1.01 | 0.63–1.64 | 0.85 | 0.51–1.42 | 0.92 | 0.55–1.54 | 0.58 |
| Fish | 1.00 | 1.26 | 0.80–2.01 | 1.07 | 0.66–1.74 | 0.86 | 0.51–1.44 | 0.71 | 0.41–1.23 | 0.06 | |
| Liver | |||||||||||
| n=582 | Poultry | 1.00 | 0.95 | 0.75–1.21 | 0.79 | 0.61–1.01 | 0.75 | 0.57–0.98 | 0.75 | 0.57–0.99 | 0.03 |
| Fish | 1.00 | 1.00 | 0.78–1.28 | 1.01 | 0.79–1.30 | 0.95 | 0.73–1.24 | 0.86 | 0.65–1.13 | 0.16 | |
| Bile ducts | |||||||||||
| n=307 | Poultry | 1.00 | 1.10 | 0.76–1.59 | 1.14 | 0.78–1.65 | 1.06 | 0.72–1.55 | 1.27 | 0.87–1.85 | 0.25 |
| Fish | 1.00 | 0.72 | 0.49–1.06 | 0.87 | 0.60–1.25 | 1.08 | 0.76–1.53 | 1.01 | 0.71–1.45 | 0.30 | |
| Pancreas | |||||||||||
| n=1,727 | Poultry | 1.00 | 1.03 | 0.89–1.20 | 1.02 | 0.87–1.19 | 1.11 | 0.95–1.30 | 1.10 | 0.94–1.29 | 0.22 |
| Fish | 1.00 | 1.07 | 0.92–1.25 | 1.00 | 0.86–1.17 | 1.08 | 0.93–1.27 | 1.12 | 0.96–1.31 | 0.15 | |
| Lung | |||||||||||
| n=9,751 | Poultry | 1.00 | 0.94 | 0.89–1.00 | 0.94 | 0.89–1.00 | 0.90 | 0.84–0.96 | 0.91 | 0.85–0.97 | 0.01 |
| Fish | 1.00 | 1.03 | 0.97–1.10 | 1.08 | 1.01–1.15 | 1.04 | 0.98–1.11 | 1.01 | 0.94–1.08 | 0.65 | |
| Pleura | |||||||||||
| n=218 | Poultry | 1.00 | 0.94 | 0.63–1.41 | 1.16 | 0.78–1.72 | 0.71 | 0.45–1.14 | 0.93 | 0.60–1.46 | 0.52 |
| Fish | 1.00 | 1.76 | 1.18–2.63 | 1.09 | 0.69–1.71 | 1.08 | 0.68–1.71 | 1.04 | 0.64–1.67 | 0.27 | |
| Bladder | |||||||||||
| n=2,296 | Poultry | 1.00 | 0.86 | 0.76–0.98 | 0.89 | 0.78–1.01 | 0.92 | 0.81–1.05 | 0.83 | 0.73–0.96 | 0.07 |
| Fish | 1.00 | 1.03 | 0.90–1.18 | 1.08 | 0.95–1.23 | 1.15 | 1.00–1.31 | 1.13 | 0.99–1.29 | 0.06 | |
| Kidney | |||||||||||
| n=2,065 | Poultry | 1.00 | 0.94 | 0.82–1.08 | 0.94 | 0.82–1.08 | 0.94 | 0.82–1.09 | 1.00 | 0.87–1.16 | 0.51 |
| Fish | 1.00 | 1.01 | 0.88–1.16 | 1.07 | 0.93–1.24 | 1.12 | 0.97–1.29 | 1.10 | 0.93–1.28 | 0.15 | |
| Thyroid | |||||||||||
| n=250 (M) | Poultryb | 1.00 | 1.23 | 10.79–1.93 | 1.23 | 0.78–1.93 | 1.46 | 0.94–2.26 | 1.74 | 1.14–2.67 | 0.005 |
| n=333 (F) | Poultryb | 1.00 | 0.73 | 0.52–1.03 | 0.68 | 0.48–0.96 | 0.69 | 0.49–0.98 | 0.82 | 0.58–1.14 | 0.66 |
| Fish | 1.00 | 1.15 | 0.88–1.51 | 1.14 | 0.87–1.50 | 1.25 | 0.95–1.64 | 1.18 | 0.90–1.55 | 0.38 | |
| Non–Hodgkin lymphoma | |||||||||||
| n=2,905 | Poultry | 1.00 | 1.04 | 0.92–1.16 | 1.06 | 0.94–1.19 | 0.97 | 0.86–1.10 | 0.99 | 0.87–1.12 | 0.51 |
| Fish | 1.00 | 0.98 | 0.87–1.11 | 1.08 | 0.96–1.22 | 1.07 | 0.95–1.20 | 1.09 | 0.97–1.23 | 0.09 | |
| Leukemia | |||||||||||
| n=1,625 | Poultry | 1.00 | 0.97 | 0.83–1.13 | 0.97 | 0.82–1.13 | 1.01 | 0.86–1.18 | 1.06 | 0.90–1.25 | 0.31 |
| Fish | 1.00 | 1.00 | 0.85–1.17 | 1.09 | 0.93–1.27 | 0.95 | 0.81–1.12 | 1.00 | 0.85–1.18 | 0.74 | |
| Melanoma | |||||||||||
| n=2,960 | Poultry | 1.00 | 1.09 | 0.96–1.23 | 1.20 | 1.06–1.35 | 1.16 | 1.02–1.31 | 1.03 | 0.91–1.17 | 0.86 |
| Fish | 1.00 | 1.10 | 0.97–1.24 | 1.12 | 0.99–1.26 | 1.17 | 1.04–1.32 | 1.19 | 1.05–1.34 | 0.01 | |
| Brain | |||||||||||
| n=749 | Poultry | 1.00 | 1.23 | 0.97–1.55 | 1.20 | 0.94–1.52 | 1.04 | 0.81–1.34 | 1.10 | 0.86–1.41 | 0.95 |
| Fish | 1.00 | 1.14 | 0.91–1.45 | 1.04 | 0.82–1.33 | 1.22 | 0.97–1.55 | 1.05 | 0.83–1.35 | 0.77 | |
| Myeloma | |||||||||||
