Abstract
Oxidative stress is well known to lead to vascular dysfunction. Thioredoxin reductase (TrxR) catalyzes the reduction of oxidized thioredoxin (Trx). Reduced Trx plays a role in cellular antioxidative defense as well as in decreasing S-nitrosylation. It is not known whether TrxR affects vascular reactivity. We hypothesized that TrxR inhibition decreases vascular relaxation via increased oxidative stress and S-nitrosylation. Aortic rings from C57BL/6 mice were treated with the TrxR inhibitor, 1-chloro-2,4-dinitrobenzene (DNCB) or auranofin for 30 min. Vascular relaxation to acetylcholine (ACh) was measured in the rings contracted with phenylephrine. DNCB and auranofin reduced relaxation compared to vehicle (vehicle Emax = 71 ± 3 %, DNCB Emax = 53 ± 3 %; p<0.05). The antioxidants, apocynin (NADPH oxidase inhibitor) and tempol (superoxide dismutase mimetic) normalized impaired relaxation by DNCB in aorta (DNCB Emax = 53 ± 3 %, DNCB+tempol Emax = 66 ± 3 %; p<0.05). In addition, DNCB reduced sodium nitroprusside (SNP)-induced relaxation. DNCB increased soluble guanylyl cyclase (sGC) S-nitrosylation and decreased sGC activity. These data suggest that TrxR regulates vascular relaxation via antioxidant defense and sGC S-nitrosylation. TrxR may be an enzyme to approach for treatment of vascular dysfunction and arterial hypertension.
Keywords: vascular relaxation, reactive oxygen species, soluble guanylyl cyclase
INTRODUCTION
Reactive oxygen species (ROS) are small molecules derived from molecular oxygen and are known to play an important role in vascular function. ROS are produced in all vascular cell types, including endothelial cells, smooth muscle cells, and adventitial fibroblasts.1, 2 Under physiological conditions, the generation of ROS in vascular cells and redox-dependent signaling are tightly regulated; however, in pathological conditions, such as cardiovascular diseases, ROS production is increased. Numerous studies indicate that ROS are associated with various mechanisms related to impair vascular function, such as endothelial dysfunction, vascular inflammation, vascular smooth muscle cell growth, and apoptosis.3, 4
An imbalance between ROS generation and antioxidant defense mechanisms results in oxidative stress. Several enzymes and enzyme systems, such as NADPH oxidase, xanthine oxidase, mitochondria, and uncoupled endothelial nitric oxide synthase (eNOS), produce ROS in the vessels. ROS are scavenged and inactivated by several antioxidant mechanisms, such as superoxide dismutase, catalase, glutathione peroxidase, and the thioredoxin (Trx) system. The Trx system plays a role in the degradation of ROS and other diverse cellular functions.5 However, the role of the Trx system in the vasculature is unclear and is the focus of this study.
The Trx system consists of Trx and Trx reductase (TrxR). TrxR is a redox-based enzyme and catalyzes the NADPH-dependent reduction of the oxidized redox protein Trx. Mammalian TrxR can reduce several substrates, such as glutaredoxin and glutathione,6, 7 but reduces mainly Trx. As an antioxidant, TrxR reduces hydrogen peroxide (H2O2) and lipid hydroperoxides.8, 9 And also, TrxR regenerates other small antioxidant molecules, such as vitamin C and vitamin E.10, 11 In addition to an antioxidant mechanism, the reduced Trx by TrxR functions in cell growth by inhibiting apoptosis, and gene transcription.5 Additionally, a newly defined function of TrxR is denitrosylation, which is degradation of protein S-nitrosylation in the cell.12 S-Nitrosylation is a redox-based and post-translational modification of a cysteine residue on a target protein by nitric oxide (NO) to form S-nitrosothiol (SNO).13
NO is a key regulator of vascular tone, which is determined by the balance of vasodilator and vasoconstrictor stimuli. In the vasculature during relaxation, NO is released from endothelial cells and stimulates endothelium-dependent vasodilation via diffusion into the smooth muscle. NO activates soluble guanylyl cyclase (sGC) to form cyclic 3′,5′-guanosine monophosphate (cGMP) from guanosine triphosphate (GTP) and cGMP results in vascular relaxation via decreased cytosolic calcium levels, protein phosphorylation, and myosin light chain dephosphorylation.14
In this study, the hypothesis was that TrxR inhibition attenuates vascular relaxation via increased oxidative stress and S-nitrosylation. In order to test this hypothesis, we examined whether TrxR plays a role in vascular reactivity and signaling by investigating vascular relaxation in mouse aorta in the presence or absence of a TrxR inhibitor.
