Abstract
Advances in understanding of the maintenance of the cardiac valves during normal cardiac function and response to injury have lead to several novel findings, including that there is contribution of extra-cardiac cells to the major cellular population of the valve: the valve interstitial cell (VIC). While suggested to occur in human heart studies, we have been able to experimentally demonstrate, using a mouse model, that cells of bone marrow hematopoietic stem cell origin engraft into the valves and synthesize collagen type I. Based on these initial findings, we sought to further characterize this cell population in terms of its similarity to VICs and begin to elucidate its contribution to valve homeostasis. To accomplish this, chimeric mice whose bone marrow was repopulated with enhanced green fluorescent protein (EGFP) expressing total nucleated bone marrow cells were used to establish a profile of EGFP+ valve cells in terms of their expression of hematopoietic antigens, progenitor markers, fibroblast- and myofibroblast-related molecules, as well as their distribution within the valves. Using this profile, we show that normal (non-irradiated, non-transplanted) mice have BM-derived cell populations that exhibit identical morphology and phenotype to those observed in transplanted mice. Collectively, our findings establish that the engraftment of bone marrow-derived cells occurs as part of normal valve homeostasis. Further, our efforts demonstrate that the use of myeloablative irradiation, which is commonly employed in studies involving bone marrow transplantation, does not elicit changes in the bone marrow-derived VIC phenotype in recipient mice.
Keywords: heart valve homeostasis, bone marrow, EGFP lineage tracing, CD45 positive cells
Introduction
Heart valves are dynamic structures located at the inlet and outlet segments of the left and right ventricles where they maintain unidirectional blood flow during early developmental stages and throughout post-natal life. The post-natal heart valves consist of an overlying endocardium that surrounds an inner extracellular matrix that is organized into three distinct layers: the collagen type I-rich fibrosa, the GAGs-rich spongiosa and the elastic layer, which contains a network of elastic fibers [1–3]. Valve interstitial cells (VICs) are the predominant cell population of the heart valves. They maintain this trilaminar organization of the extracellular matrix of the valve that is required for their proper function. VICs are a heterogenous cell population both in terms of their origin and their phenotype [2, 4, 5]. While the specific contribution of these cells to valve plasticity during both homeostasis and response to injury is incompletely understood, recent studies have provided new clues to the ability of VICs to respond to changes in composition of their extracellular matrix, cytokine signaling and biomechanical forces [6–8]. Based on these observations, VICs have been subclassifed based on their specific contribution to valve development, homeostasis and pathological remodeling [4].
An emerging concept is that cells of extra-cardiac origin contribute to maintenance of the post-natal VIC population. This was first suggested in studies analyzing gender mismatched homograft valve transplants. These studies showed that the donor heart valve fibroblasts were replaced over time by fibroblasts of host origin [9–12]. That circulating cells derived from the bone marrow contribute to the post-natal VIC population was indicated in human studies where donor cells were detected in the valves of individuals that had received gender mismatched bone marrow transplantation [13]. As we have previously shown in a mouse model that transplanted bone marrow-derived EGFP+ cells can give rise to VICs with fibroblast-like properties [14], we sought to further characterize these cells in the adult heart valves under homeostatic conditions. We first examined the distribution of EGFP+ bone marrow-derived cells (BMDCs) within the valves in irradiated-transplanted mice, then profiled these EGFP+ cells for expression of hematopoietic antigens, progenitor markers, fibroblast- and myofibroblast-related molecules. Using this profile generated from irradiated EGFP+ transplanted mice, we show that the valves of control (non-irradiated, non-transplanted) mice have identical BM-derived VIC populations. Collectively, our findings indicate that the recruitment of BM-derived cells that give rise to VICs is a normal homeostatic process.
Material and methods
Animals
All aspects of animal research have been conducted in accordance with guidelines set by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. Ten- to twelve-week old female EGFP-C57BL/6-Ly5.2 mice [15] were used as donors of EGFP+-BM-total nucleated cells (BM-TNCs) for transplantation. Donor cells were transplanted into ten- to fourteen-week old lethally-irradiated male C57BL/6-Ly5.1 mice. Highly engrafted mice were selected for subsequent analyses as previously described. Valves from non-transplanted age-matched Ly5.1 mice were used as controls.
Reagents
The following primary antibodies were used in this study: rat anti-mouse CD45, rat anti-mouse CD31/PECAM-1, rat anti-mouse CD34, rat anti-mouse CD45R/B220, rat anti-mouse Ly-6G and Ly-6C/Gr-1, hamster anti-mouse CD3e, hamster anti-mouse CD11c, rat anti-mouse I-A/I-E (BD Pharmingen, San Jose, CA), goat anti-mouse CD45 (R&D Systems, Minneapolis, MN), polyclonal chicken anti-GFP (AbCam, Cambridge, MA), rat anti-mouse CD11b/Mac-1, rat anti-mouse F4/80 (AbD Serotec, Raleigh, NC), mouse monoclonal anti-α-smooth muscle actin (Cy3 conjugate, Sigma Aldrich, Minn., MN), mouse monoclonal anti-Hsp47 (Calbiochem, Darmstadt, Germany). Heat-mediated antigen retrieval in citrate buffer (pH=6.0) (Vector Laboratories, Burlingame, CA) was used in some immunofluorescence experiments. All fluorochrome-conjugated secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA) and used at a final concentration of 7.0 μg/ml.
Bone Marrow Transplantation
A single bolus of 2.0 × 105 bone marrow total nucleated cells (BM-TNC) prepared from EGFP mice was injected into the tail veins of congenic mice that had received a single 850-cGy dose of whole-body irradiation using a 4 × 106 V linear accelerator. The level of blood chimerism was assayed one month after transplantation of EGFP+ cells and flow cytometric lineage analysis of hematopoietic engraftment was performed at two months as previously described.[14, 17–19]. Four- to six-month old transplanted mice that exhibited high levels of multi-lineage engraftment were used in our study (Supplemental Figure 1).
Histological Analyses of Valve Interstitial Cells
Hearts from wild-type/non-transplanted (n = 10) and chimeric/transplanted mice (n = 20) were prepared for paraffin sectioning as previously described [14], with the following modifications: fixation was performed by perfusion with Zinc-formalin (Anatech LTD, Battle Creek, MI) followed by a 30 minute post-harvest fixation at ambient temperature or overnight at 4°C. The left lung, left inferior hepatic lobe, spleen, kidney and a portion of the small intestine were isolated from all mice and were used as positive controls for immunofluorescence analyses.