| n=893 | Poultry | 1.00 | 1.09 | 0.88–1.33 | 0.98 | 0.79–1.21 | 0.92 | 0.73–1.14 | 0.93 | 0.74–1.15 | 0.19 |
| Fish | 1.00 | 0.94 | 0.76–1.16 | 0.94 | 0.76–1.16 | 1.01 | 0.82–1.25 | 1.03 | 0.84–1.28 | 0.47 | |
| Male breast | |||||||||||
| n=129 | Poultry | 1.00 | 1.08 | 0.60–1.97 | 1.41 | 0.79–2.52 | 1.46 | 0.81–2.63 | 1.69 | 0.95–3.02 | 0.06 |
| Fish | 1.00 | 1.14 | 0.67–1.94 | 0.74 | 0.41–1.35 | 0.97 | 0.56–1.70 | 0.89 | 0.51–1.58 | 0.66 | |
| Prostate | |||||||||||
| n=23,453 | Poultry | 1.00 | 1.06 | 1.02–1.10 | 1.04 | 1.00–1.09 | 1.04 | 0.99–1.08 | 1.05 | 1.00–1.09 | 0.23 |
| Fish | 1.00 | 1.03 | 0.99–1.07 | 1.04 | 1.00–1.09 | 1.05 | 1.00–1.09 | 1.02 | 0.98–1.06 | 0.67 | |
| Female breast, post–menopausal | |||||||||||
| n=7,181 | Poultry | 1.00 | 0.97 | 0.90–1.04 | 0.98 | 0.91–1.06 | 0.99 | 0.92–1.07 | 0.98 | 0.90–1.06 | 0.92 |
| Fish | 1.00 | 1.05 | 0.98–1.14 | 1.05 | 0.98–1.14 | 1.07 | 0.99–1.15 | 1.05 | 0.97–1.14 | 0.40 | |
| Endometrium | |||||||||||
| n=1,593 | Poultry | 1.00 | 1.05 | 0.89–1.24 | 1.09 | 0.93–1.28 | 1.02 | 0.87–1.21 | 0.93 | 0.78–1.10 | 0.15 |
| Fish | 1.00 | 1.04 | 0.88–1.22 | 0.97 | 0.83–1.15 | 1.15 | 0.98–1.35 | 1.12 | 0.95–1.32 | 0.06 | |
| Ovary | |||||||||||
| n=758 | Poultry | 1.00 | 1.10 | 0.87–1.39 | 1.17 | 0.93–1.48 | 1.07 | 0.84–1.36 | 1.10 | 0.87–1.36 | 0.75 |
| Fish | 1.00 | 1.00 | 0.80–1.26 | 0.86 | 0.68–1.09 | 1.04 | 0.83–1.31 | 1.04 | 0.83–1.32 | 0.41 | |
| Cervix | |||||||||||
| n=147 | Poultry | 1.00 | 0.81 | 0.47–1.40 | 1.33 | 0.81–2.19 | 0.97 | 0.56–1.67 | 1.10 | 0.65–1.88 | 0.66 |
| Fish | 1.00 | 0.91 | 0.55–1.53 | 0.88 | 0.53–1.49 | 0.91 | 0.54–1.54 | 0.90 | 0.54–1.52 | 0.82 | |
N= 293,466 men; 198,720 women
Abbreviations: M, male; F, female
Addition model: adjusted for red meat intake, age, sex, education, marital status, family history of cancer, race, body mass index, smoking status, frequency of vigorous physical activity, menopausal hormone therapy in women, and intake of alcohol, fruit, vegetables, and total energy; mutually adjusted for intake of fish or poultry
Statistically significant interaction with sex
Total fish intake was associated with higher risk of melanoma (Table 2); this appeared to be driven by intake of canned tuna [HR and 95% for fifth versus first quintile: 1.30 (1.16–1.46); Ptrend<0.0001; data in text only]. Similarly, we observed a suggestive increased risk for canned tuna intake and bladder cancer [1.13 (0.99–1.28); Ptrend=0.04], as well as ovarian cancer [1.28 (1.02–1.61); Ptrend=0.05]. Conversely, intake of canned tuna was associated with lower risk of liver cancer [0.74 (0.55–0.96); Ptrend=0.05; data in text only].
Various subanalyses including a lag analysis excluding the first two years of follow-up and stratified analyses by smoking status produced comparable results. We also did not observe any striking differences in risk across cancer-sites for intake of processed poultry, poultry consumed with or without skin, or fish intake excluding deep-fried fish compared to the overall results presented.
Discussion