MATERIALS AND METHODS
Drugs and reagents
1-Chloro-2,4-dinitrobenzene (DNCB) and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Auranofin was obtained from BioMol (Plymouth Meeting, PA).
Animals
Male, 12-14 weeks-old, C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were used in this study. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Institutional Animal Care and Use Committee (IACUC) and Georgia Health Sciences University Committee on the Use of Animals in Research and Education. The animals were housed on a 12-hour light/dark cycle and fed a standard chow diet with water ad libitum.
Isolation of aortic rings and functional studies
After mice were euthanized using carbon dioxide (CO2), thoracic aortas were excised, cleaned from fat tissue and cut into 2 mm-length rings in an ice-cold physiological salt solution consisting of the following: 130 mmol/L NaCl, 4.7 mmol/L KCl, 1.18 mmol/L KH2PO4, 1.18 mmol/L MgSO4·7H2O, 1.56 mmol/L CaCl2·2H2O, 14.9 mmol/L NaHCO3, 5.6 mmol/L glucose, and 0.03 mmol/L EDTA. Aortic rings were mounted in a myograph (Danish Myo Technology A/S, Aarhus, Denmark) containing warmed (37°C), oxygenated (95% O2/5% CO2) physiological salt solution. The preparations were equilibrated for at least 1 hour under a passive force of 5 mN. After the equilibration period, arterial integrity was assessed by stimulation of vessels with 120 mmol/L KCl and, after contraction reached a plateau, the rings were washed. Subsequently, the rings were stimulated with phenylephrine (PE, 0.1 μmol/L) followed by relaxation with acetylcholine (ACh, 1 μmol/L), which was used as an evidence of an intact endothelium. Some aortic rings were incubated with DNCB (4 μmol/L) or auranofin (1 μmol/L) for 30 min, apocynin (100 μmol/L) for 40 min, or tempol (1 mmol/L) for 20 min. Endothelium-dependent relaxation was performed on PE-contracted rings followed by cumulative concentration-response curves to ACh (10−9 to 3×10−5 mol/L), and endothelium-independent relaxation was tested with sodium nitroprusside (SNP; 10−11 to 10−6 mol/L) and BAY41-2272, a sGC activator (10−9 to 3×10−6 mol/L).
Extracellular H2O2 assay
H2O2 production was measured using an Amplex red H2O2 assay kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol.15 Briefly, cleaned aorta were equilibrated for 1 h at 37 °C in a modified Krebs-HEPES buffer (containing 20 mmol/L HEPES, 119 mmol/L NaCl, 4.6 mmol/L KCl, 1.0 mmol/L MgSO4·7H2O, 0.15 mmol/L Na2HPO4, 0.4 mmol/L KH2PO4, 5 mmol/L NaHCO3, 1.2 mmol/L CaCl2, and 5.5 mmol/L dextrose, pH 7.4) and then incubated with Amplex red working solution (100 μmol/L) at 37°C for 1 h. The supernatant was then transferred to a 96-well plate, and absorbance was measured (562 nm) on a μQuant plate reader (Bio-Tek Instruments, Inc., Winooski, VT). A H2O2 standard curve constructed on the same 96-well plate was incubated with Amplex red working solution (100 μmol/L) at 37°C at the same time as the tissues and was used to determine H2O2 concentrations from samples. After each experiment, tissue total protein was determined with the BCA protein assay and used for normalization.
Detection of S-nitrosylation
Aortas were treated with vehicle (ethanol) or DNCB (4 μmol/L) for 1 h at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM). The biotin-switch method to detect S-nitrosylation was performed with a S-nitrosylated protein detection assay kit (Cayman Chemical, Ann Arbor, MI) modified from the method of Jaffrey et al.16 Biotin-labeled proteins were used for western blot analysis or pulled down by streptavidin-agarose beads (Thermo Scientific, Rockford, IL) to specifically detect sGC S-nitrosylation, followed by western blot analysis. Ten percent of biotin-labeled proteins were used to measure total sGC protein expression.
