Abstract
Ubiquitin (Ub) conjugation to a substrate protein is a widely used cellular mechanism for control of protein stability and function, modulation of signal transduction pathways and antiviral responses. Identification and characterization of ubiquitinated viral proteins is an important step in understanding novel mechanisms of viral protein regulation as well as elucidating cellular antiviral strategies. Here we describe a protocol to easily detect and characterize the ubiquitination status of a viral substrate protein expressed either during infection or ectopically expressed as a fusion with a biotinylatable epitope tag. This tag provides advantages over current immunoprecipitation techniques by making use of the extremely tight biotin-streptavidin interaction. We provide an example of this protocol using the nonstructural protein 5 (NS5) from Langat virus (LGTV), a member of the tick-borne encephalitis virus (TBEV) serocomplex within the Flavivirus genus. Using the protocols outlined here, we describe some of the pitfalls inherent in determination of Ub linkage and demonstrate that NS5 is modified by at least two distinct ubiquitination types, multiubiquitination and K48-linked polyubiquitin chains.
Keywords: flavivirus, NS5, ubiquitin, Langat, biotin, streptavidin
1. Introduction
1.1. Viruses and ubiquitin
The limited size of many virus genomes necessitates efficient strategies to encode and express proteins that perform multiple functions in the viral replication cycle, from genome replication to immune evasion. Viruses achieve this through various mechanisms including utilizing ambisense transcription, transcriptional editing and encoding for overlapping open reading frames. Following expression, viral proteins can bestow multiple biological functions, in part through the use of reversible post-translational modifications (PTMs). The covalent attachment of the 76 amino acid polypeptide ubiquitin (Ub) is one such PTM that can markedly affect the function, cellular localization, protein-protein interactions or stability of the target protein. Indeed, ubiquitination is involved in most cellular processes ranging from protein degradation and receptor trafficking to innate immunity [1–5]. Not surprisingly, viruses have evolved strategies to use host processes of ubiquitination to regulate viral proteins for the benefit of viral replication [6–11]. Conversely, ubiquitination is also used by host antiviral effector molecules as part of innate defenses to accelerate degradation of key viral proteins as a means to restrict viral replication [12,13]. Increasing evidence suggests viruses have evolved evasion strategies that specifically use or disable Ub-dependent responses through expression of viral Ub-like molecules, Ub ligases and deubiquitinases (DUBs) [12,14,15]. Thus, identifying the Ub conjugation status of viral proteins is an important step in understanding the broader roles of these proteins in virus-host cell interactions.
1.2. Ubiquitination process
The impact of Ub on its target protein is determined by its conjugation status. Ub can be attached as a monomer at one (monoubiquitination) or more (multiubiquitination) sites. Ligation usually occurs at internal lysine residues within the substrate, though cysteine, serine, threonine and terminal amino groups may serve as Ub acceptor residues. These noncanonical acceptor sites are commonly found in viral proteins or are ubiquitinated by viral E3 Ub ligases [15–20]. Alternatively, a single Ub molecule can act as a substrate for additional molecules to form polyubiquitin chains. The three-dimensional structure of the chain is determined by the linkage of Ub; the carboxy-terminal glycine is linked to one of seven lysine residues integral to Ub (K6, K11, K27, K29, K33, K48 and K63) or to the amino-terminal methionine (M1; termed linear chains) [1,21]. Both the configuration of Ub conjugation and the selection of the target protein are mediated by E3 Ub ligases that coordinate with E2 Ub conjugation enzymes to transfer activated Ub molecules (Figure 1A). This process results in the reversible covalent linkage of the 8.5 kDa Ub moiety onto a target protein. As this PTM is of considerable size relative to a phosphorylation or acetylation event, ubiquitination can have dramatic steric and functional effects on target proteins. Like many biological systems, it is getting more difficult to generalize the roles of monoubiquitination or specific polyubiquitin chain formation in determining the fate of the targeted protein [1,22]. The most commonly associated modification types and associated functions are represented graphically in Figure 1B. Generally, K48-linked polyubiquitin chains facilitate protein degradation via the proteasome [23,24] whereas K63-linked chains enable signal transduction by recruiting multi-protein signaling complexes [2,5,25]. Monoubiquitination can regulate a wide variety of cellular processes by facilitating protein-protein interactions, altering cellular localization or directing membrane protein endocytosis [26].
Figure 1.