Whole mount immunolabeling
Harvested valves (Supplemental Figure 2) were permeabilized in a 0.1% Triton X-100 in PBS for ten minutes at ambient temperature. After copious washing in PBS, the tissues were incubated in Background Buster (Innovex Biosciences, Richmond, CA) for one hour at ambient temperature with gentle agitation. After washing in PBSA (PBS + 0.01% sodium Azide) (5 × 20 minutes), valves were incubated with primary antibodies diluted in 1.0% BSA in PBSA overnight, at ambient temperature with gentle agitation. After washing in PBSA (5 × 20 minutes), tissues were incubated with coordinate secondary antibodies diluted in 1.0% BSA (in PBSA) overnight at ambient temperature with mixing. After copious washing in PBSA, nuclei were counterstained with Hoechst 33342 (Molecular Probes/Invitrogen, Eugene, OR) diluted in PBSA for 30 minutes at ambient temperature. Following the final wash procedure, individual valve leaflets were isolated and mounted onto glass slides using Fluoro-Gel (Electron Microscopy Sciences, Hatfield, PA). For sectional analysis, bisected hearts were labeled with primary antibodies, washed in PBSA and fixed in Zinc-formalin. Seven- micron sections were cut, cleared in Histoclear II (National Diagnostics, Atlanta, GA), and rehydrated through graded ethanols to PBSA. Primary antibodies were immunolocalized by incubation with coordinate secondary antibodies, diluted in 1.0% BSA in PBS, for one hour. After copious washing in PBSA, nuclear counterstaining was performed with Hoechst 33342 as described above. After washing, sections were mounted in Fluoro-Gel (as above) and imaged.
Immunofluorescence analysis
Both whole-mount valve leaflets and sections were imaged using a TCS SP5 AOBS laser scanning confocal microscope (Leica Microsystems, Inc., Exton, PA). For excitation, we used the Diode laser (405 nm, 50 mW continuous wave), the HeNe Orange laser (594 nm, 2.5 mW, continuous wave) and the HeNe Red laser (633 nm, 10 mW, continuous wave). Seven to fifteen optical sections were captured in the Z-axis from every sample with an AOTF 4 channel detector (spectral range: 400–800 nm) and a K-scanner with two independent galvanometers. The thickness of individual optical sections was 2.5 μm for objective 10X (HC PL APO CS, air, numeric aperture: 0.40) and 1.0 μm for 63X (HCX PL APO CS, oil, numeric aperture: 1.4-0.6). Samples were analyzed using bidirectional scanning with four-time lineage average to generate 2048×2048 pixel images. Images were exported in tiff format and optical sections were projected using Image J (National Institutes of Health, Bethesda, MD). In the case of high magnification confocal scans (63X), confocal scans three consecutive optical layers were projected. Images were processed using Adobe Photoshop CS4 (Adobe Systems, Inc., San Jose, CA).
Results
Bone marrow-derived GFP+ exhibit specific distribution cells in the heart valves of transplanted mice
Based on our previous report that EGFP+ HSCs contribute to the valve interstitial cell population in the post-natal mouse valve leaflets [14], we sought to further characterize their phenotype and distribution in the post-natal leaflets using both a model of EGFP+ bone marrow transplantation as well as complementary immunofluorescence analysis in unmanipulated (non-transplanted) mice. First, to evaluate bone marrow-derived cell (BMDC) contribution to the heart valve interstitial cell populations under non-pathological conditions, we transplanted bone marrow total nucleated cells (BM-TNCs) from EGFP+ donor mice into lethally irradiated congenic, non-EGFP adult mice (hereafter referred to as transplanted mice). Two months after BM transplantation, we prepared four-chamber view sections of the hearts from transplanted mice and immunolabeled them with an antibody to GFP.
Figure 1 provides an overview of the atrioventricular (mitral valve and tricuspid valve, Figure 1A) and semilunar valves (aortic valve, Figure 1B) and pulmonary valve, Figure 1C) from a transplanted mouse. GFP+ BMDCs are observed throughout the cardiac tissues, as can be seen in the interatrial and interventricular septae (Figure 1A). Importantly, BMDCs engraft with a specific localization pattern in the heart valves. In the AV valves, the GFP+ BMDCs are localized predominantly at the atrial sides of the leaflets (Figure 1D, E), while in the semilunar valves they are mostly at the ventricular sides (Figure 1F, G). Toward the distal end (free edge) of the individual valve leaflets, the GFP+ BMDCs exhibit a less restricted localization.
Figure 1. BMDCs (GFP+) in the transplanted mouse heart valves exhibit specific localization.
A four-chamber view of cardiac valves from a chimeric heart immunolabeled with anti-GFP (green) and counterstained with Hoechst 33342 nuclear stain(blue). (A) Montage depicting the atrioventricular valves: (B) the aortic valve and (C) the pulmonary valve. (D) and (E) are high magnifications of the boxed areas on the mural mitral and septal tricuspid leaflets showing that the majority of the GFP+ cells in the atrioventricular valves are localized to the atrial side (a. s.). (F,G) are high magnifications of the boxed areas on the left aortic cusp (from B) and right pulmonary cusp (from C) showing that the GFP+ cells are predominantly localized to the ventricular side (v.s.) of the semilunar valves. LA-left atrium, LV-left ventricle, RA-right atrium, RV-right ventricle, ias-interatrial septum, ivs-interventricular septum, Ao-aorta, Pu-pulmonary trunk, ao.s.-aortic side, pu.s.-pulmonary side. Scale bars: 500 μm (A–C), 50 μm (D–G).
Bone marrow-derived GFP+ cells in the heart valves express CD45 and represent two morphologically distinct cell populations
For a detailed characterization of the GFP+ BMDCs in the valve leaflets, we chose to investigate their distribution and phenotype in the aortic (anterior) leaflet of the mitral valve. All results presented herein were confirmed in the leaflets of the other cardiac valves (not shown).
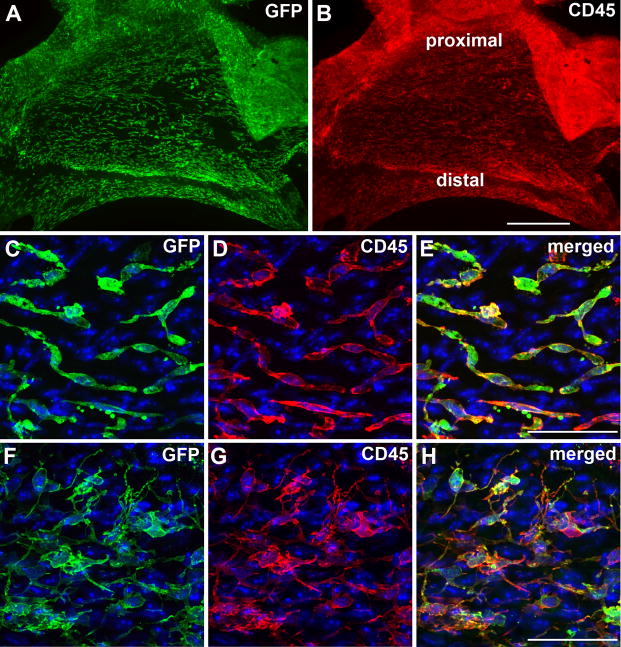
First, to investigate which compartment of the bone marrow (hematopoietic or stromal/mesenchymal) is the source of the GFP+ cells in the heart valves, we used anti-CD45 immunofluorescence analysis combined with anti-GFP in whole-mount valve leaflets. As seen in Figure 2, GFP+ (Fig. 2A) and CD45+ (Fig. 2B) cells exhibit overlapping distribution in the valve leaflets. At higher magnification, two observations can be made. First, all BMDCs (GFP+ cells) express the hematopoietic and pan-leukocyte marker CD45 (Figure 2E, H), thus identifying their hematopoietic origin. Second, two morphologically distinct GFP+/CD45+ populations are observed: spindle-shaped cells (Figure 2C–E) that are preferentially located at the proximal portion (toward the valve annulus) and dendritic-like cells (Figure 2F–H) that are located at the distal portion (at the free edge) of the leaflet. Importantly, while all GFP+ cells were CD45+, we occasionally observed GFP−/CD45+ cells (Fig 2E).
