Abstract
Transient, repetitive ischemia (RI) stimulates coronary collateral growth (CCG) in normal, healthy (SD) rats, which requires p38 MAPK activation. In contrast, RI does not induce CCG in the metabolic syndrome (JCR) rats, which is associated with lack of p38 MAPK activation. The functional consequences of p38 MAPK activation in CCG remain unknown. Theoretically, effective collateral growth would require extracellular matrix remodeling; however, direct assessment as well as identification of proteases responsible for this degradation are lacking. In this study, we investigated the role of p38 MAPK in the regulation of matrix metalloproteinases 2 and 9 (MMPs 2 and 9) and their requirement for CCG in SD vs. JCR rats. The rats underwent the RI protocol (8 LAD occlusions, 40 sec each, every 20 min, in 8 hr cycles for 0, 3, 6, or 9 days). MMP expression was measured in the ischemic, collateral-dependent zone (CZ) and the normal zone (NZ) by Western blot, and MMP activity by zymography. Expression and activation of MMP 2 and 9 were significantly increased (~3.5 fold) on day 3 of RI in the CZ of SD rats. In vivo p38 MAPK inhibition completely blocked RI-induced MMP 2 and 9 expression and activation. MMP activation correlated with increased degradation of components of the basement membrane and the vascular elastic laminae: elastin (~3 fold), laminin (~3 fold) and type IV collagen (~2 fold). This was blocked by MMP 2 and 9 inhibition, which also abolished RI-induced CCG. In contrast, in JCR rats, RI did not induce expression or activation of MMP 2 or 9 and there was no associated degradation of elastin, laminin or type IV collagen. In conclusion, MMP 2 and 9 activation is essential for CCG and is mediated, in part, by p38 MAPK. Furthermore, compromised CCG in the metabolic syndrome may be partially due to the lack of p38 MAPK-dependent activation of MMP 2 and 9 and resultant decreased extracellular matrix degradation.
Keywords: p38 MAPK, MMPs, ECM remodeling, coronary collateral growth
Introduction
Myocardial ischemia-reperfusion injury is a well-known phenomenon resulting from prolonged periods of ischemia followed by re-oxygenation. However, short, repetitive periods of ischemia followed by reperfusion can lead to adaptive responses and render the myocardium tolerant to longer periods of ischemia, in part through promoting collateral development [1]. Stable angina pectoris is a consequence of significant coronary artery constriction, and is characterized by transient periods of ischemia, upon increased myocardial metabolic demand followed by reperfusion at rest. Coronary collateral growth (CCG) is an adaptive response to transient, repetitive myocardial ischemia (RI). Clinically, patients with stable angina have a decreased incidence of fatal myocardial infarction, which is associated with better developed collateral networks [2]. In contrast, CCG has been shown to be severely impaired in patients suffering from type II diabetes [3] and the metabolic syndrome [4]. Likewise, CCG is impaired in our metabolic syndrome rat model (JCR:LA-cp or JCR) [5]. The JCR rat is obese, dyslipidemic (low HDL, high LDL, VLDL, and triglycerides) [5], insulin resistant with impaired glucose tolerance [6], and hypertensive [5], and thus, mimics the complex pathology of the human metabolic syndrome.
The process of CCG involves endothelial and vascular smooth muscle cell (VSMC) proliferation and migration, as well as extracellular matrix (ECM) remodeling. The early phase of collateral growth is associated with inward remodeling, in which cells migrate across the internal elastic lamina and the basement membrane, into the lumen of the pre-existing native collaterals. This is followed by outward remodeling in which cells migrate across the external elastic lamina into the vascular adventitia and the surrounding myocardium, thus allowing for vessel expansion and significant increases in blood flow [7–9]. Consequently, reorganization of the ECM, including ECM degradation, is a presumed integral part of collateral remodeling. However, direct measurements of this process during collateral growth have never been reported.
ECM degradation requires matrix metalloproteinases (MMPs), zinc-dependent endopeptidases capable of degrading extracellular matrix proteins. MMPs can be separated based on substrate specificity into interstitial collageneases (MMPs 1, 8 and 13), broad specificity MMPs (MMPs 3 and 7), metalloelastases (MMP 12), membrane-bound MMPs (MMP 14 (MT1-MMP) and MMP 17), and gelatinases (MMP 2 and 9). MMP 2 and 9 have been shown to degrade type IV collagen, laminin and elastin, the primary components of the vascular basement membrane and the internal and external elastic laminae, in vitro [10–13]. They are known to play a role in cell proliferation, migration, differentiation, angiogenesis associated with cancer metasthesis, neointima formation following vascular injury and aneurysim formation and rupture [14–16]. Although degradation of the basement membrane and the vascular elastic laminae is a common aspect shared between these processes and collateral remodeling, they are not identical, and conclusions drawn from these studies do not uniformly apply to collateral growth. Increased MMP 2 and 9 expression has been associated with collateral growth, but the results are not entirely in agreement. In one study, during the early, inward remodeling phase in growing coronary collaterals, the neointima showed high expression of MMPs 2 and 9 while mature collaterals expressed low levels of these MMPs [17]. On the other hand, MMP 2 but not MMP 9 expression and activity were increased in mesenteric collateral vessels [18]. Importantly, a conclusive requirement for MMP 2 and 9 activation in CCG has not been shown. Furthermore, it is unknown whether MMP 2 and/or 9 regulation is altered in the metabolic syndrome, where CCG is impaired.
MMPs are regulated at the level of both expression and activation. Several signaling pathways have been shown to regulate MMP expression and/or activation. Among these are the mitogen activated protein kinases (MAPKs). MAPKs can be divided into the extracellular signal-regulated kinases (ERK1/2), p38 MAPK, and c-Jun N-terminal kinase (JNK) [19]. The ERK1/2 pathway has been shown to regulate expression and activation of many MMPs including MMP 9 [20]. p38 MAPK, when activated during inflammation or the innate immune response, lead to the activation of MMP 9 [21], and a single study implicates p38 MAPK in the regulation of MMP 9 activation in cultured airway smooth muscle cells [22]. However, it is not known which signaling pathways may regulate MMP expression and/or activation in collateral growth.
We have previously shown that transient p38 MAPK activation on day 3 of RI is required for CCG in the normal healthy rat model where inhibition of p38 MAPK resulted in ~60% reduction in RI-induced CCG [23]. In addition, we have shown that RI-induced CCG was severely compromised in the metabolic syndrome JCR rat model, and that this correlated with lack of RI-induced p38 MAPK activation [24]. However, the functional consequence of p38 MAPK activation in collateral growth remained unknown. Therefore the goals of this study were to determine: 1) whether RI-induced activation of p38 MAPK regulated the development of coronary collaterals through the activation of MMP 2 and 9 and the degradation of their ECM substrates, 2) whether MMP 2 and 9 were required for this ECM degradation and CCG, and 3) whether MMP 2 and/or 9 expression and/or activity were altered in the metabolic syndrome.
