Abstract
Aims
Dual epidemics of HIV and alcohol use disorders, and a dearth of professional resources for behavioral treatment in sub-Saharan Africa, suggest the need for development of culturally relevant and feasible interventions. The purpose of this study was to test the preliminary efficacy of a culturally adapted 6-session gender-stratified group cognitive-behavioral therapy (CBT) intervention delivered by paraprofessionals to reduce alcohol use among HIV-infected outpatients in Eldoret, Kenya.
Design
Randomized clinical trial comparing CBT against a usual care assessment only control
Setting
A large HIV outpatient clinic in Eldoret, Kenya, part of the Academic Model for Providing Access to Healthcare collaboration
Participants
75 HIV-infected outpatients who were antiretroviral (ARV)-initiated or ARV-eligible and who reported hazardous or binge drinking
Measurements
Percent drinking days (PDD) and mean drinks per drinking days (DDD) measured continuously using the Timeline Followback
Findings
There were 299 ineligible and 102 eligible outpatients with 12 refusals. Effect sizes of the change in alcohol use since baseline between the two conditions at the 30-day follow-up were large (d=.95, p=.0002, mean difference=24.93 (95% CI: 12.43, 37.43) PDD; d=.76, p=.002, mean difference=2.88 (95% CI: 1.05, 4.70) DDD). Randomized participants attended 93% of the 6 CBT sessions offered. Reported alcohol abstinence at the 90-day follow-up was 69.4% (CBT) and 37.5% (usual care). Paraprofessional counselors achieved independent ratings of adherence and competence equivalent to college-educated therapists in the U.S. Treatment effect sizes were comparable to alcohol intervention studies conducted in the U.S.
Conclusions
Cognitive-behavioral therapy can be successfully adapted to group paraprofessional delivery in Kenya and may be effective in reducing alcohol use among HIV-infected Kenyan outpatients.
Introduction
Approximately two-thirds of the world’s 33.2 million individuals infected with the HIV virus live in sub-Saharan Africa. Several Africa-based studies have demonstrated a high rate of alcohol dependence (1–3), often involving the consumption of inexpensive local brew with high ethanol content (4). In Eldoret, Kenya, our work has also shown that an average chang’aa drink (locally made spirit) is equal to two U.S. standard drinks (4), and prevalence of hazardous drinking was reported among HIV (53%) and general medicine (68%) outpatients (5). Alcohol use displays a dose-response association with imperfect adherence to antiretrovirals (ARVs) (6), with comorbid medical diseases and AIDS-defining conditions (7) and has been associated with increased risk of unprotected sex (8;9). In Kenya, alcohol use correlates with HIV infection (10;11) and with risk of sexually transmitted infections (12;13). There is growing evidence in both the U.S. and Africa that heavy drinking limits the success of HIV prevention efforts (14–16). Hence, effective alcohol interventions are needed in sub-Saharan Africa.
In this report we describe a randomized clinical trial (RCT) of a culturally adapted Cognitive-Behavioral Therapy (CBT) to reduce alcohol use among HIV-infected outpatients in Eldoret, Kenya. CBT is a highly structured, skills-based approach largely informed by social-cognitive theory (17;18), which construes the maintenance of addictive behaviors at least in part as learned behaviors to cope with stress and problems (19). CBT was selected for this Kenyan adaptation because of its strong empirical support in both individual and group formats to reduce substance abuse (20–22), durability of treatment effects (23;24), and prior successful applications in sub-Saharan Africa to reduce risky sexual behaviors among HIV-infected Zambian couples (25) and to improve mood among Nigerian surgical patients (26). Furthermore, because of its highly structured format, we felt CBT was feasible for training paraprofessionals and for delivery to those with limited formal education. We first describe the methodology for the RCT, which we conceptualized as a Stage 1 trial. The Stage Model of behavioral therapy research highlights the importance of developmental research (Stage 1) to establish the feasibility and promise of novel behavioral therapies prior to large efficacy (Stage 2) and transportability trials (Stage 3) (27). We then present treatment integrity and alcohol outcome results. We hypothesized that there would be a greater reduction in alcohol use (percent drinking days (PDD) and mean drinks per drinking day (DDD)) in the CBT condition relative to the usual care condition at the 30-day follow-up. We also conducted exploratory analyses to model the trajectory of alcohol use over time using repeated measures regression.
Methods
Setting
Kenya is a country in East Africa with 39 million citizens. Kiswahili is the national language. HIV prevalence was estimated to be 7.4% in 2007 (28). There are few professional resources for treating alcohol use disorders; in 2005, there were 47 psychiatrists serving the entire country (29).
The Kenya Health Behavior Study
This trial was the first behavioral therapy study performed within the clinical services of the Academic Model Providing Access to Healthcare (AMPATH), a multinational collaboration in western Kenya (30), which provides HIV care for more than 70,000 current HIV-infected outpatients in 25 clinics in western Kenya and utilizes an electronic medical records system (31). The adaptation of CBT for use in this study and results of feasibility testing have been described in more detail elsewhere (4;32). The study protocol was reviewed and approved by IRBs at all affiliated universities. The purpose of the project was to ascertain whether CBT, which has well-documented efficacy for reducing alcohol use in Western settings, could be adapted to the Kenyan culture, language and group paraprofessional delivery. The team first adapted CBT and the research methods to the cultural context, then trained counselors according to methods described in our previous report (32).