(A) Ubiquitination. Schematic representation of the necessary steps for Ub ligation onto a substrate protein. 1. Activation. In an ATP-dependent process, free Ub is activated by an E1 Ub activating enzyme. 2. Conjugation. The activated Ub molecule in conjugated to an E2 Ub conjugating enzyme and is now ready for transfer to a substrate. 3. Ligation. Specific interaction between an E3 Ub ligase enzyme and substrate facilitates transfer of Ub from the E2 enzyme to the substrate protein. (B) Illustration of 4 types of Ub modifications: monoubiquitination, multiubiquitination, K48-linked Ub chains, and K63-linked Ub chains, and general functional outcomes observed with the modification type.
1.3. The multifunctional flavivirus nonstructural protein 5 (NS5)
The nonstructural protein 5 (NS5) of flaviviruses, including dengue virus, tick-borne encephalitis virus (TBEV) and West Nile virus, is of utmost importance to virus replication owing to the fact that it encodes the viral methyltransferase and RNA-dependent RNA polymerase [27]. It also encodes the major antagonist of type I interferon (IFN) signaling and, in the case of TBEV, is known to interact with at least two pathways associated with degradation, the proteasome and the lysosome [28,29]. In addition IFN antagonism by dengue virus NS5 is dependent upon the ubiquitin-proteasome system [30]. However, little is known about the precise PTM that contribute to these processes and almost nothing is known about the ubiquitination status of flavivirus NS5. Therefore, we used NS5 derived from Langat virus (LGTV), a member of the TBEV serogroup, as an example of a multifunctional viral protein to examine its conjugation to Ub and highlight some of the complexities in assessing this critical PTM.
2. Methods and Results
2.1. Reagents
2.1.1. Antibodies
The following antibodies were obtained from commercial sources and used for western blotting at the indicated dilutions: −actin (A5441, Sigma, 1:10,000); −V5 (R960-25, Invitrogen, 1:5000); −HA (16B12, Covance, 1:1000); −Ub (P4D1, sc-8017, Santa Cruz Biotechnology, 1:500); −polyubiquitin FK1 (PW8805, Enzo, 1:1000); −poly/monoubiquitin FK2 (PW8810, Enzo, 1:1000); −Ub-K48 (05-1307, Millipore, 1:1000); and −Ub-K63 (PW0600, Enzo, 1:500). Affinity purified chicken antibodies specific for LGTV NS5 (peptide sequence: CZ DRHDLHWELKLESSIF, 1:200) and control IgY antisera were custom prepared by Aves Labs.
2.1.2. Buffers
Immunoprecipitation (IP) buffer: 50mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% Na-deoxycholate.
Radioimmunoprecipitation assay (RIPA) buffer: 50mM Tris-HCl, 150 mM NaCl, 0.1 % sodium dodecyl sulfate (SDS), 1% NP-40, 0.5% Na-deoxycholate.
DNAse buffer (10X): 100mM Tris-HCl pH 7.5, 25mM MgCl2, 5 mM CaCl2.
Sample buffer (2X): 125 mM TRIS pH 6.8, 20% glycerol, 30 mM EDTA, 8% 2-mercaptoethanol, 4% SDS and bromophenol blue.
20% SDS in distilled water.
2.1.3. Chemicals and beads
The following reagents were obtained from commercial sources: Lipofectamine LTX with Plus Reagent (Invitrogen); N-ethylmaleimide (NEM, E3876, Sigma); MG132 (C2211, Sigma); lactacystin (L6785, Sigma); dimethyl sulfoxide (DMSO, D2650, Sigma); DNase I (10104159001, Roche); Complete Protease Inhibitor Cocktail (04693132001, Roche); agarose coupled goat anti-Chicken (PrecipHen, P-1010, Aves Labs); streptavidin agarose (S951, Invitrogen); unconjugated agarose beads (CL4B200, Sigma), Protein G or A agarose beads (11719416001 or 11719408001, Roche). Dulbecco’s Phosphate-Buffered Saline (DPBS), Dulbecco’s Modified Eagle Medium (DMEM), and OptiMEM were obtained from Invitrogen.
2.1.4. Cells, plasmids and viruses
HEK 293 (293) cells were obtained from ATCC and were cultured in DMEM supplemented with 10% (vol/vol) fetal calf serum (Invitrogen), 100 units/ml penicillin, and 100 µg/ml streptomycin (Invitrogen) in an atmosphere of 5% CO2 at 37°C. The NS5 entry vector for Gateway cloning (Invitrogen) was described previously [28]. The NS5 expression plasmid was obtained by recombination from the entry vector into the Gateway destination vector pcDNA-3.2/capTEV-CT/V5-DEST (C-terminal V5/AP tag, BN3002, Invitrogen) according to manufacturer’s instructions. Known NS5 functions (interferon antagonism) and protein interactions were confirmed with this expression construct (data not shown). pRK5-HA-Ub-WT (Addgene plasmid 17608), K48 (Addgene plasmid 17605), K63 (Addgene plasmid 17606), K48R (Addgene plasmid 17604) and K0 (Addgene plasmid 17603) plasmids were provided by Ted Dawson (Johns Hopkins University, MD) [31]. Propagation and titration of LGTV on Vero cells was described previously [32].