Materials and Methods
Rat model of coronary collateral growth/RI
Male, 10–12 week old Sprague-Dawley (SD) (300–350g) or obese JCR:LA-cp rats (JCR) (650–700g) were used for chronic implantation of a pneumatic occluder over the left anterior descending coronary artery (LAD), as described previously [25]. The RI protocol for rats consisted of 8–40s occlusions, one every 20min over 2h 20min followed by a period of “rest” for 5h 40min. This 8h cycle was repeated three times per day for 0–9 days. The specific inhibitors of p38 MAPK, SB203580 (3.2 mg/kg/day, Calbiochem), and of MMP 2 and 9, SB-3CT (25 mg/kg/day, Enzo Life Sciences), were delivered once/day on days 2 and 3 of the RI protocol i.v. via a jugular vein catheter implanted at the time of LAD occluder implantation. All surgical procedures were performed in accordance with the Animal Welfare Act and are approved by the IACUC of the University of South Alabama.
Myocardial and collateral-dependent blood flow measurements were performed as described previously [26]. Color microspheres (5×105, 15μM diameter) labeled with gold (at day 0 of RI (initial surgery)) or samarium (at day 10 of RI) were injected into the left ventricle (LV) during LAD occlusion. Arterial reference blood samples (carotid) and heart tissue from the normal, non-ischemic zone (NZ) and the collateral-dependent, ischemic zone (CZ), were collected, weighed and sent to BioPal (Worcester, MA) for analysis. Blood flows to the normal (NZ) and collateral-dependent (CZ) zones (ml/min/g) were calculated from the formula: Blood Flow=[(radioactive counts in myocardial tissue)×(blood reference withdrawal rate)/(radioactive count in blood reference)]/(weight of myocardial tissue). Blood flows were measured in the following groups of animals: SD sham, SD sham + SB-3CT, SD RI and SD RI + SB-3CT. Results were expressed as the CZ/NZ flow ratio at day 10 of RI. All experiments were n=5. Results were analyzed by 2-way ANOVA followed by Bonferroni correction. p<0.05 determined statistical significance.
Western blot analysis
Hearts were excised, and the LAD-dependent, collateral-dependent zone (CZ) was separated from the non-ischemic normal zone (NZ) then snap-frozen in liquid nitrogen before homogenization in lysis buffer containing 0.1% SDS and 1% Triton as previously described [25]. Equal amounts of protein (30 or 50 μg) were separated by SDS-PAGE, and transferred to Hybound-ECL nitrocellulose membranes. Anti-MMP 2 (Anaspec), anti-MMP 9 (Milipore), phospho-specific anti-p38 MAPK and anti-MK2 (Cell Signaling) were used for Western blot analysis. Bands were visualized by enhanced chemiluminescence (Amersham) and quantified using Un-Scan-It Image software (Silk Scientific Corporation). Experiments were n=5 animals per group, and analyzed by two-way ANOVA followed by the Bonferroni post-hoc analysis. p<0.05 determined statistical significance.
Gelatin Zymography
Hearts were excised, the CZ separated from the NZ, and snap-frozen in liquid nitrogen before homogenization in lysis buffer containing 4% SDS and 25% glycerol, followed by incubation at 37°C for 20 minute. Equal amounts of protein (30 μg) were separated by SDS-PAGE gels containing 1 mg/ml gelatin (Bio-Rad 161–1167). The gels were then incubated in renaturing buffer (2.5% X-100) twice for 20 minutes and washed after each incubation period with ddH2O, then incubated in the developing buffer (50 mM Tris-HCl, pH 7.6, 0.2 M NaCl, 5 mM CaCl2, and 0.02% BriJ 35) overnight at 37ºC in a shaking water bath. After being rinsed with ddH2O, gels were stained with Coomassie brilliant blue for 30 minutes to 1 hour then partially de-stained using a 10% acetic acid/20% methanol/70% ddH2O solution for 30–45 minutes. Gels were scanned using HP Scanjet G4050 and bands quantified using UnScan-It Image software. Experiments were n=5 animals per group, and analyzed by two-way ANOVA followed by the Bonferroni post-hoc analysis. p<0.05 determined statistical significance.
ECM Degradation
Type IV collagen, laminin and elastin degradation in tissue lysates was evaluated according to similar protocols [27–29]. Hearts were excised, and the CZ was separated from the NZ then snap-frozen in liquid nitrogen before homogenization in lysis buffer containing 0.1% SDS and 1% Triton as previously described [25]. The hearts were not rinsed so that the samples were comprised of both tissue and whole blood contained within the coronary vasculature at the time of sacrifice. Equal amounts of protein (30 or 50 μg) were separated by SDS-PAGE (4–15% acrylamide gradient gels, BioRad), and transferred to Hybound-ECL nitrocellulose membranes. Anti-elastin (Abcam), anti-type IV collagen (Milipore), and anti-laminin (R&D Systems) were used for Western blot analysis. Bands were visualized by enhanced chemiluminescence (Amersham) and quantified using Un-Scan-It Image software (Silk Scientific Corporation). Experiments were n=5 animals per group, and analyzed by two-way ANOVA followed by the Bonferroni post-hoc analysis. p<0.05 determined statistical significance.