Participants
Inclusion/exclusion criteria
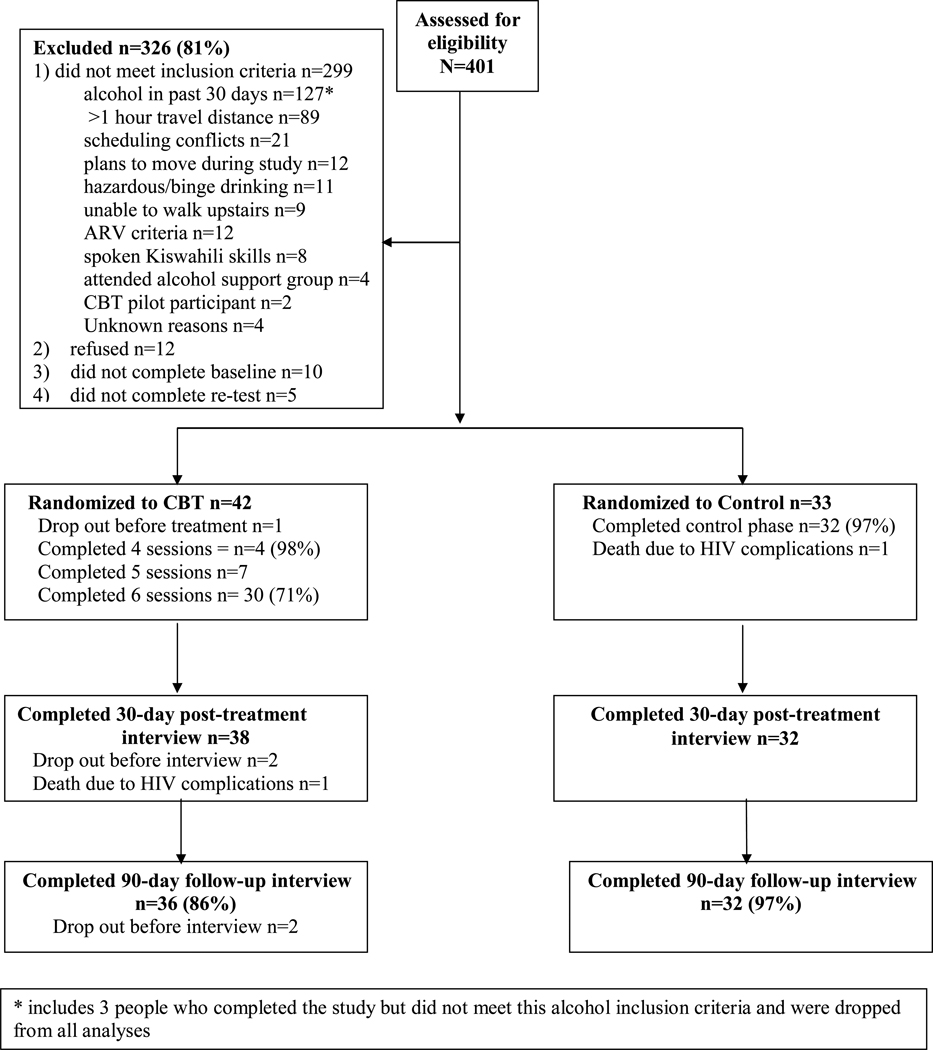
Inclusion criteria were: age ≥ 18 years, enrollment as an AMPATH HIV outpatient attending the Eldoret clinic affiliated with Moi Teaching and Referral Hospital, hazardous or binge drinking criteria (score ≥ 3 on the AUDIT-C (33;34), or ≥ 6 drinks per occasion at least monthly), any alcohol use in the past 30 days, being ARV-eligible or ARV-initiated in the past 12 months (to capture a “teachable moment” for quitting drinking and to enhance retention due to monthly clinic appointments to retrieve ARVs), spoken knowledge of Kiswahili, living within one hour travel distance from the clinic, no plans to move further away during the study period and being available during the weekly group time (see CONSORT diagram, Figure 1). Exclusion criteria included active psychosis or suicidality, attendance in the past year at an existing AMPATH alcohol peer support group, or participation in the study’s group CBT pre-pilot development.
Figure 1.
CONSORT diagram of eligibility, enrollment, randomization, treatment, and follow-up rates. CBT=cognitive behavioral therapy; Control=usual care condition.
Assessment of outcome
Alcohol use
Alcohol use was assessed for the past 30 days at baseline, then consecutively thereafter during weekly interviews during the 6-week treatment phase, and at 30-, 60- and 90-day post-treatment interviews. If a participant missed a previous interview, the days since the last interview were assessed at the subsequent interview. Based on our previous work (4), we estimated use of local brew (chang’aa, spirit, and busaa, maize beer) by asking participants how much money they spent on personal consumption, and use of commercial drink by asking volume drunk for the respective time periods. We used the adapted Timeline Followback (TLFB), a well-established, reliable and valid retrospective calendar-based measure employing memory cues to assess alcohol use (35–38). Reported cost and volume were then converted into grams of ethanol and divided by 14 grams, to achieve equivalence to a U.S. standard drink. The first 50% of each of the 6 cohorts (n=39) were selected to complete a 7-day retest of the survey. Seven-day retest reliability using the adapted TLFB was .88 for PDD and .92 for DDD (Pearson r, p<.0001 for both analyses). At every visit, we also assessed withdrawal symptoms using the validated CIWA-Ar (39) and objective alcohol consumption using the Alco Screen® saliva tests donated by Chematics, Inc. This assay assesses alcohol consumed approximately in the last 1 to 6 hours at indications of .02, .04, .08 or .30% as reflected by swab color (40). A positive saliva test precluded CBT participants from attending the CBT group due to the possibility of creating alcohol triggers for other participants. Additionally, usual care participants were not permitted to complete the interview with a positive test due to concerns for reporting validity. Participants whose score on the CIWA was 10 or more were provided with medical assessment and free medications, if needed. The CIWA-Ar and TLFB were adapted to the culture and Kiswahili language using World Health Organization (WHO)-modified methods (41).
Counselor CBT integrity
The Yale Adherence and Competence Scale, a reliable and valid therapist integrity rating system for several psychosocial addiction treatments (YACS, (42;43), was modified for group delivery in Kenya. This scale, completed by independent raters, included 10 CBT-consistent items (e.g., focus on high risk situations/triggers) and 1 item to capture any CBT-inconsistent categories (e.g., confronting participants). Each type of behavior was rated using a 7-point Likert-type scale on two dimensions: adherence (i.e., frequency and extensiveness; 1=not present, 7=extensively) and competence (i.e., skillfulness; 1=very poor, 7=excellent), for a total of 22 items.
