Abstract
Inhibitors of cyclooxygenase (COX) indicate that up-regulation of inflammatory eicosanoids produced by COX, and in particular prostaglandin E2 (PGE2), are early events in the development of colorectal cancer (CRC). Ginger has demonstrated down regulation of COX in vitro and decreased incidence/ multiplicity of adenomas in rats. This study was conducted to determine if 2.0 g/day of ginger could decrease the levels of PGE2, 13-hydroxy-octadecadienoic acids (13-HODE), and 5-, 12-, & 15-hydroxyeicosatetraenoic acid (5-, 12-, & 15-HETE), in the colon mucosa of healthy volunteers. To investigate this aim we randomized 30 subjects to 2.0 g/day ginger or placebo for 28 days. Flexible sigmoidoscopy at baseline and day 28 was used to obtain colon biopsies. A liquid chromatography mass spectrometry method was used to determine eicosanoid levels in the biopsies, and levels were expressed per protein or per free arachidonic acid. There were no significant differences in mean percent change between baseline and day 28 for any of the eicosanoids, when normalized to protein. There was a significant decrease in mean percent change in PGE2 (p=0.05) and 5-HETE (p=0.04), and a trend toward significant decreases in 12-HETE (p=0.09) and 15-HETE (p=0.06) normalized to free arachidonic acid. There was no difference between the groups in terms of total adverse events (AE) (p=0.55). Based on these results, it appears that Ginger has the potential to decrease eicosanoid levels, perhaps by inhibiting their synthesis from arachidonic acid. Ginger also appeared to be tolerable and safe. Further investigation in people at high risk for CRC seems warranted.
Keywords: Cancer Risk Reductive, Eicosanoids, Colorectal Cancer, Inflammation, and Ginger
INTRODUCTION
Anti-inflammatory agents such as aspirin and related non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclooxygenase (COX) enzymes and decrease the levels of the inflammatory prostaglandin E2 (PGE2) appear to be promising colorectal cancer (CRC) chemopreventive agents.1 Aspirin and related NSAIDs have been shown to prevent the development of adenomas and CRC in both animal models of CRC and in numerous epidemiological studies.1 While aspirin and related NSAIDs are encouraging chemopreventive agents, there is some speculation that the inhibition of COX enzymes could cause the shunting of arachidonic acid (AA), the substrate for COX, towards the production of other inflammatory eicosanoids.2
The lipoxygenase (LOX) enzymes also use AA as a substrate to produce eicosanoids. Eicosanoids products of 5-, 12-, & 15-lipoxygenase (5-, 12-, & 15-LOX) are 5-, 12-, & 15-hydroxyeicosatetraenoic acid (HETEs).3 There is some evidence that that the simultaneous inhibition of COX-2 and 5-LOX causes greater inhibition of tumor growth and decreased concentrations of PGE2 compared to inhibition of COX-2 or 5-LOX alone.4 While evidence is strongest for the role of PGE2 in colon tumor initiation and progression 5-HETE and 12-HETE have also been implicated in the development of CRC.5 Soumaoro and colleagues demonstrated that 5-LOX expression and 5-HETE concentrations are up-regulated in human colorectal cancer specimens, and are correlated with tumor size, depth, and vessel invasion.6, 7 12-HETE was found to stimulate the proliferation of both HT-29 and HCT-15 human colon carcinoma cells.8 Increased production of 5-HETE and 12-HETE has also been reported in the mucosa of colon cancer patients.9
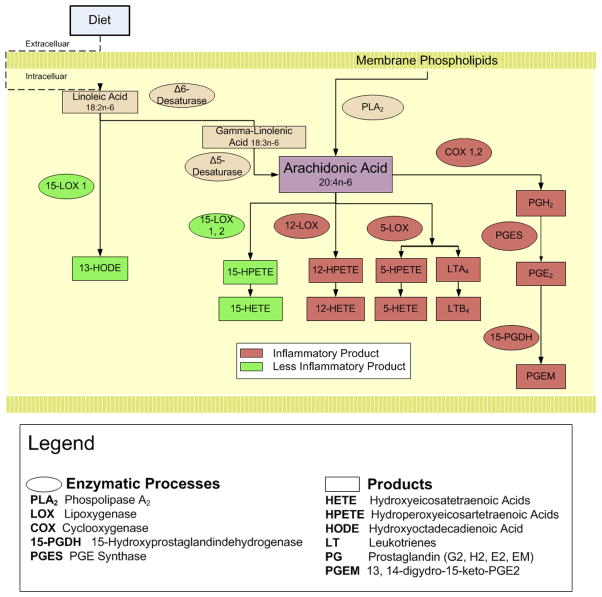
Complicating this picture is the production of putative anti-tumor/anti-inflammatory eicosanoids in colorectal tumorigenesis such as 15-HETE and 13-HODE.10,11 While both 15-HETE and 13-HODE are catalyzed from different substrates (AA for 15-HETE and linoleic acid for 13-HODE) by 15-LOX, they are produced by different isoforms of 15-LOX, with15-LOX-1 metabolizing linoleic acid and 15-LOX-2 metabolizing AA (see Figure 1).3, 10 Consequently, an attractive colorectal chemopreventive agent would impact not just the COX enzymes, but the balance of products from differing eicosanoid enzymes, potentially shifting the eicosanoid system toward a local anti-inflammatory state.
Figure 1.