2.2. Detection of ubiquitinated viral proteins
2.2.1. General considerations
The ubiquitination enzymatic reaction can be performed in vitro using defined reagents and purified proteins in a cell-free system [33]. However, the protocols described herein in vivo, are cell-based ubiquitination assays that rely upon cellular expression of the necessary components. Following expression of individual proteins, identification of Ub modification can be readily accomplished using standard co-immunoprecipitation (co-IP) protocols (protocol discussed first in 2.2.2.). A concern with this approach is the relatively high background and risk of detection of co-precipitating proteins carrying their own Ub moieties. Aside from the abundant protein-protein interactions inherent to large multifunctional viral proteins, many cellular proteins contain Ub interacting motifs (UIMs) that avidly bind Ub. To help overcome these issues, we use expression vectors that enable substrate tagging and biotinylation by the eukaryotic cellular machinery during expression to greatly simplify the purification of ubiquitinated proteins when coupled with streptavidin-conjugated agarose (referred to as V5/affinity purification AP tag). The biotin-streptavidin interaction can withstand high detergent concentrations and stringent wash conditions. Additionally, as Ub ligation is a covalent process, substrates can be heated prior to precipitation without risk of losing the PTM or the need for a second precipitation step. Combined, these conditions (described in 2.2.4) ensure the elimination of binding partners and UIM-containing proteins that may have their own Ub conjugations and therefore mask the true status of the substrate. It is important to note that while Ub conjugation is a covalent linkage, it is reversible by the activity of ubiquitin-specific proteases/DUBs. NEM, a nonreversable DUB active site inhibitor, should be added to lysates to prevent deubiquitination. Cell lysates and IP samples should be kept on ice between steps to minimize protein degradation.
2.2.2. Preparation and IP of endogenous Ub-modified protein precipitates from virus infected cells
Seed 293 cells (1 × 106 cells/well) into 6 well tissue culture dishes one day prior to infection.
Infect cells at a relatively high multiplicity of infection (e.g. 5 focus forming units (FFU)/cell LGTV) to ensure a synchronous infection.
At 24 hours post infection (hpi), gently wash the infected 293 cells twice with DPBS and lyse by adding 1 ml of IP buffer with NEM (10 mM) and protease inhibitor cocktail. Scrape cells into this lysis buffer, transfer to a 1.5 ml centrifuge tube and successively freeze/thaw 3 times.
DNAse treat samples by adding 5 µg DNase I and 100 µl DNAse buffer to sample for 30 min at 37°C to eliminate DNA contamination and centrifuge for 10 min at 16,000 × g in a microcentrifuge to remove cellular debris.
Preclear lysates by adding 25 µl agarose beads (PrecipHen is used specifically for antibodies raised in chickens. For all other antibodies add Protein G or Protein A conjugated agarose beads) with rotation for 3 h at 4°C.
Remove beads by brief centrifugation (1,000 × g) and add 2 µg anti-substrate (NS5) or control antibody to each lysate and rotate for 1 h at 4°C.
Add 25 µl agarose beads again and incubate lysates with rotation for at least 3 h or overnight at 4°C.
Discard lysates following centrifugation (1,000 × g) and wash beads twice with 1 ml IP buffer and twice with RIPA buffer with a brief centrifugation step between washes (30 s at 1,000 × g). Use a narrow gel loading tip to remove the remaining wash buffer from beads.
Elute proteins from beads by incubation at 95°C for 5 min in 25 µl 2X sample buffer.
Visualize immunoprecipitates by western blot using substrate and Ub-specific antibodies.