Results
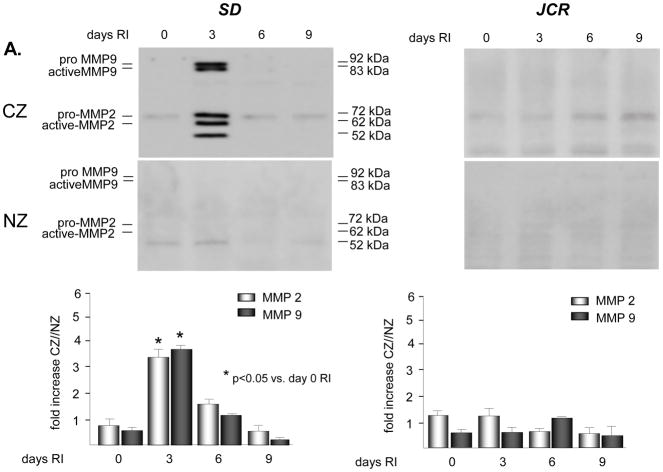
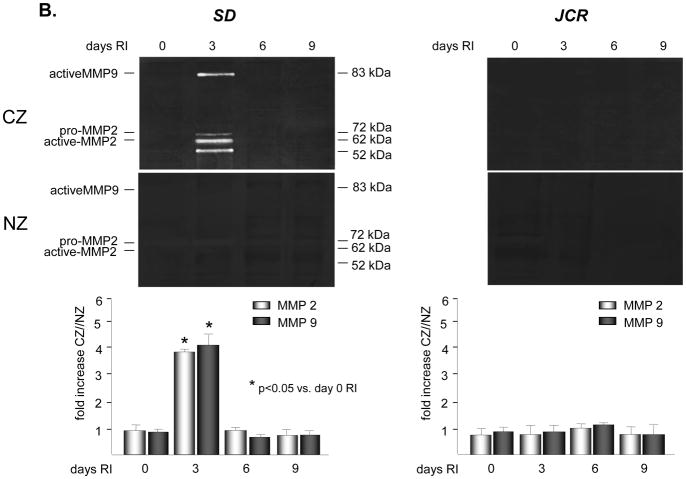
MMP 2 and 9 expression and activation are increased in response to repetitive ischemia in normal, healthy animals but not in the metabolic syndrome
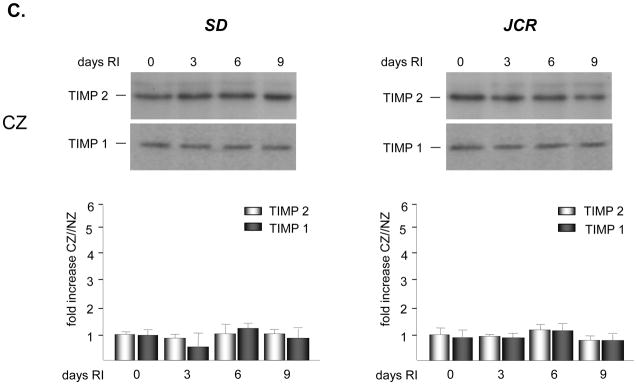
No significant basal expression or activation of either MMP2 or 9 was observed in either rat phenotype. Western blot analysis demonstrated an increase in expression of MMP 2 and 9 in the SD animals specifically confined to the collateral dependent zone (CZ) on day 3 of the RI protocol (3.3 ± 0.3 fold vs. NZ for MMP 2; 3.6 ± 0.2 fold vs. NZ for MMP 9) (Figure 1A). This increase in expression in the collateral dependent zone corroborates our previously published data that showed increased p38 MAPK activation specifically on day 3 of the RI protocol [23]. Similarly, RI significantly increased the activation of MMP 2 and 9 in the SD animals, specifically in the collateral dependent zone on day 3 of the RI protocol correlating with p38 MAPK activation (3.9 ± 0.1 fold vs. NZ for MMP 2; 4.1 ± 0.3 fold vs. NZ for MMP 9) (Figure 1B). In contrast, RI did not induce MMP 2 or 9 expression or activation in the CZ of JCR rats at any day of the RI protocol (Figures 1A and 1B), correlating with lack of RI-induced p38 MAPK activation in JCR rats [5]. Basal MMP 2 and 9 expression and activity were not significantly different between the SD and JCR phenotypes. Likewise, RI did not alter MMP 2 or 9 expression or activation in the NZ. Finally, RI did not alter TIMP1 or TIMP2 expression nor was there any difference in their baseline expression between the SD and JCR phenotypes (Figure 1C).
Figure 1.
SD or JCR rats were sacrificed on day 0, 3, 6 or 9 of RI. Tissue samples were collected from the non-ischemic normal zone (NZ) or the ischemic LAD/collateral-dependent zone (CZ). MMP expression was determined by Western Blot and activity by Zymography. A. Top: Representative Western blot using anti-MMP 2 and 9 antibodies. Bottom: Cumulative data, *p<0.05, n=5. B. Top: Representative gelatin zymogram for MMP 2 and 9. Bottom: Cumulative data, *p<0.05, n=5. C. Top: Representative Western blot using anti-TIMP1 and 2 antibodies. Bottom: Cumulative data, *p<0.05, n=5.
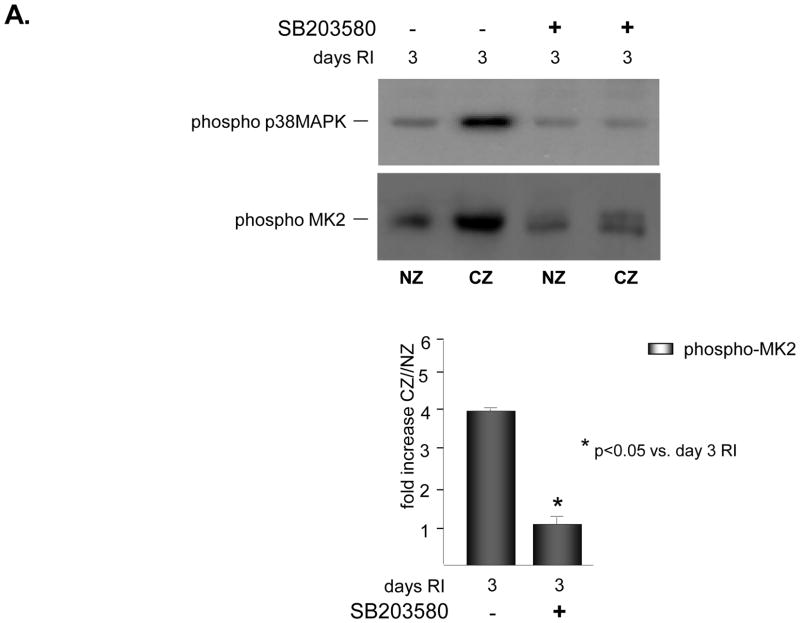
Inhibition of p38 MAPK attenuates MMP 2 and 9 expression and activation
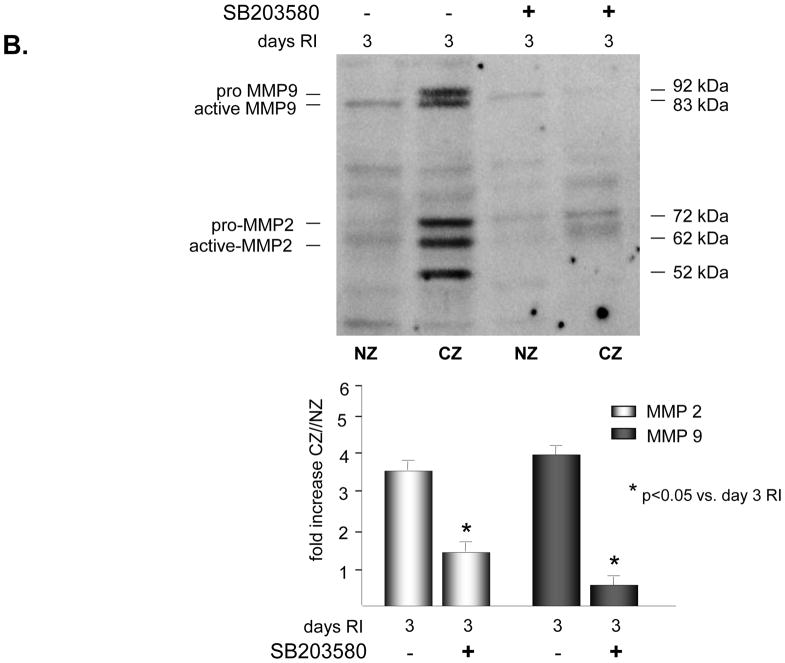
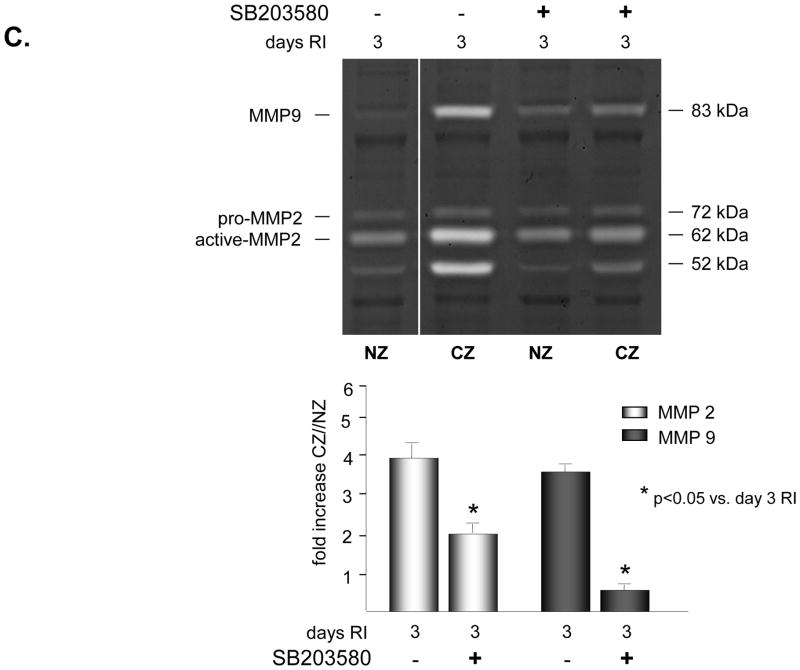
To determine whether p38 MAPK was required for increased expression and activation of MMP2 and/or MMP9 in response to RI, rats were treated in vivo with a specific p38 MAPK inhibitor, SB203580 (3.2mg/kg/day, i.v.) on days 2 and 3 of the RI protocol. This treatment protocol was selected because we have previously shown that p38 MAPK was specifically activated in the CZ on days 2 (~1.5 fold vs. NZ) and 3 (~3 fold vs. NZ) of the RI protocol only, and inhibition of its activity with SB203580 resulted in ~60% reduction in RI-induced CCG [23]. Furthermore, there was no difference in CCG whether the animals were treated with SB203580 on days 