Western blot analysis
After precipitation with streptavidin, proteins were eluted by boiling in Laemmli sample buffer (Bio-Rad, Hercules, CA). The eluted samples or biotin-labeled proteins were separated by electrophoresis on a 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membranes were blocked with 5% skim milk in Tris-buffered saline solution with 0.1% Tween-20 for 1 h at room-temperature. Membranes were then incubated with anti-sGCβ1 primary antibody (Cayman Chemical), anti-phospho-eNOS (Ser 1177), or anti-eNOS antibody (Cell Signaling Technology, Inc., Danvers, MA) overnight at 4°C. After incubation with secondary antibody, signals were developed with chemiluminescence, visualized by autoradiography, and quantified densitometrically.
cGMP assay
Cleaned aortas were incubated with vehicle (ethanol) or DNCB (4 μmol/L) for 1 h at 37 °C in DMEM and then treated with SNP (3×10−8 mol/L) for 20 min. cGMP was measured using a commercially available cGMP enzyme-linked immunoassay (cGMP EIA kit) according to the manufacturer’s instructions (Cayman Chemical, Ann arbor, MI). The measured cGMP level was normalized by protein concentration using BCA protein assay.
Statistical analysis
Values are means ± standard error of the mean (SEM), and n represents the number of animals used in the experiments. Experimental values were calculated relative to the maximal changes from the contraction produced by PE in each segment, which was taken as 0%. The baseline tension before addition of PE was considered as 100%. Concentration-response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 4.0; GraphPad Software, San Diego, CA), and two pharmacological parameters were obtained: the maximal effect generated by the agonist (or Emax) and EC50 (molar concentration of agonist producing 50% of the maximum response) or pD2 (−log EC50). Statistical differences were calculated by Student’s t-test or one-way ANOVA. A P value less than 0.05 was considered to be statistically significant.
RESULTS
Effect of TrxR inhibition on endothelium-dependent relaxation
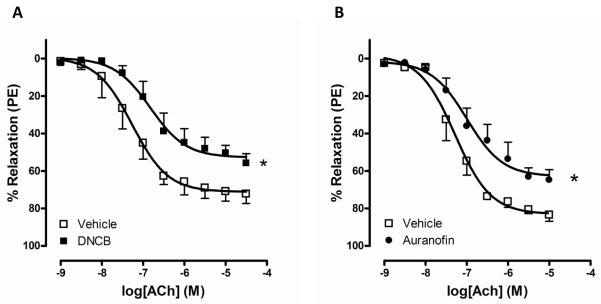
To test the hypothesis that inhibition of TrxR decreases vascular relaxation in mouse aorta, we first determined ACh-induced relaxation in the presence of the TrxR inhibitor, DNCB or auranofin.12, 17 Aortic rings incubated with a TrxR inhibitor exhibited significantly attenuated relaxation to ACh compared to the vehicle control group (Figure 1; Table 1).
Figure 1.
Vascular relaxation to ACh is attenuated by DNCB and auranofin. Concentration-dependent responses to ACh were measured after incubation with 4 μmol/L DNCB (A; vehicle n=6, DNCB n=5) or 1 μmol/L auranofin (B; vehicle n=5, auranofin n=6) for 30 min in mouse aorta. Relaxation responses were calculated relative to the maximal PE contraction, which was taken as 100%. Results are presented as mean ± SEM in each experimental group (n=5 to 6). *p<0.001 compared with vehicle.
Table 1.
Emax and pD2 values for ACh, SNP and BAY41-2272-induced responses in aortic rings
| Vehicle |
DNCB |
|||
|---|---|---|---|---|
| Agonist | Emax | pD2 | Emax | pD2 |
| ACh | 71±3 | 7.3±0.2 | 53±3* | 6.8±0.2 |
| ACh + Apocynin | 71±2 | 6.7±0.1** | 71±4† | 6.7±0.1** |
| ACh + Tempol | 68±3 | 7.4±0.1 | 69±3† | 7.4±0.1 |
| SNP | 98±2 | 9.0±0.1 | 95±2 | 8.7±0.1* |
| BAY41-2272 | 102±1 | 6.7±0.1 | 98±2 | 6.4±0.1* |
Values are mean ± SEM for experiments in each group (n=5 to 7). Relaxation induced by ACh, SNP and BAY41-2272 was calculated relative to the maximal changes from the contraction produced by PE and is represented as percentage of relaxation.
p<0.05 vs vehicle in same agonist;
p<0.05 vs ACh-induced response in DNCB;
p<0.05 vs ACh-induced response in vehicle.