2.2.3. Application of Ub Co-IP (Figure 2A)
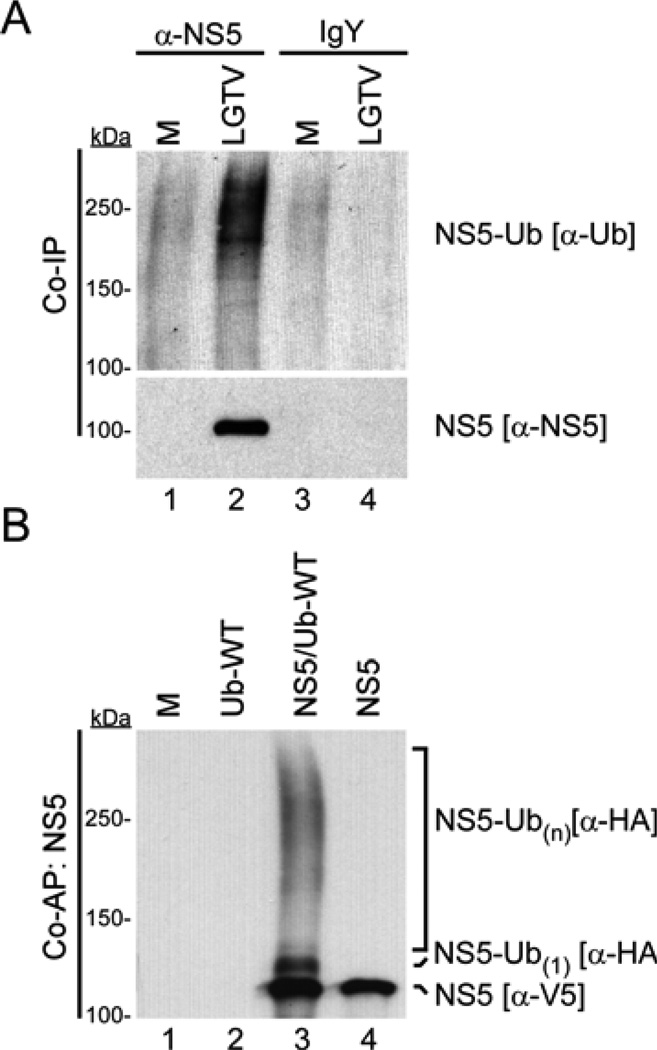
Figure 2.
LGTV NS5 is ubiquitinated during infection and following ectopic expression. (A) IP of NS5 from lysates of 293 cells infected with LGTV (MOI 5 FFU/cell) for 24 h. Total and ubiquitinated NS5 were examined by western blot using NS5 (−NS5) and Ub (−Ub P4D1) antibodies. (B) Ubiquitination assay for total and ubiquitin-modified NS5. NS5 was affinity purified from lysates of 293 cells expressing NS5-V5/AP with or without HA-Ub-WT. Total and ubiquitinated NS5 were examined by western blot using tag-specific antibodies for NS5 (−V5) and Ub (−HA) on the same blot. M, mock infection or transfection.
The protocol described in 2.2.2. was used for IP of NS5 from LGTV infected cells (Figure 2A). Use of a custom prepared chicken NS5 antibody, and not the IgY antibody control, revealed a Ub enriched sample when western blots were probed with −Ub (P4D1) antibody. This protocol is a useful starting point to assess the potential of a given virus protein to be ubiquitinated. However, using this protocol, it is difficult to confidently claim NS5 alone, and not interacting proteins, is the substrate for the Ub observed upon IP. Additionally, while clearly demonstrating high molecular weight NS5-Ub species, NS5 modified by one or a few Ub moieties is difficult to visualize with this antibody. Thus, we performed additional experiments using a more stringent purification protocol, described in 2.2.4, to confirm the ubiquitination of NS5 as well as characterize the type of ubiquitin modification.
2.2.4. Preparation and precipitation of in vivo expressed HA-Ub-modified proteins using streptavidin affinity purification (AP)
Seed 293 cells (1 × 106 cells/well) into 6 well tissue culture dishes one day prior to transfection.
Transfect cells with pcDNA3.2/capTEV-CT-NS5-V5/AP (1 µg/well) and pRK5-HA-Ub plasmid (1 µg/well) using Lipofectamine LTX with Plus Reagent according to manufacturer’s protocol.
At 24 h post transfection, carefully wash the transfected 293 cells twice with DPBS and lyse by adding 1 ml of IP buffer with NEM (10 mM) and protease inhibitor cocktail. Scrape cells into this lysis buffer, transfer to a 1.5 ml centrifuge tube and successively freeze/thaw 3 times.
DNAse treat samples by adding 5 µg DNase I and 100 µl DNAse buffer to sample for 30 min at 37°C to eliminate DNA contamination and centrifuge for 10 min at 16,000 × g in a microcentrifuge to remove cellular debris.
Add 55 µl of 20% SDS (to 1% final concentration) to lysates and heat to 95°C for 5 min.
Preclear samples with 25 µl unconjugated agarose beads with rotation for 3 h at 4°C.
Briefly centrifuge (30 s at 1,000 × g) and transfer lysate to a new tube. Add 25 µl streptavidin-conjugated agarose beads to each lysate and rotate overnight at 4°C.
Wash the streptavidin-conjugated agarose beads twice with 1 ml IP buffer and twice with RIPA buffer, with a brief centrifugation step between washes (1,000 × g).
Elute proteins from beads by incubation at 95°C for 5 min in 25 µl of 2X sample buffer.