In this large U.S. cohort, poultry and fish intake was associated with lower risk of digestive and respiratory cancers. In analyses holding total meat intake constant, such that higher intake of poultry and fish coincides with lower intake of red meat, we observed inverse associations for cancers of the esophagus, liver, colon, rectum, anus, lung, and pleura. With red meat intake held constant, poultry intake remained inversely associated with esophageal squamous cell carcinoma, liver and lung cancer, while associations with fish intake were mixed. Although we cannot completely rule out the possibility of residual confounding by smoking status or other factors, similar associations were observed among never-smokers. Our findings for poultry and fish intake and cancer risk seemingly reflect the importance of red meat substitution, but are also suggestive of an effect independent of red meat intake.
The inverse associations we observed for poultry and fish intake with lower gastrointestinal cancers appeared to largely driven by the red meat substitution effect. Consistent with our findings, the ratio of [higher] red meat to [lower] poultry and fish intake has been previously associated with an increased risk of colorectal cancer 18–19, while null findings in the additive analysis are consistent with summary data from a range of prospective cohorts 20. A number of studies provide possible mechanisms for these results. Poultry and fish, relative to red meat, are lower in saturated fat and heme iron, potential inducers of oxidative stress and DNA damage 21. Consumption of white meat is also likely to result in significantly lower exposure to carcinogenic N-nitroso compounds (NOCs) 22, which form more readily with red and processed meat intake due to the higher concentration of heme iron 23. Human feeding studies have shown that throughout the gastrointestinal tract intake of white meat versus red meat results in lower levels of DNA strand breaks, ileal and fecal apparent total NOCs, and DNA adducts 24–26.
Significant findings for poultry intake and lower risk of esophageal squamous cell carcinoma, liver and lung cancer in both the substitution and addition analyses suggest that other components of a high white meat diet, independent of red meat, may also be important. These findings are consistent with previous reports for total white meat intake in this cohort 27–28. However, poultry intake was positively associated with esophageal cancer 29 and not related to lung cancer risk 30 in the European Prospective Investigation into Cancer and Nutrition (EPIC). Residual confounding by smoking status does not appear to have greatly influenced our findings, as poultry intake remained consistently associated with lower risk of lung cancer in analyses restricted to never smokers. Lower levels of pro-oxidant and mutagenic activity related to intake of white versus red meat may be relevant for lung and other smoking-related cancers 31–32, but there is little mechanistic research in this area. Poultry intake was also inversely associated with laryngeal cancer in women, but positively associated with thyroid and male breast cancer in men in this cohort. However, scant evidence from retrospective studies provide little support for these findings 33–35 and we cannot rule out the possibility of residual confounding by hereditary and other environmental factors.