Metabolism of Linoleic and Arachidonic Acid
Ginger root (Zingiber officinale Roscoe, Zingiberaceae) is one of the most heavily consumed dietary substances in the world and is one of the top selling dietary supplements in the United States.12, 13 Ginger’s mechanism of anticarcinogenesis action is not entirely known, but appears to be associated with the anti-oxidant and anti-inflammatory actions of its non-volatile pungent components the gingerols and shogaols.12 Ginger inhibits 5-LOX14–17 and COX-1 & -2;14, 18–20 decreases inflammation in various murine models,12, 16, 21–23 and reduces serum concentrations of PGE2 in rats.23 Several studies of chemically-induced colon carcinogenesis have demonstrated that ginger is preventive24–26 When ginger was administered in the post-initiation stage, however, it did not suppress aberrant crypt foci formation nor did ginger significantly change the proliferative or apoptotic indexes of the colonic crypt.27
The purpose of this study was to examine the effect of 2.0 g of ginger taken daily for 28 days compared to placebo on eicosanoids in the colon mucosa of people at normal risk for developing colorectal cancer. Secondary objectives were to evaluate the safety, tolerability, adherence and blinding success of ginger given orally for 28 days.
METHODS
Participants
A total of 33 participants were recruited from the surrounding community through fliers or word-of-mouth between April 2007 and May 2008. To be eligible for the study, participants had to be 18 years or older and in good health as defined by an unremarkable medical history, physical and screening blood work (chemistry screen, complete blood count) within 60 days of study entry. No chronic medication use was allowed and participants could not have taken aspirin or related NSAIDs during the study or 14 days before the first dose of the study medication. Participants also had to be classified as being at normal-risk for developing colorectal cancer. Normal-risk was defined as having; no history of familial colorectal cancer syndromes; no first-degree relatives with colon cancer diagnosed before the age of 60; no personal history of colorectal cancer and no adenomas >1 cm in size or containing carcinoma in situ. Exclusion criteria for the study included: (1) a history of peptic ulcer disease, gastrointestinal bleeding from gastric or duodenal ulcers, or gastrin secreting tumors; (2) pregnant or lactating women; (3) history of cardiovascular disease; (4) lactose intolerance; (5) or an allergy to ginger. Participants were asked to avoid all foods containing ginger within the 14 days prior to drug administration. This was confirmed by having participants complete a food checklist to verify that they were not consuming any ginger-rich foods such as ginger ale or Japanese food. All of the participants were reimbursed for their time. All study procedures were administered at the University of Michigan Clinical Research Unit (MCRU) after the participant gave written, informed consent. The study was approved by the University of Michigan Institutional Review Board.
Drug
The ginger product used in this study was manufactured by Pure Encapsulations® (Sudbury, MA). Pure Encapsulation’s® ginger (Z. officinale) powder was processed using Good Manufacturing Procedures (GMP). Each capsule contained 250 mg dry extract of ginger root [10:1 (v/v) extraction solvent (ethanol 50 %): root] normalized to 15 mg (5%) of total gingerols. Based on high performance liquid chromatography (HPLC) analysis, a 250 mg capsule of ginger extract (from both batches) contained 5.38 mg (2.15%) 6-gingerol, 1.80 mg (0.72%) 8-gingerol, 4.19 mg (1.78%) 10-gingerol, and 0.92 mg (0.37%) 6-shogaol. Gingerol and shogaol content was verified by an independent laboratory using appropriate HPLC techniques (Integrated Biomolecule Corporation: Tucson, Arizona). The study was conducted using two batches (ZO/06006 and ZO/07006) of ginger powder extract, both of which were tested for gingerols and shogaol content.
The 2.0 g dose used in the study was chosen based on the highest tolerated amount of ginger extract in a phase I dose escalation study in healthy volunteers.28 Also, 2.0 g of ginger extract is equivalent to 20 g of raw ginger root, which would be a large but not unreasonable amount to consume through the diet. Placebo consisted of lactose powder. Ginger powder and lactose were placed into identical opaque red capsules. Placebo and ginger capsules were assembled, stored and dispensed by the Investigational Drug Service of the University of Michigan (U of M IDS). The participants were instructed to take eight 250 mg capsules daily with food and to bring any unused capsules to the final (28 day) study visit.
Randomization, Allocation and Blinding
Eligible participants were randomized equally to one of two groups: placebo or ginger extract (2.0 g). The randomization code was computer-generated by the study biostatistician. The randomization code was kept by the University of Michigan (U of M IDS), which assigned the next available randomization number as the study team informed them of eligible participants. Study participants and all study personnel who assessed outcomes, worked with study data or administered tests or questionnaires were unaware of the randomization list or treatment assignment.
Adherence and Assessment of Blinding
Participants were assessed for adherence by a research coordinator through weekly telephone calls, self-report, and pill countsat the end of the study. Adherencewas defined as taking the capsules within 4 hours of the agreed upon time, twice daily. Participants were classifiedas adherent if the adherence monitoring suggested that 80% or more of the doses were taken as prescribed.
Blinding was assessed by asking the participants during their final visit which treatment they believed they received (“ginger”, “placebo” or “don’t know”). Participants were also asked the reason for their response, e.g., “was it the way the capsule smelled?”
Toxicity Assessment
Participants were assessed for toxicity by direct questioning in person, by email or by telephone at weekly intervals. The National Cancer Institute (NCI) Common Toxicity Scale V 3.0 (Regulatory Affairs Branch, Cancer Therapy Evaluation program, Division of Cancer Treatment, Diagnosis, and Centers, NCI, Bethesda, MD)29 was used to quantify toxicity.
Flexible Sigmoidoscopy and Tissue Collection
Participants underwent two flexible sigmoidoscopies, one before drug treatment and the second 28 days after ginger extract treatment commenced. The second procedure was performed at a time as close as possible to 24 hours after the participant took the final ginger dose. The participants were not prepared for the procedure with any enemas. Participants were, however, asked to evacuate their rectum within12 hours of the procedure, but to not take any laxatives to enhance evacuation.
Participants were placed in a left lateral decubitus position and a flexible sigmoidoscope was passed to 20 to 25 cm from the anal sphincter. Four tissue samples were taken by opening and pressing the biopsy forceps perpendicular to the mucosal surface with mild pressure. Each biopsy specimen was taken approximately 2 cm or more from other biopsy sites in distal sigmoid colonic mucosa that had no visual appearance of trauma or recent biopsy.