Figure 2. BMDCs in the heart valves of transplanted mice express CD45 and can be divided into two morphologically distinct cell populations.
(A) Low power en face image of the mitral aortic (anterior) leaflet, viewed from atrial side, showing the GFP+ BMDCs (green). (B) CD45 (red) immunolabeling in the same leaflet. (C–H) High magnification images from A & B, depicting GFP+ (green)/CD45+ (red) spindle-shaped cells in the proximal (toward the annulus) portion (C–E) and dendritic-like cells of the distal (toward the free edge) portion (F–H) of the leaflet. (C–H) Counterstained with Hoechst 33342. Scale bars: 500 μm (A,B), 50 μm (C–H).
The majority of the BMDCs are localized to the subendocardially aspect of the leaflet, while a subpopulation of the BMDCs resides deeper in the valve interstitium
Having noted a difference in the morphology of EGFP+ BMDCs distributed along the proximo-distal axis of the leaflets, we next sought to determine if there was a difference in the BMDCs with respect to the atrio-ventricular axis. To accomplish this, valves from transplanted mice, immunolabeled with antibodies to EGFP and CD45, were analyzed by laser scanning confocal microscopy. Figure 3 depicts a section of the aortic mitral valve leaflet that has been double immunolabeled with antibodies against GFP and CD45 (Figure 3A, F). BMDCs on the atrial side of the leaflet exhibit stratified localization. While the majority of the GFP+/CD45+ cells are very close to the atrial aspect of the leaflets (arrows on Figure 3F), a subpopulation of them resides deeper in the valve interstitium (arrowheads on Figure 3F).
Figure 3. The majority of GFP+/CD45+ BMDCs reside immediately sub-adjacent to the valve endocardium.
(A–C) Section of the mitral aortic (anterior) leaflet from a transplanted mouse immunolabeled with antibodies to GFP and CD45. (A) EGFP (green), (B) CD45 (red) and (C) superimposition of (A) and (B). (D–F) Higher magnification of the boxed area in (C) showing that most of the GFP+ (green, D) and CD45+ (red, E) BMDCs are positioned toward the atrial side of the mitral leaflet (arrowheads on F), while some GFP+ cells are found deeper (arrow on F) in the valve interstitium. (G–I) En face images from LSCM Z-series (10 sections) of the mitral aortic leaflet immunolabeled with antibodies to GFP (green, G), CD31 (red, H). (I) Superimposition of (G) and (H) shows no co-localization of the BMDC lineage marker (GFP, green) with the valve endocardium marker (CD31, red). (J–L) Dual-immunofluorescence for GFP (green, J) and CD31 (red, K) in a section from the mitral aortic leaflet. Superimposition of (J) and (K) shows that the majority of the BMDCs are located toward the atrial side of the leaflet, immediately underneath the CD31+ valve endocardium. Nuclei were counterstained with Hoechst 33342. Scale bars: 200 μm (AC), 50 μm (D–L).
Given the close proximity of the more superficially positioned GFP+/CD45+ BMDCs to the overlying endocardium, we next sought to investigate whether these cells are part of the valve endocardium. To accomplish this, anti-GFP and anti-CD31 (Pecam-1, endothelial marker) double immunolabelings on both whole mount valves (Figure 3G–I) and sections (Figure 3J–L) were performed. Analyses of these specimens showed that none of the GFP+ cells exhibited codistribution with CD31+ cells, supporting our conclusion that BMDCs are localized to the valve interstitium sub-adjacent to the overlying valve endocardium. Consistent with this finding, we detected no EGFP+/CD31+ valvular endocardial cells in all transplanted mice examined.
The spindle-shaped BMDCs are distinct from the dendritic-like cells and represent a non-leukocytic cell popuation in the heart valves
Considering that CD45 is a pan-leukocyte marker that is expressed by all cells of hematopoietic origin, we next evaluated whether BMDCs present in the heart valves might express other more specific leukocyte markers. When examined for expression of the dendritic cell-specific antigens CD11c (Figure 4A–C) and MHC II (Figure 4G–I), we observed that only the distally positioned GFP+ BMDCs with dendritic-like morphology are CD11c+/MHC II+. Additionally, CD11b (Mac-1, a differentiation antigen expressed by granulocytes, monocytes and tissue macrophages, dendritic cells, and NK cells) was also co-expressed by GFP+ dendritic-like cells (Supplemental Figure 3). However, neither the GFP+ dendritic-like nor the GFP+ spindle-shaped BMDCs (Supplemental Figure 3) were immunopositive for the murine macrophage marker F4/80. Thus, the distal multipolar GFP+ stellate cells appear to be a dendritic cell population that engrafted into the valve interstitium subsequent to repopulation of the bone marrow with EGFP+ donor HSCs. Additional immunolabeling with antibodies against granulocytes (GR-1), B-lymphocytes (B220) and T-lymphocytes (CD3) revealed the complete absence of these cells in normal homeostatic heart valves (data not shown). Importantly, the proximally positioned GFP+/CD45+ spindle-shaped BMDCs were negative for all leukocyte lineage markers used in this study (Figures 4D–F, J–L and Supplemental Figures 3). This suggests that, while the spindle-shaped GFP+/CD45+ BMDCs, which are preferentially located to the proximal part of the valves, are of (CD45+) hemopoietic origin, they represent a distinct, non-leukocytic cell type.
Figure 4. Detection of dentritic cell markers is limited to the distally localized BMDCs in the heart valves.
En face images of the aortic mitral leaflets from transplanted mice immunolabeled with (A–F) anti-GFP and anti-CD11c, (G–L) anti-GFP and anti-MHC class II. (A,D,G,J) EGFP (green), (B,E) CD11c (red), (H,K) MHC class II (red). Merged images show that only the distally localized BMDCs that exhibit dendritic-like morphology in the valves of transplanted mice are GFP+/CD11c+ (C) and GFP+/MHC class II+ (I), while the proximally localized spindle-shaped BMDCs are GFP+/CD11c− (F) and GFP+/MHC class II− (L). All images are counterstained with Hoechst 33342 (blue). Scale bars: 30 μm.
Bone marrow-derived subendocardial spindle-shaped cells express CD133 but not CD34
After determining that the spindle-shaped BMDCs in the heart valves do not represent differentiated leukocyte populations, we next investigated whether these spindle-shaped GFP+/CD45+ cells might express progenitor markers. First we performed anti-CD34 (an endothelial and hematopoietic progenitor marker) and anti-GFP double immunolabelings on both sectioned (Figure 5A–F) and whole mounted valves (Figure 5G–I). Using LSCM, we observed that, although the majority of the BMDCs were CD34 positive, both the valve endocardial cells and the GFP+ cells within the valve interstitium were CD34 negative. Next, sections were labeled with an antibody to the hematopoietic stem cell and progenitor marker CD133 (Prominin-1). Using LSCM analysis, we observed that the majority of the superficially positioned spindle-shaped GFP+/CD45+ BMDCs were CD133 positive. While some GFP+ BMDCs were CD133−, we also found GFP−/CD133+ cells (Figure 5J–L). Importantly, the distal bone marrow-derived dendritic-like (GFP+/CD11c+/MHCII+/CD11b+) cells in the homeostatic heart valves completely lacked the expression of these progenitor markers (data not shown).
