Abstract
This is a review of research that supports a hypothesis regarding early restriction of gene expression in the vertebrate embryo. We hypothesize that vertebrate retinoic acid receptors (RARs for several vertebrates but rars for zebrafish) are part of an embryonic, epigenetic switch whose default position, at the time of fertilization is “OFF”. This is due to the assemblage of a rar-corepressor-histone deacetylase complex on retinoic acid response elements (RAREs) in regulatory regions of a subset of genes. In addition, selective and precise allocation of retinoic acid during early development through the interaction of Phase I enzymes throws the switch “ON” in a predictable, developmental manner. We are proposing that this is a basic, early embryonic switch that can cause the initiation of cascades of gene expression that are responsible for at least some early, diversification of cell phenotypes. Dehydrogenases and a subset of cytochrome p450 genes (cyp26a1, cyp26b1, and cyp26c1) play the major role in providing the retinoic acid and limiting its access. We also suggest that this mechanism may be playing a significant role in the repression of genes in undifferentiated stem cells.
Keywords: retinoic acid, retinoic acid receptor, epigenetic, corepressor
I. Introduction
The purpose behind this review is to present the hypothesis that a naturally occurring complex involving nuclear receptors could very well provide an epigenetic mechanism for initiating the diversification of cell phenotypes in the developing embryo. Why is this important? We argue that this is important because of the role that somewhat normal or “house-keeping” genes play in facilitating the mechanism. From the viewpoint of a neurotoxicologist, this implies and predicts that compounds: environmental compounds, hormones, and pharmaceuticals that interact with these mechanism-supportive gene products could have a deleterious effect upon the developing organism. Also, this hypothesis may change the way we consider the effects of teratogens and result in the formulation of new predictions and hypotheses about how the developing embryo is affected by selective groups of compounds. This hypothesis could provide a mechanism for the well-studied teratogenicity of retinoic acid, illustrated by the Shenefelt study (Shenefelt RE 1972) and accidentally and tragically in humans (Lammer EJ, et al. 1985). We are suggesting through this hypothesis that retinoic acid acts a teratogen by ectopically turning on genes that are being precisely repressed by a retinoic acid receptor mediated epigenetic switch. While we will use zebrafish as a model to outline this hypothesis, there is a vast amount of excellent research in many different vertebrate model systems that also support this hypothesis.
We start with a hypothesis, but also present a review of a complex field of work developed by many laboratories. When considering these data together they provide a basis of support for the hypothesis that a RAR- based epigenetic switch functions early in development. While this work will summarize a considerable amount of research done by many laboratories, we will use our own experience to emphasize the work done with the zebrafish model since it allows unique entre into the very earliest times of embryogenesis.
The focus with be the role of the retinoic acid receptors (RARs) (Giguere V, et al. 1987, Petkovich M, et al. 1987) or rars (as lower case for the proper useage for zebrafish genes) as part of complexes that could be considered an “OFF”<>“ON” switch for gene expression and importantly that we hypothesize that the “OFF” position is the default position for vertebrate embryos at fertilization. This switch is illustrated in Figure 1.
Figure 1.
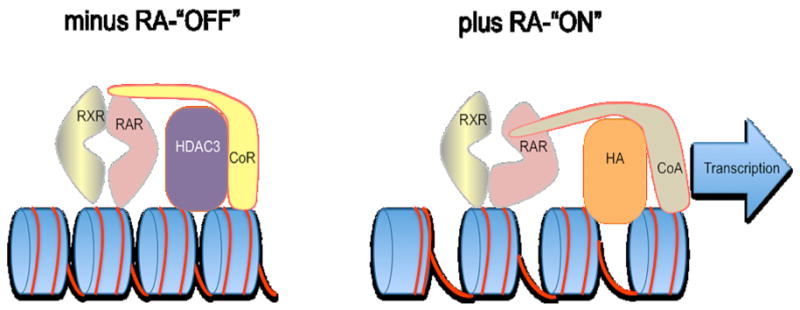
A model of the epigenetic switch. To the left is the “OFF” position where the RAR/RXR heterodimer is binding to a Retinoic Acid Response Element (RARE) in the absence of the retinoic acid ligand. This creates a corepressor binding site on the RAR (CoR binding site) and the corepressor has a histone deacetylase (HDAC3) binding site. As described in the text, this causes both a condensation of the chromatin and a repression of transcription. To the right is the “ON” position. When the retinoic acid ligand (RA) binds to the retinoic acid receptor (RAR) the receptor goes through an allosteric change eliminating the corepressor binding site and creating an association site for a coactivator (CoA). This also brings in a histone acetyltransferase (HA) that helps to open up the chromatin. The coactivator provides the association with the transcription complex to facilitate transcription. This model is derivative of several studies on individual components of the RAR corepressor complex in cell culture.
A succinct description of the elements of this hypothesis are: a) that the retinoic acid receptors bind to their retinoic acid response elements (RAREs) (Umesono K, et al. 1991) in the absence of ligand (and they do this as a heterodimeric partner with retinoid-X receptors (RXRs) (Mangelsdorf DJ, et al. 1990, Yu VC, et al. 1991)); b) in this context they provide a binding site for one of two retinoic acid receptor/thyroid hormone receptor corepressors, NCor (Horlein AJ, et al. 1995) or Smrt (Chen JD and Evans RM 1995, Ordentlich P, et al. 1999) (also called Ncor2); c) the corepressor has a binding site for histone deacetylase (HDAC) (Nagy L, et al. 1997) that can deacetylate chromatin and thus condense it; d) when retinoic acid is available to the RAR, the receptor undergoes a conformational change that eliminates the corepressor binding site and creates a transcriptional activator binding site (le Maire A, et al. 2010). The transcriptional activator also brings in a histone transacetylase that opens up the chromatin structure while the coactivator facilitates transcriptional activation of the gene (see review by Rosenfeld MG, et al. 2006).
While these are all observations that have been reported, here we emphasize that the early embryo has this epigenetic switch in the OFF position, and the precise expression of retinal dehydrogenases play a crucial role in apportioning retinoic acid at specific times and places as the embryo develops. In concert with this, a family of cytochrome p450s, cyp26a1, cyp26b1 and cyp26c1 (Dobbs-McAuliffe B, et al. 2004) (Hernandez RE, et al. 2007) (White JA, et al. 1997) (Nelson DR 1999) (Zhao Q, et al. 2005) (Gu X, et al. 2005) (White JA, et al. 1996) (White RJ, et al. 2007) are also precisely expressed and their presence metabolizes retinoic acid to a non-functional ligand thereby “painting” borders of retinoic acid availability and ultimately affecting the fate of cells. While this can affect many different tissues (Shenefelt RE 1972) the effects are marked in the nervous system.