The mechanism whereby poultry intake alone may modulate cancer risk remains unclear. We examined and adjusted for a multitude of key confounders; however, as high intake of poultry and fish often clusters with a healthier overall eating pattern and lifestyle 36, the possibility of residual confounding by other factors remains. With intake of red meat, fish, fruit, vegetables, alcohol, and total energy held constant in multivariate additive models, it is plausible that an increase in poultry intake may represent displacement of other foods within the diet, such as refined grains and desserts. Varied risks by sex for some cancers may be related, at least in part, to overall differences in reporting, diet and lifestyle habits 36–37 and/or preferences related to the way the meat is prepared and consumed 38–40.
Consistent with many prospective cohort studies 4, 20, 41–42, we found little evidence of a protective role for total fish intake and cancer risk, perhaps surprising as experimental evidence suggests that the anti-inflammatory, long-chain n-3 fatty acids in concentrated doses of fish oil inhibit cancer development and progression 5. However the participants in our study and U.S. consumers, in general, eat very little fish 3. Additionally, some species of fish may be a source of potential carcinogens, such as polychlorinated biphenyls, dioxins, and mercury 6–7. Queries for fish intake on the standardized dietary questionnaire included little detail, and with the exception of canned tuna, did not allow us to differentiate fish types. Canned tuna intake was inversely associated with liver cancer, but positively associated with ovarian cancer and melanoma in this cohort. There is little prospective evidence to support a role of fish intake in ovarian cancer 43–45; however, one other study observed a positive association between intake of cod-liver oil and melanoma risk 46. Without adjustment for all the pertinent risk factors, particularly sun exposure for melanoma risk, confounding may explain the associations we observed.
The NIH-AARP cohort presented a sufficiently large sample to investigate the role of poultry and fish intake and cancer risk across a range of high- and low-incidence malignancies in U.S. men and women. With over 70,000 cancer cases, it is unlikely that any substantial effects of white meat intake on cancer risk may have been missed. The prospective design avoids potential biases due to recall and selection that may have affected the results of previous case-control studies. However, diet and lifestyle information ascertained in the baseline questionnaire among older adults may not be entirely reflective of lifelong cumulative exposures or the most pertinent time period for cancer etiology. Due to a large number of comparisons, it is possible that some of the more modestly significant results may be attributable to chance and results for cancers, particularly those with a small numbers of cases, should be interpreted with caution.
In the largest U.S. prospective investigation of white meat and cancer risk to-date, intake of poultry and fish, mainly as a substitute for red meat, was associated with lower risk of cancers of the esophagus, liver, colon, rectum, anus, pleura, and lung. Independent of red meat intake, we also observed inverse associations between poultry intake and risk of esophageal squamous cell carcinoma, liver and lung cancer. Consistent with several other cohorts, we found little convincing evidence for total fish intake and cancer risk. Our findings generally support the American Cancer Society’s dietary guidelines, which recommend limiting intake of red and processed meats and choosing poultry and fish, as lean alternatives 2. In light of the growing popularity of poultry in the U.S. and the paucity of prospective evidence, additional detailed investigations are needed to clarify the role of white meat intake in the etiology of cancer.