Figure 5. All valve BMDCs are CD34 negative, while CD133 is detected in subendocardial, proximally localized BMDCs.
(A–I) Anti-GFP and anti-CD34 immunolabeling in (A–F) sectioned and (G–I) whole mount mitral aortic leaflet. (A,D,G) EGFP (green), (B,E,H) CD34 (red). Superimposition of the low magnification images (C) shows that the majority of VICs are CD34+, but higher magnification (F) of the boxed area in (C) and the whole mount (I) reveals that the EGFP+ BMDCs are CD34−. (J–L) En face image of the mitral aortic leaflet depicting GFP (green, J) and CD133 (red, K) immunofluorescence. Merged image (L) shows EGFP and CD133 co-expression (arrowheads). Nuclei are counterstained with Hoechst 33342 (blue). Scale bars: 100 μm (A–C), 50 μm (D–L).
A subpopulation of the deeper spindle-shaped BMDCs show a synthetic profile similar to homeostatic VICs
To further characterize the phenotype of the spindle-shaped BMDC in the heart valves, we used antibodies against fibroblast/myofibroblast markers, including vimentin (an intermediate filament of the connective tissue cells), Hsp47 (an intracellular chaperon of collagen types I-IV synthesis), periostin (an extracellular matrix protein secreted by VICs) and alpha smooth muscle actin (a myofibroblast marker). Virtually all interstitial cells of the heart valves, both GFP+ and GFP−, expressed vimentin (not shown). However, alpha smooth muscle actin was completely absent in all homeostatic mouse heart valves (data not shown). GFP and Hsp47 were co-localized in a sub-population of spindle-shaped BMDCs localized deeper in the valves (Figure 6A–C), demonstrating active involvement of these cells in collagen synthesis. GFP+ spindle-shaped cells were also immunopositive for periostin (Figure 6D, F), further indicating a fibroblast synthetic profile. Importantly, a subpopulation of GFP− VICs also expressed Hsp47 and periostin, suggesting a shared synthetic profile of EGFP− VICs and EGFP+ BMDCs in the valve interstitium. This is also consistent with the established role of periostin to promote collagen secretion.
Figure 6. A subpopulation of the spindle-shaped BMDCs that are located deeper in the proximal portion of the leaflets express Hsp47 and periostin.
En face images of the aortic mitral leaflets from transplanted mice immunolabeled with antibodies against GFP and Hsp47 (A–C) and GFP and periostin (D–F). (A,D) GFP (green), (B) Hsp47 (red) and (E) periostin (red). Superimposition of the respective images shows that some of the BMDCs from deeper in the valve insterstitium are GFP+/Hsp47+ (arrows on C) and GFP+/Periostin+ (arrows on F). All images are counterstained with Hoechst 33342 (blue). Scale bars: 30 μm.
Whole-body irradiation required for BM-TNC transplantation does not influence the distribution pattern of the CD45+ cells in the heart valves
Having established that GFP+ BMDCs in the heart valve of the transplanted mice express CD45, we next sought to determine whether the presence of this CD45+ cell population was an effect of the myeloablative irradiation required for preparation of bone marrow recipient mice or is part of a normal homeostatic process. To accomplish this, we evaluated whether a similar (CD45 positive) BMDC population is present in the valves of non-irradiated (non-tranplanted) mice. As seen in the LSCM images of the non-irradiated aortic leaflet of the mitral valve from a non-transplanted mouse, viewed from the atrial surface, CD45+ cells were present throughout the leaflet (Figure 7A). Moreover, as in the case of GFP+/CD45+ BMDCs in the valve leaflets of transplanted (irradiated and BM transplanted) mice, the same two morphologically distinct CD45+ cell populations were observed: the proximally localized spindle-shaped cells (Figure 7B), and the dendritic-like cells at the distal portions of the valves (Figure 7C). Further, as was observed with the GFP+/CD45+ BMDCs in the valves of tranplanted mice, CD45+ cells in the non-irradiated valves exhibited the same specific localization pattern: the atrial side of the atrioventricular valves (Figure 7D, E) and ventricular side of the semilunar valves (not shown).
Figure 7. CD45-expressing cells are detected in the valves of control (non-irradiated) mice and their phenotype is not influenced by lethal irradiation.
(A,C,D) En face views of the aortic leaflet of the mitral valve from control mouse immunolabeled with antibodies to CD45. (A) Low magnification image reveals the presence of numerous CD45+ cells (red). Higher magnification images of the proximal spindle-shaped CD45+ cells (B) and the distal dendritic-like CD45+ cells (C). (D,E) Histological section of a mitral valve leaflet immunolabeled with antibodies to CD45. (D) CD45 (red) immunofluorescence reveals a distribution of bone marrow-derived VICs that is similar to distribution of EGFP+/CD45+ cells observed in the valves of transplanted mice. (E) Higher magnification view of the boxed area in (D), depicting a subendocardial distribution of CD45+ cells that is similar to that observed in transplanted mice. (F–R) En face views of the mitral valve from a control mouse immunolabeled with antibodies to CD45 and CD34. (F) CD45 (green), (G) CD34 (red) and (H) superimposition of CD45 and CD34 immunofluorescence showing that, as was the case in transplanted mice, CD45+ cells in control mice do not express CD34. (I–K) En face views of the atrial side of mitral valve from control mouse immunolabeled with antibodies to CD45 and CD133. (I) CD45 (green), (J) CD133 (red) and (K) superimposition of CD45 and CD133 immunofluorescence showing that a subpopulation of the CD45+ cells express CD133 (arrowheads). (L–R) En face views of the atrial side of mitral valves immunolabeled with antibodies to (L,O) CD45 (red,green), (M) Hsp47 (green) and (P) periostin (red). Merged images show that a subpopulation of CD45+ cells express Hsp47 (N) and periostin (R). (B–R) Hoechst 33342 (blue) nuclear counterstain. Scale bars: 500 μm (A); 50 μm (B,C,E); 50 μm (D); 30 μm (F–R).
The phenotype of the CD45+ cells in the non-irradiated heart valves is the same as was observed in the valves of transplanted mice
After determining that the same CD45+ BMDC populations were present in the leaflets of valves from transplanted and non-transplanted mice, we next sought to phenotypically compare the CD45+ BMSC population in the leaflets of non-transplanted mice to that in the transplanted cohort. As in the valve leaflets from transplanted mice, we observed no signal for the F4/80 (macrophage), GR-1 (granulocyte), B220 (B-lymphocyte) and CD3 (T-lymphocyte) antigens in the leaflets from non-irradiated mice (data not shown), indicating that the presence of CD45+ cells in the non-irradiated heart valves, with the same distribution as was observed for the GFP+/CD45+ BMDCS in the irradiated valves, is absolutely not induced by irradiation injury.
