Abstract
Although the cochlear implant is already the world's most successful neural prosthesis, opportunities for further improvement abound. Promising areas of current research include work on improving the biological infrastructure in the implanted cochlea to optimize reception of cochlear implant stimulation and on designing the pattern of electrical stimulation to take maximal advantage of conditions in the implanted cochlea. In this review we summarize what is currently known about conditions in the cochlea of deaf, implanted humans and then review recent work from our animal laboratory investigating the effects of preserving or reinnervating tissues on psychophysical and electrophysiological measures of implant function. Additionally we review work from our human laboratory on optimizing the pattern of electrical stimulation to better utilize strengths in the cochlear infrastructure. Histological studies of human temporal bones from implant users and from people who would have been candidates for implants show a range of pathologic conditions including spiral ganglion cell counts ranging from approximately 2% to 92% of normal and partial hair cell survival in some cases. To duplicate these conditions in a guinea pig model, we use a variety of deafening and implantation procedures as well as post1-deafening therapies designed to protect neurons and/or regenerate neurites. Across populations of human patients, relationships between nerve survival and functional measures such as speech have been difficult to demonstrate, possibly due to the numerous subject variables that can affect implant function and the elapsed time between functional measures and postmortem histology. However, psychophysical studies across stimulation sites within individual human subjects suggest that biological conditions near the implanted electrodes contribute significantly to implant function, and this is supported by studies in animal models comparing histological findings to psychophysical and electrophysiological data. Results of these studies support the efforts to improve the biological infrastructure in the implanted ear and guide strategies which optimize stimulation patterns to match patient-specific conditions in the cochlea.
Keywords: Cochlear implant, Auditory psychophysics, Cochlear histopathology, Ensemble spontaneous activity, Electrically-evoked compound action potential, Neurotorphins
1. Introduction
These are very exciting times for the Auditory Prosthesis community: for patients, researchers, and cochlear-implant companies. We are seeing exponential growth in research on cochlear implants and in opportunities to further improve an already amazingly successful neural prosthesis. There are two key classes of variables that influence the performance of cochlear implant users: (1) the ability of the processor to deliver appropriate content in the electrical signal, and (2) the ability of the subject to receive and process that input. In the past decades, significant advances have been made in the fields of electrical engineering, signal processing and computer science which have facilitated the development of faster, more complex, and more power-efficient processors for auditory prostheses. These advances give us a great deal more flexibility in processing-strategy design and stimulus delivery. The challenges now are to determine the limits of the human listener to receive the advanced electrical signals, engineer the biological infrastructure to reduce these limits, and configure the electrical signal to interface optimally with the individual implant user.
In several respects, the technical capabilities of current cochlear prosthesis systems exceed the capacity of the patient to receive and process electrical signals. In the spectral domain, current implants have up to 22 intracochlear electrodes and the technology exists to develop implants with much higher numbers of stimulation sites (Wise et al., 2008). However, the capacity of patients to use multiple channels typically asymptotes at around 8 channels or less (Friesen et al., 2001). This is probably due to channel interaction that depends on conditions in the implanted cochlea and the condition of the surviving neural population. In the temporal domain, current implants are capable of delivering very high pulse rates, interleaved across multiple channels. High pulse rates are potentially advantageous because they allow a more detailed representation of temporal information in the envelope of the modulated pulse train. However, in reality, high carrier rates have proved suboptimal for cochlear implant users. Modulation detection thresholds for carrier-pulse rates above about 2000 pulses per second are often poorer than when lower pulse rate carriers are used (Galvin and Fu, 2005; Pfingst et al., 2007) as is the cortical representation of modulation (Middlebrooks, 2004). This is probably because the representation of the carrier in the auditory nerve and higher auditory centers is poorer at the high pulse rates than at the low pulse rates. Thus, in order to optimally utilize the information provided by the currently available technology, the physiology and biophysical conditions in the implanted cochlea must be taken into account.
The ability of the auditory pathway to receive and process complex electrical signals depends in part on the conditions in the implanted cochlea, which vary considerably from subject to subject and from one stimulation site to another in a hearing impaired patient. Recent advances in cell and molecular biology offer many potential opportunities to modify the biological infrastructure in the implanted cochlea and improve the interface between the cochlear implant and the stimulated neural pathways. To optimize this tissue engineering requires an ability to estimate morphological and physiological conditions in the implanted ear, preferably on a site by site basis, as well as an understanding of the functional implications of those conditions.
In this review, we will first consider the current state of knowledge about the infrastructure of the human cochlea in deaf and implanted ears. We will then review the relationships between cochlear conditions and functional data from humans and from animal models that attempt to replicate the human conditions. Finally, we will discuss the implications of our current state of knowledge for treatment of hearing impaired patients with cochlear implants, and the research that our laboratories are undertaking to apply these data to rehabilitation strategies for these patients.
2. Histological stat us of deaf and implanted ears in humans
In order to design and interpret experiments on the effects of cochlear infrastructure on implant function in humans and in animal models, we need to consider the condition of the cochlea in humans who have cochlear implants. Histological analysis of temporal bones generally assesses the preservation of tissues at three levels: first, the survival of the sensory epithelium (inner and outer hair cells); second, survival of peripheral processes of the auditory nerve; and third, the number of spiral ganglion cells remaining in Rosenthal's canal. Additionally, analysis must consider new bone and fibrous tissue growth in the implanted cochlea.
Conditions in the implanted human cochlea have been estimated in two ways: First by postmortem examination of temporal bones from people who would have been candidates for an implant based on their audiological records; and second, by postmortem examination of temporal bones of actual cochlear implant users. To validate the first approach, it is important to compare deaf implanted and deaf non-implanted cochleae from the same individual. Implantation often results in a characteristic pattern of injury to the lateral wall in the 8-15mm region, and is often accompanied by new fibrous tissue and bone growth, which may impact current conduction paths during electrical stimulation (Nadol, 1997). When comparing the deaf implanted cochlea to the deaf non-implanted cochlea, the basal turn is the area of greatest difference in terms of hair cell counts, peripheral processes, spiral ligament and stria vascularis (Fayad et al., 2009). What remains less clear is the effect of implantation on spiral ganglion cell counts. In two studies, total spiral ganglion cell counts were modestly lower in implanted deaf ears when compared to non-implanted deaf ears, but this difference was not statistically significant (Khan et al., 2005b; Fayad and Linthicum, 2006). Other studies suggest that spiral ganglion cell counts in the basal segments may actually be higher in implanted ears than in non-implanted ears, the hypothesis being that stimulation provides some protection against neuronal degeneration (Nadol et al., 2001; Khan et al., 2005b). This hypothesis is supported by studies in guinea pigs demonstrating enhanced spiral ganglion cell survival in stimulated ears when compared to non-stimulated ears (Mitchell et al., 1997; Miller, 2001).
Several theories exist about the degeneration of neuronal elements in the cochlea after damage to the organ of Corti. It is generally believed that when hair cells are lost and sensory afferent information ceases to be transmitted, subsequent retrograde degeneration of nerve fibers toward the spiral ganglion cell bodies ensues. Spoendlin (1966), Johnsson (1974) and others have correlated loss of spiral ganglion cells with loss of inner hair cells. Others have suggested a relation between loss of spiral ganglion cells and loss of the supporting cells (Schuknecht, 1953; Otte et al., 1978). In mammalian animal models, there is significant loss of spiral ganglion cells after damage to the cochlea (Webster and Webster, 1981). However, in contrast to animal models, human temporal bone studies sometimes reveal relatively good spiral ganglion cell survival despite near total organ of Corti destruction and sufficient variability across cases that conclusions about these degenerative patterns are difficult (Hinojosa and Marion, 1983).