Acknowledgments
This study was funded by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDC) under contract with the Florida Department of Health (FDOH). The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis. We wish to acknowledge the loss and memory of Dr. Arthur Schatzkin, our leader, colleague, mentor, and friend.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans. 6 ed. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 2.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, Gansler T, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention: Reducing the Risk of Cancer With Healthy Food Choices and Physical Activity. CA Cancer J Clin. 2006 September 1;56(5):254–281. doi: 10.3322/canjclin.56.5.254. 2006. [DOI] [PubMed] [Google Scholar]
- 3.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14(04):575–583. doi: 10.1017/S1368980010002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 5.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004 Jun;79(6):935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 6.Groth Iii E. Ranking the contributions of commercial fish and shellfish varieties to mercury exposure in the United States: Implications for risk communication. Environ Res. 2010;110(3):226–236. doi: 10.1016/j.envres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Rimm EB. Fish Intake, Contaminants, and Human Health: Evaluating the Risks and the Benefits. JAMA. 2006 October 18;296(15):1885–1899. doi: 10.1001/jama.296.15.1885. 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A Prospective Study of Red and Processed Meat Intake in Relation to Cancer Risk. PLoS Med. 2007 December 01;4(12):e325. doi: 10.1371/journal.pmed.0040325. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009 Mar 23;169(6):562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001 Dec 15;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 11.Thompson FE, Kipnis V, Midthune D, Freedman LS, Carroll RJ, Subar AF, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008 Feb;11(2):183–195. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 12.Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, Kipnis V, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000 Aug 1;152(3):279–286. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 13.Michaud DS, Midthune D, Hermansen S, Leitzmann MF, Harlan LC, et al. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. Journal of Registry Management. 2005;32:70–77. [Google Scholar]
- 14.International Classification of Diseases for Oncology. Third edition. Geneva: World Health Organization; 2000. [Google Scholar]
- 15.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986 Jul;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 16.Kulldorff M, Sinha R, Chow W-H, Rothman N. Comparing odds ratios for nested subsets of dietary components. International Journal of Epidemiology. 2000 December 1;29(6):1060–1064. doi: 10.1093/ije/29.6.1060. 2000. [DOI] [PubMed] [Google Scholar]
- 17.Friday JE, Bowman SA. MyPyramid Equivalents Database for USDA Survey Food Codes, 1994–2002 Version 1.0. [Online] Beltsville MD: USDA, ARS, Community Nutrition Research Group; 2006. [cited January 2009]; Available from: http://www.ars.usda.gov/ba/bhnrc/fsrg/. [Google Scholar]
- 18.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, et al. Meat Consumption and Risk of Colorectal Cancer. JAMA. 2005 January 12;293(2):172–182. doi: 10.1001/jama.293.2.172. 2005. [DOI] [PubMed] [Google Scholar]
- 19.English DR, MacInnis RJ, Hodge AM, Hopper JL, Haydon AM, Giles GG. Red Meat, Chicken, and Fish Consumption and Risk of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2004 September 1;13(9):1509–1514. 2004. [PubMed] [Google Scholar]
- 20.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: A quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125(1):171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 21.Tappel A. Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med Hypotheses. 2007;68(3):562–564. doi: 10.1016/j.mehy.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Joosen AMCP, Kuhnle GGC, Aspinall SM, Barrow TM, Lecommandeur E, Azqueta A, et al. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis. 2009 August 1;30(8):1402–1407. doi: 10.1093/carcin/bgp130. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003 May 15;63(10):2358–2360. [PubMed] [Google Scholar]
- 24.Kuhnle GG, Bingham SA. Dietary meat, endogenous nitrosation and colorectal cancer. Biochem Soc Trans. 2007 Nov;35(Pt 5):1355–1357. doi: 10.1042/BST0351355. [DOI] [PubMed] [Google Scholar]
- 25.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr. 2002 Nov;132(11 Suppl):3522S–3525S. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- 26.Joosen AMCP, Lecommandeur E, Kuhnle GGC, Aspinall SM, Kap L, Rodwell SA. Effect of dietary meat and fish on endogenous nitrosation, inflammation and genotoxicity of faecal water. Mutagenesis. 2010 May 1;25(3):243–247. doi: 10.1093/mutage/gep070. 2010. [DOI] [PubMed] [Google Scholar]