2 and 3 only, or for the duration of the RI protocol (data not shown). Inhibition of p38 MAPK by SB203580 was confirmed by evaluating the phosphorylaiton of the immediate and specific downstream target of p38 MAPK, MK2. MK2 phosphorylation was increased ~ 4 fold in the CZ only at day 3 of RI as expected, corresponding with increased p38 MAPK activation, and this increase was completely blocked in animals treated with SB203580 (Figure 2A). SB203580 did not effect MK2 expression (data not shown). Importantly, p38 MAPK inhibition resulted in a significant decrease in MMP 2 (58 ± 6% vs. RI) and MMP 9 (89 ± 5% vs. RI) expression (Figure 2B) and activation (49 ± 4% vs. RI for MMP 2; 75 ± 5% vs. RI for MMP 9) (Figure 2C) in the collateral-dependent zone on day 3 of the RI protocol. SB203580 did not alter p38 MAPK, MK2, MMP 2 or 9 expression or activation in the NZ, as shown (Figure 2).
Figure 2.
SD rats were treated with the p38 MAPK inhibitor, SB203589 as indicated, and sacrificed on day 0 or 3 of RI. Tissue samples were collected from the non-ischemic normal zone (NZ) or the ischemic LAD/collateral-dependent zone (CZ). MMP expression and MK2 activation were determined by Western Blot and MMP activity by zymography. A. Top: Representative Western blot using phospho-specific anti-MK2 antibodies. Bottom: Cumulative data, *p<0.05, n=5. B. Top: Representative Western blot using anti-MMP 2 and 9 antibodies. Bottom: Cumulative data, *p<0.05, n=5. C. Top: Representative gelatin zymogram for MMP 2 and 9 (all lanes are from the same gel). Bottom: Cumulative data, *p<0.05, n=5.
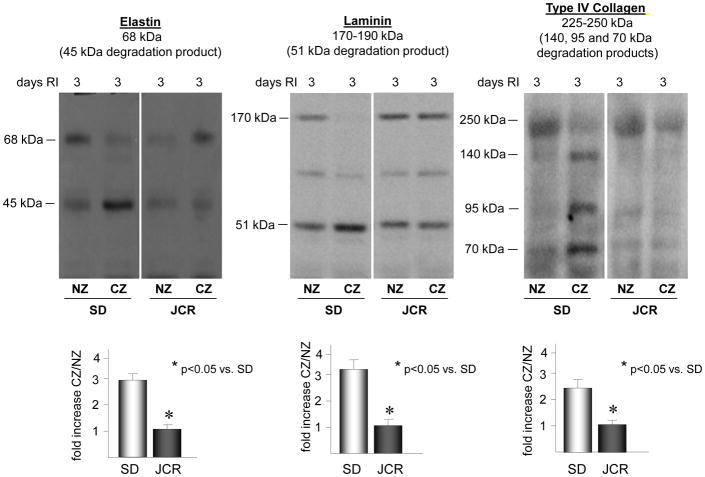
Repetitive ischemia induces degradation of laminin, elastin and type IV collagen in normal, healthy animals but not in the metabolic syndrome
To assess whether the early phase of coronary collateral growth is associated with degradation of the basement membrane and the elastic laminae in our model, we evaluated the degradation of laminin, elastin and type IV collagen in the collateral dependent zone vs. the normal zone. Western blot analysis was used to detect the degradation products of laminin (51 kDa), elastin (45 kDa), and type IV collagen (140, 95 or 70 kDa) [30–32]. RI induced a significant increase in laminin (3.2 ± 0.2 fold vs. NZ), elastin (2.9 ± 0.3 fold vs. NZ) and type IV collagen (2.2 ± 0.2 fold vs. NZ) degradation at day 3 of the RI protocol, which corresponded with decreased amounts of intact laminin (170–190 kDa), elastin (68 kDa) and type IV collagen (225–250 kDa), specifically confined to the CZ, thus corresponding with increased p38 MAPK and MMP 2 and 9 activation (Figure 3A). No significant degradation of these ECM components was observed during the later stages of CCG (days 6 and 9 of RI) (data not shown). In contrast, there was no increase in degradation of these ECM components in the CZ of JCR rats at any day of the RI protocol corresponding with lack of MMP 2 and 9 activation by RI in the JCR animals (Figure 3B). RI did not induce laminin, elastin or type IV collagen degradation in the NZ, as shown (Figure 3).