A wide range of spiral ganglion cell survival has been observed in the deaf human cochlea, as illustrated in Table 1. Spiral ganglion cell counts in the normal human cochlea range from 29,802 – 39,520, with an average of 33,915 cells (Hinojosa and Marion, 1983). In this review we use these numbers to calculate survival percentages from the data presented in the literature. In deaf, but non-implanted cochlea, the range of spiral ganglion survival ranged from approximately 4% to 92% (Hinojosa and Marion, 1983; Incesulu and Nadol, 1998). In the deaf, implanted cochlea, the range was modestly lower; approximately 2% to 72% (Nadol et al, 2001; Fayad and Linthicum, 2006). On average, spiral ganglion cell survival was around 25% of normal, yet even in the deaf implanted ear there were some cases of spiral ganglion cell survival greater than 85% (Fayad and Linthicum, 2006). The greatest number of peripheral fibers and spiral ganglion cells were usually found in those few a reas where inner hair cells were still present (Hinojosa and Marion, 1983; Zimmermann et al., 1995).
Table 1.
Summary of Literature on Spiral Ganglion Cell Counts
| Subject Category | Number of Cases | Range of SGC Survival | Mean SGC Survival | Reference |
|---|---|---|---|---|
| Normal Adults | 12 | 29,802 – 39,520 | 33,915 | Hinojosa and Marion, 1983 |
| Potential Implant Candidates | 26* | 1,444 – 31,284 | 12,021 | Incesulu and Nadol, 1998 |
| 15** | 7,305 – 25,783 | 16,318 | Hinojosa and Marion, 1983 | |
| 11** | 4,752 – 18,936 | 11,884 | Khan et al., 2005b | |
| Deaf, Implanted Adults | 14 | 2,286 – 24,536 | 11,761 | Fayad and Linthicum, 2006 |
| 11 | 2,471 – 15,023 | 8,688 | Khan et al., 2005b | |
| 10 | 1,701 – 17,901 | 8,469 | Fayad et al., 2009 | |
| 15 | 1,443 – 15,023 | 7,969 | Khan et al., 2005a | |
| 8 | 391 – 17,418 | 6,872 | Nadol et al., 2001 |
Criteria for potential implant candidacy:
Pure tone average (PTA) of 70 dB or greater and speech discrimination < 30%
PTA of 90 dB or greater and no speech discrimination.
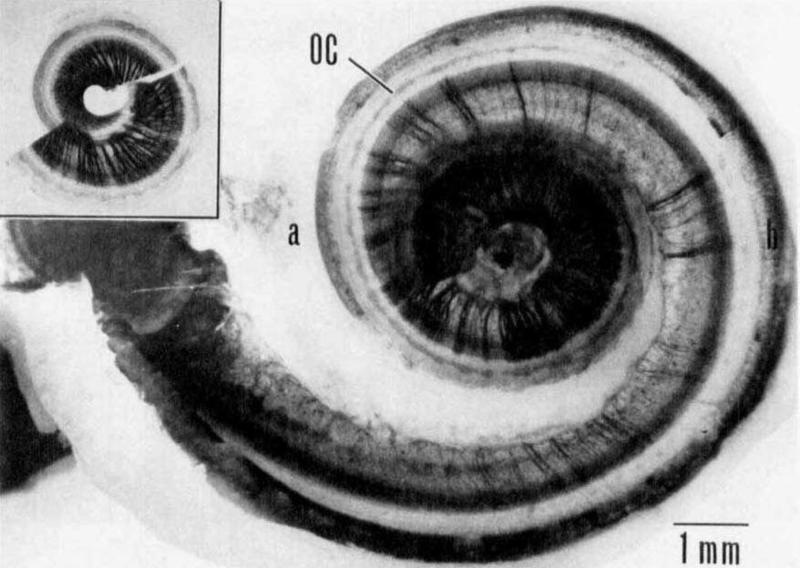
In addition to the range of spiral ganglion survival observed across patients, it is also valuable to consider the variation in nerve survival along the tonotopic axis for an individual cochlea. This is especially important when interpreting psychophysical measures in humans across electrode stimulation sites (as discussed in Section 3.2) because variations in nerve survival along the electrode array imply that individual stimulation sites contact differing numbers of surviving sensorineural elements. Hinojosa and Marion (1983) reported spiral ganglion cell survival as low as 20% in the base, while near 100% in the apex in the non-implanted deaf ear. This gradient of survival from base to apex is generally supported by the findings of Zimmermann and colleagues (1995), Nadol (1997) and others, regardless of etiology. However, there are cases which demonstrate patchy survival of sensorineural elements, such as that illustrated in Fig. 1 (Johnsson et al., 1981). After examining five ototoxically damaged human cochleae, Johnsson and colleagues concluded that cases with mild ototoxic damage demonstrated a base-to-apex gradient of survival, cases with moderate ototoxic damage demonstrated patchy asymmetric survival, and the most severe cases of damage resulted in extensive, bilaterally symmetric, neuronal destruction. The trend across etiologies including labrynthitis, and otosclerosis appears to be that milder insults often result in more patchy and asymmetric damage when compared to severe insults. This is especially important to keep in mind given that the criteria for cochlear implant candidacy have broadened considerably in the past 20 years. People with considerable residual acoustic hearing can be candidates if they are experiencing less than 60% speech recognition in the best acoustically aided ear. Therefore it is reasonable to expect that conditions in the implanted cochlea on average will be better than those reported in most studies currently in the literature, and perhaps the histological picture may be even more varied along the tonotopic axis than demonstrated in prior histological studies.
Fig. 1.
Example of patchy nerve survival in an ototoxically deafened human cochlea as seen in a surface preparation by Johnsson and colleagues (1981). The inset shows the upper half of middle and apical turns of the cochlea. Degeneration is more severe in the basal turns (main figure). Nerve fibers in the osseous spiral lamina are stained with osmium tetroxide. OC = supporting cells in the Organ of Corti. No hair cells were found. Reproduced, with permission, from Acta Oto-Laryngologica, 1981, Supplement 383, page 12, Figure 9.
Certainly the histological picture of hearing loss in the human involves variable loss of inner and outer hair cells, correlated with more or less severe degeneration of peripheral fibers and spiral ganglion cells. In our aminoglycoside-deafened and implanted guinea pigs, spiral ganglion cell survival has ranged from 1.6% to 20%, which is in the lower end of the spectrum of survival seen in deaf human ears. Better spiral ganglion cell survival can be obtained by implanting animals without pre-deafening, creating a model which may more accurately reflect the most recent implant patients who still have various degrees of residual hearing (Kang et al., 2010). Perhaps the most representative animal model of the median human histological picture is the deafened, implanted ear that has been treated with neurotrophins. Multiple studies suggest locally delivered neurotrophins encourage peripheral nerve fiber growth, and maintain spiral ganglion survival, even in the absence of hair cells (Wise et al., 2005; Agterberg et al., 2008; Scheper et al., 2009; Shibata et al., 2010).