With this summary, we will proceed with more specific descriptions and examples of how this works through experiments performed in this laboratory and references to the work of others. However, it should be emphasized that many laboratories, some working with cell culture and some working with a variety of vertebrate model systems (mouse, chick, Xenopus, zebrafish and rat to name a few) have contributed to investigative elements that we are putting together as this hypothesis. For a review on some of the effects of retinoic acid on adult brain one is referred to (Drager UC 2006).
2. Methods
Zebrafish work was done through institutionally approved IACUC protocols. Past transgenic zebrafish were constructed and screened using procedures described in (Linney E and Udvadia AJ 2004). More recent transgenic construction and production were performed using a modified miniTol2 vector (Balciunas D, et al. 2006) with integration facilitated via the Tol2 transposase. In situ hybridization analysis was a modification the (Thisse C and Thisse B 2008) protocol as described in (Dobbs-McAuliffe B, et al. 2004) and (Zhao Q, et al. 2005). This included digoxigenin-labeled antisense probes. The probes for raraa and rarab were selected to minimize homology to 40% or less of each other.
The analysis and quantification of neuron number via a retinoic acid pulse was performed in the following manner: Embryo fixation and whole mount immunohistochemistry was performed as described previously in (Stemple DL, et al. 1996). The antibody 39.4D5 (islet 1/2) (Developmental Hybridoma Studies Bank) was used at 1:1 as described for a hybridoma supernatant. Our only modification was to use an Alexa 488, fluorescent secondary antibody (Molecular Probes), at a ratio of 1:500, rather than use the ABC method. As a counter-stain, samples were incubated in 1μg/ml propidium iodide (red) in PBT for 10 minutes, washed 10 minutes in PBT, and placed in fresh PBT. Embryos were de-yolked, flat mounted and visualized with a Nikon TE300 microscope using laser scanning confocal microscopy. Optical stacks were captured at 1μm intervals through the depth of tissue containing the neuron type of interest using EZ-C1 software. Counts of islet positive Rohon-Beard neurons and motor neurons were performed by scrolling through the stacks. Data were analyzed using an unpaired student's t test (n = 10). Images of Rohon-Beard neurons and motor neurons are maximum projections of the optical stacks containing those cells.
3. Retinoic Acid Receptors
3.1 Basic retinoic acid receptor biology
The retinoic acid receptors have the typical steroid superfamily receptor domain structure shown in Figure 2A. In mammals there are three RAR genes: alpha, beta, and gamma—each of which has two promoters that produce two isoforms, with differing amino termini. Zebrafish, with a further genome duplication and loss, does not have an rar beta, but has two rar alphas (rar aa and rar ab) and two rar gammas (rar ga and rar gb) (Joore J, et al. 1994) (White JA, et al. 1994), Hale LA, et al. 2006, Waxman JS and Yelon D 2007). Similarly, their dimerization partners, the retinoid-X receptors are present as three mammalian RXR genes: alpha, beta, and gamma and there are six zebrafish rxr genes rxr aa, rxr ab, rxr ba, rxr bb, rxr ga, and rxr gb (Jones BB, et al. 1995, Tallafuss A, et al. 2006, Waxman JS and Yelon D 2007), Figure 2B. In general, a rar/rxr heterodimer binds to a retinoic acid response element, or RARE, within the regulatory DNA regions of genes that are retinoic acid inducible, Figure 2C.
Figure 2.

Retinoic acid receptors, domains, and response elements. A. The basic domain structure of the members of the steroid superfamily including the retinoic acid receptors (RARs) and the retinoid-X receptors (RXRs). B. A comparison of the identified mouse/human RARs and RXRs and the zebrafish rars and rxrs. Because the fish went through an additional genomic duplication, some genes in zebrafish have duplicates, such as the rar a and rar g genes. In addition no rar b genes have been identified for zebrafish. C. The DNA binding sequences that RAR/RXR heterodimers bind to are named RAREs. In the genome, these can take many forms but in many cases they are direct repeats. A prototypical RARE is shown and based upon a study by (Umesono K, et al. 1991).
As with most things in biology, there are exceptions to this rule, but for the most part this describes associations of the receptors with their DNA response elements. The RARs are members of the steroid superfamily that bind to their RAREs in the absence of ligand. The majority of receptors in this family do not do this. And in fact, this property is essential to their role in this hypothesis. It should also be emphasized that the structure of the RAR is different whether or not it is bound to ligand. In the absence of ligand its carboxyl region provides a binding site for a corepressor and in the presence of ligand the receptor goes through an allosteric change that eliminates the corepressor binding site and creates a binding site for a transcriptional co-activator. It has been suggested that the alpha form of the mammalian RAR shows the highest affinity for this corepressor binding (Farboud B, et al. 2003).
Regarding the relevance of this to other species, several studies involving mouse knockout technology have focused upon knocking out all of the mouse RARs and RXRs. Rarely does a single receptor knockout give a distinct phenotype, but double and triple knockouts create developmental phenotypes representing the important, but overlapping functional roles of these receptors (see Mark M, et al. 1999, for a review of this field).
3.2 Corepressor biology
In the 1990s, after RARs and RXRs were identified, large corepressor molecules were identified that bind to RARs and the thyroid hormone receptors, NCor (Horlein AJ, et al. 1995) and Smrt (Chen JD and Evans RM 1995) (also called Ncor2). The first Smrt cDNA that was isolated was considerably smaller that NCor but later, a full-length cDNA was isolated and this cDNA (Ordentlich P, et al. 1999) was similar in size to NCor. These were shown to have binding domains to several proteins including the nuclear receptors and histone deacetylase. In some cases they were isolated or characterized using yeast two-hybrid systems with a receptor as “bait” for proteins from a cDNA library that were expressed in yeast. The technique was designed to allow one to isolate clones that bound to the bait, or in this case, RAR or THR. Through these and other functional binding assays it was determined that the corepressor would bind to an RAR in the absence of ligand, but not in its presence. Further studies indicated that RAR alpha had a stronger binding affinity to the Smrt corepressor than RAR beta and gamma (Farboud B, et al. 2003).
An allosteric shift provides a mechanism for the switch from repressed to de-repressed state. Thus, an RAR alpha without ligand has a binding site for a corepressor and when bound to ligand this site is eliminated and a coactivator site created. The coactivator then brings in a histone acetyl transferase to open up the local chromatin (le Maire A, et al. 2010). These actions are depicted in Figure 1.