TrxR inhibition and ROS
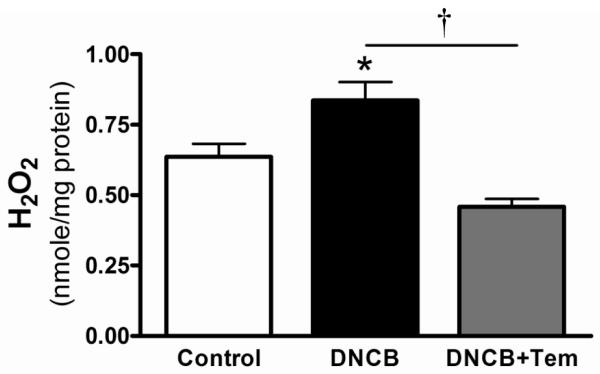
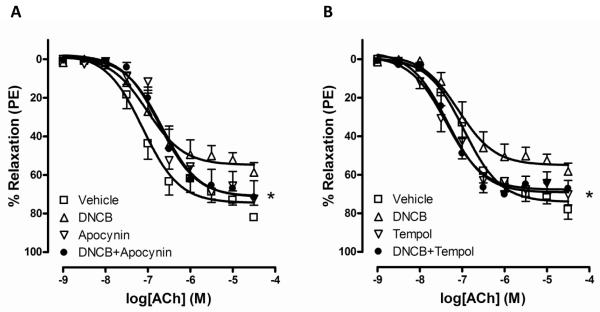
One of the functions of TrxR is to reduce ROS; therefore we measured whether TrxR inhibition increases ROS in mouse aorta. Figure 2 shows that DNCB increased H2O2 production from aorta. Tempol is used for an antioxidant and tempol treatment reduced H2O2 production increased by DNCB. To test whether increased ROS by TrxR inhibition contributes to impaired relaxation to ACh, we used two antioxidants, a NADPH oxidase inhibitor (apocynin) and a superoxide dismutase mimetic (tempol), to attenuate ROS concentration. These antioxidants normalized ACh-induced relaxation decreased by DNCB (Figure 3; Table 1).
Figure 2.
DNCB increased H2O2 levels in mouse aorta. H2O2 concentration was measured in the presence or absence of DNCB (4 μmol/L, 1 h) or/and tempol (Tem; 1 mmol/L, 1 h) in mouse aorta using Amplex red as described in the Methods. Results are presented as mean ± SEM for n=3 in control and n=4 in DNCB group. *p<0.05 compared with control. †p<0.05 DNCB vs. DNCB+Tem.
Figure 3.
Antioxidants normalized impaired relaxation by DNCB. Concentration-dependent relaxations to ACh were measured after incubation of aortic rings with A: vehicle, DNCB (4 μmol/L, 30 min), apocynin (100 μmol/L, 40 min), and DNCB plus apocynin. B: vehicle, DNCB (4 μmol/L, 30 min), tempol (1 mmol/L, 20 min), and DNCB plus tempol. Relaxation responses were calculated relative to the maximal PE contraction, which was taken as 100%. Results are presented as mean ± SEM in each experimental group (n=6). *p<0.05 DNCB vs. DNCB plus apocynin (A) or DNCB plus tempol (B).
Effect of TrxR inhibition on endothelium-independent relaxation
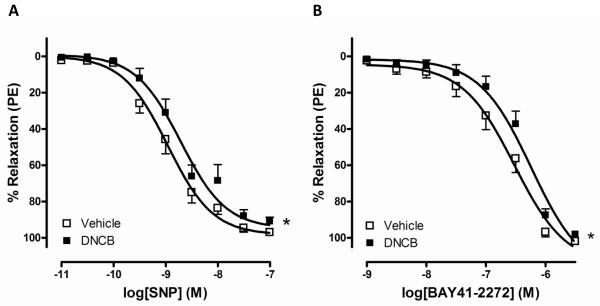
To consider whether TrxR inhibition contributes to relaxation via an endothelium-independent mechanism, we measured relaxation to SNP diffused directly into smooth muscle. DNCB shifted the concentration-response relaxation to SNP to the right in mouse aorta (Figure 4A; Table 1). To further test relaxation to NO downstream, we investigated a sGC activator (BAY41-2272)-induced relaxation responses. DNCB shifted the relaxation curves to BAY41-2272 to the right compared to vehicle (Figure 4B; Table 1).