Double immunolabeling of anti-CD45 with anti-CD34 or anti CD133 in the heart valves from the non-irradiated mice produced the same result as those observed in the transplanted mice. While a large portion of the VICs in both irradiated and non-irradiated mice are CD34+, CD45+ BMDCs and the overlying valve endocardium were CD34− (Figure 7F–H). The majority of the CD45+ cells in the non-irradiated valves, similar to our observations in the valves from transplanted mice, were also CD133+ (Figure 7I–K). Similarly, as in transplanted mice, the distally positioned dendritic-like BMDCs expressed the dendritic markers CD11c+/MHCII+/CD11b+ (not shown).
When we next examined the valve leaflets from non-transplanted mice for co-expression of CD45 and myo/fibroblastic markers, we again observed that a subpopulation of the spindle-shaped CD45+ cells were both Hsp47+ (Figure 7L–N) and periostin+ (Figure 7O–R). As in the case of the transplanted mice, we did not observe any alpha smooth muscle actin positive cells in the non-irradiated valves, but all valve cells (CD45+ and CD45−) were vimentin+ (not shown).
Quantitative assessment of VIC sub-populations in the anterior mitral leaflet
After identifying identical populations BM-derived cells in the valve leaflets leaflet based on their expression of specific lineage markers, we next sought to quantitatively evaluate the different VIC subpopulations in the leaflets. As above, we present our findings for the anterior mitral valve leafet (Figure 8).
Figure 8. Quantitative assessment of VIC sub-populations.
Fluorescently labeled VICs were enumerated at five section intervals through the anterior mitral leaflets from control (non-irradiated, non-transplanted) mice. All bone marrow hematopoietic cell-derived populations are in boxes. 28.9% of VICs were CD45+/CD34− and 70.73% were CD45−/CD34+ (n = 5, SD is ± 2.2%). In total, 20.02% of VICs were CD133+ (n = 2, SD is ± 4.71%), 12.94% of total VICs were CD45+ and 7.08% were CD45−. All CD11c+/MHCII+ VICs (14.16%, n = 4, SD ± 1.34%) were CD45+. In total, 6.17% (n=2, SD ± 0.71%) of VICs exhibited synthetic phenotype; 1.8% of total VICs were CD45+/Hsp47+/Postn+ and 4.37% of total VICs were CD45−/Hsp47+/Postn+.
By combining anti-CD45 and anti-CD34 immunolabeling on serial sections of wild type (non-irradiated, non-transplanted) mice, we determined that hematopoietic bone marrow-derived CD45+/CD34− cells represent 28.9% of the total VIC population, and 70.73% of them are CD45−/CD34+ cells. 20.02% of total VICs were CD133+ (12.94% were CD45+/CD133+ and 7.08 were CD45−/CD133+). The dendritic markers CD11c and MHC II were detected in 14.16% of VICs, and all of these cells were in the CD45+ population. In order to evaluate the amount of the VICs that exhibit synthetic phenotpye, we combined anti-Hsp47 and anti-Periostin immunolabelings with anti-CD45 immunolabeling. As a result, we found that 6.17% of VICs exhibits a synthetic phenotype (1.8% of them co-expressed CD45 and 4.37% were CD45−). These quantitative analyses confirm our findings, described above, which show that both the progenitor and synthetic VIC populations are mixed in terms of their expression of the CD45 antigen.
Discussion
Valve interstitial cells are the major cell population of the cardiac valve leaflets. These cells perform the complex biological processes that contribute to the maintenance and function of the valves through synthesis of extracellular matrix (ECM), communication with one another and the overlying valvular endocardium and response to changes in their environment. In recent years, advances in our understanding of the molecular control of valve interstitial cells in homeostasis and response to valve injury have lead to number of interesting discoveries, including contribution of extra-cardiac cells to this cell population [20, 21]. We have previously demonstrated, using clonal EGFP+ bone marrow HSC transplantation, that cells derived from the donor HSC contribute to the collagen producing cell population (valve interstitial cells) in the heart valves [14]. More recently, a number of reports have identified extra-cardiac sources of VICs in both healthy and diseased valves [22]. These non-native VICs are often referred to as valve progenitor cells [4]. The collective importance of these findings is that they suggest that valves are dynamic tissues whose constituent cells normally maintain some degree of plasticity throughout post-natal life. One concern of our previously published work was whether the use of irradiation, which is commonly used in bone marrow transplantation studies, might specifically recruit BMDCs to the valves as part of a radiation-induced injury response. Therefore, in the current study, we sought to compare outcomes in age-matched populations of irradiated, bone marrow transplanted mice to non-irradiated, non-transplanted mice. This afforded us the opportunity to not only to determine whether irradiation affects the distribution of BMDCs in the cardiac valves but also perform phenotypic comparison of endogenous VICs to those of extracardiac (bone marrow) origin. Additionally, in the current study, we employed bone marrow total nucleated cell transplantation (i.e., a cell population that contains both HSCs and MSCs) to extend our investigation to the specific contribution of bone marrow subpopulations to the cardiac valve interstitial cell population.
To determine the cellular origin of BMDCs detected in the valves, we used the HSC marker CD45 in combination with EGFP expression. Interestingly, all GFP+ BMDCs were immunopositive for CD45, leading to the conclusion that all BMDCs detected in the post-natal valves are of HSC origin with limited or no contribution by the CD45− mesenchymal stem cell (MSC) population of the bone marrow, at least in the murine model. Based on the fact that CD45 was described as the leukocyte common antigen expressed from very early stages of hematopoiesis [23], we focused first on identification of the GFP+/CD45+ BMDCs in heart valves using markers of the differentiated leukocyte (WBC) lineages. Our results clearly show that only the distally localized BMDCs represent a leukocytic cell population: a CD11c+/MHCII+ dendritic cell population that was previously described by Choi et et al. [24]. Based on the complete absence of F4/80 positive cells in the valves, we conclude that macrophages are rarely present in the normal mouse valves, which is supported by the report of Ghosh and colleagues [25]. While Tanaka and colleagues alternatively concluded that the MOMA-2-positive cells they observed in their study represented an F4/80-negative macrophage population, it is important to remember that expression of the MOMA-2 antigen has been reported in dendritic cells and their precursors in addition to monocytes/macrophage [26, 27]. Therefore, we conclude that the CD11b (Mac-1) expression that we observe in the distally localized GFP+/CD45+/F4/80− BMDCs identifies a dendritic cell population. The absence of GR-1+, B220+ and CD3+ cells is consistent with reports in the literature that indicate that leukocytes belonging to the granolucytic and lymphocytic lineages are not normally present in the adult homeostatic heart valves [25, 28, 29]. Importantly, no expression of leukocytic markers was detected in the proximally-located CD45+ spindle-shaped BMDC population. Thus, one of the most important findings of our study is that in homeostatic adult mouse heart valves, we have identified a previously unrecognized CD45+ BMDC population that exhibits a non-leukocytic phenotype, is localized mostly to the proximal areas of the valves and has a spindle-shaped morphology.