A zebrafish ncor has been isolated and identified (Xu F, et al. 2009). Also, our own laboratory has used yeast two-hybrid selection to isolate zebrafish smrt and has shown its retinoic acid sensitive binding to raraa [unpublished results]. The zebrafish ncor study, through the use of antisense ncor morpholinos, demonstrated that ncor was required for normal embryonic development. In fact, these embryos had phenotypes resembling retinoic acid induced teratogenicity, which is predicted by our hypothesis. To examine whether the machinery for repressing retinoic acid inducible genes is available at fertilization, we have performed in situ hybridization with z smrt, z ncor, z rar aa, and zrar ab (Figure 3) in 8 cell embryos (a stage at which the embryonic genome is not yet transcriptionally active). Their presence at this early stage supports our hypothesis that the default position at fertilization is “OFF” for this potential epigenetic switch. We have confirmed their presence in oocytes along with z hdac3, rxr ga and rxr ab. Therefore, the molecular machinery for keeping genes repressed is apparent at the time of fertilization and we would speculate that this is probably the case for embryonic stem cells, given their sensitivity to retinoic acid and the sensitivity of embryonal carcinoma cells to retinoic acid (Espeseth AS, et al. 1989, McBurney MW, et al. 1982).
Figure 3.

Identification of mRNAs for z smrt, z ncor, z rar aa and z rar ab in 8-cell embryos before the embryonic genome turns on. This figure plus separate in situ hybridizations with z rxr ab, z rxr ga, zebrafish histone deacetylase 3 (z hdac3) in 8-cell embryos and oocytes support the hypothesis that the molecular machinery for repression is available at the time of fertilization. The z rar aa and z rar ab in situs are consistent with PCR data of Waxman and Yelon (Waxman JS and Yelon D 2007).
That these corepressive mechanisms may be significant to mammalian embryos is suggested by mouse gene knockouts. The mouse Smrt has been knocked out resulting in embryonic fatality mid-gestation most probably due to a heart defect (Jepsen K, et al. 2007). However, when functional Smrt was directed to myocytes in a Smrt-/- embryo to overcome this mid-gestation block, the animals could survive birth. Analysis of the brains in this myocyte-specific rescue showed a distinct effect upon the development of the brain (Jepsen K, et al. 2007). Mouse NCor knockouts appear to affect erythrocyte and thymocyte differentiation (Jepsen K, et al. 2000)] along with effects upon neural stem cells (Hermanson O, et al. 2002).
It should be emphasized that the corepressor molecules bind to several different factors in addition to RARs (Chen JD and Evans RM 1995, Horlein AJ, et al. 1995, Privalsky ML 2004). Therefore, knocking out or knocking down their expression and any associated developmental phenotype could be due to their interactions with other factors (though in the Xu F, et al. 2009, zebrafish ncor study, sufficient controls were incorporated to show a retinoic acid related phenotype. Also, the lack of any distinct phenotype could be due, as is the case for RARs, to overlapping expression and function of the two co-repressor molecules.
3.3 Visualizing epigenetic switching: transgenic retinoic acid activity indicator embryos
At the time that retinoic acid receptors were identified and shown to bind to specific sequences in promoters, several laboratories, including our own, made retinoic acid indicator transgenics (Balkan W, et al. 1992, Rossant J, et al. 1991) to try to identify the regions in developing embryos where retinoic acid receptor activity occurred. In these cases retinoic acid response elements (RAREs) were coupled to basal promoters to drive a beta-galactosidase indicator gene. This allowed one to capture developmental snapshots of the regions of retinoic acid activity from dissected, fixed, and processed embryos.
While these transgenic indicator mice were made with the caveat that expression would be dependent upon the promoter to which the RARE was attached, transgenics derived in different laboratories with different basal promoters did show overlap of expression. When our laboratory switched from using mouse as a vertebrate model to zebrafish, we used fluorescent reporters as transgenic indicators so that we could follow live gene expression instead of viewing snapshots of isolated, fixed, and processed mouse embryos. A comparison of our mouse RA indicator transgenic (Balkan W, et al. 1992) with one of our zebrafish RA indicator transgenics (Perz-Edwards A, et al. 2001) is shown in Figure 4. Studies of transgene expression in mice along with those in zebrafish revealed the homology of regional retinoic acid receptor activity in the two species. It should be stressed that these indicator lines reveal a subset of tissues where retinoic acid activated gene expression occurs. A transgene using an endogenous retinoic acid regulated promoter that was made in this laboratory (Hu P, et al. 2008) reveals a very different pattern. However, using relatively neutral, basal promoters allowed us to develop ideas concerning at least a subset of where retinoic acid receptor activity occurred.
Figure 4.

Transgenic indicator embryos for the developmental detection of retinoic acid receptor activity. The transgenes are identical except for the reporter. Both have 3 RARE elements coupled the Herpes simplex virus thymidine kinase promoter. The mouse transgene (left) is driving the bacterial beta-galactosidase gene while the zebraifish transgene is driving a Green Fluorescent Protein gene. Note that both are expressed in the neural tube. For details of developmental expression see (Balkan W, et al. 1992) and (Perz-Edwards A, et al. 2001) studies.
When we began to work with zebrafish so that we could look at early developmental events not easily viewed in placental embryos, we used the same RARE sequences to make a retinoic acid responsive transgenic line, but in this case with a fluorescent reporter so that we could watch, via time-lapse, the development of retinoic acid responsiveness. The parallel patterns of retinoic acid responsiveness in mouse and zebrafish confirmed at least partial regulatory homology (and one is referred to the original studies, Balkan W, et al. 1992, Perz-Edwards A, et al. 2001), for more developmental patterns of transgene expression in the mouse and zebrafish). Fortunately, some of the regions of expression in the transgenics were already known to express retinaldehyde dehydrogenase within or near the site of transgene signal where expression are patterns observed. This allowed for investigations of genes and gene families that might play a role in the presentation and restriction of retinoic acid in the developing embryo.