Tissue Handling and Disposition
Biopsy samples were placed into a sterile1.5-ml Eppendorf tube and frozen in liquid nitrogen at exactly 50seconds after the time the biopsy forceps were closed. The specimens were stored at -70°C until immediately before analysis.
Frozen biopsy samples weighed approximately 5 mg and yielded between 400 and600 μg protein. Triplicate assays for the eicosanoids required approximately 10–20 μg of colon tissue. The remaining frozen tissue samples were stored at −70°C for future use.
Analytical Methods
Eicosanoids (PGE2, 5-HETE, 12-HETE, 15-HETE and 13-HODE)
All eicosanoids and deuterated internal standards used in this study were purchased from Cayman Chemical Co. (Ann Arbor, MI). Arachidonate, butylated hydroxytoluene (BHT), citric acid, and EDTA were obtained from Sigma Chemical Co. (St. Louis, MO). All Burdick and Jackson brand HPLC-grade solvents were purchased from Fisher Scientific Co. (Fair Lawn, NJ).
Reverse-phase LC electrospray ionization mass spectrometry (LC/MS/MS) analyses were used for quantitation of PGE2, 5-HETE, 12-HETE, 15-HETE and 13-HODE as described previously.30, 31 Two frozen colonic biopsy specimens from the same participant and time point were removed from the freezer, combined and ground to a fine powder using a liquid-nitrogen-cooled mortar. Samples were then transferred to sealed microcentrifuge tubes, and three volumes of ice-cold PBS buffer containing 0.1% BHT and 1mM EDTA were added. The samples were then homogenized by an Ultrasonic Processor (Misonix, Farmingdale, NJ) at 0 C for 3min. A 100-μl aliquot of the homogenate was transferred to a glass tube (13×100mm) for extraction of eicosanoids. Briefly, 20-μl aliquots of 1N citric acid and 10μl of deuterated PGE2; 5-, 12-, or 15-HETE; or 13-HODE (100ng/ml) were added to the samples. Eicosanoids were then extracted with 1ml of hexane:ethyl acetate (1:1, v/v) and vortexed for 2 minutes. All extraction procedures were performed at minimum light levels under cold conditions (4 C). Samples were centrifuged at 1800×g for 10min at 4 C. The upper organic layer was collected, and the organic phases from three extractions were combined prior to drying under a stream of nitrogen at room temperature. Samples were then reconstituted in 100μl of methanol:ammonium acetate buffer (10mM at pH 8.5; 70:30, v:v) before LC/MS/MS analysis. The protein concentration in the homogenate was determined by a Bradford protein assay (Bio-Rad, Hercules, CA).
LC/MS/MS analyses were performed using a Quattro Ultima tandem mass spectrometer (Micromass, Beverly, MA) equipped with an Agilent HP 1100 binary pump HPLC inlet. Eicosanoids were separated using a Luna 3μ Phenyl-Hexyl 2×150mm LC column (Phenomenex, Torrance, CA). The mobile phase consisted of 10 mM ammonium acetate (pH 8.5) and methanol. For the analysis of PGE2, HETEs and 13-HODE, the separation was achieved using a linear methanol gradient from 40% to 60% over 18 minutes followed by a methanol flush. The flow rate was 250μl/minute with a column temperature of 50 C. The sample injection volume was 25μl. Samples were kept at 4 C during the analysis. All eicosanoids were detected using electrospray negative ionization and multiple-reaction monitoring of the transition ions for the metabolites and their internal standards.32
The mass spectrometer (Thermo Finnigan TSQ Quantum, San Jose, CA) was operated in the electrospray negative ion mode with a cone voltage of 2300V, a cone gas flow rate of 117l/h, and a devolution gas flow rate of 998l/h. The temperature of the desolvation region was 350 C, and the temperature of the source region was 120 C. Fragmentation for all compounds was performed using argon as the collision gas at a collision cell pressure of 2.10×10−3Torr. The collision energy ranged from 16 to31V depending on the analyte. The results were either expressed as nanogram (ng) of eicosanoid per milligram (mg) of protein or as ng of eicosanoid per microgram (μg) of free AA. All of the biopsy samples from a given individual were assayed in the same batch to eliminate any batch effects on changes over time. Four batches were assayed for this study. The within-day coefficients of variation (CV) of the assay, based on three injections of the same sample on the same day, for PGE2, 5-, 12-, 15-HETE, 13-HODE, and AA were 3.8%, 13.2%, 15.4%, 13.2%, 12.2% and 2.5 %, respectively and the between day CV for PGE2, 5-, 12-, 15-HETE, 13-HODE and AA were 5.3%, 18.9%, 16.0%, 34.2%, 28.4 and 7.0 %, respectively.
Statistical Methods and Sample Size
Statistical analyses were conducted using SPSS software version 18.0 (Somers, NY). Baseline characteristics were analyzed, stratified by treatment group, using means and SDs for continuous variables, and counts and percentages for categorical variables. Balance between treatment groups on baseline characteristics was tested using independent sample t-tests for continuous variables and Pearson’s Chi-square and Fisher exact tests, as appropriate, for categorical variables.
Mean percent change from baseline to day 28 for each treatment group for PGE2, 5-HETE, 12-HETE, 15-HETE and 13-HODE was calculated (e.g., [PGE2 at day 28 PGE2 at baseline]/ PGE2 at baseline). Also, given the large batch-to-batch variability of the eicosanoid assays we investigated the effect of batch on mean percent change using general linear models. A Kolmogorov-Smirnov-Lilliefors’ (KSL) test for normality was conducted to determine if either treatment groups were normally distributed. Depending on the results of the KSL test, independent sample t-tests were used to calculate the differences between treatment groups for mean percent change when normally distributed and the Mann-Whitney U test was used when not normally distributed. Results are reported as means ± standard deviations. Adverse events, blinding and adherence between groups were analyzed using Pearson’s Chi-square or Fisher’s exact test as appropriate. A p-value of ≤ 0.05 was considered statistically significant.