Figure 3.
SD or JCR rats were sacrificed on day 0, 3, 6 or 9 of RI. Tissue samples were collected from the non-ischemic normal zone (NZ) or the ischemic LAD/collateral-dependent zone (CZ). Elastin, laminin and type IV collagen degradation was determined by Western blot. Top: Representative Western blots detecting intact elastin (68 kDa), laminin (170–190 kDa) and type IV collagen (225–250 kDa) and degradation products of elastin (45 kDa), laminin (51 kDa) and type IV collagen (140, 95 and 75 kDa) (all lanes are from the same gel). Bottom: Cumulative data for degradation products, *p<0.05, n=5.
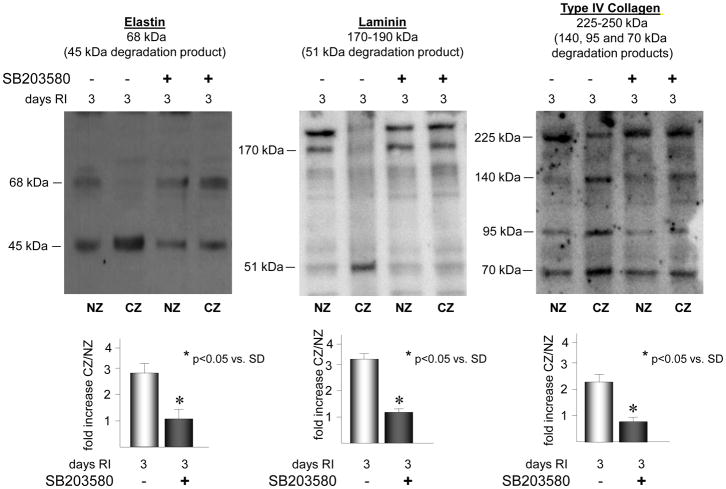
Inhibition of p38 MAPK attenuates RI-induced degradation of laminin, elastin and type IV collagen
To definitively assess whether the degradation of the ECM components of the basement membrane and the elastic laminae (laminin, elastin, and type IV collagen) is dependent on p38 MAPK activation, p38 MAPK was inhibited with SB203580 on days 2 and 3 of RI in the SD rats. p38 MAPK inhibition resulted in a significant decrease in the abundance of laminin (56 ± 3% vs. RI) elastin (62 ± 3% vs. RI) and type IV collagen (48 ± 3% vs. RI) degradation products and a corresponding increase in intact proteins in the CZ, compared to non-SB203580 treated animals (Figure 4).
Figure 4.
SD rats were treated with the p38 MAPK inhibitor, SB203589 as indicated, and sacrificed on day 0 or 3 of RI. Tissue samples were collected from the non-ischemic normal zone (NZ) or the ischemic LAD/collateral-dependent zone (CZ). Elastin, laminin and type IV collagen degradation was determined by Western blot. Top: Representative Western blots detecting intact elastin (68 kDa), laminin (170–190 kDa) and type IV collagen (225–250 kDa) and degradation products of elastin (45 kDa), laminin (51 kDa) and type IV collagen (140, 95 and 75 kDa). Bottom: Cumulative data for degradation products, *p<0.05, n=5.
Inhibition of MMP 2 and 9 activation blocks RI-induced degradation of laminin, elastin and type IV collagen and attenuates RI-induced CCG
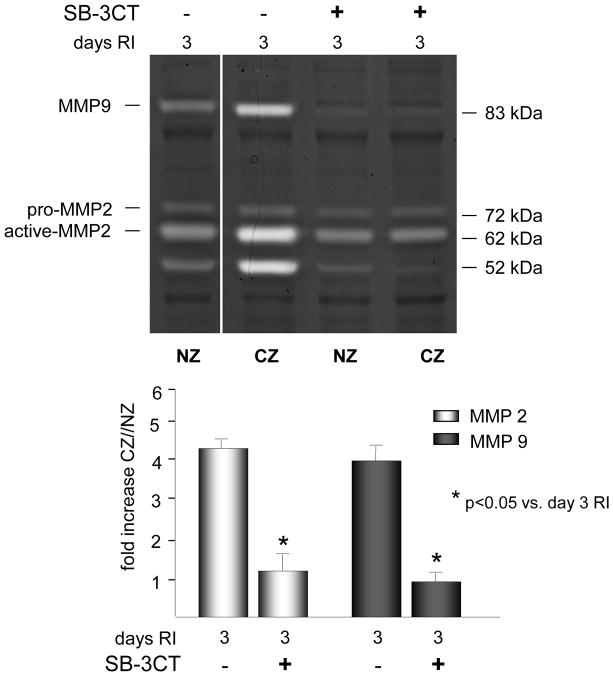
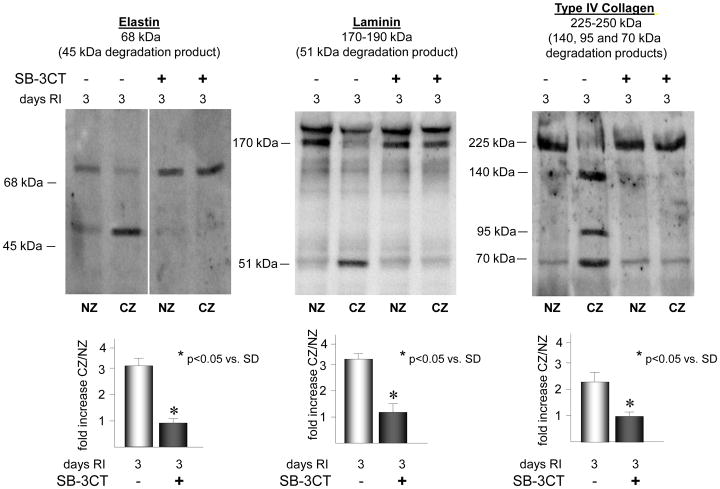
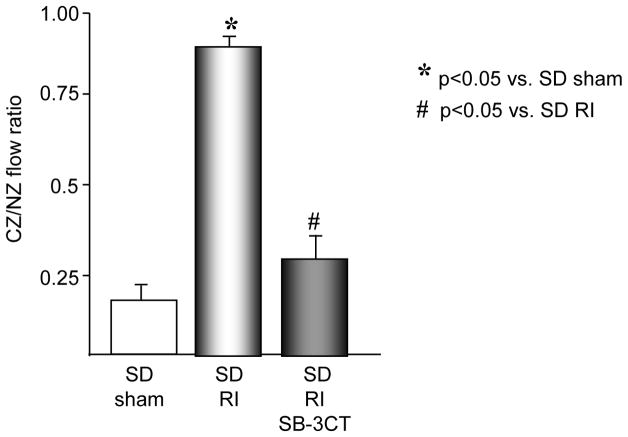
To definitively determine whether MMP 2 and 9 activation was required for RI-induced degradation of the ECM components of the basement membrane and the elastic laminae, MMP 2 and 9 activity was inhibited with a gelatinase-specific inhibitor SB-3CT. SB-3CT inhibits gelatinases (MMP 2 and 9) in the nanomolar range (Ki for MMP2=14nM, for MMP9=600nM) and does inhibit other MMPs as well as ADAMs but in the micromolar range. At the dose of 25 mg/kg, the calculated average molar plasma concentration of SB-3CT is 19.6 nM. This dose has been shown to be effective against both gelatinases in vivo. SB-3CT has been used in vivo at concentrations ranging from 5 to 25 mg/kg [29, 33–35]. In our study, SB-3CT (25 mg/kg/day, i.v.) was administered on days 2 and 3 of RI to the SD rats and completely blocked RI-induced MMP 2 and 9 activity (Figure 5) without altering MMP 2 and/or 9 expression or activity of other MMPs, including MMPs 1, 2, 3, 7, 8, 12, 13 and MT1-MMP (data not shown). As expected, MMP 2 and 9 inhibition resulted in a complete blockade of RI-induced laminin, elastin and type IV collagen degradation (Figure 6). Importantly, blockade of MMP 2 and 9 activation resulted in near complete abolition of RI-induced CCG (at day 10 of RI CZ/NZ flow ratio was 0.27±0.08 vs. 0.87±0.04 in SD RI) (Figure 7). SB-3CT did not alter basal coronary blood flows (data not shown).