3.4 How is the switch thrown—retinaldehyde dehydrogenases
The illustration of retinoic acid responsive areas in developing embryos begs the question of how they might be created. Clearly the protein components for activating the switch are necessary, but the important controlling elements that regulate the allocation of the retinoic acid ligand to different embryonic regions at specific developmental times are equally important. This might have been expected based upon the very long history of retinoic acid teratogenesis. Retinoic acid can affect many different organ systems, both excellently documented in the golden hamster (Shenefelt RE 1972) and unfortunately experienced through accidental exposure of human fetuses through retinoid based acne medicine (Lammer EJ, et al. 1985). And perhaps to toxicologists and teratologists, one of the most important points of this review is that the phase I enzymes involved in the synthesis, metabolism and distribution play a primary role in determining the position (ON<>OFF) of this proposed epigenetic switch, i.e. sub-families of dehydrogenases and cytochrome p450 genes. The final enzymatic activity required to produce and distribute retinoic acid is retinaldehyde dehydrogenase (raldh). Enzymes upstream from this include retinol dehydrogenase, bcox (Lampert JM, et al. 2003) and dhrs3a (Feng L, et al. 2010) that oxidize beta-carotene to retinal and reduce retinal to retinol, respectively. A major source of retinoic acid in the early embryo comes from raldh2 activity (though additional raldh genes are expressed). As the trunk neural tube grows caudally, the adjacent somites have Raldh2 activity, where RA is produced and then diffuses to the developing neural tube. Additional raldh genes turn on at specific developmental times and regions to synthesize retinoic acid that can then create the allosteric change in rars found in adjacent cells causing them to activate genes. The story becomes more complex in non-teleosts where an intronic enhancer in the Raldh2 gene promotes expression in the roof-plate of the neural tube, where it can also provide a source of retinoic acid (Castillo HA, et al. 2010). In Figure 5 we illustrate just one aspect of the precise developmental regulation of the raldh2 gene, the expression in the somites that allows for retinoic acid to reach the adjacent developing neural tube. In the model below, we demonstrate this expression in somites (with other sites of expression not noted).
Figure 5.
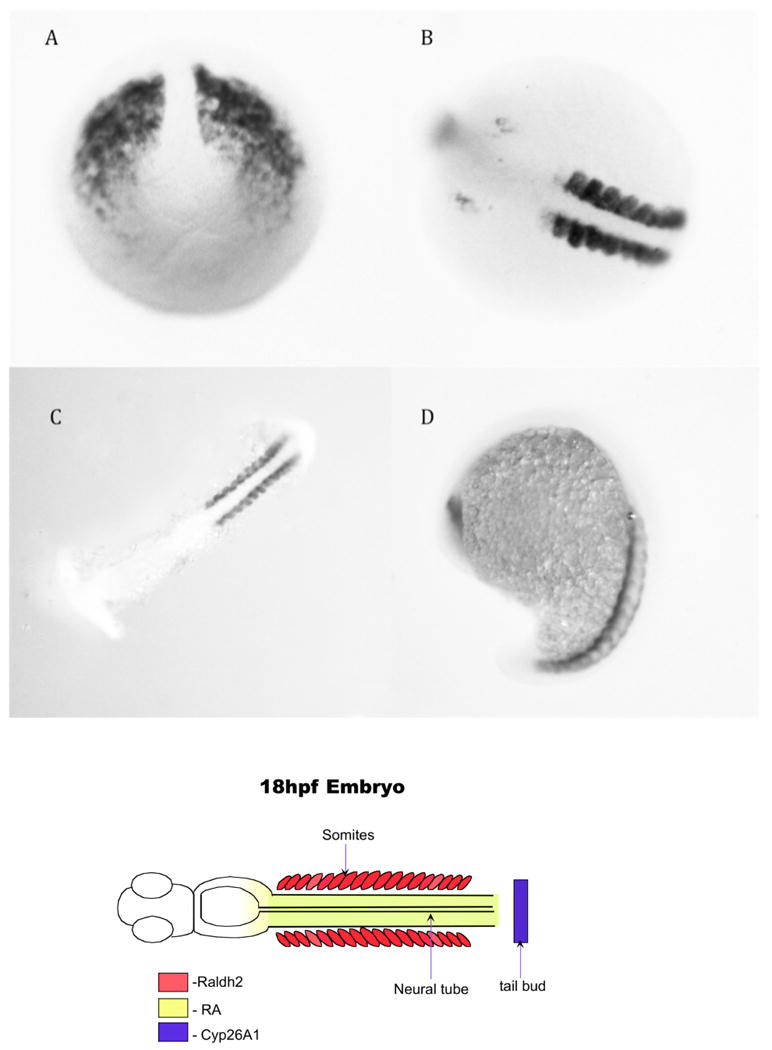
Raldh2 in situ hybridization at different developmental stages. A. Tailbud stage. B.10 somite stage-note the distinct localization in the somites adjacent to the neural tube. C.14 somite stage, flatmount. D. 20 somites stage, side view. The cartoon below represents the expression of the raldh2 in the somites—its expression in the brain is not included in the cartoon.
However, the important point for this review is that the developmental presentation of retinoic acid to the embryo is very precisely and developmentally controlled. If one examines Figure 6 one can see that there are several different pathways by which retinoic acid is produced, and how it can be limited, both by reduction of retinal by the Dhrs3a (which is upregulated by retinoic acid, (Feng L, et al. 2010)) and through its oxidation by the cyp26 enzymes.
Figure 6.
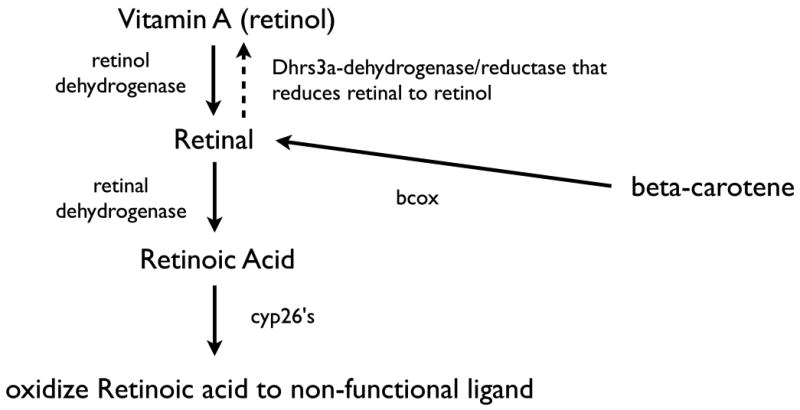
Retinoid pathways for the synthesis and oxidation of retinoic acid. Retinoic acid comes from either vitamin A or beta-carotene that is converted to retinal via retinol dehydrogenases. Retinoic acid is produced by the action of one of several retinal dehydrogenases, the major early embryonic enzyme being retinaldehyde dehydrogenase 2.