The sample size needed for the study was determined using published data on PGE2 levels.33 PGE2 concentration in human colon tissue at baseline had a mean and standard deviation of 11.7 pg/mg, ± 1.7 pg/mg. Based on this PGE2 level, we calculated that a sample size of 15/treatment group would have better than 80% power to detect a reduction in PGE2 level of at least 25%. A post-hoc power analysis was also performed for PGE2. The analysis was based on the observed data using a two-sample t-test of percent change for PGE2 to determine the sample size needed for 80% power.
RESULTS
Subjects and Toxicity
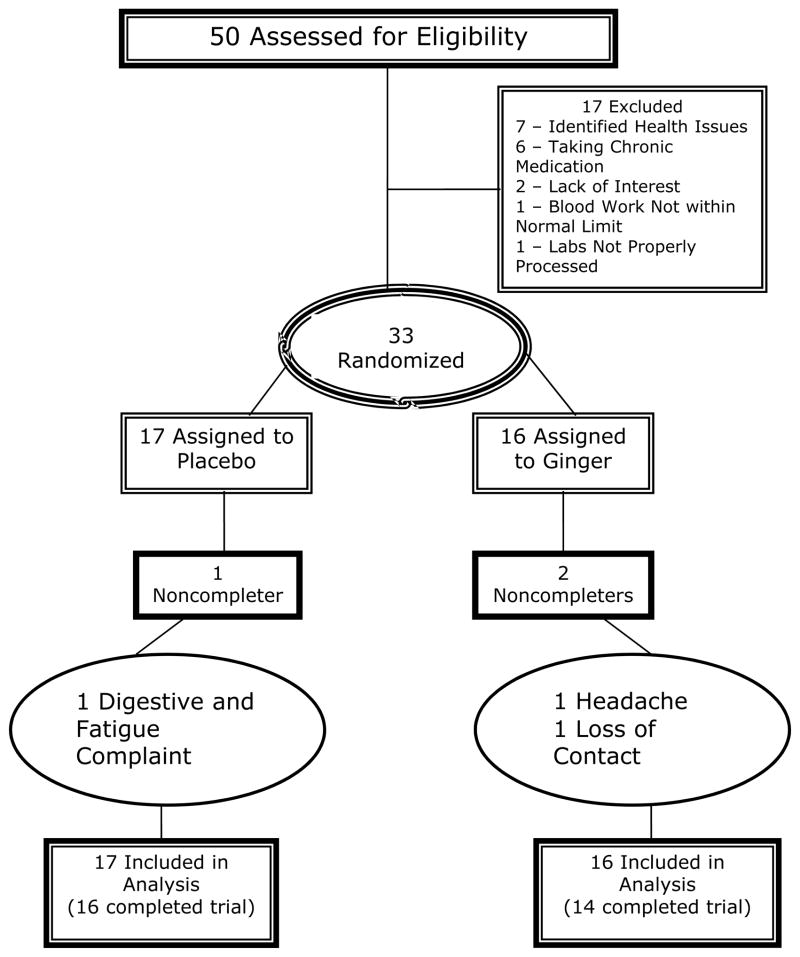
We screened 50 people between January 2007 to June 2008, of whom 33 met all eligibility criteria and were randomized: 17 to placebo and 16 to 2.0 g ginger, for 28 days. Figure 2 documents the numbers of participants, reasons for exclusions and reasons for discontinuing the intervention. There was no significant difference between treatment groups for any demographic or clinical characteristic. Less than one-half of the participants were male (N=16, 48.5%) with mean (±SD) age of 33.9±11.5 (range 20 – 59 years), and over one-half (N=21, 63.6%) of the participants were Caucasian. Less than one-fifth were African American (N=6, 18.2%) and only 3% (N=1) of participants reported being of Hispanic ethnicity. The mean body mass index (BMI) was 25.9 ± 5.0 (range 18.2 to 39.3).
Figure 2.
Patient Flow in the Randomized Controlled Trial
All toxicities reported are shown in Table 1. No toxicities greater than NCI Common Toxicity Criteria (v. 3.0) Grade 1 was reported.29 There was no difference between the groups in terms of total adverse events (p=0.55) or specific types of adverse events such as gastrointestinal (GI) toxicities (p=0.71).
Table 1.
Adverse Events Reported by Person (All Adverse Events are NCI grade 1)
| Adverse Events (AE) | Ginger, (n=14) | Placebo, (n=16) | P-Valuea |
|---|---|---|---|
| All Participants with any AE, No. (%) | 5 (35.7) | 7 (43.8) | 0.55 |
| GIb | 5 (35.7) | 6 (37.5) | 0.71 |
| Headache | 1 (7.14) | 1 (6.25) | 0.83 |
| Fatigue | 0 (0) | 2 (12.5) | 0.47 |
| Otherc | 0 (0) | 2 (12.5) | 0.47 |
P-Value: Chi-Square or Fisher’s exact test as appropriate
GI symptoms include: bloating, gas, nausea, heartburn, mouth burning, rectal itching, stomach pain, IBS
Other includes: flu symptoms, pain in leg
Eicosanoids (PGE2, 5-HETE, 12-HETE, 15-HETE and 13-HODE)
The baseline values of PGE2 5-HETE, 12-HETE, 15-HETE and 13-HODE in colon biopsies across both groups were 10.8 ± 10.3, 0.7 ± 0.5, 0.8 ± 0.6, 7.8 ± 5.0, and 27.1 ± 19.1 pg/μg protein, respectively (mean ± SD, n=30). Table 2, presents all continuous outcomes (PGE2, 5-, 12-, 15-HETE, and 13-HODE, AA), and mean percent change from baseline to day 28 of PGE2, 5-, 12-, 15-HETE, 13-HODE and AA. Table 2 presents eicosanoid concentrations normalized by protein mass, the, the conventional method used to report ELISA results, and normalized by the mass of AA. The LC/MS/MS method also detects and quantifies AA.