3. Dependence of cochlear implant function on cochlear infrastructure
3.1 Relation of histology to functional data from patients
Postmortem examination of temporal bones from cochlear implant users has yielded some unexpected results with regard to implant functional data collected months to years prior to death. It was believed that spiral ganglion cell count would correlate with implant functional outcome measures such as speech perception. As both spiral ganglion cell counts and speech perception decrease with increasing duration of deafness, theoretically these two variables could be correlated (Nadol, 1997; Khan et al., 2005a). However in multichannel cochlear implant users there is poor correlation between spiral ganglion cell survival and speech recognition (Nadol et al., 2001; Fayad and Linthicum, 2006). Examination of 14 patients with multichannel Nucleus or Ineraid cochlear implants by Khan and colleagues (2005a) concluded that increased numbers of spiral ganglion cells does not predict implant success when using scores on the NU-6 word recognition test. Examination of eight patients with multichannel implants by Nadol and colleagues (2001) showed an apparent negative correlation between spiral ganglion cell counts and NU-6 scores. The two patients with the lowest percent survival (< 15%) had the better NU-6 scores (30%) while the two patients with the greatest percent survival (50%, and 32%) had the worst NU-6 scores (< 15%). While counterintuitive, these results could be due to other variables such as aberrant current paths, central processing deficits, and lower verbal abilities, which have been correlated with lower NU-6 scores (Fayad and Linthicum, 2006). It is also important to keep in mind the limitations of the NU-6 test itself, as it is performed in quiet, and is widely used in clinical fitting verification so patients have increasing familiarity with the test over time.
As speech recognition is believed to depend in part on comfort levels and dynamic ranges for individual implant users, Kawano and colleagues (1998) studied five temporal bones in order to correlate thresholds, comfort levels and dynamic ranges with spiral ganglion cell survival and new tissue growth. That study found a statistically significant positive correlation between comfort levels, dynamic ranges and spiral ganglion cell survival. However, psychophysical detection threshold levels were not strongly correlated with spiral ganglion cell survival across patients. While spiral ganglion survival might not predict cochlear implant success for speech recognition, it can perhaps help inform the selection of implant stimulation parameters. There are some limits to the data from the aforementioned histological analyses. Age at implantation, etiology of deafness, duration of deafness, duration of implant use, and cause of death are important variables that play a role in histological appearance. As continued degeneration of sensory elements is expected, the histological picture obtained after death will likely overestimate the degree of pathology present during the time of implant use by variable degrees, depending on many uncontrolled variables. An advantage of animal models is that they allow greater control of these variables and standardization of time intervals between implantation, functional measurement, and death.
No study has yet examined the r elationship of peripheral fiber survival specifically to cochlear implant function in humans. It is hypothesized that with more robust peripheral fibers, the proximity of the nerve endings to the site of stimulation would allow for better spatial resolution and possibly decreased detection thresholds for cochlear implant stimulation. The functional implications of peripheral fiber survival is of particular interest in our model of cochlear implantation in the setting of neurotrophin treatment as we can facilitate peripheral fiber regeneration through the osseous spiral lamina and into the basilar membrane area (Shibata et al., 2010).
3.2. Evidence from psychophysical studies in human subjects
There is little doubt that multiple variables contribute to the performance of individuals with cochlear implants. These include conditions in the cochlea that affect the current pathways from the electrodes to the neurons, the locations and conditions of surviving neurons, the condition of central auditory pathways and synapses, and cognitive variables. The large number of variables that can influence implant performance makes it difficult to interpret the comparisons across subjects between cochlear infrastructure and implant function, particularly for complex functional measures such as speech recognition as reviewed in Section 3.1.
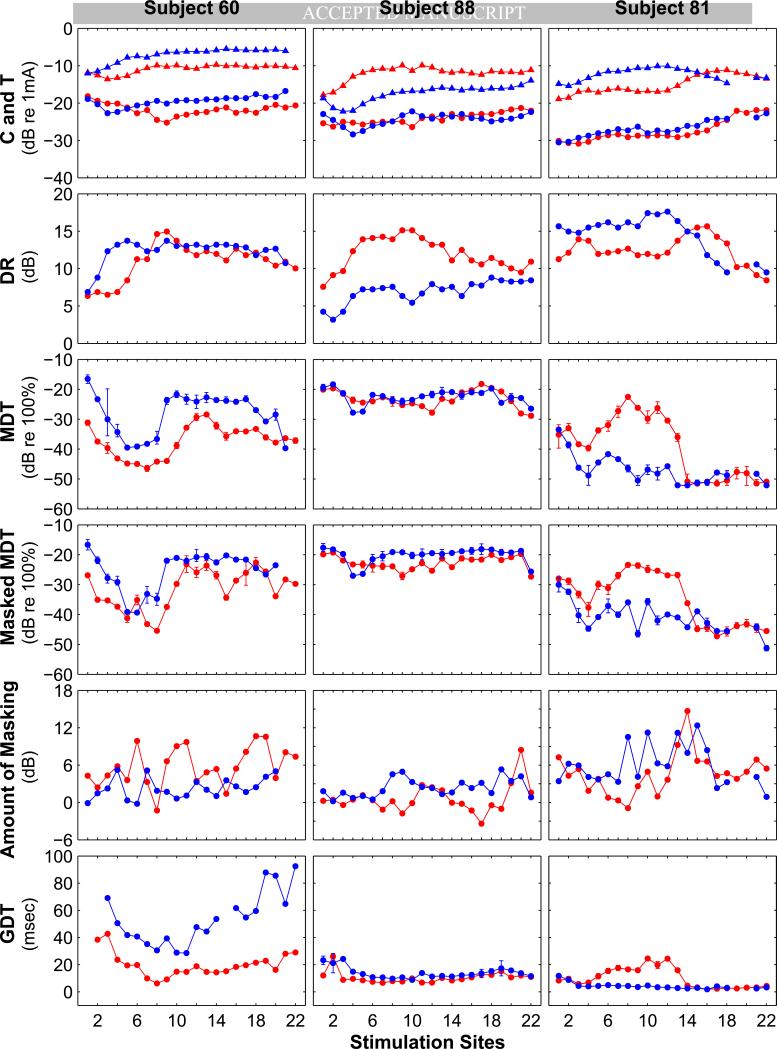
Evidence that conditions in the cochlea near the stimulating electrodes affect implant function comes from psychophysical studies of perceptual acuity for stimulation at individual sites in the electrode array. These studies show large variations in performance across stimulation sites (e.g., Donaldson et al., 1997; Pfingst and Xu, 2004; Bierer, 2007; Pfingst et al., 2008; Garadat and Pfingst, 2011). A reasonable interpretation of these findings is that nerve survival and other conditions vary from one stimulation site to the next and lead to across-site variation in perception. Examples of such variation across 22 stimulation sites for three human subjects are shown in Fig. 2. Several points are worth noting. First, for all of the psychophysical measures tested, performance levels vary from one stimulation site to the next, although the magnitude of variation is listener and mea sure specific. Across-site variation in the results for these measures suggests that the electrical hearing is dependent in part on conditions near the stimulated electrodes, not solely on a more global variable such as the subject's cognitive ability. Second, the across-site patterns for any given measure are not consistent across subjects. For example, for Subject 60, modulation detection thresholds are lowest in the region around Electrodes 5 to 9 in the right ear whereas for the right ear of Subject 81 they are highest in that region. These across-listener differences in across-site patterns suggest that the across-site variation is not due to normal variation in morphology and physiology along the tonotopic axis of the cochlea but rather that it is based on patterns of variation in pathology that are specific to each subject, or to subject-specific electrode placement. Third, the across-site patterns are not consistent within subjects across all psychophysical measures. For example, for the right ear of Subject 60, compare the across-site pattern of modulation detection thresholds (MDTs; a measure of temporal acuity) with the across-site pattern of Amount of Masking of modulation detection (a measure of channel interaction). In Subject 60, and other subjects, the across-site patterns for the two temporal measures, MDTs and gap detection thresholds (GDTs), are more similar to each other than either of them is to the channel interaction measure, Amount of Masking.