3.5 Painting borders of retinoic acid availability, the cyp26s
The role of the cytochrome p450, cyp26a1 was first described in 1996 (White JA, et al. 1996 and White JA, et al. 1997). Since that time two other cyp26 genes have been isolated and identified in several species and have distinct embryonic expression patterns and functions that play a significant role in embryogenesis. As can be seen in Figure 6 these enzymes oxidize retinoic acid so that their cellular localization and developmental expression can limit the availability of functional retinoic acid ligand to particular tissues. Genetic evidence that cyp26a1 creates a non-functional RAR ligand (Niederreither K, et al. 2002) indicates the importance of these genes in limiting the regions of retinoic acid availability in the embryo. It should be noted that while cells that produce retinoic acid can provide an extracellular source of retinoic acid to adjacent or cells close by, cells containing cyp26 activity create cellular borders blocking further diffusion. Therefore, the expression of cyp26 in cells can delimit the effects of cellular sources of retinoic acid. When one examines the developmental localization of cyp26a1, b1 and c1 it is clear that these are intricately arranged and by implication, can create dramatic effects upon which cells receive retinoic acid. In Figure 7 we illustrate just a few stages where the distribution of cyp26b1 mRNA is quite distinct, suggesting that its expression pattern could easily play a significant role in restricting the availability of retinoic acid to embryonic tissue in the normal developing embryo. Note the intricate pattern in the brain. This is certainly not unique to zebrafish, but is common to other vertebrate embryos. In addition, cyp26a1 and cyp26b1 show distinct and defining expression patterns in zebrafish (Dobbs-McAuliffe B, et al. 2004, Hernandez RE, et al. 2007, Zhao Q, et al. 2005) and in other vertebrate species. This has been elegantly described for all three cyp26 genes in the zebrafish hindbrain (Hernandez RE, et al. 2007).
Figure 7.

Cyp26b1 (blue)/Raldh2 (red) expression at 30h and 48hpf. Lateral views of in situ hybridization localization of the cyp26b1 and ralhd2 mRNAs in A. 30hpf embryo and B. 48hpf embryo. Note the discrete localization of cyp26b1 in the brain and the expression of raldh2 in the eye. More detailed expression of both of these can be found in the (Dobbs-McAuliffe B, et al. 2004) and (Zhao Q, et al. 2005) studies from this laboratory and a more detailed study showed the expression of all three of the cyp26 mRNAs can be found in (Hernandez RE, et al. 2007) study.
If one adds to this the developmental regulation of the Dhrs3a gene (Feng L, et al. 2010) that reduces retinal to retinol and whose expression is upregulated by retinoic acid, one sees a very comprehensive series of regulatory feedback mechanisms. Therefore, retinoic acid regulates its own production, reduction and consequentially its own developmental restriction.
3.6 A hypothetical situation
One can see from the above sections that built into normal development is exquisite developmental control of retinoic acid availability. We are suggesting, perhaps not surprisingly, that this system, early in development, plays a critical role in epigenetically controlling early gene expression that results in the subsequent diversification of cell types. While it might not play a specific role in defining individual cell types, we suspect that it releases the expression of genes that do initiate positive and negative controls on cascades of gene expression that do define cell phenotypes.
Independent of the hypothesis, if one examines the data collected thus far including the function of the genes and the suggested models of regulation, a logical description of what might be happening would be the following:
There are a number of genes that have retinoic acid response elements in their regulatory regions. In the oocyte and/or 8 cell stage of zebrafish when the embryonic genome has yet to turn on, we have detected, by in situ hybridization, mRNAs for rar aa, rar ab, rxr ab, rxr ga, hdac3, and the two corepressors z ncor and z smrt. In Figure 3 we illustrated the expression of rar aa, rar ab, and the two zebrafish rar/thr corepressors smrt and ncor. Not shown are our studies with oocytes and 8 cell embryos that illustrate the expression of these genes plus rxr ab, rxr ga, and hdac3. These observations are supported, in part, by the work of others (Bertrand S, et al. 2007, Waxman JS and Yelon D 2007, Xu F, et al. 2009).
These results show that the basic machinery for keeping these genes “OFF” exists before embryonic gene activation. Therefore, we propose the default position of an epigenetic switch involved in later cellular phenotype determination is “OFF”. As the embryo develops, the expression of raldh or raldh's turn on at discrete embryonic sites and times to selectively throw the switch “ON” and the degree or extent of the domain of retinoic acid distribution is limited by the selective expression of one or all 3 of the cyp26 genes. This is happening in a distinct manner during the development of the vertebrate hindbrain (Hernandez RE, et al. 2007, White RJ, et al. 2007). However, given the dramatic teratogenicity of retinoic acid (Shenefelt RE 1972) it would not be surprising if several other distinct and subtle switching events would be happening in other parts of the developing embryo.
As an example that supports this hypothesis we have pulsed zebrafish embryos with retinoic acid for 4 hours from 10hpf to 14hpf (the period of time when somitogenesis begins and trunk neural tube grows caudally). At 24 hours the embryos were fixed and processed for the identification of neurons and using confocal sectioning and reconstruction, Rohon-Beard neurons were counted from somites 6-17 of untreated and treated embryos. The results are shown in Figure 8 illustrating a dramatic increase in the numbers of neurons in the trunk neural tube of the pulsed embryos. As one can see from the images of the embryos, this pulse also had teratogenic effects on the morphology of the embryo. However, it points out a dramatic change as the “switch” is thrown on in an ectopic manner.
Figure 8.

A retinoic acid pulse increases the number of neurons in the embryo. Upper right is the experimental timing. 10-6 M retinoic acid was used to exposed embryos from 10hpf to 14hpf. They were washed out of the retinoic acid, allowed to grow to 24hpf fertilization, fixed and quantified for neuron numbers from somites 8-17.
Obviously, this potential switch is not alone in determining the complexity of the many, many different cellular phenotypes and, in fact, the switching, in itself may turn on or off other repressive or systems as the complexity of the differentiation increases. Clearly there is good evidence that expression of a “proneural” gene neurogenin1 is influenced by retinoic acid (Sharpe C and Goldstone K 2000).
Given the nature of zebrafish development, we suspect that this switching occurs in a more complicated manner by 10hpf when the trunk neural tube starts extending as somitogenesis begins.
4. Discussion
The biology of repression is clearly a very complex field. One only needs to examine general reviews of the variety of repression mechanisms (Rosenfeld MG, et al. 2006) in cells, some of which go considerably beyond the RAR-corepressor aspect of this manuscript. In addition, while we do not claim unique ownership of the ideas presented here we have tried to put, in one place, how this type of mechanism might play a role in teratogenesis and might bring out areas of vulnerability to neurotoxicological events.