Examine Ub-modified proteins by western blotting using substrate (−V5) and Ub (−HA) specific antibodies.
2.2.5. Application of Ub Co-AP (Figure 2B)
LGTV NS5 was expressed and precipitated using the protocol described in 2.2.4 (Figure 2B). This experiment included a mock transfected and a HA-Ub only control, that highlight the low background achieved in this assay, as opposed to standard IP (Figure 2A). Comparison of NS5 expressed with HA-Ub-WT (lane 3) to NS5 alone (lane 4) revealed a band that migrated slightly slower than NS5 (this comparison was made by probing the same blot concurrently with −V5 and −HA antibodies). This band represents NS5 protein modified by a single Ub moiety (monoubiquitination). NS5 modified by more than 1 Ub moiety (either as a polyubiquitin chain or multiubiquitination) is denoted as NS5-Ub(n), where n is greater than 1 Ub molecule. Since this protocol includes high detergent and high temperatures prior to precipitation, the presence of −HA reactive bands on this blot indicate NS5 is specifically ubiquitinated. Thus these data confirm that LGTV NS5 is a ubiquitinated protein.
2.3. Determination of Ub modification type and linkage
2.3.1. General considerations
Once ubiquitination of a given protein is confirmed, it is important to determine the type of Ub modification, as this can influence many different biological outcomes. A two-fold strategy using Ub lysine to arginine (K-R) mutants and chain-specific antibodies are standard approaches that reliably define Ub status on substrate proteins. A well-characterized pair of antibodies, used in the following protocol, differentiate polyubiquitin chains (FK1) and monoubiquination in addition to polyubiquitin chains (FK2)[34]. Commercial antibodies are also becoming available that recognize polymeric Ub in a linkage-specific fashion, for example K48- and K63-linked polyubiquitin specific antibodies [35]. Additionally, as demonstrated in 2.3.4, it may be necessary to inhibit proteasome-dependent degradation to stabilize specific Ub-modified proteins, such as proteins conjugated to K48-linked polyubiquitin chains.
2.3.2. Differentiation of Ub chain linkage using ubiquitin K-R variants
The protocol is modified from the description in 2.2.4. by the transfection of pRK5-Ub K-R mutants and visualization of western blot using Ub type-specific antibodies. The following K-R Ub mutants were used: Ub-K48 (all lysines mutated to arginine except K48; forms only K48-linked chains); Ub-K63 (all lysines mutated to arginine except K63; forms only K63-linked chains); Ub-K0 (all 7 lysines mutated to arginine; can be attached as monoubiquitin but cannot form lysine-linked chains); Ub-K48R (only K48 mutated to arginine; can form all chain types except K48-linked chains).
2.3.3. Application of this technique (Figure 3A)
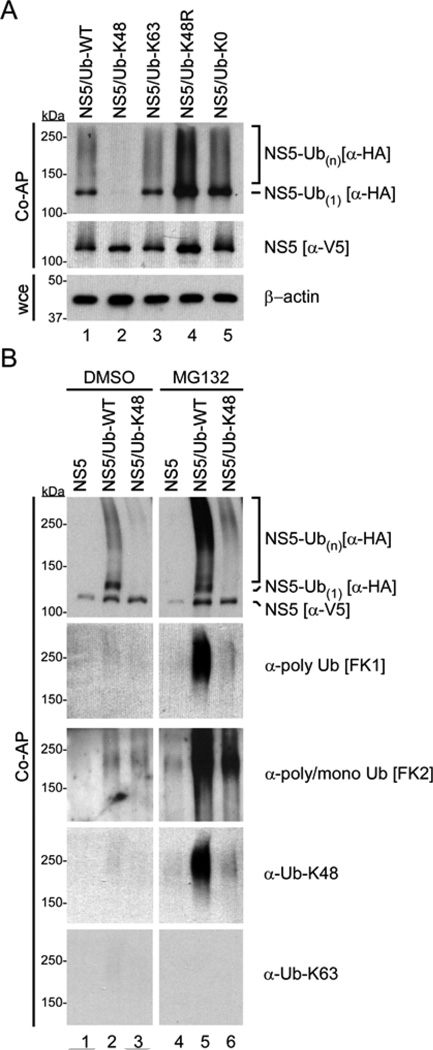
Figure 3.