Fig. 2.
Across-site patterns of various psychophysical measures for three human subjects with Nucleus CI24R(CS) or Freedom implants (one subject per column). All three subjects had bilateral implants. Functions for the right ear are shown in red and those for the left ear are shown in blue. Across site patterns for 7 psychophysical measures are shown. Monopolar stimulation with 500 ms trains of 50 μs/phase pulses at 900 pulses/s (pps) was used in all cases except gap detection where the pulse rate was 1000 pps. Top row: Triangles = maximum comfortable loudness levels (C levels); Circles = detection threshold levels (T levels). Second row: Dynamic ranges (C levels minus T levels). Third row: Modulation detection thresholds (MDTs) measured at 50% of the dynamic range (50% DR). Sinusoidal modulation of phase duration at 10 Hz around a mean of 50 μs/phase was used. Units for MDTs are modulation depth in dB re 100% modulation. Fourth row: MDTs measured in the presence of an unmodulated pulse train (masker) presented at 50% DR on an adjacent channel and interleaved with the modulated pulse train. The masker site was the next apical site to the site where the MDTs were measured in all cases except when the MDTs were measured for site 22 and the masker was on the adjacent basal site. Fifth row: Amount of masking = masked MDTs (data from row 4) minus nonmasked MDTs (data from row 3). Sixth row: Gap detection thresholds (GDTs) measured at 50% DR. Details o f the psychophysical procedures are similar to those reported in Pfingst et al., 2008 and Garadat and Pfingst, 2011.
The fact that different psychophysical measures show different patterns of across-site variation in a given subject suggests that the underlying mechanisms that affect perception are not the same for all measures of perception. Further evidence for measure-specific mechanisms comes from studies of the effects of electrode configuration on the across-site variation in performance. Specifically, electrode configuration has a dramatic effect on the across-site variance of detection threshold levels with the variance being much lower for monopolar stimulation than for narrow bipolar or tripolar stimulation (Pfingst and Xu, 2004; Bierer, 2007) but electrode configuration has little or no effect on across site variation in modulation detection thresholds (Pfingst, in press). Finally, it is interesting to compare the across-site patterns for a given measure in the left and right ears of subjects with bilateral cochlear implants. In some cases these patterns are strikingly similar for the two ears (e.g., the across site patterns of MDTs in Subject 60), suggesting that the conditions in the two ears were affected in a similar manner by a common (in this case genetic) etiology.
Overall, the across-site patterns of psychophysical performance are consistent with the idea that local conditions in the implanted cochlea affect the perception of electrical stimuli. Further evidence for this comes from animal studies described in Section 3.3. The importance of the individual psychophysical measures for perception of multichannel stimuli such as speech signals can be assessed by site-selection experiments that are described in Section 4.
3.3 Functional and histological data from animals
Studies in animals allow a more direct comparison between conditions in the implanted cochlea and cochlear implant function than is possible in human subjects. In animals, histological analysis can be obtained within a short time after obtaining detailed electrophysiological and psychophysical data. Recent work from our laboratory has used a guinea pig model to examine the relationship between nerve survival patterns in the cochlea and cochlear implant function. A variety of well developed assessment tools are available for these experiments, many of which were initiated or refined over the last 50 years by faculty and staff at the Kresge Hearing Research Institute. These include animal psychophysics procedures (Stebbins, 1970); procedures for protection and regrowth of auditory nerve (Miller et al., 2002); assessment of ensemble spontaneous activity in the auditory nerve (Dolan et al., 1990); use of multichannel thin film electrode arrays for recording neural discharges simultaneously from multiple sites along the tonotopic axis in the central nervous system in response to cochlear implant stimulation (Middlebrooks and Bierer, 2002; Anderson, 2008); gene therapy procedures for drug delivery (Raphael and Yagi, 1998); and surface preparations and other histological procedures for assessment of cochlear pathology (Johnsson and Hawkins, 1967).
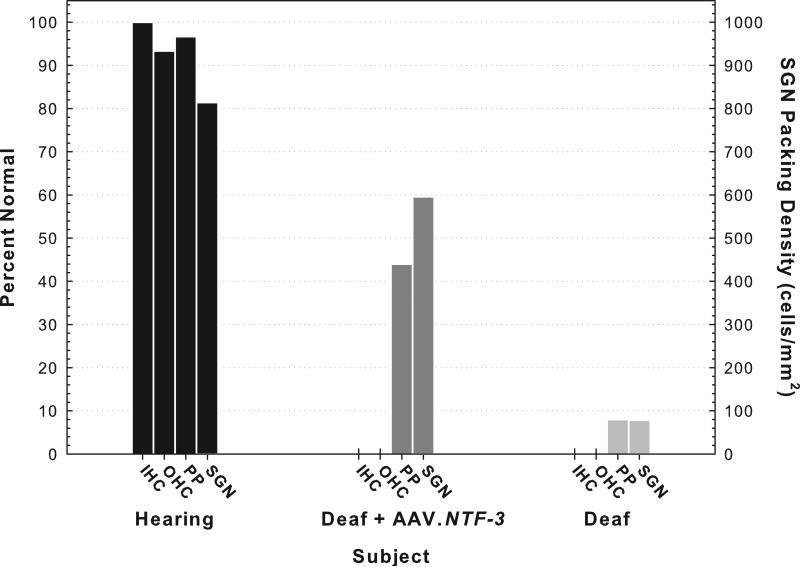
To study the relationship between cochlear infrastructure and prosthesis function, we used a variety of implantation and treatment procedures, resulting in a wide range of conditions in the implanted cochlea. Results for three cases are shown in Figs. 3 and following. Results for these cases are representative of those seen in larger groups of animals from experiments previously published (Chikar et al., 2008; Kang et al., 2010), presented (Pfingst et al., 2010) or in progress. Some animals were implanted with a cochlear implant electrode array in a hearing ear. In most cases using this procedure, some residual hearing remained in the tonotopic region occupied by the implant (around 16 kHz) and considerable hearing remained apical to the implant. On average there was about a 40 dB hearing loss at 16 kHz after implantation. These animals typically had some surviving inner and outer hair cells, auditory nerve peripheral processes, and spiral ganglion cell-body packing densities that were moderate to near normal (e.g., Kang et al., 2010 and the “Hearing” animal in Fig. 3). Another group of animals was deafened prior to implantation by injection of neomycin sulfate into the scala tympani. These animals showed a complete absence of all hair cells, few peripheral processes, and greatly reduced spiral ganglion neuron (SGN) cell-body packing densities (e.g., Kang et al., 2010 and the “Deaf” animal in Fig. 3). A third group of animals was deafened with neomycin or systemic kanamycin and ethacrynic acid and then the cochleae were inoculated with an adeno-associated virus carrying a neurotrophin gene (BDNF or NTF-3). The adeno-associated virus transfected cells remaining in the basilar membrane area and upregulated the secretion of the neurotorphin proteins. This inoculation resulted in regrowth of peripheral processes toward the basilar membrane area and retarded the degeneration of SGN cell bodies (e.g., Shibata et al., 2010 and the “Deaf + AAV.NTF-3” animal in Fig. 3).