- 27.Freedman ND, Cross AJ, McGlynn KA, Abnet CC, Park Y, Hollenbeck AR, et al. Association of Meat and Fat Intake With Liver Disease and Hepatocellular Carcinoma in the NIH-AARP Cohort. J Natl Cancer Inst. 2010 September 8;102(17):1354–1365. doi: 10.1093/jnci/djq301. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, et al. Meat Consumption and Risk of Esophageal and Gastric Cancer in a Large Prospective Study. Am J Gastroenterol. 2010 doi: 10.1038/ajg.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, et al. Meat Intake and Risk of Stomach and Esophageal Adenocarcinoma Within the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006 March 1;98(5):345–354. doi: 10.1093/jnci/djj071. 2006. [DOI] [PubMed] [Google Scholar]
- 30.Linseisen J, Rohrmann S, Bueno-de-Mesquita B, Büchner F, Boshuizen H, Agudo A, et al. Consumption of meat and fish and risk of lung cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control. 2011:1–10. doi: 10.1007/s10552-011-9764-1. [DOI] [PubMed] [Google Scholar]
- 31.Tasevska N, Sinha R, Kipnis V, Subar AF, Leitzmann MF, Hollenbeck AR, et al. A prospective study of meat, cooking methods, meat mutagens, heme iron, and lung cancer risks. Am J Clin Nutr. 2009 Apr 15; doi: 10.3945/ajcn.2008.27272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vardavas CI, Flouris AD, Tsatsakis A, Kafatos AG, Saris WHM. Does adherence to the Mediterranean diet have a protective effect against active and passive smoking? Public Health. 2011;125(3):121–128. doi: 10.1016/j.puhe.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Bosetti C, La Vecchia C, Talamini R, Negri E, Levi F, Dal Maso L, et al. Food groups and laryngeal cancer risk: A case-control study from Italy and Switzerland. Int J Cancer. 2002;100(3):355–360. doi: 10.1002/ijc.10485. [DOI] [PubMed] [Google Scholar]
- 34.Weiss JR, Moysich KB, Swede H. Epidemiology of Male Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2005 January 1;14(1):20–26. 2005. [PubMed] [Google Scholar]
- 35.Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20(1):75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 36.Flood A, Rastogi T, Wirfalt E, Mitrou PN, Reedy J, Subar AF, et al. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr. 2008 Jul;88(1):176–184. doi: 10.1093/ajcn/88.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker AH, Wardle J. Sex differences in fruit and vegetable intake in older adults. Appetite. 2003;40(3):269–275. doi: 10.1016/s0195-6663(03)00014-x. [DOI] [PubMed] [Google Scholar]
- 38.Frandsen H. Biomonitoring of urinary metabolites of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) following human consumption of cooked chicken. Food Chem Toxicol. 2008;46(9):3200–3205. doi: 10.1016/j.fct.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Iwasaki M, Kataoka H, Ishihara J, Takachi R, Hamada GS, Sharma S, et al. Heterocyclic amines content of meat and fish cooked by Brazilian methods. J Food Compost Anal. 2010 Feb 1;23(1):61–69. doi: 10.1016/j.jfca.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol. 2001 May;39(5):423–436. doi: 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 41.Spencer EA, Key TJ, Appleby PN, Dahm CC, Keogh RH, Fentiman IS, et al. Meat, poultry and fish and risk of colorectal cancer: pooled analysis of data from the UK dietary cohort consortium. Cancer Causes Control. 2010 May 1; doi: 10.1007/s10552-010-9569-7. [DOI] [PubMed] [Google Scholar]
- 42.Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr. 2010 November 1;92(5):1223–1233. doi: 10.3945/ajcn.2010.29530. 2010. [DOI] [PubMed] [Google Scholar]
- 43.Kushi LH, Mink PJ, Folsom AR, Anderson KE, Zheng W, Lazovich D, et al. Prospective Study of Diet and Ovarian Cancer. Am J Epidemiol. 1999 January 1;149(1):21–31. doi: 10.1093/oxfordjournals.aje.a009723. 1999. [DOI] [PubMed] [Google Scholar]
- 44.Schulz M, Nöthlings U, Allen N, Onland-Moret NC, Agnoli C, Engeset D, et al. No Association of Consumption of Animal Foods with Risk of Ovarian Cancer. Cancer Epidemiol Biomarkers Prev. 2007 April 1;16(4):852–855. doi: 10.1158/1055-9965.EPI-07-0054. 2007. [DOI] [PubMed] [Google Scholar]
- 45.Larsson SC, Wolk A. No association of meat, fish, and egg consumption with ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 2005 Apr;14(4):1024–1025. doi: 10.1158/1055-9965.EPI-04-0795. [DOI] [PubMed] [Google Scholar]
- 46.Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: A prospective study of 50,757 Norwegian men and women. Int J Cancer. 1997;71(4):600–604. doi: 10.1002/(sici)1097-0215(19970516)71:4<600::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 47.Kolahdooz F, van der Pols JC, Bain CJ, Marks GC, Hughes MC, Whiteman DC, et al. Meat, fish, and ovarian cancer risk: results from 2 Australian case-control studies, a systematic review, and meta-analysis. Am J Clin Nutr. 2010 June 1;91(6):1752–1763. doi: 10.3945/ajcn.2009.28415. 2010. [DOI] [PubMed] [Google Scholar]
- 48.Hu J, La Vecchia C, DesMeules M, Negri E, Mery L Group CCRER. Meat and Fish Consumption and Cancer in Canada. Nutr Cancer. 2008;60(3):313–324. doi: 10.1080/01635580701759724. [DOI] [PubMed] [Google Scholar]