Procedures
Recruitment
Eldoret HIV outpatients who had previously reported alcohol use during their first clinic visit were approached by same-sex research staff and asked for verbal consent for a brief interview to describe a health behavior study and to determine eligibility. Written informed consent was obtained from all eligible and interested participants. Recruitment of participants occurred over a sixth-month period from February to July 2009, and follow-ups were completed in December 2009. As a pilot study, our sample size was intended to provide adequate treatment delivery experience for counselors, as well as provide an estimate of CBT treatment effect.
Randomization
A stratified simple randomization procedure was used to form gender-stratified cohorts. Within gender-based cohorts, participants were randomly assigned until a minimum was achieved of 7 CBT and 5 usual care participants, thereby creating some waiting time. A group of 7 was required for CBT to enhance participation, while fewer were required for the individual usual care condition to minimize waiting time before treatment initiation. Each participant was randomized after she or he drew from a jar a paper with the name of the condition. The papers were prepared by study administrators to conceal the name of the condition during the drawing, which was supervised by staff.
Interviews
All participant interviews were audiotaped and conducted by same-sex research staff in a private setting using a computer survey interface. Interviewers were trained for a minimum of 30 hours in survey administration followed by a minimum of 2 observed interviews with mock patients. For each interviewer, all surveys were reviewed for procedural adherence and typographical errors until four perfect administrations were demonstrated. Thereafter, one in four surveys was randomly selected for full review (with default to consecutive surveys if an error was observed). Performance feedback was given weekly for the first several months and after that only if an error was noted. Nonblinded research assistants both recruited and interviewed participants; none delivered study interventions.
Counselor training, supervision and integrity
Treatment was delivered by two counselors with no prior CBT experience, one with a high school diploma and no counseling experience, and one with a 2-year post-high school counseling diploma and minimal counseling experience. They were trained as described in our previous report (32). All CBT group sessions were videotaped and monitored weekly by RP, with translational support provided as needed. Supervision was conducted via telephone during the latter stages of trial. Fifty percent of sessions with men and women, respectively (n=18 sessions), were randomly selected, translated into English, with random back translational verification, and rated by two highly experienced YACS raters from the Yale Psychotherapy Development Center.
Data and Safety Monitoring
The study included a Data and Safety Monitoring Board with representatives from affiliated universities. Adverse events were monitored during the study and reported to RP by research staff.
Treatment conditions
CBT
The CBT condition consisted of 6 weekly 90-minute group sessions conducted in Kiswahili. As described in our previous report (32), the treatment protocol was highly structured and based on a manual, with a recommended alcohol quit date following the second session. Because of the adverse effects of alcohol among HIV-infected individuals (6;7), abstinence was described as the goal, and successive approximations to abstinence were reinforced. Groups were closed and gender-stratified due to issues of stigma and the consecutive building of knowledge across sessions (Table 1).
Table 1.
Kenya Health Behavior Study CBT protocol
| CBT SESSION CONTENT |
|---|
SESSION 1
|
SESSION 2
|
| QUIT DAY |
SESSION 3
|
SESSION 4
|
SESSION 5
|
SESSION 6
|
Usual care
The usual care condition consisted of routine medical care provided in the AMPATH clinic. While participants had the option to attend the onsite peer-led HIV support group, attendance to which was restricted in the treatment condition, only one participant attended the group. All safety, monitoring and assessment procedures were identical across the two conditions.
Data analysis
Individuals assigned to the two treatment conditions were compared on demographic and other descriptive variables using independent t- or chi-squared tests. To test our hypotheses, we tested the change in alcohol use (PDD and DDD) from baseline to the 30-day follow-up between conditions using independent t-tests. Cohens d effect sizes (44) were also calculated from the change in alcohol use between conditions from baseline to the 30-day follow-up, as well as at 3 additional study timepoints: post-treatment, 60- and 90-day follow-ups. We also examined between-condition differences in the percent reporting 100% abstinence at the 4 study timepoints using chi-squared tests.
In order to better understand trajectories of reported alcohol use over time, we fit repeated measures regression models to mean PDD and DDD. The mean trajectories were characterized using 3-piece linear trends, separately by treatment arm. This structure was selected to reflect rate of change during three key periods following baseline: an initial reactivity effect (lasting up to two weeks), an active treatment effect (between weeks 2 and 6, during which the intervention is being actively administered), and then a follow-up effect. The 3-piece model was based on conceptual differences between initial reactivity, active treatment and follow-up periods, which can reflect different rates of change due to survey reactivity (45;46), variability in reinforcement rates and level of extra-session generalization of treatment (47;48).
We assumed a random effects variance structure with random effects for intercept and each of the slopes; we also used robust standard errors to account for the possible misspecification of the variance structure. This model is based on likelihood and therefore provides valid inferences under the missing-at-random assumption (49), so long as it is properly specified (6% missing observations). The fitted model was compared to observed time-specific (nonstandardized) means.
Results
Recruitment, retention and adverse events
A total of 401 participants were screened for eligibility, and 326 were excluded (Figure 1), primarily because they did not drink alcohol in the past 30 days or lived more than one hour travel distance from the clinic. No participants were excluded from the study due to psychiatric criteria. Seventy-five participants were randomized, 42 to CBT and 33 to usual care. The number of participants in each cohort ranged from 12 to 15. Waiting time for initiation of the treatment phase was on average 23.26 days (SD=11.84) and did not vary according to participant gender (p= 0.10) or condition (p=0.69). Six CBT groups were run (3 for women). Of those randomized to CBT, participants attended 93% of the 6 sessions offered (M=5.6, SD=0.66), excluding one drop out before treatment. Serious adverse events consisted of two deaths from HIV complications, one from each condition.
Participant baseline characteristics
Average age was 37.07 years (SD=8.40) and highest mean year of education completed was 8.03 (SD=4.15). Participants were diagnosed with HIV on average 1.35 years ago (SD=1.51) and had a mean CD4 count of 284.09 (SD=172.37), which is indicative of advanced immunosuppression according to WHO guidelines (50). In the total sample in the past 30 days, number of drinking days ranged from 1 to 30 (M=9.99, SD=7.89), mean number of drinks per drinking day ranged from .84 to 21.84 (M=5.68, SD=4.01), with 76% of participants reporting drinking chang’aa. There were no significant differences between conditions on any outcome variables or covariates (Table 2).