Figure 5.
SD rats were treated with the MMP 2 and 9 inhibitor, SB-3CT as indicated, and sacrificed on day 0 or 3 of RI. Tissue samples were collected from the non-ischemic normal zone (NZ) or the ischemic LAD/collateral-dependent zone (CZ). MMP activity was determined by zymography. Top: Representative gelatin zymogram for MMP 2 and 9 (all lanes are from the same gel). Bottom: Cumulative data, *p<0.05, n=5.
Figure 6.
SD rats were treated with the MMP 2 and 9 inhibitor, SB-3CT as indicated, and sacrificed on day 0 or 3 of RI. Tissue samples were collected from the non-ischemic normal zone (NZ) or the ischemic LAD/collateral-dependent zone (CZ). Elastin, laminin and type IV collagen degradation was determined by Western blot. Top: Representative Western blots detecting intact elastin (68 kDa), laminin (170–190 kDa) and type IV collagen (225–250 kDa) and degradation products of elastin (45 kDa), laminin (51 kDa) and type IV collagen (140, 95 and 75 kDa) (all lanes are from the same gel). Bottom: Cumulative data for degradation products, *p<0.05, n=5.
Figure 7.
SD rats were treated the MMP 2 and 9 inhibitor, SB-3CT as indicated and underwent 10 days of RI. Coronary blood flow was measured in the CZ and the NZ using microspheres during LAD occlusion and is expressed as the ratio between CZ and NZ flows at day 10 of RI. *p<0.05 vs. SD sham, #p<0.05 vs. SD RI.
Discussion
The major novel findings in this study are that: 1) RI induces the expression and activation of MMP 2 and 9, which correlates with degradation of components of the basement membrane and the elastic laminae, type IV collagen, laminin and elastin, 2) RI-induced activation of MMP 2 and 9 is severely compromised in the metabolic syndrome, which is associated with the absence of type IV collagen, laminin and elastin degradation, 3) MMP 2 and 9 expression and activation by RI as well as type IV collagen, laminin and elastin degradation during CCG are dependent on activation of p38 MAPK, and 4) MMP 2 and 9 activation is required for CCG.
MMPs play an important role in tissue remodeling associated with various physiological and pathological processes such morphogenesis, angiogenesis, wound repair, arthritis, metastasis, neointima formation and restenosis, aneurysm formation and rupture and cardiac remodeling during the progression to heart failure [14]. MMP 2 and 9 have been most extensively studied in cancer and are thought to be the MMPs critical for metastasis. They are known to degrade type IV collagen, a major protein component of basement membranes, which allows for tumor cell migration into the circulatory system, transport to distal sites and cancer progression [14]. Degradation of the basement membrane and the elastic laminae by MMPs also plays a critical role in cardiovascular disease. Numerous studies have demonstrated increased levels of MMPs, especially MMP 9, at sites of atherosclerotic plaques and aneurysms [14]. MMP activity has been associated with outward remodeling and thinning of the vascular wall in response to elevated blood pressure by fragmenting the internal and external elastic laminae [17].