Figure 4.
DNCB shifted vascular relaxation to SNP (A) or BAY41-2272 (B) to the right. Concentration-dependent relaxations to SNP or BAY41-2272 were measured after incubation with DNCB (4 μmol/L, 30 min) in aortic rings. Relaxation responses were calculated relative to the maximal PE contraction, which was taken as 100%. Results are presented as mean ± SEM in each experimental group (A; vehicle n=7, DNCB n=6, B; n=6). *p<0.05 compared with vehicle.
TrxR and S-nitrosylation
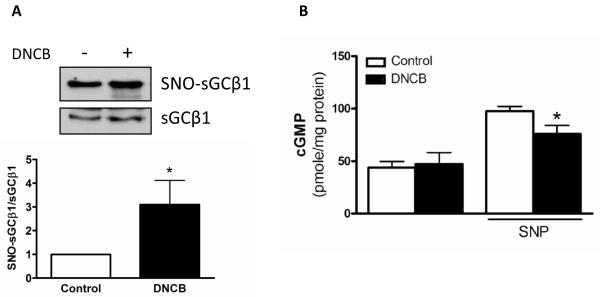
In addition to its antioxidant effect, a denitrosylation function of TrxR was confirmed via measurement of S-nitrosylation levels in the previous study.18 Since using a TrxR inhibitor DNCB, protein S-nitrosylation is stabilized, we determined whether DNCB affects sGC S-nitrosylation levels related to relaxation signaling protein in mouse aorta. One of the sGC subunits, β1 showed increased S-nitrosylation modification in the presence of DNCB compared to control without DNCB (Figure 5A). cGMP, which is downstream of sGC, was measured in mouse aorta, and SNP-stimulated cGMP levels were reduced by DNCB, but not basal levels (Figure 5B).
Figure 5.
sGC S-nitrosylation was increased and its activity was decreased by DNCB. A: sGCβ1 S-nitrosylation (SNO-sGCβ1) was detected using the biotin-switch method, streptavidin-precipitation, and western blot analysis with anti-sGCβ1 antibody. Total sGCβ1 expression (sGCβ1) was detected with biotin-labeled proteins using western blot analysis. Representative western blot images (Top). Bar graphs showing the relative expression of SNO-sGCβ1 after normalization to sGCβ1 (Bottom). *p<0.05 compared to control (n=5). B: cGMP levels were measured in mouse aorta to determine sGC activity. Aorta was incubated with DNCB (4 μmol/L, 1 h) in the presence or absence of SNP (3×10−8 mol/L, 20 min). Results are presented as mean ± SEM in each experimental group. *p<0.05 compared to control with SNP (n=4 to 6).
DISCUSSION
The main finding of the present study is that the inhibition of TrxR leads to impairment in aortic relaxation, which is associated with increased H2O2 and sGC S-nitrosylation. TrxR is an important antioxidant enzyme and also has other cellular functions; however, the relationship between TrxR and vascular reactivity has not been elucidated. In the present study, we investigated whether TrxR plays a role in vascular relaxation.
Several studies show that oxidative stress in cardiovascular disease contributes to endothelial dysfunction,19-23 which refers to impaired endothelium-dependent vascular relaxation. Increased ROS production scavenges NO and generates oxidants, resulting in loss of NO bioactivity. TrxR is an antioxidant enzyme that scavenges ROS, and this function was confirmed (Figure 2). ROS concentration is increased by TrxR inhibition, and thus, NO may be reduced rapidly by ROS. Additionally, since increased ROS impairs vascular reactivity, reducing ROS levels by treatment with apocynin and tempol normalized the decreased endothelium-dependent relaxation observed with inhibition of TrxR. These data indicate that TrxR regulates vascular relaxation via an antioxidant function. Apocynin is widely used as an inhibitor of NADPH oxidase, but Heumuller at al.24 showed that apocynin is an antioxidant not just a blocker of NADPH oxidase. Ray et al.25 recently published that endothelial NADPH oxidase-4 enhances vasodilation, which contrasts with reports for NADPH oxidase-1 and -2 and in Figure 3, apocynin shifted ACh-induced relaxation to the right compared to control. Since apocynin itself has antioxidant activity and each NADPH oxidase differently works in the vasculature, effect of apocynin needs to further investigate in vascular function.