Table 2.
Baseline demographic and clinical characteristics of participants by study condition
| Total | CBT | Control | Test Statistic | |
|---|---|---|---|---|
| N (%) | 75 | 42 (56.00) | 33 (44.00) | |
| Age M(SD) | 37.07 (8.40) | 35.55 (7.57) | 39.00 (9.11) | t(73) = −1.79, p = .08 |
| Education, highest year completed M(SD) | 8.03 (4.15) | 8.52 (3.93) | 7.39 (4.38) | t(73) = 1.17, p = .24 |
| Married N(%) | 43 (57.33) | 22 (52.38) | 21 (63.64) | χ2(1) = 0.96, p = .33 |
| ARV-initiated N(%) | 46 (61.33) | 23 (54.76) | 23 (69.70) | χ2(1) = 1.74, p = .19 |
| Time since HIV diagnosis (years) | 1.36 (1.51) | 1.49 (1.72) | 1.20 (1.20) | t(73) = 0.81, p = .42 |
| Drank chang'aa (spirit) past 30 days N(%) | 57 (76.0) | 31 (73.81) | 26 (78.79) | χ2(1) = 0.25, p = .62 |
| Days of alcohol use past 30 days M(SD) | 9.99 (7.89) | 10.57 (7.72) | 9.24 (8.17) | t(73) = 0.72, p = .47 |
| Drinks per drinking day (14 grams etoh) M(SD) | 5.68 (4.01) | 6.02 (4.48) | 5.26 (3.33) | t(73) = 0.81, p = .42 |
| Tobacco use in past 30 days N(%) | 21 (28.00) | 15 (35.71) | 6 (18.18) | χ2(1) = 2.82, p = .09 |
| Number of days smoked cigarettes in the past 30 days M(SD) (N = 21) | 25.67 (8.91) | 25.47 (9.05) | 26.17 (9.39) | t(19) = −0.16, p = .88 |
| Number of cigarettes on days smoked M(SD) | 4.95 (3.92) | 4.80 (4.39) | 5.33 (2.66) | t(19) = −0.28, p = .79 |
| Marijuana use in past 30 days N(%) | 2 (2.68) | 0 | 2 (6.06) | χ2(1) = 2.62, p = .11 |
| Khat (stimulant leaf) use in past 30 days N(%) | 5 (6.76) | 2 (4.88) | 3 (9.09) | χ2(1) = 0.52, p = .47 |
CBT integrity
An initial sample of 6 tapes was rated by two independent raters and indicated a high level of inter-rater reliability (mean intraclass correlation coefficients) across both adherence (M=0.98) and competence (M=0.95) (51). Mean ratings of 18 tapes suggested high levels of CBT adherence (M=5.26, SD=0.95) and CBT competence (M=5.71, SD=0.56). There was no skill rating of less than 4, average skill, for any item. No CBT-inconsistent ratings were endorsed by raters, suggesting that treatment was highly focused on CBT delivery without the use of incompatible techniques. Adherence (p=0.39) and competence (p=0.60) scores did not differ significantly by gender of the treatment group. The most frequently seen interventions on the tapes reviewed were discussion of high risk situations/triggers (M=6.67, SD=0.77), reflective statements (M=5.78, SD=1.83), and pros/cons/ambivalence about drinking (M=5.72, SD=1.78), which is highly consistent with the treatment manual guidelines.
Alcohol use outcomes
Overall level of drinking was low in the trial, i.e., at the 90-day follow-up, 69% of CBT participants reported abstinence and PDD was 5% (Table 3). There were 6 positive saliva tests, 3 in CBT and 3 in usual care; 5 occurred during the treatment phase. Three CBT participants and 1 baseline participant reported withdrawal symptoms requiring use of benzodiazepines. Results of t-tests showed that at 30-days post-treatment, reductions since baseline were significantly larger in the CBT condition compared to the usual care condition for both PDD and DDD. Cohen’s d effect sizes of reductions since baseline compared between conditions at 30-days post-treatment were large (d=.95 PDD; d=.76 DDD) and at the 90-day follow-up were moderate (d=.60 PDD; d=.56 DDD). More CBT than control participants reported abstinence at all follow-ups (e.g., 30 days, 63% versus 25%, χ2=10.19, p=.001; 90 days, 69% versus 38%, χ2=6.97, p=.008).
Table 3.