Another important finding of our studies is the very specific localization pattern of the GFP+/CD45+ BMDCs in the heart valves. They are found at the atrial side of the atrioventricular valves (mitral and tricuspid) and ventricular side of the semilunar valves (aortic and pulmonary). While a large population of the GFP+/CD45+ BMDCs were located superficially, we never detected co-expression of the endothelial/endocardial marker CD31. Tanaka et al. [22], using a similar experimental system (transplanting GFP+ bone marrow into wild type mice), reported that most of the GFP+ cells appeared to be endothelial cells in the aortic valves of aged mice. The explanation for this discrepancy may be either related to the age of the mice or may be purely technical. In their study, Tanaka’s group performed their immunolabelings for GFP and CD31 on separate (adjacent) sections then merged the fluorescence images. Our analyses were performed using double immunofluorescent labeling on the same section. As subtle changes to valve histology during processing can affect interpretation of the discrete localization of reporter signal in superimposed adjacent sections, we thought it was important to perform our double immunofluorescence analyses in individual sections in order to avoid difficulties in the interpretation of the data. Our analyses, using both transplanted and non-transplanted mice combined with high magnification epifluorescence analyses, suggest that there is no contribution of BMSCs to the valvular endocardium during normal tissue homeostasis.
It has been reported that the adult heart valves contain a population of multipotent progenitors that express CD133/prominin-1 and CD34 in porcine [30] and human valves [21]. We similarly identified CD133+ cells in the adult homeostatic mouse valves and further determined that its expression is limited to the spindle-shaped BMDC population. The CD133 antigen was first identified as a marker of hematopoietic progenitor cells [31]. Subsequently, reports from Peichev [32] and Tondreau [33] identified CD133 as a marker of progenitor cells with broad mesenchymal plasticity. Analysis of CD34 expression by VICs in the murine valves led to intriguing finding that neither EGFP+/CD45+ VICs in transplanted mice nor CD45+ VICs in control mice expressed CD34. However CD34 expression was broadly distributed on CD45− cells. That we would detect CD34+ VICs in the spongiosa and fibrosa of mouse valves was expected, as CD34+ VICs had previously been found in the spongiosa and fibrosa layers of human cardiac valves [34, 35]. However, the lack of CD34 expression in murine HSC-derived VICs is consistent with our previous findings showing that a Lin− CD34−, Sca-1+, Side Population of HSCs capable of long-term repopulation can gave rise to EGFP+ VICs [14]. Differences in murine and human CD34 expression have been reported previously [36–38]. Taken together our studies suggest that the CD45+/CD34− VICs that we observed are derived from CD34− long-term repopulating HSCs [38–40]. Whether there are overall differences in the plasticity of CD45+/CD34−/CD133+ progenitor population and the less frequently observed CD45−/CD34+/CD133+ cells remains to be determined.
During normal conditions, VICS are considered to be fibroblast-like cells that are highly responsive to changes in their surrounding ECM, mechanical forces and signaling molecules [41]. Given their morphological similarity to endogenous VICs, we sought to determine the contribution of the proximally positioned, spindle-shaped BMDCs to valve maintenance during normal homeostasis based on their exhibiting a fibroblastic phenotype that is similar to neighboring CD45- VICs. During embryonic morphogenesis, VICs maintain valve structure and function by synthesizing and secreting the ECM components that surround them in the valve interstitium [41]. During post-natal life, maintenance of this ECM preserves valve form and function [41]. As was the case with CD45- VICs, we found that a portion of the spindle-shaped GFP+/CD45+ BMDC population in the valves express Hsp47 and periostin. Hsp47 is an intracellular chaperone of collagen types I-IV synthesis; collagen I is a major constituent of the fibrosum of the cardiac valves. Periostin is a matricellular protein that is expressed as early as ED9.5 in the mesenchyme of the developing endocardial cushions and continues to be expressed by the valve interstitial cells throughout postnatal life. [42–44]. Its expression in the embryonic cushion mesenchyme serves not only as a marker of what will become a fibroblastic cell population, but has additionally been shown to actively drive pro-fibroblastic differentiation and secretion of collagen in this multipotential mesenchymal cell population [43, 45]. Collectively, these similarities in distribution, morphology, absence of leukocytic markers and synthesis of fibroblastic proteins demonstrates that BMDCs exhibit a VIC phenotype that mirrors the phenotype of CD45− cells in the valve interstitium.
When considering their bone marrow origin in combination with their expression of CD45 and Hsp47, the valvular BMDCs that we identified in the current study fit the description of the fibrocyte, a circulating bone marrow-derived cell that becomes fibroblast-like after tissue engraftment. Moreover, fibrocytes are identified by dual expression of CD45 or CD34 and collagen type I or pro-collagen I [46–48]. While the origin of the mesenchymal cell lineages has traditionally been ascribed to the mesenchymal stem cell (MSC) compartment of the bone marrow [49, 50], an increasing numbers of studies, including those from our group using single donor hematopoietic stem cell transplantation, provide compelling evidence for the hematopoietic stem cell origin of post-natal mesenchymal cell populations including the kidney mesangial cell [51], CNS microglia [52], hepatic stellate cells [53] and cardiac interstital fibroblasts [16]. Moreover, based on our transplantation studies, the EGFP+ BM-derived CD45+/CD34−/Hsp47+ VICs are a dynamic valve population, as evidenced by the fact that all EGFP+ VICs observed in the valves had engrafted within the three months following transplantation. Based on the dynamic properties of these cells, we speculate that the primary role of CD45+/CD34−/Hsp47+ VICs’ may be to provide a responsive population of synthetic VICs in the context of injury. Such a role for the CD45+/CD34−/Hsp47+ VICs is consistent with our previous findings showing that HSC-derived cell numbers are dramatically increased in the brain following stroke [52] and myocardial infarction [16]. Current efforts are focused on elucidation of the contribution of bone marrow-derived VICs to pathological remodeling of the valve leaflets.
Conclusions
Collectively, our findings support the conclusion that the GFP+/CD45+ BMDCs observed in the valve leaflets from the hearts of transplanted mice are phenotypicaly identical to the CD45+ cells in the valve leaflets of non-transplanted (non-irradiated) mice. Further, myeloablative irradiation does not influence either the localization or phenotype of the (GFP+/CD45+) BMDCs in the heart valves of transplanted mice, as they appear to be identical to the CD45+ cells observed in non-transplanted, non-irradiated mice. This study provides evidence that contribution of BM-derived cells to the VIC population is a normal homeostatic process and neither a response to potential valve injury associated with irradiation nor is such injury required for this engraftment.
Supplementary Material
(B) Blood cells labeled with isotype control antibodies. Reconstitution of Gr-1+/Mac1+ cells, B220+ cells and Thy1.2+ cells by EGFP+ donor cells is shown in B, C and D, respectively. The percentages of engrafting cells in the granulocyte/macrophage, B-cell and T-cell lineages were 65%, 92% and 36%, respectively.
(A) Stereomicroscopic image of a dissected mouse heart illustrating the isolation of the valve leaflets depicted in whole mount in Figures 1 and 5. After fixation and washing, the heart was placed on its ventral surface onto a Silguard-coated petri dish. The atrial wall was removed (remnants are indicated by arrowheads). The left ventricle was opened by cutting longitudinally through the mural leaflet (indicated by asterisks). The heart was immobilized using insect pins (ip) and the mitral leaflet was excised by cutting along the dotted line, maintaining a portion of both the anterior (APM) and posterior (PPM) papillary muscles for handling. (B) Differential interference contrast micrograph of the whole mount excised valve. Scale bar: 200 mm.