Fig. 3.
Histological results for three representative animals differing in the treatment and implantation procedures. All three animals received an eight-electrode Nucleus animal cochlear implant (Cochlear Corp., Lane Cove, Australia) in their left ear. The implants were inserted through a cochleostomy made approximately 0.7 mm apical to the round window. The electrodes were spaced at approximately 0.75 mm intervals and were labeled A through H with A being the most apical. The “Hearing” animal received the cochlear implant in an ear that had normal hearing prior to implantation. After implantation while pulse rate data in Fig. 6 were collected, thresholds for 16 kHz pure tones were elevated by 8.1 dB relative to the pre-implant thresholds. The “Deaf + AAV.NTF-3” animal was deafened prior to implantation by systemic administration of kanamycin (400 mg/kg) and ethacrynic acid (35 mg/kg). Seven days later, the left ear was inoculated with AAV.NTF-3 (5 μL) injected into the scala tympani through the cochleostomy and then, 20 minutes after the inoculation, the cochlear implant was inserted. The “Deaf” animal was deafened in the left ear by local injection of neomycin sulfate (60 μl, 10% w/v) through the round window prior to implantation. Histological data were obtained from peri-midmodiolar sections that were centered at the location of the primary electrode used for psychophysical and electrophysiological data collection (Electrode B, which was located in the first half of the basal turn approximately 3.8 mm on average from the round window). Five 2.5 μm sections were analyzed and spaced at intervals of 25 μm. Means of results for these five sections are shown. Inner hair cells (IHC) were counted only if a nucleus was present. Percent normal (left ordinate) is shown where normal equals one hair cell per section. The outer hair cells (OHC) were counted if any part of the cell was visible and normal equals 3 hair cells per section. Individual peripheral processes could not be counted in the peri-midmodiolar sections so density of peripheral processes was estimated on a scale of 0 to 3, with 3 being normal. Spiral ganglion cell packing density (right ordinate) was estimated by counting the number of cells with a nucleus in the cross section of Rosenthal's canal and dividing by the area of that cross section. Histological results for the hearing animal and those for the deaf animal are similar to those reported by Kang and colleagues (2010).
The mere presence of SGN neurons wit h a nucleus, as observed under the light microscope, does not guarantee that the neurons are functional at a physiological level. We have used several functional measures to assess functional differences among populations of surviving neurons in the region occupied by the cochlear-implant electrode array.
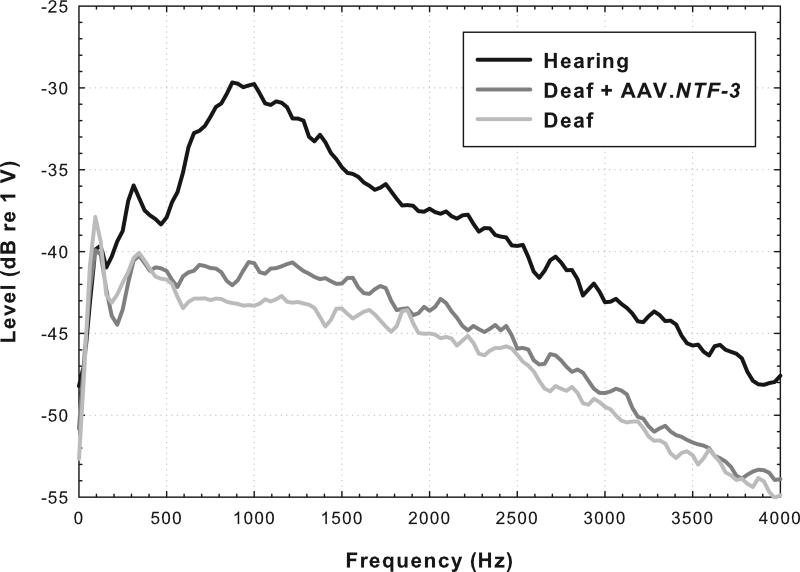
Spontaneous activity in surviving auditory nerve fibers, which depends in large part on the presence of functional inner hair cells, can have marked effects on the temporal properties of the neural response to electrical stimulation (Hu et al., 2003). We have assessed spontaneous activity in our animal preparation using the ensemble spontaneous activity (ESA; aka round-window noise) measure originally developed by Dolan and colleagues (1990) and later elaborated by Searchfield and colleagues (2004). We have recorded this activity from the cochlear implant focusing on the electrodes that were used for primary psychophysical data collection. Details of the procedure are given in Kang et al., 2010. The animals that were implanted in a hearing ear and that still had residual acoustic hearing after implantation showed peaks in the ESA spectrum around 900 Hz, indicative of spontaneous activity in the auditory nerve (e.g., trace shown in black in Fig. 4). Animals deafened with neomycin prior to implantation showed no such peaks (e.g., light gray trace in Fig. 4). Animals deafened prior to implantation and treated with AAV.NTF-3 are of particular interest. The ESA spectrum for these animals failed to show a clear peak in the 900 Hz region, but the voltages for these tracings were higher in that region than those for the tracings for the deaf animals (e.g., dark gray trace in Fig. 4). The voltages in the AAV.NTF-3 treated animals suggest that there might be some spontaneous activity in the auditory nerve of these animals despite the lack of hair cells, but further research is needed to determine if this is the case.
Fig. 4.
Spectra for ensemble spontaneous activity recorded from Electrode B for the three animals for which histology is shown in Fig. 3. A peak in the spectrum near 900 Hz (-29.7 dB re 1 V) is typical of animals with residual acoustic hearing and presumed spontaneous activity in the auditory nerve (See Kang et al., 2010: mean = -33.4 dB, s.d. = 1.84, n = 11). The lack of a distinct peak and the low potentials (max -42.9 dB) near 900 Hz in the deaf animal is also similar to data reported previously (Kang et al., 2010: mean = -44.4 dB, s.d. = 2.5, n = 4). The intermediate voltage (-40.6 dB) recorded for the Deaf + AAV.NTF-3 treated animal is similar to that found in four other animals that were treated with neomycin followed by AAV.NTF-3 (unpublished results: mean = -41.1 dB, s.d. = 2.2 , n = 4).