Changes in post-baseline reported alcohol use data
| Study timepoint | CBT | Control | Between-group difference |
|||
|---|---|---|---|---|---|---|
| Change from baseline |
Point Prevalence |
Change from baseline |
Point Prevalence |
Change from baseline |
Cohens d effect size |
|
| Percent drinking days M(SD) or M(95% CI) | ||||||
| 6-week treatment phasea | −23.26 (21.75) | 10.57 (11.23) | −3.19 (21.57) | 27.62 (26.71) | 20.07 (9.97, 30.17) | 0.93 |
| 30-day follow-up (past 30 days)b | −29.59 (23.37) | 5.08 (9.74) | −4.66 (29.06) | 27.01 (29.21) | 24.93 (12.43, 37.43) | 0.95 |
| 60-day follow-up (past 30 days)b | −27.71 (28.19) | 6.95 (16.14) | −5.84 (27.58) | 25.82 (28.37) | 21.86 (8.50, 35.23) | 0.78 |
| 90-day follow-up (past 30 days)c | −29.95 (27.81) | 5.06 (13.18) | −13.03 (28.97) | 18.64 (24.03) | 16.93 (3.17, 30.68) | 0.60 |
| Drinks per drinking day (14 grams etoh) M(SD) or M(95% CI) | ||||||
| 6-week treatment phasea | −1.99 (3.46) | 4.07 (4.06) | −0.95 (3.00) | 4.30 (3.48) | 1.04 (−0.48, 2.56) | 0.32 |
| 30-day follow-up (past 30 days)b | −4.26 (4.43) | 1.83 (3.35) | −1.38 (2.9) | 3.93 (3.56) | 2.88 (1.05, 4.70) | 0.76 |
| 60-day follow-up (past 30 days)b | −4.71 (5.01) | 1.38 (2.55) | −1.21 (3.71) | 4.11 (4.14) | 3.50 (1.37, 5.64) | 0.78 |
| 90-day follow-up (past 30 days)c | −4.36 (5.04) | 1.9 (3.87) | −1.85 (3.72) | 3.46 (3.78) | 2.51 (0.35, 4.68) | 0.56 |
(CBT n=41, UC n=33)
(CBT n=38, UC n=32)
(CBT n=36, UC n=32)
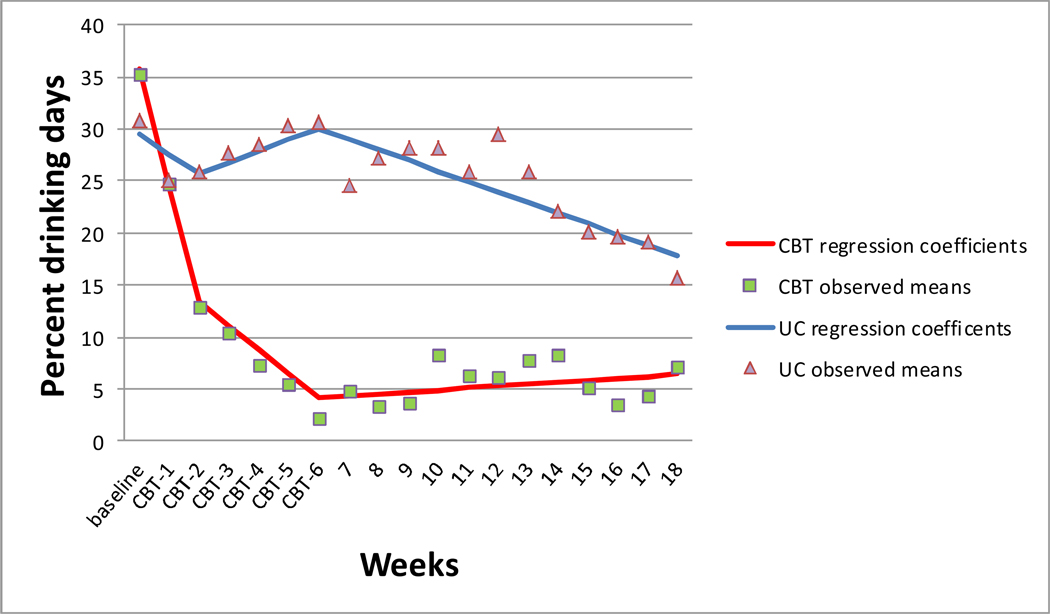
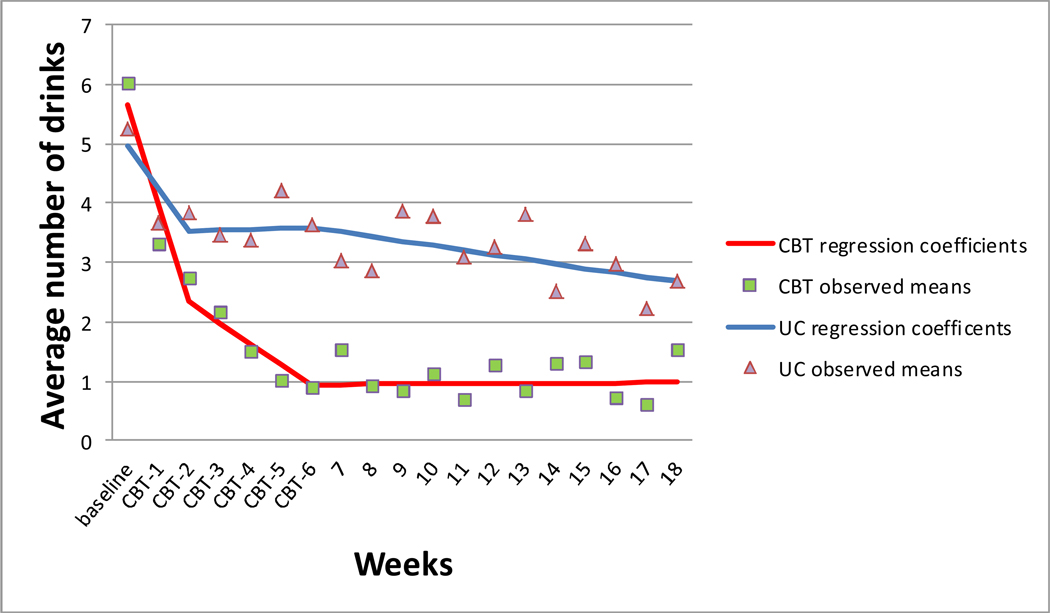
Results of the repeated measures regression models were similar for PDD and DDD. Scores indicated an initial reactivity effect (i.e., alcohol reduction) beginning after administration of the baseline survey and before treatment initiation across both conditions. During the treatment phase, CBT participants reported reducing alcohol use at a faster rate than control participants, as evidenced by the difference between slopes between conditions during the active treatment phase (−3.41, PDD; −0.36, DDD) (Table 4). During the follow-up phase, CBT participants maintained reductions while control participants continued to report gradual reductions over time. Review of the observed (nonstandardized) mean scores showed a good fit to the data (Figures 2 and 3).1
Table 4.