ACh acts as a vasodilator via NO production, which is induced by activation of the ACh receptor and subsequently eNOS in endothelial cells. NO activates sGC in vascular smooth muscle to produce cGMP, and then, cGMP plays a critical role to lead to smooth muscle relaxation.26 In Figure 1A and 4A, a TrxR inhibitor (DNCB) impaired vascular relaxation to ACh and SNP. These data suggest that some factors in vascular smooth muscle have important function related to TrxR. We propose that one of these factors may be sGC. Accordingly, TrxR is associated with sGC signaling in the relaxation of mouse aorta, as was demonstrated by reduced relaxation in DNCB-treated rings stimulated with the sGC activator (BAY41-2272). In addition to its antioxidant effect, TrxR is an enzyme that decreases protein S-nitrosylation.12 Studies show that sGC is desensitized and inhibited by increasing S-nitrosylation.27, 28 We confirmed this effect using the biotin-switch method and a cGMP assay in the presence of DNCB instead of using S-nitrosocysteine (NO donor). As shown in Figure 5, sGCβ1 S-nitrosylation was increased and sGC activity (cGMP levels) was reduced in the presence of DNCB, suggesting that vascular relaxation was attenuated by TrxR inhibition through sGC S-nitrosylation. In addition to ROS function in the vasculature, this finding provides a new concept that TrxR affects cellular signaling in aorta via sGC S-nitrosylation.
In vascular smooth muscle cell, NO functions in relaxation signaling to form cGMP in cGMP-dependent signaling29 as well as to induce various signaling in cGMP-independent mechanisms. Protein S-nitrosylation could be one of the cGMP-independent actions by NO to modify the target protein.30 The cGMP-independent mechanisms involving S-nitrosylation remains to be further investigated in the vasculature. Since NO and cGMP play an important role in transduction of signaling, the present study suggests that it is possible to manage vascular signaling with sGC via both cGMP-dependent and -independent mechanisms.
It is known that S-nitrosylation modifies several target proteins including channels, transporters, kinases, signaling proteins, and transcription factors.31 One of the important proteins in vascular relaxation signaling, eNOS is modified by S-nitrosylation and its activity is inhibited.32 In our results, eNOS phosphorylation at serine 1177, which represents eNOS activation, and S-nitrosylation was not different between the control and DNCB treatment groups (data not shown). These data suggest that while TrxR inhibition using DNCB stabilizes S-nitrosylation, this is not sufficient to increase eNOS S-nitrosylation and inactivate eNOS. On the other hand, we showed that endothelium-dependent relaxation was impaired by DNCB, and thus, we indicate that these data were due to reduced NO bioavailability by increased oxidative stress. In addition, decreased sGC activity could affect these impaired relaxation responses.
Furthermore, we tested concentration-dependent contractile responses to PE in the presence or absence of DNCB, a TrxR inhibitor, but DNCB did not change contraction to PE in mouse aorta (data not shown). Thus, we investigated relaxation using aortic rings contracted by PE in this study. In a previous study, we observed that protein kinase C-mediated contraction was slightly attenuated by DNCB.18 Therefore, we speculate that TrxR effect on S-nitrosylation is associated with several vascular signaling processes, and further study will be necessary to fully understand the mechanism of TrxR and vascular reactivity.
In summary, the present study shows that a TrxR inhibitor impaired both endothelium-dependent and -independent vascular relaxation. These impaired relaxations by the TrxR inhibitor correlate with increased oxidative stress and sGC S-nitrosylation and inactivation. Therefore, TrxR modulates relaxation in mouse aorta by an antioxidant function and a denitrosylation mechanism.