Table 2.
Eicasanoids Levels in Normal Mucosa in Participants at Normal Risk for Colorectal Cancer {Mean (SD)a}
| Placebo, n =16 | Ginger, n =14 | ||||||
|---|---|---|---|---|---|---|---|
| Eicosanoid | Baseline | 28-day follow-up | Mean % Change (SD) BTWN Baseline and Week 4e | Baseline | 28-day follow-up | Mean % Change (SD) BTWN Baseline and Week 4 | p-value |
| Normalized to Protein (pg/μg)
| |||||||
| PGE2 | 9.1 (10.5) | 8.6 (6.9) | 31.9 (89.8) | 12.9 (10.1) | 10.8 (10.0) | −6.7 (51.6) | 0.16b |
| 5-HETE | 0.6 (0.4) | 0.9 (1.1) | 54.9 (190.5) | 0.8 (0.7) | 0.9 (0.9) | 29.8 (110.7) | 0.67b |
| 12-HETE | 0.8 (0.7) | 1.5 (2.8) | 71.5 (158.8) | 0.8 (0.6) | 1.3 (2.1) | 47.5 (175.4) | 0.70b |
| 15-HETE | 7.4 (5.0) | 12.7 (22.0) | 63.3 (171.0) | 8.2 (5.1) | 11.4 (19.3) | 15.3 (109.8) | 0.31c |
| 13-HODE | 22.1 (15.4) | 22.4 (15.3) | 17.7 (75.0) | 32.8 (21.8) | 27.7 (23.1) | 2.1 (80.1) | 0.34c |
| AA (ng/pg) | 0.7 (0.4) | 0.8 (0.6) | 24.1 (84.7) | 0.7 (0.3) | 1.3 (1.3) | 163.6 (384.8) | 0.17b |
|
| |||||||
| Normalized to Arachidonic Acid (ng/μg)
| |||||||
| PGE2 | 13.1 (11.5) | 11.5 (7.6) | 26.4 (96.0) | 18.2 (12.1) | 12.9 (12.2) | −28.0 (37.9) | 0.05b |
| 5-HETE | 1.0 (1.0) | 1.0 (0.6) | 21.5 (58.5) | 1.2 (0.8) | 10.4 (0.7) | −1.9 (51.5) | 0.09c |
| 12-HETE | 1.2 (1.0) | 1.4 (1.0) | 41.0 (57.8) | 1.2 (0.8) | 1.3 (1.1) | 7.8 (77.9) | 0.06c |
| 15-HETE | 9.3 (5.9) | 11.2 (7.9) | 26.7 (61.7) | 11.0 (6.1) | 9.6 (8.2) | −15.8 (43.2) | 0.04c |
| 13-HODE | 37.1 (34.2) | 32.5 (20.8) | 9.7 (69.8) | 50.6 (34.4) | 38.1 (32.3) | −16.1 (43.8) | 0.24b |
SD = ± standard deviation
Independent t-test or
Mann-Whitney U test, as appropriate, on the difference between mean percent change between baseline and day 28
A = Arachidonic acid; PGE2 = prostaglandin E2; 5-HETE, 12-HETE, 15-HETE = 5-, 12- & 15-hydroxyeicosatetraenoic acid; 13-HODE = 13-hydroxy-octadecadienoic acids
Mean percent change between baseline and week 4 is calculated as ((eicosanoid at time 2/ eicosanoid at time 1)/eicosanoid at time 1)) per participant and then an average is obtained. Mean percent change may not appear reflective of change in baseline and 28-day follow-up mean values. This is due to the large amount of variability in the baseline measures.
The mean percent change in PGE2 in the colon mucosa after 28-days, as compared to placebo, was significantly lower (−28% versus +26%, p=0.05), as shown in Table 2. When PGE2 was normalized to protein the results were not significant (p=0.16), but there was a trend suggesting that ginger decreased PGE2 compared to placebo (7% vs. +32%, Table 2).
Changes in the other eicosanoids also were more evident when normalized to AA levels. There were no significant differences in mean percent change between baseline and day 28 for any of the other eicosanoids (HETE-5, -12, -15 and 13-HODE), when normalized to protein. In contrast, there was significant decrease in 5-HETE (p=0.04) compared to placebo and trends toward significant decreases in 12-HETE (p=0.09) and 15-HETE (p=0.06) when eicosanoid concentrations were normalized to AA. There was no significant effect of batch (p=0.47–0.95) on mean percent change for any eicosanoid whether normalized to protein or free AA.
Blinding and Adherence
Participants were able to determine whether or not they had received ginger compared to placebo (p=0.02). Participants who were randomized to placebo were unable to correctly guess their group assignment (44% guessed they were taking ginger). In contrast, participants who received ginger correctly guessed “ginger” 86% of the time. We also asked participants, “Was it the way the capsule worked, tasted, looked or smelled?” that helped you guess what you were taking? Only the way in which the capsule tasted was significantly different (p=0.01) between treatment groups.
All participants were adherent per our definition of taking at least 80% of their capsules. Participants on average took 100 ± 9.9% of their capsules and there was no difference between study groups (p = 0.15).