Although these events and collateral remodeling are vastly different processes, they share a common characteristic relevant to MMP 2 and 9 functions, namely the expected requirement for degradation of the basement membrane and/or the elastic laminae. Early phases of collateral growth have been associated with neointima formation characterized by proliferation and migration of endothelial and vascular smooth muscle cells across the basement membrane and into the lumen of the vessel [7, 36, 37]. This is followed by outward remodeling and vessel expansion and maturation to form a functional conduit collateral vessel. It is important to note that in contrast to metastasis and aneurysm formation, it is reasonable to predict that MMP activation during CCG would have to be transient. Components of the ECM would have to be degraded to allow for proliferation and migration of cells to the site of growth; however, there would have to be a point where this degradation would stop to allow for ECM synthesis and reorganization and collateral vessel maturation. Indeed, elevated MMP 2 and 9 activity has been demonstrated in the neointima of growing but not mature collaterals in a dog model of CCG [17]. Our finding that MMP 2 and 9 expression and activation were significantly and maximally increased at day 3 of RI and returned to baseline by day 6 of RI (Figure 1) is in agreement with these results and supports our hypothesis that MMP activation in CCG must be transient. Importantly, our study is the first to demonstrate a definitive requirement for MMP 2 and 9 activation in collateral growth, since specific MMP 2 and 9 inhibition abolished RI-induced CCG (Figure 7). Furthermore, the complete blockade of RI-induced CCG by MMP 2 and 9 inhibition suggests that their activation may be a rate-limiting step in this remodeling process. However, a limitation of this interpretation is that the specificity of MMP 2 and 9 inhibition was not directly demonstrated but rather extrapolated from the Ki values for the pharmacological inhibitor. Furthermore, the calculated in vivo concentration of the inhibitor falls below the Ki for MMP 9 leaving a possibility that MMP 9 may not have been effectively inhibited. The other possibility is that SB-3CT does not distribute among the bodily fluid compartments uniformly, rendering the actual in vivo concentration of the inhibitor an approximation.
Although MMP 2 and 9 have been shown to degrade type IV collagen in vivo [38–40], their ability to degrade laminin, also a critical component of the basement membrane, and elastin has been confined to in vitro studies [41–43]. In addition, no study has assessed whether these ECM components were in fact degraded during collateral remodeling or which proteases were responsible for this theoretical degradation. Our results demonstrate a significant increase in type IV collagen, laminin and elastin degradation at day 3 of RI in the normal, healthy animals, correlating with maximal MMP 2 and 9 activation (Figure 3). Importantly, our findings demonstrate that specific inhibition of MMP 2 and 9 results in complete blockade of RI-induced degradation of these ECM components (Figure 6). Together, these results provide the first conclusive evidence that these ECM components are in fact degraded during the process of collateral growth and that MMP 2 and 9 are the proteases responsible for their degradation. In contrast, in the rat model of the metabolic syndrome, consistent with lack of MMP 2 and 9 activation, RI failed to induce the degradation of type IV collagen, laminin and elastin, the expression or activation of MMP 2 and 9 (Figures 1 and 3) and coronary collateral growth [5]. Reduced expression of interstitial collagenases and corresponding increased fibrosis have been previously documented in JCR animals [6]; however, our study provides the first evidence that lack of MMP-dependant ECM remodeling may also underlay aberrant collateral remodeling in the metabolic syndrome.
MMPs are regulated at the level of both expression and activation. MMP 9 expression has been shown to be regulated by NF-κB [21, 44–46] and the signal transducer and activator of transcription (STAT) pathway [19]. Post-transcription, MMPs can be regulated by phosphoryation [47] or, in some cell types by other proteases, including plasmin and other MMPs, especially MT1-MMP [48]. However, tissue inhibitors of metalloproteinases (TIMPs) are the most important post-transcriptional regulators of MMP activity [49]. Our results demonstrate that TIMP1 and 2 expression did not change during the entire course of CCG. This is in agreement with a previous study in which TIMP1 expression was slightly and insignificantly elevated and TIMP2 expression was not detectable in the neointima of growing collaterals [17]. The difference in TIMP2 expression between the two studies may be due to the fact that TIMP2 expression is not localized to the neointima or collateral vessels but may instead be confined to the perivascular space. The perivascular space is included in the samples prepared from the collateral-dependant zone of rat hearts because the anatomy of the coronary circulation prevents isolation of collateral vessels. However, isolating collateral vessels is not a limitation of the dog model; thus, these samples do not include the perivascular space. In combination, these observations do not indicate a critical role for TIMPs in the regulation of MMP 2 and 9 activity during CCG. In fact, our results further support this notion since we observed no additional increase in RI-induced MMP 2 or 9 activity than that which could be accounted for by observed increases in MMP 2 and 9 expression alone (Figure 1).
Several signaling intermediates have been shown to regulate MMP 2 and 9 expression and activation, including the MAPK pathways. ERK1/2 MAPKs mediate the activation of several MMPs including MMP 9 [20]. p38 MAPK activation in response to inflammation or the innate immune response can activate MMP 9 [21]. Overexpression of the downstream effector of p38 MAPK, MK2, was associated with MMP 2 and 9 activation in transitional cell carcinoma [50]. MMP 9 activation was p38 MAPK-dependant in airway smooth muscle [22]. We have previously shown that p38 MAPK was required for coronary collateral growth [23]; however, those studies did not provide any mechanistic insight into the downstream effects of this activation. p38 MAPK mediates a variety of cellular effects involved in collateral remodeling, including cell migration, proliferation and survival [51], as well as biosynthesis of inflammatory cytokines including tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), prostaglandins (via COX-2) [52], and growth factors including the vascular endothelial growth factor (VEGF) [53]. Duration of p38 MAPK activation seems to be a critical determinant of its downstream effects. A unique feature of p38 activation with regards to angiogenesis is that it’s been reported to be both pro[54, 55]- and anti[55, 56]-angiogenic in vitro. Specifically, its early and transient activation promoted the acquisition of a malignant, migratory phenotype and tumor angiogenesis [57], while prolonged activation lead to cell death and blocked tumor angiogenesis [58].
Accumulating evidence indicates that the oxidative state of the cell plays an important role in MMP regulation in various cellular processes including proliferation, angiogenesis, apoptosis, invasion and metastasis [59]. MMP 2 and 9 contain a redox-sensitive disulfide bond, which may influence their activity [60]. Our previously published data demonstrated that an optimal amount of reactive oxygen species (ROS), or the optimal oxidative (redox) state, was required for CCG [23]. Reduction in RI-induced ROS abrogated CCG in normal, healthy animals, but markedly improved CCG in the metabolic syndrome animals, which exhibit elevated basal and RI-induced ROS [23]. Similarly, while reduction in RI-induced ROS prevented p38 MAPK activation in normal, healthy animals, it induced p38 MAPK activation in the metabolic syndrome animals [23]. This redox-dependence of p38 MAPK activation is in agreement with multiple cell culture studies, including those in cardiac [61] and vascular [62] cells. Therefore a question remained whether these MMPs were regulated by ROS directly or via p38 MAPK. In this study, increased expression and activation of MMP 2 and 9 only on day 3 of the RI protocol in the normal, healthy rats (Figure 1) correlated with transient p38 MAPK activation by RI in the normal but not in the metabolic syndrome rats [23]. Furthermore, p38 MAPK inhibition significantly decreased RI-induced MMP 2 activation (~50%) and completely abolished RI-induced MMP 9 activation, as well as the degradation of elastin, laminin and type IV collagen (Figure 4). These results indicate that the p38 MAPK pathway rather than the altered oxidative state alone is in large part directly responsible for decreased MMP 2 and 9 activation in the metabolic syndrome.