Slopes of alcohol use using repeated measures regression at 3 study phases: Initial Reactivity (Baseline-Week 2), Treatment (Weeks 3–6) and follow-up (Weeks 7–18)
| Variable | CBT (95% CI) | Control (95% CI) | Difference (95% CI) |
|---|---|---|---|
| Percent drinking days | |||
| Baseline mean | 35.76 (27.68, 43.83) | 29.56 (20.47, 38.65) | 6.20 (−5.96, 18.35) |
| Slope 1 | −11.18 (−14.89, −7.47) | −1.95 (−6.37, 2.47) | −9.23 (−15.00, −3.46) |
| Slope 2 | −2.31 (−3.42, −1.20) | 1.10 (−0.99, 3.19) | −3.41 (−5.77, −1.04) |
| Slope 3 | 0.19 (−0.17, 0.55) | −1.02 (−1.64, −0.39) | 1.20 (0.49, 1.92) |
| Average number of drinks per drinking day (14 grams etoh) | |||
| Baseline mean | 5.67 (4.40, 6.93) | 4.97 (3.86, 6.09) | 0.70 (−0.99, 2.38) |
| Slope 1 | −1.67 (−2.24, −1.10) | −0.72 (−1.25, −0.19) | −0.95 (−1.73, −0.17) |
| Slope 2 | −0.35 (−0.58, −0.12) | −0.01 (−0.27, 0.29) | −0.36 (−0.72, 0.002) |
| Slope 3 | 0.003 (−0.04, 0.05) | −0.08 (−0.16, 0.007) | 0.08 (−0.02, 0.17) |
Figure 2.
Repeated measures regression coefficients and observed means of percent drinking days across three study phases: Initial Reactivity (Baseline-Week 2), Treatment (Weeks 3–6) and follow-up (Weeks 7–18).
CBT=cognitive behavioral therapy UC=usual care control.
Note: baseline represents previous 30 days
Figure 3.
Repeated measures regression coefficients and observed means of average drinks per drinking day across three study phases: Initial Reactivity (Baseline-Week 2), Treatment (Weeks 3–6) and follow-up (Weeks 7–18).
CBT=cognitive behavioral therapy UC=usual care control.
Note: baseline represents previous 30 days
Discussion
To our knowledge, this is the first published randomized clinical trial of CBT to reduce alcohol use in sub-Saharan Africa. Results of this trial suggest that CBT can be successfully adapted to group paraprofessional delivery and may be effective in reducing alcohol use in this sample. Our hypothesis that CBT would be more effective than usual care in reducing reported alcohol use at the 30-day follow-up was supported for both primary outcomes (PDD and DDD). Large effects were sustained between CBT and usual care between baseline and the 30-day follow-up and moderate effects were sustained at the 90-day follow-up. These effect sizes are comparable to alcohol intervention studies conducted in the U.S. (52). Repeated measures regression analyses revealed a possible assessment reactivity effect (45;46), wherein participants across both conditions reported a reduction in alcohol use beginning after administration of the baseline survey. This transient effect is believed to be associated with self-monitoring and self-evaluation (53) and warrants further study. During the treatment phase, CBT participants reported more rapid reductions in alcohol use than control participants, while during the follow-up phase, CBT participants maintained reductions while control participants continued to report gradual reductions in use.
Because this design did not involve an outcome period beyond 90 days, it is not known whether differences between conditions increased or decreased beyond that period. Independent ratings of CBT integrity among paraprofessionals showed acceptable adherence and skill ratings, suggesting a successful treatment delivery approach. The group paraprofessional delivery model may provide a useful tool in settings with few professional resources by expanding available treatments for counteracting public health crises.
While our study results suggest a promise of efficacy for CBT in this setting, the study was exploratory and the sample was small. Hence, there was insufficient power to examine mediator and moderator analyses associated with behavior change (e.g., use/quality of CBT coping skills). From a qualitative standpoint, our treatment protocol provided education about biopsychosocial consequences of alcohol use, including the harmful effects of alcohol on HIV complications. Participants reported in debriefings that mitigation of these effects was a motivation for change. Further, our methods of assessing alcohol by money spent were also reported in debriefings to increase awareness of financial costs of drinking, and to act as a motivator for change, particularly for men. Because the adapted CBT manual was not compared to an active control condition, it did not control for increased attention. It is possible that increased attention from counselors resulted in decreased report of use due to social desirability. It should be noted that alcohol use was assessed by research assistants rather than counselors. Our design also did not control for group factors such as cohesion and support, and so the impact of nonspecific factors (i.e., those unrelated to CBT) cannot be ruled out as impacting reported use. These factors may have biased alcohol report and maximized group differences in favor of CBT.
This study represents a preliminary step to counteract the dual public health crises of alcohol use disorders and HIV transmission in sub-Saharan Africa. Consistent with an emerging literature suggesting that heavy drinking limits the success of HIV prevention efforts (14–16), future research is needed to determine whether reduction of heavy drinking is a successful strategy for reducing sexual risk behaviors. Additionally, future research examining the efficacy of enhanced interventions that co-target both heavy drinking and sexual risk behaviors would be useful. Strengths of our study were the use of standardized protocols for treatment, training and integrity ratings; the feasibility of treatment delivery by paraprofessionals; and the systematic cultural adaptation of the intervention. Limitations of the study include the small sample size, reliance on self-report of alcohol use, a relatively brief follow-up, assessment by nonblinded research assistants, and the lack of an active control group. Although we employed objective alcohol saliva tests for corroboration of self-reports of abstinence, the assay provides only a point-prevalence verification over the past 6 hours. Data on changes in sexual risk behaviors and other health outcomes were also not assessed. Because of these limitations, generalizability of study results awaits empirical demonstration. Future research employing an active control group and a longer follow-up period in a larger sample is needed to confirm efficacy, to examine the durability of this intervention over time on multiple risk behaviors, and to explore mediators of behavior change.
Acknowledgments
This research was sponsored by NIAAA-funded R21AA016884. It was also supported in part by a grant to the USAID-AMPATH Partnership from the United States Agency for International Development as part of the President’s Emergency Plan for AIDS Relief and by P50DA09241. We would like to thank Joanne Corvino and Karen Hunkele for providing consultation about treatment integrity, Traci Green, Charla Nich and Jing Zhang for providing consultation about statistical analyses, and Tobista Nafula for her assistance with treatment delivery and data quality monitoring. We also thank Robert Skipworth Comer from the Indiana University School of Informatics for the contribution and development of locally relevant CBT illustrations. We extend our appreciation to Chematics, Inc. of North Webster, Indiana for the generous donation of alcohol saliva tests for this project. We thank the AMPATH clinic staff for facilitating this research. Finally, we thank the participants in this project for providing contributions to the development of this intervention.