Before aspects of this model and its implications are discussed, we will repeat our basic thoughts and description of the model: We are proposing that there is a family of genes, some of which are very important to the initiation of cellular phenotype diversification in the developing embryo, that are being repressed via RAR/RXR corepressor complexes that are present before the embryonic genome is turned on (and we suspect this is also true in embryonic stem cells). We are calling this the OFF position of an epigenetic switch. As the early embryo develops, Phase I gene products are expressed and a subset act on available retinoic acid precursors to “present” retinoic acid at specific times and places in development to basically throw the “switch” ON. The retinoic acid sources are cells with both access to retinoic acid precursors and cells that are expressing one of several retinaldehyde dehydrogenase genes. Once retinoic acid leaves the cells, it can then diffuse to nearby cells, enter, and “throw the switch”. An important and limiting control on which cells “see” retinoic acid are the cells that are producing cyp26a1, cyp26b1, or cyp26c1 activity. This activity oxidizes retinoic acid to a non-functional ligand, thereby “painting” regions and developmental times where retinoic acid can or cannot bind RARs. Once retinoic acid is available to cells, it can play a role in initiating different cascades of signaling, beginning processes of diversification of cell phenotypes.
We stress that this is a hypothesis based upon the observations of many laboratories and borrowing models based in the main on mammalian cell culture work. As a hypothesis it is expected to be challenged. For example, in a recent publication (Alexa K, et al. 2009) a series of experiments suggest strongly that raldh2 mRNA is maternally inherited and functional at some time during embryogenesis. Its message was detectable in 3 hour, post-fertilization embryos. The work supported the maternal acquisition of this mRNA. Therefore, it is possible that raldh2 protein could be present in the early embryo and available to act on any retinal substrates for the production of retinoic acid. Availability of retinoic acid at this time would be an argument against the hypothesis. On the otherhand, in an examination of the expression of the zebrafish family of cytochrome p450 genes in the early zebrafish embryo (Goldstone JV, et al.) it was clearly shown that cyp26a1 was also present by 3 hours post-fertilization. Therefore, if these mRNAs are translated, the cyp26a1 gene product could easily “protect” the embryo from the effects of any retinoic acid that raldh2 might produce. However, it remains to be shown when and where either of these different transcripts are translated-a reason for why we are defining this as a hypothesis.
In this discussion we will summarize some of the implications of this hypothesis: 1) the importance of Phase 1 enzymes that are specifically involved in the switching mechanism; 2) the roles and significance this might have in stem cell biology; 3) the possibility that similar repressive events might be mediated by thyroid hormone-corepressor mechanisms; and 4) some of the questions the hypothesis leads us to regarding the development of the nervous system and its possible vulnerability.
As the fields of epigenetics and epigenomics develop there is the implication that environmental effects may be leaving non-genetic “memory” on the genome. While we suspect that this switch plays a primary role in early definition of cellular phenotypes and not necessarily in transgenerational “memory” (unless perhaps a premature exposure of primordial germ cells to retinoic acid occurs) it does point out a mechanism for both marking the chromatin and reversing the mark via the influence of small molecules and gene products common to other processes in biology, namely the dehydrogenases and cyp26's. It also raises a question concerning the stability of these reversible complexes in cell types that do not divide. For example, does a complex that represses certain genes in embryonic cells leave it association site in differentiated cells, a more of less “hit and run” defining moment that eliminates the possibility of reversing the switch?
Could this switching paradigm be indicative of additional processes of repression that work through transcriptional activators, including those studied in environmental work such as the aryl hydrocarbon receptor? Certainly the enzymes that “paint” the borders of retinoic acid distribution are targets of pharmaceuticals, such as cyp26a1 inhibitors like liarozole (Stoppie P, et al. 2000). If it is found that more common gene products have a dual role, a role in triggering differentiation in early development and a separate role in the adult then we might have more caution concerning those individuals that might be taking pharmaceuticals that affect elements of the switch. If these repressive mechanisms play an additional role during adulthood, one will have to use more caution and consideration of secondary effects of developed drugs.
Since embryonic stem cells are recovered from embryos, a distinct and strong possibility is that this hypothesized switch is acting on stem cells to play a role in retaining their pluripotency. This is suggested by years of research leading up to and after embryonic stem cells were isolated and identified from embryos. The conceptual precursor of embryonic stem cells, embryonal carcinoma cells (EC cells), were themselves isolated from gonadal tumors that differentiated with the EC cell being a stem cell that was either limited or pluripotent in its potential. At least two lines of EC cells were shown to be induced to differentiate with retinoic acid, F9 EC cells (Espeseth AS, et al. 1989) and P19 EC cells (McBurney MW, et al. 1982), and we showed through dominant negative RAR transfection experiments that repressing RAR function inhibited retinoic acid induced differentiation (Espeseth AS, et al. 1989). There are numerous reports of the effects of retinoic acid on embryonic stem cells supporting the basic idea that RARs may repress gene expression in embryonic stem cells. Understanding the processes and the control of the processes may aid in the development of procedures for improving the direction of differentiation of the cells. Since there are serious attempts for exploring histone deacetylase inhibitors for cancer research (Jones PA and Baylin SB 2007), and since this switch involves histone deacetylase activity, the implication of this area of research stretches from embryogenesis to stem cells to cancer control.
While we have not covered it in this review, the development of the corepressor field involved not just RAR-NCor or RAR-Smrt interaction but also interaction of these corepressors with the thyroid hormone receptor (Horlein AJ, et al. 1995). There is evidence in zebrafish that thyroid hormone receptor RNA is present in oocytes (Takayama S, et al. 2008), including a receptor that we have shown is hormone sensitive in its association with the z smrt gene we isolated [Linney et al. unpublished]. So there exists the possibility that a parallel system of embryonic corepression may exist for the thyroid hormone receptor. This might then implicate genes that are involved in thyroid hormone synthesis, metabolism, and distribution of thyroid hormone.
While we hypothesize that this is a teratogenic paradigm, we have not gone into detail with connections we could draw. Many developmental and teratogenic studies have involved both vitamin A and when it was discovered as a more potent teratogen, with retinoic acid (Kochar D 1967). Several experimental studies ensued including the Shenefelt study (Shenefelt RE 1972) as an excellent example of how widespread a teratogen retinoic acid can be. We believe this hypothesis, when extended to the whole embryo, makes it easy to imagine why retinoic acid is such a potent teratogen-and not a paradox. When one exposes retinoic acid to the embryo they are not only throwing many “switches” that were not meant to thrown at that developmental time and place, but they are disrupting a very carefully modulated system of feedback control of enzymes that either are in the pathway of synthesis or of oxidation of retinoic acid. One of the steps in that process is to change the structure of the retinoic acid receptor to remove the corepressor and its associated histone deacetylase. As it turns out, valproic acid, a pharmaceutical that is also a teratogen, is a direct inhibitor of histone deacetylase activity (Gurvich N, et al. 2005, Phiel CJ, et al. 2001) showing a potential overlap in the teratogenic function of retinoic acid and valproic acid. While other teratogens might act through different paradigms, this at least suggests a mechanistic basis for the teratogenic effects of these two compounds. It also points out that any chemical or pharmaceutical perturbation of these Phase I enzyme gene products in the embryo may result in developmental disruptions.