DISCUSSION
We found a significant effect of a ginger root extract, in the dose and formulation used, to decrease our primary endpoint, the mean percent change in PGE2 levels in colon biopsies from subjects at normal risk for developing colorectal cancer when normalized to free AA. We did not, however, find a significant difference in PGE2 concentrations when normalized to protein. Similarly, we found no difference in the concentrations of 5-, 12-, 15-HETE, or 13-HODE when normalized per protein. However, when normalized per free AA there was a significance decrease in 5-HETE and decreases in both 12-, & 15-HETE approached significance. Eicosanoid levels per amount of protein reflect absolute concentrations of eicosanoids in the tissue; however, eicosanoid levels per amount of free AA could reflect enzymatic activity of the COX and LOX enzymes. In essence, when the catalytic enzymes, i.e. COX are blocked, less substrate is metabolized increasing the amount of AA and decreasing the eicosanoids. This may possibly imply some inhibition of COX-1, LOX-5, -12 and LOX-15-2 enzymes by ginger extract. However, rigorous kinetic experiments assessing COX and LOX enzymatic activity would need to be conducted to confirm this hypothesis. Linoleic acid was not quantified, making interpretation of 13-HODE levels difficult.
This study observed a 28.0% mean decrease in PGE2 normalized to free AA and a roughly 7% decrease when normalized to protein from baseline colon mucosal levels. To date, how much PGE2 concentration needs to be decreased in human colonic mucosa to prevent the occurrence of adenomas is unknown. While aspirin has been shown to both prevent adenomas and decrease colonic mucosal PGE2 no studies have combined these endpoints. Several studies have examined the effect of aspirin on production of PGE2 in human colonic mucosa showing anywhere from no reduction to an 85% decrease in PGE2 in colonic mucosa.33–37 Unlike aspirin, a study examining sulindac, another NSAID, did examine the effect on mucosal prostanoids and polyp occurrence in patients with genotypically affected familial adenomatous polyposis (FAP). On average, PGE2 concentrations in rectal biopsies in participants that received sulindac decreased significantly by 19.2% from baseline levels when taken for 48 months at doses of either 75 to 150 mg daily.38 In the sulindac arm, those participants that did not develop an adenoma had a 33.9% mean reduction in baseline PGE2 rectal mucosal concentrations compared to baseline levels, while those who received sulindac and developed a polyp had a slight increase of 2.4% from baseline PGE2 levels.38 In contrast, taking difluoromethylornithine/sulindac for 3 years resulted in a 70 to 90% reduction in the recurrence of colorectal adenomas but this was not correlated with reductions in mucosal PGE2, although higher baseline levels of PGE2 were associated with higher recurrence rates.39 Our results are more modest than those observed for aspirin, but only slightly lower than those observed for sulindac. Our results are most likely attenuated by lower baseline PGE2 levels because of our healthy normal risk for CRC sample and relatively short study duration of 28 days. Longer-term use in high-risk patients could possibly maximize the effect of ginger.
Previous to this study, ginger and ginger constituents’ anti-inflammatory effects on COX and LOX enzymes and their products had only been observed ex vivo.40 The only exception is in one study of rats where decreased serum levels of PGE2 were observed with ginger treatment.22 The present study indicates that oral ginger could have inhibitory effects on colon tissue COX and LOX enzymes in humans.
This study had several limitations. We had a small sample size of only 30 participants and this study was intended as a pilot to determine if a larger study with ginger extract was warranted. Also, our results had much larger standard deviations for all of the eicosanoids than anticipated, and as such we had inadequate sample size to detect meaningful changes in colon eicosanoid concentrations in several instances, especially when normalized to protein. The sample size of this study was based on the mean and standard deviation of PGE2 concentrations in human colon tissue determined by our group’s previous study using enzyme-linked immunosorbent assay (ELISA).33 The ELISA assay results indicated standard deviation of around 10% of the mean. In contrast, the LC/MS/MS assay, employed in our study had standard deviations that exceeded 100% of the mean. With this standard deviation, a post-hoc sample size analysis indicated that 61 subjects would be needed to detect a significant difference in PGE2 levels normalized to protein.
Despite the variability of the LC/MS/MS assay it provided several advantages over an ELISA. Mainly, with LC/MS/MS we could measure numerous eicosanoids and free AA simultaneously. The LC/MS/MS method is also more specific for a given analyte than ELISA as it avoids cross-reactivity issues inherent in ELISAs. Importantly, however, we did determine that our mean baseline PGE2, 12-, 15-HETE and 13-HODE concentrations per protein derived from LC/MS/MS were similar to other studies,11, 32, 36 which used other methods to determine eicosanoid concentrations. Other studies using ELISA and gas chromatography-mass spectrometry also found high amounts of variability in colonic PGE2 concentrations, not dissimilar to our results.38, 41 One explanation for the high level of variability in our eicosanoid assays is the >15% between-day CV42 for all the eicosanoids except PGE2 and AA. However, assay batch had no significant effect on mean percent change for any eicosanoid when examined in linear models, and was thus not added to the final analysis. Another source of variability is the considerable dissimilarity of eicosanoids at different locations of the colon both between and within people.41 To help address this, we combined two biopsies from the same participant at the same time point, but it was in the same section of the colon.
Participants reported a high level of adherence in this study with an average intake of 100% of study medication, making it an unlikely source of variability. A recent study has also found that adenoma risk was not significantly associated with genetic variation in PGE2 synthase and prostaglandin dehydrogenase, however genetic variations in these key enzymes and associations with variation in levels of PGE2 were not examined.43 Similarly, no significant associations were found between age, body mass index, percentage of body fat, NSAID drug use, history of adenomas and family history of colon cancer with either baseline levels of mucosal PGE2 or change of PGE2 through time.41 Another potential source of variability could be due to differences in absorption of key ginger constituents in human tissue. Limited research has been conducted examining the pharmacokinetics of ginger constituents in human blood and tissue. In one study a dose of 2.0 g of ginger extract led to detectable levels of all four of the main ginger constituents (6-, 8-, and 10-gingerols and 6-shogaols) in human plasma after a single oral dose.28 Some normal colon tissue samples were also determined to have detectable levels of 10-gingerols glucuronide and sulfate within 24 hours of the last dose of ingesting 2.0 g of ginger extract for 28 days. Presence of gingerols in tissue were affected by the length of time form the last dose of ginger extract due to the fast half-lives (between 1 to 3 hours) and clearance of the gingerols and shogaol in humans.44 These findings argue for large sample sizes, careful recording of when ginger was last consumed and the use of colonic biopsies taken at multiple time points to help draw meaningful conclusions that would otherwise be masked by the considerable variability in this marker.