Footnotes
Conflict of interest declaration: None
Because of the small sample size and limited variability in drinking behavior at follow-up, we were unable to examine the possible effect of group membership on alcohol report (i.e., correlations between members of the same group when compared between groups).
Reference List
- 1.Othieno CJ, Kathuku DM, Ndetei DM. Substance abuse in outpatients attending rural and urban health centres in Kenya. East African Medical Journal. 2000;77(11):592–595. doi: 10.4314/eamj.v77i11.46728. [DOI] [PubMed] [Google Scholar]
- 2.Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-I. Addiction. 1993;88:349–362. doi: 10.1111/j.1360-0443.1993.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Status Report on Alcohol 2004. [accessed 2010 Nov 1]; http://www.who.int/substance_abuse/publications/global_status_report_2004_overview.pdf (Archived by WebCite® at http://www webcitation org/5uKxBgKuh)
- 4.Papas RK, Sidle JE, Wamalwa ES, Okumu TO, Bryant KL, Goulet JL, et al. Estimating alcohol content of traditional brew in western Kenya using culturally relevant methods: The case for cost over volume. AIDS and Behavior. 2010 Aug;14(4):836–844. doi: 10.1007/s10461-008-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaffer DN, Njeri R, Justice AC, Odero WW, Tierney WM. Alcohol abuse among patients with and without HIV infection attending public clinics in western Kenya. East African Medical Journal. 2004 Nov;81(11):594–598. [PubMed] [Google Scholar]
- 6.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcoholism: Clinical & Experimental Research. 2005 Jul;29(7):1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 7.Justice AC, Lasky E, McGinnis KA, Griffith T, Skanderson M, Conigliaro J, et al. Comorbid disease and alcohol use among veterans with HIV infection: a comparison of measurement strategies. Medical Care. 2006;44(8 Suppl 2):S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 8.Apostolopoulos Y, Sonmez S, Yu CH. HIV-risk behaviors of American spring break vacationers: A case of situational disinhibition? International Journal of STD & AIDS. 2002;13:733–743. doi: 10.1258/095646202320753673. [DOI] [PubMed] [Google Scholar]
- 9.Seage GR3, Holte S, Gross M, et al. Case-crossover study of partner and situational factors for unprotected sex. Journal of Acquired Immune Deficiency Syndrome. 2002;31:432–439. doi: 10.1097/00126334-200212010-00010. [DOI] [PubMed] [Google Scholar]
- 10.Ayisi JG, van Eijk AM, ter Kuil OF, Kolczak MS, Otieno JA, Misore AO, et al. Risk factors for HIV infection among asymptomatic pregnant women attending an antenatal clinic in western Kenya. International Journal of STD & AIDS. 2000;11:393–401. doi: 10.1258/0956462001916119. [DOI] [PubMed] [Google Scholar]
- 11.Hargreves JR. Socioeconomic status and risk of HIV infection in an urban population in Kenya. Tropical Medicine and International Health. 2002;7:793–802. doi: 10.1046/j.1365-3156.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- 12.Feldblum PJ, Kuyoh M, Omari M, et al. Baseline STD prevalence in a community intervention trial of the female condom in Kenya. Sexually Transmitted Infections. 2000;76:454–456. doi: 10.1136/sti.76.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavreys L. Human herpesvirus 8: seroprevalence and correlates in prostitutes in Mombasa, Kenya. Journal of Infectious Disease. 2003;187:359–363. doi: 10.1086/367703. [DOI] [PubMed] [Google Scholar]
- 14.Bedoya CA, Mimiaga MJ, Beauchamp G, Donnell D, Mayer KH, Safren SA. Predictors of HIV transmission risk behavior and seroconversion among Latino men who have sex with men in Project EXPLORE. AIDS and Behavior. 2011 Mar 10; doi: 10.1007/s10461-011-9911-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritz K, McFarland W, Wyrod R, Chasakara C, Makumbe K, Chirowodza A, et al. Evaluation of a peer network-based sexual risk reduction intervention for men in beer halls in Zimbabwe: Results from a randomized controlled trial. AIDS and Behavior. 2011 Mar 5; doi: 10.1007/s10461-011-9922-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalichman SC, Simbayi LC, Vermaak R, Cain D, Smith G, Mthebu J, et al. Randomized trial of a community-based alcohol-related HIV risk-reduction intervention for men and women in Cape Town South Africa. Annals of Behavioral Medicine. 2008;36:270–279. doi: 10.1007/s12160-008-9067-2. [DOI] [PubMed] [Google Scholar]
- 17.Bandura A. Principles of Behavior Modification. New York: Holt, Rinehart & Winston; 1969. [Google Scholar]
- 18.Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, N.J: Prentice-Hall; 1986. [Google Scholar]
- 19.Monti PM, Kadden RM, Rohsenow DJ, Cooney NL, Abrams DB. Treating Alcohol Dependence: A Coping Skills Training Guide. 2nd ed. New York: The Guilford Press; 2002. [Google Scholar]
- 20.Kadden RM, Cooney NL, Getter H, Litt MD. Matching alcoholics to coping skills or interactional therapy: Posttreatment results. Journal of Consulting and Clinical Psychology. 1989;57:698–704. doi: 10.1037//0022-006x.57.6.698. [DOI] [PubMed] [Google Scholar]
- 21.Miller WR, Zweben J, Johnson WR. Evidence-based treatment: Why, what, where, when and how? Journal of Substance Abuse Treatment. 2005;29:267–276. doi: 10.1016/j.jsat.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Project Match Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol. 1997;58(1):7–29. [PubMed] [Google Scholar]
- 23.Carroll KM, Nich C, McCance-Katz EF, Frankforter T, Rounsaville BJ. One year follow-up of disulfiram and psychotherapy for cocaine-alcohol abusers: Sustained effects of treatment. Addiction. 2000;95:1335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- 24.McKay JR, Alterman AI, Cacciola JS, O'Brien CP, Koppenhaver J, Shepard DS. Continuing care for cocaine dependence: Comprehensive 2-year outcomes. Journal of Consulting and Clinical Psychology. 1999;63:70–78. doi: 10.1037//0022-006x.67.3.420. [DOI] [PubMed] [Google Scholar]