LGTV NS5 is modified by multiubiquitination and labile K48-linked polyubiquitin chains. (A) Ubiquitination assay for total and Ub-modified NS5. NS5 was affinity purified from lysates of 293 cells expressing NS5-V5/AP with HA-Ub-WT or the indicated HA-Ub mutants. Total and ubiquitinated NS5 were examined by western blot using tag-specific antibodies for NS5 (−V5) and Ub (−HA). Whole cell extracts (wce) were probed with −actin antibody. (B) NS5 was affinity purified from lysates of 293 cells expressing NS5-V5/AP, with or without HA-Ub-WT or HA-Ub-K48 and treated with DMSO or MG132 (10 M) for 4 h prior to harvest of cell lysates. Total and ubiquitinated NS5 were examined by western blot using tag-specific antibodies for NS5 (−V5) and Ub (−HA), as well as antibodies specific for polyubiquitin (FK1), poly/monoubiquitin (FK2), K48-polyubiquitin or K63 polyubiquitin.
After confirming that LGTV NS5 is ubiquitinated, we next determined the type of Ub modification by co-expressing NS5 with mutant Ub proteins and comparing the Ub profile to NS5-Ub-WT (Figure 3A, lane 1). Expression of Ub-K48 (lane 2) and Ub-K63 (lane 3) resulted in different Ub profiles; NS5-Ub-K48 was not detected here, although NS5-Ub-K63 was similar to NS5-Ub-WT. Given that K48-linked chains regulate protein degradation through the proteasome, the lack of NS5-Ub-K48 could be a result of enhanced turnover, and therefore not be detectable in this experiment. Expression of Ub-K48R prevents only K48-linked chain formation and therefore may stabilize NS5-Ub that is normally degraded by this mechanism. Consistent with this possibility, Ub-K48R (lane 4) expression enhanced NS5 ubiquitination above Ub-WT and the other Ub mutant proteins. Taken together, these results suggest that NS5 is conjugated to K48-linked Ub chains.
The similar profile of NS5-Ub-WT and NS5-Ub-K63 might suggest that NS5 is also conjugated to K63-linked Ub chains. However, since all K-R Ub mutant proteins can be conjugated to a substrate in a monoubiquitin form, it is possible that the high molecular mass smear observed in these two samples resulted from multiple Ub moieties conjugated in a monoubiquitin fashion (multiubiquitation). Hence, we next expressed Ub-K0 that can form monoubiquitin but not lysine-dependent polyubiquitin chains. This resulted in enhanced levels of NS5-Ub (lane 5) suggesting that modification of NS5 by Ub does not require lysine-linked Ub chains and that the predominant visible modification is likely multiubiquitination. Without the inclusion of Ub-K0 and Ub-K48R, these data might be interpreted differently (i.e. the presence of K63-linked Ub chains).
2.3.4. Visualizing labile Ub forms using specific antibodies coupled with proteasome inhibition
Expression of Ub species that direct targeted proteins for degradation by either proteasome or lysosome may require stabilization to be observed by western blotting. As observed in Figure 3A, Ub-K48 expression may enrich for proteins targeted by K48-linked chains and therefore accelerate degradation. Common inhibitors of the proteasome include MG132 (0.1–10 µM) and lactacystin (0.1–1 µM). To include proteasome inhibition in a ubiquitination assay, incubate transfected cells for 4–8 h with either carrier control (DMSO) or proteasome inhibitor supplemented media prior to lysis. HA-Ub modified proteins are then prepared and visualized as previously described in 2.2.4.
2.3.5. Application of proteasome inhibition (Figure 3B)
Using the procedure described in 2.3.4., we found that MG132 treatment resulted in enhanced detection of both NS5-Ub-WT (Figure 3B, compare lanes 2 and 5) and NS5-Ub-K48 (compare lanes 3 and 6), although the latter was not recovered to NS5-Ub-WT levels. Increased abundance of NS5-Ub with MG132 treatment suggests that some NS5-Ub is processed by the proteasome and that proteasome inhibition stabilizes this species of NS5. This data is supported by probing the blots with Ub-type specific antibodies. Ub chains on NS5 were detected only after proteasome inhibition using the polyubiquitin-specific FK1 and K48 polyubiquitin chain specific antibodies (lanes 5 and 6). K63 polyubiquitin chain specific antibody did not noticeably detect NS5-Ub species. In contrast, multiubiquitin forms of NS5 were detectable without proteasome inhibition by probing the blots with the poly/monoubiquitin-specific FK2 antibody. Taken together, the app roaches using Ub mutants, linkage-specific antibodies, and proteasome inhibition revealed NS5 to be modified by multiubiquitination and labile K48-linked polyubiquitin chains.