Future studies of ginger root extract should focus on examining the mechanisms of action by which ginger extract is affecting the COX and LOX enzymes involved in the production of both the inflammatory and anti-inflammatory eicosanoids. In addition, the effect of ginger on microsomal prostaglandinE2 synthase-1 (mPGEs-1) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) should be considered as the role of both of these enzymes in PGE2 production and degradation are being recognized as increasingly important to governing tissue concentrations of PGE2.45
Subsequent studies should also examine the effect of ginger extract in people at high risk for CRC to determine if there is a differential or similar effect between normal and high-risk populations.
In conclusion, ginger appeared to be well tolerated. There were no differences between placebo and ginger for total adverse events (AE) or in common AE categories including fatigue, gastrointestinal effects or headaches. Participants reported a high level of adherence with all participants reporting taking at least 80% of their study medication. Ginger extract had no significant effect on colon concentrations of AA, PGE2, 5-, 12-, & 15-HETE or 13-HODE normalized to protein when compared to the placebo group. However, ginger extract did appear to have an inhibitory effect on COX and LOX-5, 12-, & 15-2 enzymes as observed by significant or close to significant decreases in the mean percent change in PGE2, 5-, 12-, & 15-HETE normalized to AA. Consequently, it would appear that ginger extract has an anti-inflammatory effect in the colon of persons at normal risk for CRC and warrants further study.
Acknowledgments
This publication was made possible in part by Grant Number P30 CA047904, P30 CA 48592 and K07CA102592 from the National Cancer Institute (NCI) and University of Michigan Clinical Research Center UL1RR024986, and the Kutsche Family Memorial Endowment. The ginger extract was generously donated by Pure Encapsulations ® (Sudbury, MA). We would also like to thank Ananda Sen, PhD, for assistance with statistical analyses and Kate Brummett for assistance with figures.
Footnotes
ClinicalTrials.gov Identifier: NCT01344538
References
- 1.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373(9671):1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 2.Cianchi F, Cortesini C, Fantappie O, Messerini L, Sardi I, Lasagna N, et al. Cyclooxygenase-2 activation mediates the proangiogenic effect of nitric oxide in colorectal cancer. Clin Cancer Res. 2004;10(8):2694–2704. doi: 10.1158/1078-0432.ccr-03-0192. [DOI] [PubMed] [Google Scholar]
- 3.Pidgeon GP, Lysaght J, Krishnamoorthy S, Reynolds JV, O'Byrne K, Nie D, et al. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev. 2007;26(3–4):503–524. doi: 10.1007/s10555-007-9098-3. [DOI] [PubMed] [Google Scholar]
- 4.Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26(4):827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 5.Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, Strouch M, et al. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14(20):6525–6530. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- 6.Soumaoro LT, Iida S, Uetake H, Ishiguro M, Takagi Y, Higuchi T, et al. Expression of 5-lipoxygenase in human colorectal cancer. World J Gastroenterol. 2006;12(39):6355–6360. doi: 10.3748/wjg.v12.i39.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong WG, Ding XZ, Talamonti MS, Bell RH, Adrian TE. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res Commun. 2005;335(3):949–956. doi: 10.1016/j.bbrc.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 8.Bortuzzo C, Hanif R, Kashfi K, Staiano-Coico L, Shiff SJ, Rigas B. The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. Biochim Biophys Acta. 1996;1300(3):240–246. doi: 10.1016/0005-2760(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen CK, Campbell JI, Ohd JF, Morgelin M, Riesbeck K, Landberg G, et al. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res. 2005;65(3):732–742. [PubMed] [Google Scholar]
- 10.Bhattacharya S, Mathew G, Jayne D, Pelengaris S, Khan M. 15-Lipoxygenase-1 in colorectal cancer: a review. Tumor Biology. 2009;30(4):185–199. doi: 10.1159/000236864. [DOI] [PubMed] [Google Scholar]
- 11.Shureiqi I, Chen D, Day R, Zuo X, Hochman F, Ross W, et al. Profiling Lipoxygenase Metabolism in Specific Steps of Colorectal Tumorigenesis. Cancer Prevention Research. 2010;3(7):829. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutations Research. 1998;402(1–2):259–267. doi: 10.1016/s0027-5107(97)00305-9. [DOI] [PubMed] [Google Scholar]
- 13.Cavaliere C, Rea P, Lynch M, Blumenthal M. Herbal Supplement sales rise in all channels in 2009: HerbalGram [Google Scholar]
- 14.Koo KL, Ammit AJ, Tran VH, Duke CC, Roufogalis BD. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thrombosis Research. 2001;103(5):387–397. doi: 10.1016/s0049-3848(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi F, Shibuya M, Sankawa U. Inhibitors of prostaglandin biosynthesis from ginger. Chemical and Pharmaceutical Bulletin (Tokyo) 1982;30(2):754–757. doi: 10.1248/cpb.30.754. [DOI] [PubMed] [Google Scholar]
- 16.Mascolo N, Jain R, Jain SC, Capasso F. Ethnopharmacologic investigation of ginger (Zingiber officinale) Journal of Ethnopharmacology. 1989;27(1–2):129–140. doi: 10.1016/0378-8741(89)90085-8. [DOI] [PubMed] [Google Scholar]
- 17.Sharma JN, Srivastava KC, Gan EK. Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology. 1994;49(5):314–318. doi: 10.1159/000139248. [DOI] [PubMed] [Google Scholar]
- 18.Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorganic Chemistry. 2001;29(3):156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 19.Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chemical and Pharmaceutical Bulletin (Tokyo) 1992;40(2):387–391. doi: 10.1248/cpb.40.387. [DOI] [PubMed] [Google Scholar]