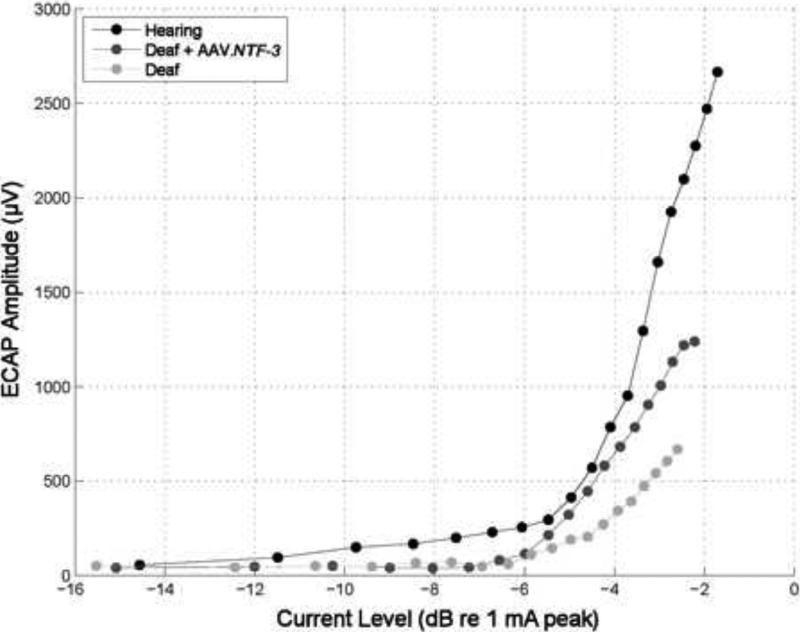
Fig. 5 shows amplitude growth functions for electrically-evoked compound action potentials (ECAPs) for animals with various degrees of nerve survival as shown in Fig. 3. The slopes of these functions vary systematically across animals with the steepest slope occurring in the animal with the best nerve survival and the shallowest slope found in the animal with a very low nerve survival. Similar differences in slopes of electrically evoked potentials have been associated with degree of nerve survival in other studies including those by Smith and Simmons (1983) and Hall (1990) using electrically-evoked auditory brainstem responses (EABRs) and Jyung and colleagues (1989) using electrically-evoked middle latency responses (EMLRs). This is in line with the theory that the slope correlates with an increase in the number of neurons that respond to every increment in stimulation level, i.e. that a steeper growth function indicates a greater number of neurons activated for every increment in stimulation level. Importantly, a recent study by Kim and colleagues (2010) found a correlation between slopes of ECAP amplitude growth functions and speech recognition in human subjects using a short (hybrid) electrode array inserted into the first half of the basal turn of the cochlea, similar to the implant placement in our guinea pig experiments.
Fig. 5.
Electrically-evoked compound action potential (ECAP) input/output functions for the three animals for which histology is given in Fig. 3. Potentials were evoked by monopolar stimulation of Electrode B and recordings were made from Electrode A. Stimuli were 25 μs/phase biphasic pulses of alternating initial-phase polarity presented at 50 pps, delivered using a MED-EL Pulsar ci100 receiver/stimulator connected to the implant through a percutaneous connector. ECAP amplitudes were calculated as the peak to peak voltages: P2 (latency ~0.3 ms) minus N2 (latency ~0.8 ms) from waveforms averaged over 20 pulses. The differences in slopes of the ECAP input/output functions across the three conditions shown here have been duplicated in preliminary data from experiments currently in progress involving three hearing animals, four deafened AAV.NTF-3 inoculated animals and two deafened AAV.empty inoculated animals.
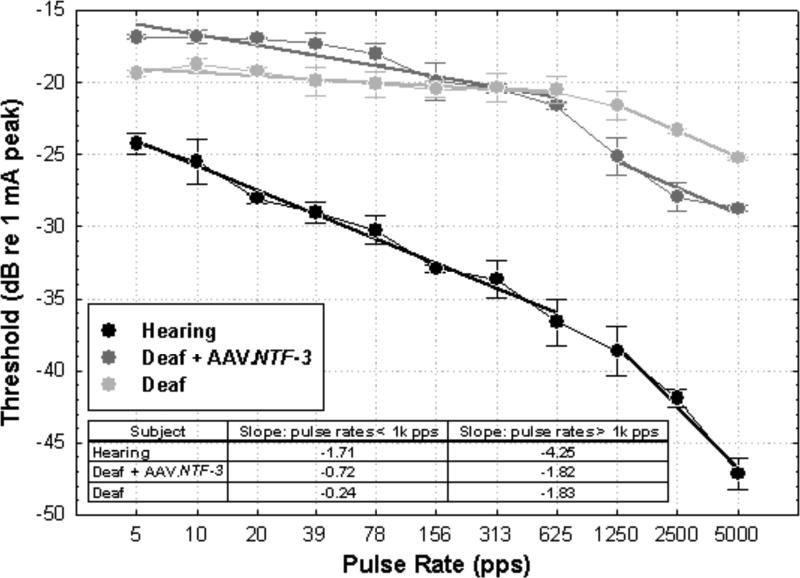
It is commonly assumed that healthy conditions in the cochlea will be associated with lower detection thresholds for cochlear implant stimulation. Lower thresholds would have several advantages for the function of the auditory prostheses including longer battery life and/or the ability to safely use electrodes with smaller surface areas to achieve better spatial resolution. However, we have found that the relationship between nerve survival and detection thresholds for electrical stimulation is not simple. In animals with good nerve survival, thresholds for short duration pulsatile stimuli (such as those commonly used in most current cochlear implants) are typically lower than in those animals with poor nerve survival, whereas for long-phase-duration signals (such as low-frequency analog stimuli), the opposite is often true (Su et al., 2008; Kang et al., 2010). The effects of phase duration probably reflect differences in membrane properties at the site of action-potential initiation. Furthermore, for pulse trains with short-duration pulses, the magnitude of difference in threshold between animals with good nerve survival and those with poor nerve survival depends on the pulse rate. This is because for animals with good nerve survival, thresholds decrease as a function of pulse rate whereas for animals with poor nerve survival, pulse rate below 1000 pulses per second has little or no effect on detection thresholds (e.g., Kang et al., 2010 and function in light gray in Fig. 6). Above 1000 pulses per second all subjects show decreases in threshold as a function of pulse rate for both psychophysical and cortical-neural thresholds (Middlebrooks, 2004; Kang et al., 2010; and examples shown in Fig. 6). Slopes at these pulse rates are probably due in part to additive effects of partial depolarization of one subthreshold pulse on the thresholds for discharge for subsequent pulses (Middlebrooks, 2004). The slopes of the psychophysical threshold versus pulse rate functions for pulse rates below 1000 pps are a particularly good indicator of a healthy infrastructure in the implanted cochlea (Kang et al., 2010 and examples in Fig. 6). The slightly steeper slope of the function for the AAV.NTF-3 treated animal compared to those typical of deaf animals is intriguing. This will be explored further in ongoing experiments.
Fig. 6.
Psychophysical detection thresholds for monopolar pulsatile stimulation of Electrode B as a function of pulse rate for the three animals for which histology is shown in Fig. 3. Stimuli were 25 μs/phase biphasic pulses delivered by a controlled-current stimulator (built in house). Stimulus levels in μA were controlled in 1 dB steps. Detection thresholds (50% correct detections) are presented in dB relative to 1 mA peak. Pulse-train duration was 200 ms. Means and standard deviations for three repeated measures are shown. Other details of the psychophysical training and testing procedures are given in Kang et al., 2010. Best-fit linear regression lines were fit to the data points below 1000 pps and separate lines were fit to the data above 1000 pps. These slopes, in dB per doubling of pulse rate, are given in the inset table. Differences between the deaf and hearing animals in the slopes and levels of these functions are similar to those reported in Kang et al., 2010. Experiments are currently in progress for two other guinea pigs that were deafened and inoculated with AAV.NTF-3. Slopes for these animals for pulse rates below 1 kpps were similar to those for the deafened AAV.NTF-3 treated animal in this graph (-0.88 and -1.36 dB/doubling).
4. Discussion
Psychophysical detection and discrimination of cochlear implant stimuli varies considerably from one stimulation site to another with across-site patterns that are specific to individual subjects, as described in Section 3.2. This suggests that conditions near each stimulation site have a strong influence on cochlear implant function. This contention is supported by research in animals (Section 3.3) where the conditions near the stimulation sites can be measured histologically in close proximity to the time of psychophysical data collection, and is consistent with the patchy nerve survival patterns along the length of the cochlea that are often observed in human temporal bones from potential or actual implant users (Section 2).