Acknowledgment
This study was supported by grants from the National Institutes of Health (R01HL071138 and R01DK083685).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Grech ED, Dodd NJ, Jackson MJ, Morrison WL, Faragher EB, Ramsdale DR. Evidence for free radical generation after primary percutaneous transluminal coronary angioplasty recanalization in acute myocardial infarction. Am J Cardiol. 1996 January 15;77(2):122–127. doi: 10.1016/s0002-9149(96)90580-9. [DOI] [PubMed] [Google Scholar]
- (2).White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, Gianturco SH, Gore J, Freeman BA. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci U S A. 1994 February 1;91(3):1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005 October;289(4):R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- (4).Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007 May;292(5):H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- (5).Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000 February 15;346(Pt 1):1–8. [PMC free article] [PubMed] [Google Scholar]
- (6).Johansson C, Lillig CH, Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem. 2004 February 27;279(9):7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- (7).Lillig CH, Lonn ME, Enoksson M, Fernandes AP, Holmgren A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc Natl Acad Sci U S A. 2004 September 7;101(36):13227–13232. doi: 10.1073/pnas.0401896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Bjornstedt M, Hamberg M, Kumar S, Xue J, Holmgren A. Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J Biol Chem. 1995 May 19;270(20):11761–11764. doi: 10.1074/jbc.270.20.11761. [DOI] [PubMed] [Google Scholar]
- (9).Zhong L, Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J Biol Chem. 2000 June 16;275(24):18121–18128. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]
- (10).May JM, Cobb CE, Mendiratta S, Hill KE, Burk RF. Reduction of the ascorbyl free radical to ascorbate by thioredoxin reductase. J Biol Chem. 1998 September 4;273(36):23039–23045. doi: 10.1074/jbc.273.36.23039. [DOI] [PubMed] [Google Scholar]
- (11).Tamura T, Gladyshev V, Liu SY, Stadtman TC. The mutual sparing effects of selenium and vitamin E in animal nutrition may be further explained by the discovery that mammalian thioredoxin reductase is a selenoenzyme. Biofactors. 1995;5(2):99–102. [PubMed] [Google Scholar]
- (12).Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008 May 23;320(5879):1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 January 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Chitaley K, Weber D, Webb RC. RhoA/Rho-kinase, vascular changes, and hypertension. Curr Hypertens Rep. 2001 April;3(2):139–144. doi: 10.1007/s11906-001-0028-4. [DOI] [PubMed] [Google Scholar]
- (15).Szasz T, Thompson JM, Watts SW. A comparison of reactive oxygen species metabolism in the rat aorta and vena cava: focus on xanthine oxidase. Am J Physiol Heart Circ Physiol. 2008 September;295(3):H1341–H1350. doi: 10.1152/ajpheart.00569.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001 June 12;2001(86):l1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- (17).Holmgren A. Biochemistry. SNO removal. Science. 2008 May 23;320(5879):1019–1020. doi: 10.1126/science.1159246. [DOI] [PubMed] [Google Scholar]
- (18).Choi H, Tostes RC, Webb RC. S-nitrosylation Inhibits Protein Kinase C-mediated Contraction in Mouse Aorta. J Cardiovasc Pharmacol. 2011 January;57(1):65–71. doi: 10.1097/FJC.0b013e3181fef9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Watt PA, Thurston H. Endothelium-dependent relaxation in resistance vessels from the spontaneously hypertensive rats. J Hypertens. 1989 August;7(8):661–666. doi: 10.1097/00004872-198908000-00010. [DOI] [PubMed] [Google Scholar]
- (20).Williams SP, Shackelford DP, Iams SG, Mustafa SJ. Endothelium-dependent relaxation in estrogen-treated spontaneously hypertensive rats. Eur J Pharmacol. 1988 January 12;145(2):205–207. doi: 10.1016/0014-2999(88)90231-2. [DOI] [PubMed] [Google Scholar]
- (21).Oyama Y, Kawasaki H, Hattori Y, Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 December 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- (22).Winquist RJ, Bunting PB, Baskin EP, Wallace AA. Decreased endothelium-dependent relaxation in New Zealand genetic hypertensive rats. J Hypertens. 1984 October;2(5):541–545. doi: 10.1097/00004872-198410000-00015. [DOI] [PubMed] [Google Scholar]
- (23).Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000 November 10;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- (24).Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008 February;51(2):211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- (25).Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, om-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH Oxidase Enhances Vasodilatation and Reduces Blood Pressure In Vivo. Arterioscler Thromb Vasc Biol. 2011 March 17; doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- (26).Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000 September;184(3):409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- (27).Sayed N, Baskaran P, Ma X, van den AF, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci U S A. 2007 July 24;104(30):12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sayed N, Kim DD, Fioramonti X, Iwahashi T, Duran WN, Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res. 2008 September 12;103(6):606–614. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Denninger JW, Marletta MA. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim Biophys Acta. 1999 May 5;1411(2-3):334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- (30).Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004 August;287(2):L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- (31).Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001 September 21;106(6):675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- (32).Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005 May 20;280(20):19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]