- 25.Jones DL, Ross D, Weiss SM, Bhat G, Chitalu N. Influence of partner participation on sexual risk behavior reduction among HIV-positive Zambian women. Journal of Urban Health. 2005 Sep;82(3 Suppl 4):92–100. doi: 10.1093/jurban/jti111. [DOI] [PubMed] [Google Scholar]
- 26.Osinowo HO, Olley BO, Adejumo AO. Evaluation of the effect of cognitive therapy on perioperative anxiety and depression among Nigerian surgical patients. West African Journal of Medicine. 2003;22(4):338–342. doi: 10.4314/wajm.v22i4.28060. [DOI] [PubMed] [Google Scholar]
- 27.Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from Stage I. Clinical Psychology: Science and Practice. 2001;8(2):133–142. [Google Scholar]
- 28.National AIDS and STI Programme, Ministry of Health Kenya. Nairobi, Kenya: Kenya AIDS Indicator Survey 2007: Preliminary Report. [Google Scholar]
- 29.Njenga FG, Kigamwa PA. London: Royal College of Psychiatrists; 2005. Mental health policy and programmes in Kenya. Report No.: 8. [PMC free article] [PubMed] [Google Scholar]
- 30.Einterz RM, Kimaiyo S, Mengech HNK, Khwa-Otsyula BO, Esamai F, Quigley F, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Academic Medicine. 2007;82(812):818. doi: 10.1097/ACM.0b013e3180cc29f1. [DOI] [PubMed] [Google Scholar]
- 31.Siika AM, Rotich JK, Simiyu CJ, et al. An electronic medical record system for ambulatory care of HIV-infected patients in Kenya. International Journal of Medical Informatics. 2005;74(5):345–355. doi: 10.1016/j.ijmedinf.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Papas RK, Sidle JE, Martino S, Baliddawa JB, Songole R, Omolo OE, et al. Systematic cultural adaptation of cognitive-behavioral therapy to reduce alcohol use among HIV-infected outpatients in western Kenya. AIDS and Behavior. 2010 Jun;14(3):669–678. doi: 10.1007/s10461-009-9647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon AJ, Maisto SA, McNeil M, Kraemer KL, Conigliaro RL, Kelley ME, et al. Three questions can detect hazardous drinkers. The Journal of Family Practice. 2001 Apr;50(4):313–320. [PubMed] [Google Scholar]
- 34.Saunders JB, Aasland OG, Babor TF, DeLaFuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 35.Sobell LC, Sobell MB. Timeline Followback: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption. 1st ed. New Jersey: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 36.Maisto SA, Sobell LC, Sobell MB. Comparison of alcoholics' self-reports of drinking behavior with reports of collateral informants. Journal of Consulting and Clinical Psychology. 1979;47(1):106–112. [PubMed] [Google Scholar]
- 37.Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behavior Research & Therapy. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 38.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988 Apr;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan JT, Sykora K, Schneiderman J, Naranjo CJ, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) British Journal of Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 40.Chematics I. Chematics I. Alco-Screen Technical Information. North Webster, Indiana: Chematics, Inc.; 2004. Ref Type: Pamphlet. [Google Scholar]
- 41.World Health Organization. WHO - Process of translation and adaptation of instruments. [accessed 2010 Nov 1]; http://www who int/substance_abuse/research_tools/translation/en/ (Archived by WebCite® at http://www webcitation org/5uKwiqRhz)
- 42.Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 43.Carroll KM, Nich C, Rounsaville BJ. Use of observer and therapist ratings to monitor delivery of coping skills treatment for cocaine abusers: Utility of therapy session checklists. Psychotherapy Research. 1998;8:307–320. [Google Scholar]
- 44.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 45.Clifford PR, Maisto SA. Alcohol treatment research assessment exposure subject reactivity effects: Part I. Alcohol use and related consequences. Journal of Studies on Alcohol and Drugs. 2007;68:519–528. doi: 10.15288/jsad.2007.68.519. [DOI] [PubMed] [Google Scholar]
- 46.Maisto SA, Clifford PR, Davis CM. Alcohol treatment research assessment exposure subject reactivity effects: Part II. Treatment engagement and involvement. Journal of Studies on Alcohol and Drugs. 2007;68:529–533. doi: 10.15288/jsad.2007.68.529. [DOI] [PubMed] [Google Scholar]
- 47.Koegel RL, Rincover A. Research on the difference between generalization and maintenance in extra-therapy responding. Journal of Applied Behavior Analysis. 1977;10:1–12. doi: 10.1901/jaba.1977.10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouton ME. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychology. 2000;19(1 Suppl):57–63. doi: 10.1037/0278-6133.19.suppl1.57. [DOI] [PubMed] [Google Scholar]
- 49.Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 50.World Health Organization. Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillance. [accessed 2010 Nov 1];African Region. http://www.who.int/hiv/pub/guidelines/WHO%20Adult%20ART%20Guidelines.pdf (Archived by WebCite® at http://www webcitation org/5uKx49UzF)
- 51.Bonett DG. Sample size requirements for estimating intraclass correlations with desired precision. Statistics in Medicine. 2002;21(9):1331–1335. doi: 10.1002/sim.1108. [DOI] [PubMed] [Google Scholar]
- 52.Magill M, Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. Journal of Studies on Alcohol. 2009;70(4):516–527. doi: 10.15288/jsad.2009.70.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clifford PR, Maisto SA. Subject reactivity effects and alcohol treatment outcome research. Journal of Studies on Alcohol and Drugs. 2000;61(6):787–793. doi: 10.15288/jsa.2000.61.787. [DOI] [PubMed] [Google Scholar]