3. Concluding remarks
Using the protocols described here, we demonstrated that NS5 from LGTV was modified by at least two distinct ubiquitination events. Multiubiquitination (enriched by Ub-K0 expression) was observed without proteasome inhibition and did not markedly affect NS5 stability, consistent with the characteristic role of single/multiple non-linked Ub moieties on substrate proteins, often regulating protein function, subcellular distribution or protein-protein interactions. The presence of a labile K48-linked Ub chain (enriched by Ub-K48 expression in conjunction with MG132), potentially reveals an unexplored cellular antiviral response, as cellular E3 Ub ligases would be required to modify and direct NS5 for degradation by the proteasome, acting as potential viral restriction factors. Alternatively, selective degradation of specific populations of NS5 may be a necessary part of the viral replication cycle. However, experiments used to define NS5 modifications in the absence of other viral proteins now need to be confirmed during a productive viral infection, as the entire compliment of viral proteins may have additional interactions with NS5 or host proteins that alter NS5 PTM.
Identification of residues critical for Ub ligation is an important step in understanding the impact of specific ubiquitination events in virus replication and pathogenesis. The mapping of residues within a target protein that are key acceptors for Ub can be attempted by mutagenesis of lysine residues to arginine. In our case, NS5 has a combination of 50 lysine residues and multiubiquitination, suggesting that a mutagenesis approach might be quite difficult. Therefore, mass spectrometry can be used to identify lysines within substrate proteins that are bound to Ub [36,37]. Once individual lysine residues are identified, point mutations within substrates can be engineered to define the effect of ubiquitination on viral protein function. Of relevance to virology, key K-R mutations could also be incorporated into recombinant viruses to examine the role of individual ubiquitination events in virus replication and pathogenesis. These types of analyses can be combined with an exciting application, Ub-mediated fluorescence complementation analysis. This method enables examination of specific Ub-modified substrate populations at the cellular level using immunofluorescence and confocal microscopy [38]. The combination of these studies will aid in the identification of virus-host interactions that could be targeted by novel therapeutics to accelerate viral protein degradation or otherwise disrupt virus replication.
Taylor and Best Research Highlights.
Viruses use ubiquitination to facilitate replication and evade innate immunity
We describe a protocol to detect and characterize ubiquitinated viral proteins
Streptavidin-based precipitations eliminate common pitfalls of ubiquitin assays
Flavivirus NS5 is a multi-functional protein known to manipulate cell processes
NS5 is modified by multiubiquitination and K48-linked polyubiquitin chains
Acknowledgements
We thank Dr. Heinz Feldmann, Dr. Shelly Robertson and Kirk Lubick for critical reviews of the manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID).
Abbreviations
- AP
affinity purification
- R
arginine
- DUB
deubiquitinase
- FFU
focus forming units
- LGTV
Langat virus
- IP
immunoprecipitation
- IFN
interferon
- K
lysine
- NEM
N-ethylmaleimide
- NS5
nonstructural protein 5
- PTM
post-translational modification
- RIPA
radioimmunoprecipitation assay
- TBEV
tick-borne encephalitis virus
- Ub
ubiquitin
- UIM
ubiquitin interacting motif
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicting interests.
References
- 1.Komander D. The emerging complexity of protein ubiquitination. Biochem. Soc. Trans. 2009;37:937. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- 2.Liu YC, Penninger J, Karin M. Immunity by ubiquitylation: a reversible process of modification. Nat. Rev. Immunol. 2005;5:941–952. doi: 10.1038/nri1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nature. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 5.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 6.Liao T-L, Wu C-Y, Su W-C, Jeng K-S, Lai MMC. Ubiquitination and deubiquitination of NP protein regulates influenza A virus RNA replication. EMBO J. 2010;29:3879–3890. doi: 10.1038/emboj.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farris KD, Fasina O, Sukhu L, Li L, Pintel DJ. Adeno-associated virus small rep proteins are modified with at least two types of polyubiquitination. J. Virol. 2010;84:1206–1211. doi: 10.1128/JVI.01660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peloponese J-M, Iha H, Yedavalli VRK, Miyazato A, Li Y, Haller K, et al. Ubiquitination of human T-cell leukemia virus type 1 tax modulates its activity. J. Virol. 2004;78:11686–11695. doi: 10.1128/JVI.78.21.11686-11695.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Si X, Gao G, Wong J, Wang Y, Zhang J, Luo H. Ubiquitination is required for effective replication of coxsackievirus B3. PLoS One. 2008;3:e2585. doi: 10.1371/journal.pone.0002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YE, Park A, Lake M, Pentecost M, Torres B, Yun TE, et al. Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding. PLoS Pathog. 2010;6:e1001186. doi: 10.1371/journal.ppat.1001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patnaik A, Chau V, Wills JW. Ubiquitin is part of the retrovirus budding machinery. Proc. Natl. Acad. Sci. USA. 2000;97:13069–13074. doi: 10.1073/pnas.97.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacson MK, Ploegh HL. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe. 2009;5:559–570. doi: 10.1016/j.chom.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nisole SEB, Stoye JP, b ASI. TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Micro. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- 14.Randow F, Lehner PJ. Viral avoidance and exploitation of the ubiquitin system. Nat. Cell. Biol. 2009;11:527–534. doi: 10.1038/ncb0509-527. [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan K, Früh K, DeFilippis V. Viral hijacking of the host ubiquitin system to evade interferon responses. Curr. Opin. Microbiol. 2010;13:517–523. doi: 10.1016/j.mib.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breitschopf K, Bengal E, Ziv T, Admon A, Ciechanover A. A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 1998;17:5964–5973. doi: 10.1093/emboj/17.20.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandimarti R, Roizman B. Us9, a stable lysine-less herpes simplex virus 1 protein, is ubiquitinated before packaging into virions and associates with proteasomes. Proc. Natl. Acad. Sci. USA. 1997;94:13973–13978. doi: 10.1073/pnas.94.25.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokarev AA, Munguia J, Guatelli JC. Serine-Threonine Ubiquitination Mediates Downregulation of BST-2/Tetherin and Relief of Restricted Virion Release by HIV-1 Vpu. J. Virol. 2010;85:51–63. doi: 10.1128/JVI.01795-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Herr RA, Chua W-J, Lybarger L, Wiertz EJHJ, Hansen TH. Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3. J. Cell Biol. 2007;177:613–624. doi: 10.1083/jcb.200611063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda F, Crosetto N, Dikic I. What determines the specificity and outcomes of ubiquitin signaling? Cell. 2010;143:677–681. doi: 10.1016/j.cell.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 24.Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu. Rev. Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Chen ZJ. Expanding role of ubiquitination in NF-κB signaling. Cell Res. 2011;21:6–21. doi: 10.1038/cr.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicke L. Protein regulation by monoubiquitin. Nature. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 27.Davidson A. Chapter 2. New insights into flavivirus nonstructural protein 5. Adv. Virus Res. 2009;74:41–101. doi: 10.1016/S0065-3527(09)74002-3. [DOI] [PubMed] [Google Scholar]
- 28.Best SM, Morris KL, Shannon JG, Robertson SJ, Mitzel DN, Park GS, et al. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 2005;79:12828–12839. doi: 10.1128/JVI.79.20.12828-12839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor RT, Lubick KJ, Robertson SJ, Broughton JP, Bloom ME, Bresnahan WA, et al. TRIM79α, a novel interferon stimulated gene, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. 2011 doi: 10.1016/j.chom.2011.08.004. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashour J, Laurent-Rolle M, Shi P, Garcia-Sastre A. NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 2009;83:5408–5418. doi: 10.1128/JVI.02188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim K, Chew K, Tan J, Wang C, Chung K, Zhang Y, et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J. Neurosci. 2005;25:2002–2009. doi: 10.1523/JNEUROSCI.4474-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitzel DN, Best SM, Masnick MF, Porcella SF, Wolfinbarger JB, Bloom ME. Identification of genetic determinants of a tick-borne flavivirus associated with host-specific adaptation and pathogenicity. Virology. 2008;381:268–276. doi: 10.1016/j.virol.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belz T, Pham A, Beisel C, Anders N, Bogin J, Kwozynski S, et al. In vitro assays to study protein ubiquitination in transcription. Methods. 2002:1–12. doi: 10.1016/S1046-2023(02)00027-0. [DOI] [PubMed] [Google Scholar]
- 34.Fugimuro M, Yokosawa H. Production of Antipolyubiquitin Monoclonal Antibodies and Their Use for Characterization and Isolation of Polyubiquitinated Proteins. Meth. Enzymol. 2005;399:75–86. doi: 10.1016/S0076-6879(05)99006-X. [DOI] [PubMed] [Google Scholar]
- 35.Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 36.Mollah S, Wertz IE, Phung Q, Arnott D, Dixit VM, Lill JR. Targeted mass spectrometric strategy for global mapping of ubiquitination on proteins. Rapid Commun. Mass Spectrom. 2007;21:3357–3364. doi: 10.1002/rcm.3227. [DOI] [PubMed] [Google Scholar]
- 37.Xu G, Paige JS, Jaffrey SR. Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat. Biotech. 2010;28:868–873. doi: 10.1038/nbt.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang D, Kerppola T. Ubiquitin-mediated fluorescence complementation reveals that Jun ubiquitinated by Itch/AIP4 is localized to lysosomes. Proc. Natl. Acad. Sci. USA. 2004:14782–14787. doi: 10.1073/pnas.0404445101. [DOI] [PMC free article] [PubMed] [Google Scholar]