(A–F) En face LSCM images of the aortic leaflet of the mitral valve from transplanted mouse immunolabeled with antibodies to GFP and CD11b/Mac-1. (A–C) proximal spindle-shaped VICs and (D–F) distal dendritic cells. EGFP immunofluorescence (A and D, green) and CD11b/Mac-1 immunofluorescence (B and E, red). Superimposition of the EGFP and CD11b/Mac-1 immunofluorescence (C and F) shows that spindle-shaped cells are CD11b−/Mac-1− (C) while dendritic cells are CD11b+/Mac-1+. (G–L) En face LSCM images of the aortic leaflet of the mitral valve from transplanted mice immunolabeled with antibodies to GFP and F4/80. (G–I) proximal spindle-shaped cells and (J–L) distal dendritic cells. anti-GFP immunofluorescence (G and J, green) and anti-F4/80 immunofluorescence (H and K, red). Superimposition of the EGFP and F4/80 immunofluorescence shows that neither the spindle-shaped or dendritic cells express F4/80. Scale bars: 30 μm.
Research highlights.
Bone marrow-derived valve interstitial cells are CD45+
CD45+ interstitial cells have distinct localization in the valves
Most of the valve CD45+ cells are progenitor cells
A subpopulation of valve CD45+ cells exhibit fibroblast-like properties
Recruitment of CD45+ cell to the valve is not a response to lethal irradiation
Acknowledgments
This work was supported by grants from the National Institute of Health RO1-HL080168 (CJD), RO1-HL033756 (RRM), the National Center for Research Resources P20-RR1-16434 (RPV), the American Heart Association 0865325E (RPV), P20RR021949-01A2 (RPV) and Leducq Foundation 07CVD04 (RPV and ZH), the Extramural Research Facilities Program of the National Center for Research Resources CO6 RR018823 (Medical University of South Carolina) and the Cardiac Developmental Biology Center Medical University of South Carolina. The authors wish to thank Haiqun Zeng for expertise with cell sorting and the staff of the Department of Radiation Oncology, MUSC for assistance in the irradiation of mice.
Abbreviations
- EGFP
enhanced green fluorescent protein
- VIC
valve interstitial cell
- CD
cluster of differentiation
- HSC
hematopoietic stem cell
- BM
bone marrow
- BM-TNC
bone marrow total nucleated cells
- LSCM
laser scanning confocal microscope
Footnotes
Author’s contribution
Z.H. designed and performed research, analyzed data and wrote the manuscript. P.A.F. performed research. S.J.R. performed research. R.R.M. analyzed data and wrote manuscript. R.P.V. designed research, analyzed data and wrote the manuscript. C.J.D. designed research, analyzed data and wrote the manuscript.
Conflict of interest
Authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harper WF. Further observations on the structure of human heart-valves. J Anat. 1940 Oct;75(Pt 1):88–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008 Oct 28;118(18):1864–80. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 3.Hinton RB, Jr, Alfieri CM, Witt SA, Glascock BJ, Khoury PR, Benson DW, et al. Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol. 2008 Jun;294(6):H2480–8. doi: 10.1152/ajpheart.91431.2007. [DOI] [PubMed] [Google Scholar]
- 4.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007 Nov;171(5):1407–18. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Xu S, Gotlieb AI. The response to valve injury. A paradigm to understand the pathogenesis of heart valve disease. Cardiovasc Pathol. 2011 May-Jun;20(3):183–90. doi: 10.1016/j.carpath.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Lester W, Rosenthal A, Granton B, Gotlieb AI. Porcine mitral valve interstitial cells in culture. Lab Invest. 1988 Nov;59(5):710–9. [PubMed] [Google Scholar]
- 7.Lester WM, Gotlieb AI. In vitro repair of the wounded porcine mitral valve. Circ Res. 1988 Apr;62(4):833–45. doi: 10.1161/01.res.62.4.833. [DOI] [PubMed] [Google Scholar]
- 8.Wang SY, Sutherland JC. Colonic perforation secondary to fecal impaction: report of a case. Dis Colon Rectum. 1977 May-Jun;20(4):355–6. doi: 10.1007/BF02586438. [DOI] [PubMed] [Google Scholar]
- 9.Armiger LC, Gavin JB, Barratt-Boyes BG. Histological assessment of orthotopic aortic valve leaflet allografts: its role in selecting graft pre-treatment. Pathology. 1983 Jan;15(1):67–73. doi: 10.3109/00313028309061405. [DOI] [PubMed] [Google Scholar]
- 10.Hazekamp MG, Koolbergen DR, Braun J, Sugihara H, Cornelisse CJ, Goffin YA, et al. In situ hybridization: a new technique to determine the origin of fibroblasts in cryopreserved aortic homograft valve explants. J Thorac Cardiovasc Surg. 1995 Jul;110(1):248–57. doi: 10.1016/S0022-5223(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 11.Braun J, Hazekamp MG, Koolbergen DR, Sugihara H, Goffin YA, Huysmans HA, et al. Identification of host and donor cells in porcine homograft heart valve explants by fluorescence in situ hybridization. J Pathol. 1997 Sep;183(1):99–104. doi: 10.1002/(SICI)1096-9896(199709)183:1<99::AID-PATH1086>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Koolbergen DR, Hazekamp MG, Kurvers M, de Heer E, Cornelisse CJ, Huysmans HA, et al. Tissue chimerism in human cryopreserved homograft valve explants demonstrated by in situ hybridization. Ann Thorac Surg. 1998 Dec;66(6 Suppl):S225–32. doi: 10.1016/s0003-4975(98)01109-6. [DOI] [PubMed] [Google Scholar]
- 13.Deb A, Wang SH, Skelding K, Miller D, Simper D, Caplice N. Bone marrow-derived myofibroblasts are present in adult human heart valves. J Heart Valve Dis. 2005 Sep;14(5):674–8. [PubMed] [Google Scholar]
- 14.Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006 Mar 17;98(5):690–6. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 15.Ikawa M, Yamada S, Nakanishi T, Okabe M. ‘Green mice’ and their potential usage in biological research. FEBS Lett. 1998 Jun 23;430(1–2):83–7. doi: 10.1016/s0014-5793(98)00593-6. [DOI] [PubMed] [Google Scholar]
- 16.Visconti RP, Markwald RR. Recruitment of new cells into the postnatal heart: potential modification of phenotype by periostin. Ann N Y Acad Sci. 2006 Oct;1080:19–33. doi: 10.1196/annals.1380.003. [DOI] [PubMed] [Google Scholar]
- 17.Masuya M, Drake CJ, Fleming PA, Reilly CM, Zeng H, Hill WD, et al. Hematopoietic origin of glomerular mesangial cells. Blood. 2003 Mar 15;101(6):2215–8. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]
- 18.Ebihara Y, Masuya M, Larue AC, Fleming PA, Visconti RP, Minamiguchi H, et al. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp Hematol. 2006 Feb;34(2):219–29. doi: 10.1016/j.exphem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 19.LaRue RC, Masuya M, Ebihara Y, Fleming PA, Visconti RP, Minamiguchi H, et al. Hematopoietic origins of fibroblasts: I. In vivo studies of fibroblasts associated with solid tumors. Exp Hemat. 2006 doi: 10.1016/j.exphem.2005.10.009. in press. [DOI] [PubMed] [Google Scholar]