There are several important implications of these findings for future treatment of hearing impairment with cochlear implants. First, they support the efforts in tissue engineering to improve the infrastructure of the implanted cochlea and they can help guide those efforts by identifying the biological elements that are most important for specific features of cochlear implant function. Some of these tissue engineering procedures are elaborated in the article by Shibata and colleagues in this special issue of Hearing Research. Determining which biological elements are most important for implant function is complicated by the fact that survival rates of multiple elements are correlated. Hair cell loss is usually followed by loss of auditory nerve peripheral processes and then cell bodies (Section 2). This makes it difficult to determine which of the elements have what effect on implant function. As Lars Johnsson observed from his work at KHRI in the 1970's: “the fact that sensory and neural degeneration rarely occur in reasonably “pure” forms may explain why it is often difficult to distinguish between them by means of audiological tests alone” (Johnsson, 1974). Now, however, the ability to regenerate neural elements in the cochlea following hair cell destruction in animal models (Shibata et al., 2010) has provided the opportunity to separately study the contributions of neural and sensory elements to cochlear implant function.
A second implication of the finding that cochlear implant performance varies from site to site along the electrode array is that many implanted subjects have both strengths and weaknesses with regard to electrical hearing that depend on the specific sites of electrical stimulation within their cochlear implant. This observation supports the strategy of selecting specific stimulation sites for the processor map based on the psychophysical data. The efficacy of such a site-selection strategy was demonstrated originally by Zwolan and colleagues (1997) and more recently, Garadat and colleagues (2011).
It is important to note that although both the animal and the human data suggest that cochlear conditions affect perception as assessed with psychophysical procedures, not all psychophysical measures of perception are affected in the same way. This is reflected in Fig. 2 where the across-site patterns differ from one measure to another in the same subject. It follows that the effectiveness of the site-selection strategy will depend on the psychophysical measure being used and on the multisite function being targeted. In our recent experiments, we have found that a psychophysical measure that a ssesses both spatial and temporal acuity is effective for identifying stimulation sites that are important for recognition of speech in a noisy background. In these experiments (Garadat et al., 2011) we selected sites for experimental processor maps based on psychophysical measures of envelope modulation detection in the presence of an unmodulated pulse train interleaved on an adjacent stimulation site. By using this measure to select stimulation sites for experimental processor maps, we were able to achieve improvements in speech recognition even with respect to the subjects’ every day processor maps, with which they had considerably more experience. Experiments are in progress to determine the extent to which training with these experimental processor maps results in further improvements in speech recognition in noise.
The within-subject across-site comparisons in humans perhaps give a clearer and more practically-useful view of the relationship between infrastructure of the implanted cochlea and cochlear implant function than can be obtained from across-subject studies. In the across-subject studies, the data are complicated by many other subject-specific variables that affect implant performance, particularly for complex functions such as speech recognition. The within-subjects approach requires two stages. First, we must determine what psychophysical or electrophysiological measures of implant performance are affected on a site-specific basis and second, we must determine how performance on those measures is related to speech recognition or other measures that require multisite stimulation. This work, including the study by Garadat and colleagues (2011) is currently in progress.
From our work in animals, we and other investigators have identified psychophysical and electrophysiological measures that are correlated with nerve survival and other conditions in the implanted cochlea. Examples include levels of ensemble spontaneous activity (Fig. 4), slopes of ECAP growth functions (Fig. 5), and slopes and levels of psychophysical detection threshold versus pulse rate functions (Fig. 6). The next critical step is to determine the relation of these measures to speech recognition in humans. Selecting stimulation sites for the speech-processor map based on these measures is a powerful experimental procedure for assessing the importance of the measures for speech recognition because it uses a within subject design that reduces confounding effects of across subject differences in other variables. Data from this approach should greatly improve our understanding of the roles of cochlear infrastructure for cochlear implant function which can then guide tissue engineering procedures to improve the infrastructure as well as site-selection procedures to take advantage of regions with the best infrastructure. This could lead to significant improvements in the treatment of hearing loss.
Acknowledgements
This work was supported financially in large part by research grants from the NIDCD (R01s DC010786, DC010412, DC007634, & DC004312), a research contract from MED-EL Corporation, and training grants from NIDCD (F32 DC010318 & T32 DC000011). We gratefully acknowledge the assistance from core facilities supported by NIDCD P30 DC005188 which included support for electronics, computer and machine shops: Chris Ellinger, David Rodgers, Dwayne Vailliencort and Jim Wiler. Special thanks to the Electrophysiology Core directed by Dr. David Dolan which performed the ESA recordings under the expert guidance of Karin Halsey and to Don Swiderski and Lisa Beyer in the Raphael laboratory for histological preparation. We appreciate the equipment, software and personnel time provided by Cochlear Corporation which facilitated work done in both the human and animal laboratories; particular thanks to Chris van den Honert, Barbara Buck and Colin Irwin from Cochlear Corporation. Thanks to Ariana Di Polo for providing the viral constructs. Consistently helpful review and guidance from Carolyn Garnham at MED-EL and from our clinical faculty, particularly Terry Zwolan and Caroline Arnedt is greatly appreciated.
List of Abbreviations
- AAV
Adeno-associated viral vector
- BDNF
Brain-derived neurotrophic factor
- C level
Maximum comfortable loudness level
- CU
Current units
- DR
Dynamic range
- EABR
Electrically-evoked auditory brainstem response
- ECAP
Electrically-evoked compound action potential
- EMLR
Electrically-evoked middle-latency response
- ESA
Ensemble spontaneous activity
- GDT
Gap-detection threshold
- IHC
Inner hair cell
- MDT
Modulation detection threshold
- n
Number of cases
- NTF-3
Neurotrophin three
- NU-6
Northwestern University Auditory Test No. 6
- OC
Organ of Corti
- OHC
Outer hair cell
- PP
Peripheral process of the spiral ganglion cell
- p-p
Peak to peak
- pps
Pulses per second
- PTA
Pure-tone average for acoustic detection thresholds in dB hearing level
- SGN
Spiral ganglion neuron
- s.d.