As the field of epigenesis and the more specific subdivision of gene imprinting and transgenerational mechanisms of marking the genome develop, it would not be surprising to have this hypothesis grow in its sophistication. The corepressor molecules that bind with higher affinity to RARs have been shown not only to bind to other receptors, but also to have a complex array of association sites for other proteins with enzymatic or association sites. Therefore, while this model follows the basic model of having a corepressor bring a histone deacetylase to the site of RAR/RXR binding, it would not be surprising to find that other systems for marking chromatin or even DNA might also be attracted to RAR-corepressor sites on RAREs.
Our future work will be directed towards defining when the retinoic acid switch can be thrown in the very early embryo, and the natural roles it might play in the beginning of the diversification of cell types in the early embryos. Clearly there are many interacting systems that are involved in such processes and we are interested in whether this switch involves the “start” of differentiation cascades that themselves refine cell types (cascades that can involve both transcriptional activators or retinoic acid induced repressors) or whether there is a direct effect upon the early neural phenotypes.
Highlights.
>We hypothesize that retinoic acid receptors are part of an embryonic, epigenetic switch. >This switch is based upon the two different forms of the retinoic acid receptor, with and without retinoic acid ligand. >Zebrafish is used to model this hypothesis.
Acknowledgments
We wish to thank Dr. Kari Yacisin for her in situ hybridization work with raldh2. The research of this laboratory leading up to this article was supported by a series of PHS grants, the latest of which was ES011375 and ES016554.
Funding Source: All sources of funding should also be acknowledged and you should declare any involvement of study sponsors in the study design; collection, analysis and interpretation of data; the writing of the manuscript; the decision to submit the manuscript for publication. If the study sponsors had no such involvement, this should be stated.
Footnotes
Declarations: Neurotoxicology and Teratology requires that all authors sign a declaration of conflicting interests. If you have nothing to declare in any of these categories then this should be stated.
Conflict of Interest: A conflicting interest exists when professional judgement concerning a primary interest (such as patient's welfare or the validity of research) may be influenced by a secondary interest (such as financial gain or personal rivalry). It may arise for the authors when they have financial interest that may influence their interpretation of their results or those of others. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexa K, Choe SK, Hirsch N, Etheridge L, Laver E, Sagerstrom CG. Maternal and zygotic aldh1a2 activity is required for pancreas development in zebrafish. PLoS One. 2009;4:e8261. doi: 10.1371/journal.pone.0008261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balciunas D, Wangensteen KJ, Wilber A, Bell J, Geurts A, Sivasubbu S, Wang X, Hackett PB, Largaespada DA, McIvor RS, et al. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2006;2:e169. doi: 10.1371/journal.pgen.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkan W, Colbert M, Bock C, Linney E. Transgenic indicator mice for studying activated retinoic acid receptors during development. Proc Natl Acad Sci U S A. 1992;89:3347–51. doi: 10.1073/pnas.89.8.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand S, Thisse B, Tavares R, Sachs L, Chaumot A, Bardet PL, Escriva H, Duffraisse M, Marchand O, Safi R, et al. Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet. 2007;3:e188. doi: 10.1371/journal.pgen.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo HA, Cravo RM, Azambuja AP, Simoes-Costa MS, Sura-Trueba S, Gonzalez J, Slonimsky E, Almeida K, Abreu JG, de Almeida MA, et al. Insights into the organization of dorsal spinal cord pathways from an evolutionarily conserved raldh2 intronic enhancer. Development. 2010;137:507–18. doi: 10.1242/dev.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–7. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- Dobbs-McAuliffe B, Zhao Q, Linney E. Feedback mechanisms regulate retinoic acid production and degradation in the zebrafish embryo. Mech Dev. 2004;121:339–50. doi: 10.1016/j.mod.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Drager UC. Retinoic acid signaling in the functioning brain. Sci STKE. 2006;2006:pe10. doi: 10.1126/stke.3242006pe10. [DOI] [PubMed] [Google Scholar]
- Espeseth AS, Murphy SP, Linney E. Retinoic acid receptor expression vector inhibits differentiation of F9 embryonal carcinoma cells. Genes Dev. 1989;3:1647–56. doi: 10.1101/gad.3.11.1647. [DOI] [PubMed] [Google Scholar]
- Farboud B, Hauksdottir H, Wu Y, Privalsky ML. Isotype-restricted corepressor recruitment: a constitutively closed helix 12 conformation in retinoic acid receptors beta and gamma interferes with corepressor recruitment and prevents transcriptional repression. Mol Cell Biol. 2003;23:2844–58. doi: 10.1128/MCB.23.8.2844-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Hernandez RE, Waxman JS, Yelon D, Moens CB. Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev Biol. 2010;338:1–14. doi: 10.1016/j.ydbio.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–9. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, Nelson DR, Stegeman JJ. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Xu F, Wang X, Gao X, Zhao Q. Molecular cloning and expression of a novel CYP26 gene (cyp26d1) during zebrafish early development. Gene Expr Patterns. 2005;5:733–9. doi: 10.1016/j.modgep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Gurvich N, Berman MG, Wittner BS, Gentleman RC, Klein PS, Green JB. Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. Faseb J. 2005;19:1166–8. doi: 10.1096/fj.04-3425fje. [DOI] [PubMed] [Google Scholar]
- Hale LA, Tallafuss A, Yan YL, Dudley L, Eisen JS, Postlethwait JH. Characterization of the retinoic acid receptor genes raraa, rarab and rarg during zebrafish development. Gene Expr Patterns. 2006;6:546–55. doi: 10.1016/j.modgep.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Hermanson O, Jepsen K, Rosenfeld MG. N-CoR controls differentiation of neural stem cells into astrocytes. Nature. 2002;419:934–9. doi: 10.1038/nature01156. [DOI] [PubMed] [Google Scholar]
- Hernandez RE, Putzke AP, Myers JP, Margaretha L, Moens CB. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134:177–87. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- Hu P, Tian M, Bao J, Xing G, Gu X, Gao X, Linney E, Zhao Q. Retinoid regulation of the zebrafish cyp26a1 promoter. Dev Dyn. 2008;237:3798–808. doi: 10.1002/dvdy.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen K, Hermanson O, Onami TM, Gleiberman AS, Lunyak V, McEvilly RJ, Kurokawa R, Kumar V, Liu F, Seto E, et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102:753–63. doi: 10.1016/s0092-8674(00)00064-7. [DOI] [PubMed] [Google Scholar]
- Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, Hermanson O, Rosenfeld MG. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450:415–9. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- Jones BB, Ohno CK, Allenby G, Boffa MB, Levin AA, Grippo JF, Petkovich M. New retinoid X receptor subtypes in zebra fish (Danio rerio) differentially modulate transcription and do not bind 9-cis retinoic acid. Mol Cell Biol. 1995;15:5226–34. doi: 10.1128/mcb.15.10.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joore J, van der Lans GB, Lanser PH, Vervaart JM, Zivkovic D, Speksnijder JE, Kruijer W. Effects of retinoic acid on the expression of retinoic acid receptors during zebrafish embryogenesis. Mech Dev. 1994;46:137–50. doi: 10.1016/0925-4773(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Kochar D. Teratogenic activity of retinoic acid. Acta Pathol Microbiol Scand. 1967;70:398–404. doi: 10.1111/j.1699-0463.1967.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, Curry CJ, Fernhoff PM, Grix AW, Jr, Lott IT, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–41. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, von Lintig J. Provitamin A conversion to retinal via the beta, beta-carotene-15,15′-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development. 2003;130:2173–86. doi: 10.1242/dev.00437. [DOI] [PubMed] [Google Scholar]
- le Maire A, Teyssier C, Erb C, Grimaldi M, Alvarez S, de Lera AR, Balaguer P, Gronemeyer H, Royer CA, Germain P, et al. A unique secondary-structure switch controls constitutive gene repression by retinoic acid receptor. Nat Struct Mol Biol. 2010;17:801–7. doi: 10.1038/nsmb.1855. [DOI] [PubMed] [Google Scholar]
- Linney E, Udvadia AJ. Construction and detection of fluorescent, germline transgenic zebrafish. Methods Mol Biol. 2004;254:271–88. doi: 10.1385/1-59259-741-6:271. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–9. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Wendling O, Dupe V, Mascrez B, Kastner P, Chambon P. A genetic dissection of the retinoid signalling pathway in the mouse. Proc Nutr Soc. 1999;58:609–13. doi: 10.1017/s0029665199000798. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299:165–7. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–80. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Nelson DR. A second CYP26 P450 in humans and zebrafish: CYP26B1. Arch Biochem Biophys. 1999;371:345–7. doi: 10.1006/abbi.1999.1438. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, Dolle P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat Genet. 2002;31:84–8. doi: 10.1038/ng876. [DOI] [PubMed] [Google Scholar]
- Ordentlich P, Downes M, Xie W, Genin A, Spinner NB, Evans RM. Unique forms of human and mouse nuclear receptor corepressor SMRT. Proc Natl Acad Sci U S A. 1999;96:2639–44. doi: 10.1073/pnas.96.6.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perz-Edwards A, Hardison NL, Linney E. Retinoic acid-mediated gene expression in transgenic reporter zebrafish. Dev Biol. 2001;229:89–101. doi: 10.1006/dbio.2000.9979. [DOI] [PubMed] [Google Scholar]
- Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–50. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol. 2004;66:315–60. doi: 10.1146/annurev.physiol.66.032802.155556. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–28. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–44. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Sharpe C, Goldstone K. Retinoid signalling acts during the gastrula stages to promote primary neurogenesis. Int J Dev Biol. 2000;44:463–70. [PubMed] [Google Scholar]
- Shenefelt RE. Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology. 1972;5:103–18. doi: 10.1002/tera.1420050115. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Solnica-Krezel L, Zwartkruis F, Neuhauss SC, Schier AF, Malicki J, Stainier DY, Abdelilah S, Rangini Z, Mountcastle-Shah E, et al. Mutations affecting development of the notochord in zebrafish. Development. 1996;123:117–28. doi: 10.1242/dev.123.1.117. [DOI] [PubMed] [Google Scholar]
- Stoppie P, Borgers M, Borghgraef P, Dillen L, Goossens J, Sanz G, Szel H, Van Hove C, Van Nyen G, Nobels G, et al. R115866 inhibits all-trans-retinoic acid metabolism and exerts retinoidal effects in rodents. J Pharmacol Exp Ther. 2000;293:304–12. [PubMed] [Google Scholar]
- Takayama S, Hostick U, Haendel M, Eisen J, Darimont B. An F-domain introduced by alternative splicing regulates activity of the zebrafish thyroid hormone receptor alpha. Gen Comp Endocrinol. 2008;155:176–89. doi: 10.1016/j.ygcen.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallafuss A, Hale LA, Yan YL, Dudley L, Eisen JS, Postlethwait JH. Characterization of retinoid-X receptor genes rxra, rxrba, rxrbb and rxrg during zebrafish development. Gene Expr Patterns. 2006;6:556–65. doi: 10.1016/j.modgep.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–66. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman JS, Yelon D. Comparison of the expression patterns of newly identified zebrafish retinoic acid and retinoid X receptors. Dev Dyn. 2007;236:587–95. doi: 10.1002/dvdy.21049. [DOI] [PubMed] [Google Scholar]
- White JA, Boffa MB, Jones B, Petkovich M. A zebrafish retinoic acid receptor expressed in the regenerating caudal fin. Development. 1994;120:1861–72. doi: 10.1242/dev.120.7.1861. [DOI] [PubMed] [Google Scholar]
- White JA, Guo YD, Baetz K, Beckett-Jones B, Bonasoro J, Hsu KE, Dilworth FJ, Jones G, Petkovich M. Identification of the retinoic acid-inducible all-trans-retinoic acid 4-hydroxylase. J Biol Chem. 1996;271:29922–7. doi: 10.1074/jbc.271.47.29922. [DOI] [PubMed] [Google Scholar]
- White JA, Beckett-Jones B, Guo YD, Dilworth FJ, Bonasoro J, Jones G, Petkovich M. cDNA cloning of human retinoic acid-metabolizing enzyme (hP450RAI) identifies a novel family of cytochromes p450. J Biol Chem. 1997;272:18538–41. doi: 10.1074/jbc.272.30.18538. [DOI] [PubMed] [Google Scholar]
- White RJ, Nie Q, Lander AD, Schilling TF. Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Li K, Tian M, Hu P, Song W, Chen J, Gao X, Zhao Q. N-CoR is required for patterning the anterior-posterior axis of zebrafish hindbrain by actively repressing retinoid signaling. Mech Dev. 2009;126:771–80. doi: 10.1016/j.mod.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Yu VC, Delsert C, Andersen B, Holloway JM, Devary OV, Naar AM, Kim SY, Boutin JM, Glass CK, Rosenfeld MG. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991;67:1251–66. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Dobbs-McAuliffe B, Linney E. Expression of cyp26b1 during zebrafish early development. Gene Expr Patterns. 2005;5:363–9. doi: 10.1016/j.modgep.2004.09.011. [DOI] [PubMed] [Google Scholar]