- 20.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007 Mar 1;109(5):1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skowasch D, Schrempf S, Wernert N, Steinmetz M, Jabs A, Tuleta I, et al. Cells of primarily extra-valvular origin in degenerative aortic valves and bioprostheses. Eur Heart J. 2005 Dec;26(23):2576–80. doi: 10.1093/eurheartj/ehi458. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Sata M, Fukuda D, Suematsu Y, Motomura N, Takamoto S, et al. Age-associated aortic stenosis in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2005 Jul 5;46(1):134–41. doi: 10.1016/j.jacc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Hills D, Taylor E, Pfeffer K, Ure J, Medvinsky A. Transgenic tools for analysis of the haematopoietic system: knock-in CD45 reporter and deletor mice. J Immunol Methods. 2008 Sep 15;337(2):81–7. doi: 10.1016/j.jim.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009 Mar 16;206(3):497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh S, Hoenerhoff MJ, Clayton N, Myers P, Stumpo DJ, Maronpot RR, et al. Left-sided cardiac valvulitis in tristetraprolin-deficient mice: the role of tumor necrosis factor alpha. Am J Pathol. 2010 Mar;176(3):1484–93. doi: 10.2353/ajpath.2010.090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Wilsem EJ, Breve J, Kleijmeer M, Kraal G. Antigen-bearing Langerhans cells in skin draining lymph nodes: phenotype and kinetics of migration. J Invest Dermatol. 1994 Aug;103(2):217–20. doi: 10.1111/1523-1747.ep12393088. [DOI] [PubMed] [Google Scholar]
- 27.Barsky AJ, Cleary PD, Wyshak G, Spitzer RL, Williams JB, Klerman GL. A structured diagnostic interview for hypochondriasis. A proposed criterion standard. J Nerv Ment Dis. 1992 Jan;180(1):20–7. doi: 10.1097/00005053-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990 Jan 1;171(1):307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roake JA, Rao AS, Morris PJ, Larsen CP, Hankins DF, Austyn JM. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995 Jun 1;181(6):2237–47. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009 Mar;174(3):1109–19. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997 Dec 15;90(12):5002–12. [PubMed] [Google Scholar]
- 32.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000 Feb 1;95(3):952–8. [PubMed] [Google Scholar]
- 33.Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005 Sep;23(8):1105–12. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 34.Barth PJ, Koster H, Moosdorf R. CD34+ fibrocytes in normal mitral valves and myxomatous mitral valve degeneration. Pathol Res Pract. 2005;201(4):301–4. doi: 10.1016/j.prp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Veinot JP, Prichett-Pejic W, Song J, Waghray G, Parks W, Mesana TG, et al. CD117-positive cells and mast cells in adult human cardiac valves--observations and implications for the creation of bioengineered grafts. Cardiovasc Pathol. 2006 Jan-Feb;15(1):36–40. doi: 10.1016/j.carpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Ito T, Tajima F, Ogawa M. Developmental changes of CD34 expression by murine hematopoietic stem cells. Exp Hematol. 2000 Nov;28(11):1269–73. doi: 10.1016/s0301-472x(00)00535-x. [DOI] [PubMed] [Google Scholar]
- 37.Tajima F, Deguchi T, Laver JH, Zeng H, Ogawa M. Reciprocal expression of CD38 and CD34 by adult murine hematopoietic stem cells. Blood. 2001 May 1;97(9):2618–24. doi: 10.1182/blood.v97.9.2618. [DOI] [PubMed] [Google Scholar]
- 38.Zanjani ED, Almeida-Porada G, Livingston AG, Zeng H, Ogawa M. Reversible expression of CD34 by adult human bone marrow long-term engrafting hematopoietic stem cells. Exp Hematol. 2003 May;31(5):406–12. doi: 10.1016/s0301-472x(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 39.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996 Jul 12;273(5272):242–5. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 40.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998 Sep;4(9):1038–45. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 41.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009 Nov 6;105(10):934–47. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borg TK, Markwald R. Periostin: more than just an adhesion molecule. Circ Res. 2007 Aug 3;101(3):230–1. doi: 10.1161/CIRCRESAHA.107.159103. [DOI] [PubMed] [Google Scholar]
- 43.Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, et al. Periostin regulates atrioventricular valve maturation. Dev Biol. 2008 Apr 15;316(2):200–13. doi: 10.1016/j.ydbio.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007 Aug 3;101(3):313–21. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008 Apr 11;102(7):752–60. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994 Nov;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 47.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010 Jul;38(7):548–56. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009 Apr 1;179(7):588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 49.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9(5):641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 50.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970 Oct;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 51.Masuya M, Drake CJ, Fleming PA, Reilly CM, Zeng H, Hill WD, et al. Hematopoietic origin of glomerular mesangial cells. Blood. 2003 Mar 15;101(6):2215–8. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]
- 52.Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, et al. Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004 Apr;186(2):134–44. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Miyata E, Masuya M, Yoshida S, Nakamura S, Kato K, Sugimoto Y, et al. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood. 2008 Feb 15;111(4):2427–35. doi: 10.1182/blood-2007-07-101261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(B) Blood cells labeled with isotype control antibodies. Reconstitution of Gr-1+/Mac1+ cells, B220+ cells and Thy1.2+ cells by EGFP+ donor cells is shown in B, C and D, respectively. The percentages of engrafting cells in the granulocyte/macrophage, B-cell and T-cell lineages were 65%, 92% and 36%, respectively.
(A) Stereomicroscopic image of a dissected mouse heart illustrating the isolation of the valve leaflets depicted in whole mount in Figures 1 and 5. After fixation and washing, the heart was placed on its ventral surface onto a Silguard-coated petri dish. The atrial wall was removed (remnants are indicated by arrowheads). The left ventricle was opened by cutting longitudinally through the mural leaflet (indicated by asterisks). The heart was immobilized using insect pins (ip) and the mitral leaflet was excised by cutting along the dotted line, maintaining a portion of both the anterior (APM) and posterior (PPM) papillary muscles for handling. (B) Differential interference contrast micrograph of the whole mount excised valve. Scale bar: 200 mm.
(A–F) En face LSCM images of the aortic leaflet of the mitral valve from transplanted mouse immunolabeled with antibodies to GFP and CD11b/Mac-1. (A–C) proximal spindle-shaped VICs and (D–F) distal dendritic cells. EGFP immunofluorescence (A and D, green) and CD11b/Mac-1 immunofluorescence (B and E, red). Superimposition of the EGFP and CD11b/Mac-1 immunofluorescence (C and F) shows that spindle-shaped cells are CD11b−/Mac-1− (C) while dendritic cells are CD11b+/Mac-1+. (G–L) En face LSCM images of the aortic leaflet of the mitral valve from transplanted mice immunolabeled with antibodies to GFP and F4/80. (G–I) proximal spindle-shaped cells and (J–L) distal dendritic cells. anti-GFP immunofluorescence (G and J, green) and anti-F4/80 immunofluorescence (H and K, red). Superimposition of the EGFP and F4/80 immunofluorescence shows that neither the spindle-shaped or dendritic cells express F4/80. Scale bars: 30 μm.