Standard deviation
- T level
Detection threshold level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agterberg M, Versnel H, de Groot J, Smoorenburg G, Albers F, Klis S. Morphological changes in spiral ganglionc cells after intracochlear application of BDNF in deafened guinea pigs. Hear. Res. 2008;244:25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Anderson DJ. Penetrating multichannel stimulation and recording electrodes in auditory prosthesis research. Hear. Res. 2008;242:31–41. doi: 10.1016/j.heares.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Bierer JA. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J. Acoust. Soc. Am. 2007;121:1642–1653. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, Polo AD, Raphael Y, Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear. Res. 2008;245:24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan DF, Nuttall AL, Avinash G. Asynchronous neural activity recorded from the round window. J Acoust. Soc. Am. 1990;87:2621–2627. doi: 10.1121/1.399054. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Viemeister NF, Nelson DA. Psychometric functions and temporal integration in electric hearing. J. Acoust. Soc. Am. 1997;101:3706–3721. doi: 10.1121/1.418330. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH., Jr. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Makarem AO, Linthicum FH., Jr. Histopathological assessment of fibrosis and new bone formation in implanted human temporal bones using 3D-reconsruction. Otolaryngol. Head Neck Surg. 2009;141:247–252. doi: 10.1016/j.otohns.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants. J. Acoust. Soc. Am. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Galvin JJ, 3rd, Fu QJ. Effects of stimulation rate, mode and level on modulation detection by cochlear implant users. J. Assoc. Res. Otolaryngol. 2005;6:269–279. doi: 10.1007/s10162-005-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garadat SN, Pfingst BE. Relationship between gap detection thresholds and loudness in cochlear-implant users. Hear. Res. 2011 doi: 10.1016/j.heares.2010.12.011. doi:10.1016/j.heares.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garadat S, Zwolan T, Pfingst B. Across-site patterns of modulation detection: relation to speech recognition. Assoc. Res. Otolaryngol. 2011 doi: 10.1121/1.3701879. Abst. 34, Abstract #213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear. Res. 1990;45:123–136. doi: 10.1016/0378-5955(90)90188-u. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Marion M. Histopathology of profound sensorineural deafness. Ann. N. Y. Acad. Sci. 1983;405:459–484. doi: 10.1111/j.1749-6632.1983.tb31662.x. [DOI] [PubMed] [Google Scholar]
- Hu N, Abbas P, Mi ller C, Robinson B, Nourski K, Jeng F, Abkes B, Nichols J. Auditory response to intracochlear electric stimuli following furosemide treatment. Hear. Res. 2003;185:77–89. doi: 10.1016/s0378-5955(03)00261-2. [DOI] [PubMed] [Google Scholar]
- Incesulu A, Nadol JB., Jr. Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann. Otol. Rhinol. Laryngol. 1998;107:906–911. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- Johnsson LG. Sequence of degeneration of Corti's organ and its first order neurons. Ann. Otol. Rhinol. Laryngol. 1974;83:294–303. doi: 10.1177/000348947408300303. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE., Jr. A direct approach to cochlear anatomy and pathology in man. Arch. Otolaryngol. 1967;85:599–613. doi: 10.1001/archotol.1967.00760040601005. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE, Jr., Kingsley TC, Black FO, Matz GJ. Aminoglycoside-induced cochlear pathology in man. Acta Otolaryngol Suppl. 1981;383:1–19. [PubMed] [Google Scholar]
- Jyung RW, Miller JM, Cannon SC. Evaluation of eighth nerve integrity by the electrically evoked middle latency response. Otolaryngol. Head Neck Surg. 1989;101:670–682. doi: 10.1177/019459988910100610. [DOI] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J. Assoc. Res. Otolaryngol. 2010;11:245–265. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta. Otolaryngol. 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB., Jr. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005a;115:672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Eddington D, Damian D, Nadol JB., Jr. Effects of cochlear implantation on residual spiral ganglion cell count as determined by comparison with the contralateral nonimplanted inner ear in humans. Ann. Otol. Rhinol. Layrngol. 2005b;114:381–385. doi: 10.1177/000348940511400508. [DOI] [PubMed] [Google Scholar]
- Kim JR, Abbas PJ, Brown CJ, Etler CP, O'Brien S, Kim LS. The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otol. Neurotol. 2010;31:1041–1048. doi: 10.1097/MAO.0b013e3181ec1d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC. Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. J. Acoust Soc. Am. 2004;116:452–468. doi: 10.1121/1.1760795. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Bierer JA. Auditory cortical images of cochlear-implant stimuli: Coding of stimulus channel and current level. J. Neurophysiol. 2002;87:493–507. doi: 10.1152/jn.00211.2001. [DOI] [PubMed] [Google Scholar]
- Miller AL. Effects of chronic sti mulation on auditory nerve survival in ototoxically deafened animals. Hear. Res. 2001;151:1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Miller JM, Miller AL, Yamagata T, Bredberg G, Altschuler RA. Protection and regrowth of the auditory nerve after deafness: neurotrophins, antioxidants and depolarization are effective in vivo. Audiol. Neurootol. 2002;7:175–179. doi: 10.1159/000058306. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear. Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol. Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr., Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland T, Shallop JK. Histopathology of cochlear implants in humans. Ann. Otol. Rhinol. Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Otte J, Schuknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88(8 Pt 1):1231–1246. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- Pfingst BE. Effects of electrode configuration on cochlear implant modulation detection thresholds. J. Acoust. Soc. Am. doi: 10.1121/1.3583543. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Burkholder-Juhasz RA, Xu L, Thompson CS. Across-site patterns of modulation detection in listeners with cochlear implants. J. Acoust. Soc. Am. 2008;123:1054–62. doi: 10.1121/1.2828051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Su GL, Middlebrooks JC, Raphael Y. Perception of pulse trains in the electrically stimulated cochlea: Effects of preserving acoustic hearing. Assoc. Res. Otolaryngol. Abst. 2010;33:118–119. [Google Scholar]
- Pfingst BE, Xu L. Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. J. Assoc. Res. Otolaryngol. 2004;5:11–24. doi: 10.1007/s10162-003-3051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS. Effects of carrier pulse rate and stimulation site on modulation detection by subjects with cochlear implants. J. Acoust. Soc. Am. 2007;121:2236–2246. doi: 10.1121/1.2537501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y, Yagi M. Gene transfer in the inner ear. Current Opinion in Otolaryngol. Head & Neck Surg. 1998;6:311–315. [Google Scholar]
- Scheper V, Paasche G, Miller JM, Warnecke A, Berkingali N, Lenarz T, Stover T. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J. Neurosci. Res. 2009;87:1389–1399. doi: 10.1002/jnr.21964. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Lesions of the organ of Corti. Trans. Am. Acad. Opthalmol. Otolaryngol. 1953;57:366–382. [PubMed] [Google Scholar]
- Searchfield GD, Munoz DJ, Thorne PR. Ensemble spontaneous activity in the guinea-pig cochlear nerve. Hear. Res. 2004;192:23–35. doi: 10.1016/j.heares.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Shibata SB, Cortez SR, Beyer LA, Wiler JA, Di Polo A, Pfingst BE, Raphael Y. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp. Neurol. 2010;223:464–472. doi: 10.1016/j.expneurol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Simmons FB. Estimating eighth nerve survival by electrical stimulation. Ann. Otol. Rhinol. Laryngol. 1983;92:19–23. doi: 10.1177/000348948309200105. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Organization of the cochlear receptor. Fortschr. Hals Nasen Ohrenheilkunde. 1966;13:1–227. [PubMed] [Google Scholar]
- Stebbins WC. Animal Psychophysics: The Design and Conduct of Sensory Experiments. Plenum Press; New York: 1970. [Google Scholar]
- Su GL, Colesa DJ, Pfingst BE. Effects of deafening and cochlear implantation procedures on postimplantation psychophysical electrical detection thresholds. Hear. Res. 2008;241:64–72. doi: 10.1016/j.heares.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M, Webster DB. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O'leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J. Comp. Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Wise KD, Bhatti PT, Wang J, Friedrich CR. High-density cochlear implants with position sensing and control. Hear. Res. 2008;242:22–30. doi: 10.1016/j.heares.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Zimmermann CE, Burgess BJ, Nadol JB., Jr. Patterns of degeneration in the human cochlear nerve. Hear. Res. 1995;90:192–201. doi: 10.1016/0378-5955(95)00165-1. [DOI] [PubMed] [Google Scholar]
- Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J. Acoust. Soc. Am. 1997;102:3673–3685. doi: 10.1121/1.420401. [DOI] [PubMed] [Google Scholar]