- 20.Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, Tran VH, Duke CC. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res. 2003;111(4–5):259–265. doi: 10.1016/j.thromres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Letters. 1998;129(2):139–144. doi: 10.1016/s0304-3835(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 22.Suekawa M, Aburada M, Hosoya E. Pharmacological studies on ginger. II. Pressor action of (6)-shogaol in anesthetized rats, or hindquarters, tail and mesenteric vascular beds of rats. J Pharmacobiodyn. 1986;9(10):842–852. doi: 10.1248/bpb1978.9.842. [DOI] [PubMed] [Google Scholar]
- 23.Ojewole JA. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res. 2006;20(9):764–772. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimi N, Wang A, Morishita Y, Tanaka T, Sugie S, Kawai K, et al. Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Jpn J Cancer Res. 1992;83(12):1273–1278. doi: 10.1111/j.1349-7006.1992.tb02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clinica Chimica Acta. 2005;358(1–2):60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124(2):264–271. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 27.Dias MC, Spinardi-Barbisan AL, Rodrigues MA, de Camargo JL, Teran E, Barbisan LF. Lack of chemopreventive effects of ginger on colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Food Chem Toxicol. 2006;44(6):877–884. doi: 10.1016/j.fct.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP, Alrawi S, et al. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1930–1936. doi: 10.1158/1055-9965.EPI-07-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. [Accessed June 10, 2009, 2007.];CTC v2.0 and Common Terminology Criteria for Adverse Events v3.0 (CTCAE) http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- 30.Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P, Chan D, Felix E, Madden T, Klein RD, Shureiqi I, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids. 2006;75(6):385–395. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem. 2007;282(31):22834–22847. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan K, Ruffin MT, Normolle D, Shureiqi I, Burney K, Bailey J, et al. Colonic mucosal prostaglandin E2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(5):447–453. [PubMed] [Google Scholar]
- 34.Frommel TO, Dyavanapalli M, Oldham T, Kazi N, Lietz H, Liao Y, et al. Effect of aspirin on prostaglandin E2 and leukotriene B4 production in human colonic mucosa from cancer patients. Clin Cancer Res. 1997;3(2):209–213. [PubMed] [Google Scholar]
- 35.Barnes CJ, Hamby-Mason RL, Hardman WE, Cameron IL, Speeg KV, Lee M. Effect of aspirin on prostaglandin E2 formation and transforming growth factor alpha expression in human rectal mucosa from individuals with a history of adenomatous polyps of the colon. Cancer Epidemiol Biomarkers Prev. 1999;8(4 Pt 1):311–315. [PubMed] [Google Scholar]
- 36.Ruffin MTt, Krishnan K, Rock CL, Normolle D, Vaerten MA, Peters-Golden M, et al. Suppression of human colorectal mucosal prostaglandins: determining the lowest effective aspirin dose. J Natl Cancer Inst. 1997;89(15):1152–1160. doi: 10.1093/jnci/89.15.1152. [DOI] [PubMed] [Google Scholar]
- 37.Venerito M, Treiber G, Wex T, Kuester D, Roessner A, Di Mario F, et al. Effects of low-dose aspirin on gastric erosions, cyclooxygenase expression and mucosal prostaglandin-E2 do not depend on Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;23(8):1225–1233. doi: 10.1111/j.1365-2036.2006.02856.x. [DOI] [PubMed] [Google Scholar]
- 38.Giardiello FM, Casero RA, Jr, Hamilton SR, Hylind LM, Trimbath JD, Geiman DE, et al. Prostanoids, ornithine decarboxylase, and polyamines in primary chemoprevention of familial adenomatous polyposis. Gastroenterology. 2004;126(2):425–431. doi: 10.1053/j.gastro.2003.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson PA, Wertheim BC, Zell JA, Chen WP, McLaren CE, LaFleur BJ, et al. Levels of rectal mucosal polyamines and prostaglandin E2 predict ability of DFMO and sulindac to prevent colorectal adenoma. Gastroenterology. 139(3):797–805. 805, e791. doi: 10.1053/j.gastro.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12(9):684–701. doi: 10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Abrahamson PE, King IB, Ulrich CM, Rudolph RE, Irwin ML, Yasui Y, et al. No effect of exercise on colon mucosal prostaglandin concentrations: a 12-month randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2351–2356. doi: 10.1158/1055-9965.EPI-07-0120. [DOI] [PubMed] [Google Scholar]
- 42. [Accessed February 21, 2011.];Guidance for Industry Bioanalytical Method Validation, United States Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research. 2011 < http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf%3E.
- 43.Poole EM, Hsu L, Xiao L, Kulmacz RJ, Carlson CS, Rabinovitch PS, et al. Genetic variation in prostaglandin E2 synthesis and signaling, prostaglandin dehydrogenase, and the risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2010;19(2):547–557. doi: 10.1158/1055-9965.EPI-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Y, Zick S, Li X, Zou P, Wright B, Sun D. Examination of the Pharmacokinetics of Active Ingredients of Ginger in Humans. AAPS J. 2011 doi: 10.1208/s12248-011-9286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim S, Cho H, Lee T, Choi C, Min Y, Kim S, et al. Impacts of Cytosolic Phospholipase A2, 15-Prostaglandin Dehydrogenase, and Cyclooxygenase-2 Expressions on Tumor Progression in Colorectal Cancer. Yonsei medical journal. 51(5):692. doi: 10.3349/ymj.2010.51.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]