We did not investigate the exact mechanism by which p38 MAPK regulates MMP 2 and 9 or whether this regulation was direct or indirect. However, several findings suggest that p38 MAPK may directly increase the expression of these MMPs. Despite the popular belief that MMP 2 is a constitutive enzyme, we observed no significant baseline expression of either MMP 2 or 9, but a significant increase in expression at day 3 of RI (Figure 1). This is in agreement with mounting evidence indicating that MMP 2 expression is in fact highly regulated in cardiac cells in response to a multitude of factors, which have been shown to play a role in collateral remodeling, including angiotensin II, inflammatory mediators (TNF-α) and growth factors (bFGF) [63]. This activation was transient and returned to baseline by day 6 of RI (Figure 1). In fact, upon closer examination of the full time-course of RI, we observed that p38 MAPK was activated on both days 2 and 3 of RI, whereas MMP 2 and 9 expression and activation demonstrated a slight trend towards an increase, which did not achieve statistical significance on day 2 of RI, reached maximum on day 3 of RI and remained elevated at ~ 40% of maximum on day 4 of RI, returning back to baseline by day 5 of RI (data not show). The relationship between these two time-courses is compatible with the idea that p38 MAPK directly regulates MMP 2 and 9 expression. In further support of this notion, when p38 MAPK was inhibited, we observed no additional inhibition in MMP 2 or 9 activation other than that which could be accounted for by the observed decrease in their expression (Figures 2 and 3). Binding sites for many of the classical transcription factors downstream of p38 MAPK, such as STAT-1, Myc, Elk-1 and ATF-2 [64, 65], have not been identified in MMP 2 and 9 promoters. However, ETS-1, also a transcription factor downstream of p38 MAPK, has been shown to upregulate MMP expression [66]. Also, it is known that p38 MAPK regulates angiogenesis and cell invasion through the regulation of MMPs, which is dependent on the transcription factor AP-1 [64].
Another limitation to our study is that we did not investigate the cellular source of MMPs. All vascular and cardiac cells are capable of synthesizing and secreting MMP 2 and 9. Since 80% of cells, by volume, in the whole heart are cardiac myocytes, the observed p38 MAPK-dependent regulation of MMP 2 and 9 in this study could be in significant part related to their activation in myocytes. Several studies support the idea that p38 MAPK activation in myocytes may play a role in activation and secretion of paracrine factors, such as MMPs. p38 MAPK has been shown to regulate secretion of some growth factors, including VEGF, from myocytes [67]. Doxorubicin stimulation resulted in p38 MAPK-dependent MMP 9 activation in isolated cardiomyocytes [68]. In addition, cardiac fibroblasts are not only the most numerous cell type in the heart but are also major producers of both ECM components and MMPs. A different form of RI, ischemic preconditioning, has been shown to stimulate p38 MAPK activation in cardiac fibroblasts. Thus p38 MAPK-dependent MMP regulation in cardiac fibroblasts likely also plays a significant role. However, inflammatory cells, including neutrophils and macrophages, are also well-known producers of MMPs [69]. For example, secretion and activation of MMPs by macrophages induces degradation of the ECM in atherosclerotic plaques and consequent plaque rupture [70]. Although significant neutrophil infiltration is not typically associated with collateral remodeling and we have not observed it in our model, in ligation-induced collateral remodeling, macrophages accumulate along collateral vessels within 12 hours, reaching a maximum around 3 days and diminishing over time [69]. Thus, MMP 2 and 9 secretion from macrophages is also highly likely to play a significant role in CCG modulation.
We have previously shown that p38 MAPK was activated by RI in normal, healthy but not in the metabolic syndrome rats, and that inhibition of p38 MAPK abrogated CCG in the normal animals [23, 24, 71]. The present study provides a mechanism through which p38 MAPK regulates CCG by identifying MMP 2 and 9 activation as a critical early step required for CCG and demonstrates that this mechanism is dysfunctional in the metabolic syndrome. Thus, this study offers new insight into the underlying reasons for the absence of coronary collateral development in the metabolic syndrome. However, specific localization of events is important for many paracrine interactions as well as for collateral growth. Thus, while we provide a novel key mechanism involved in the regulation of CCG, the implications of our study are limited by lack of ability to ascertain the exact localization of MMP 2 and 9 activation within the walls of growing coronary collaterals as well as the actual source of these MMPs. Studies conducted in larger animal models of coronary collateral growth are necessary to answer these questions.
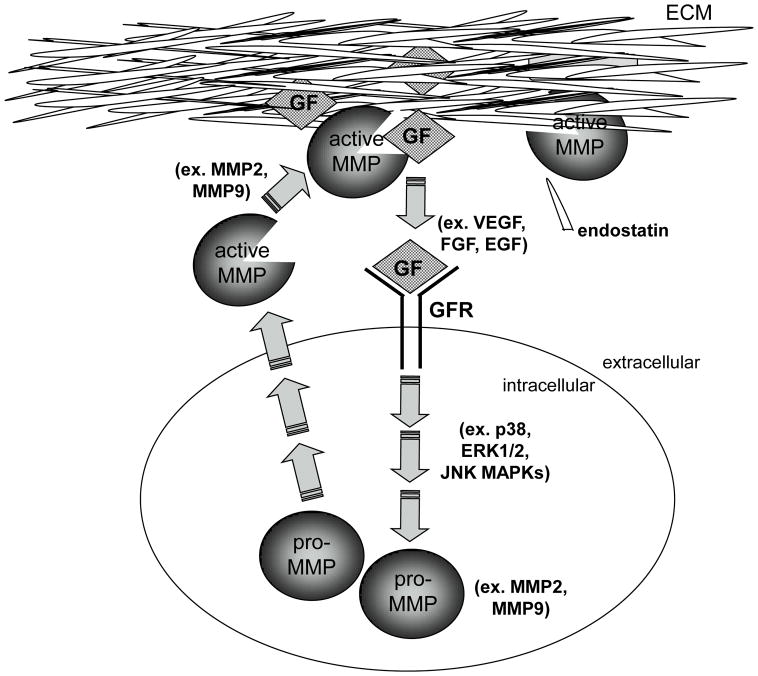
Coronary collateral remodeling in response to transient, repetitive ischemia is a complex process, which involves a coordinated interplay between multiple cell types, growth factors, inflammatory cytokines and the ECM. The relationship between these factors in collateral growth remains largely unknown, but some inferences may be made from in vitro and cancer studies. Inflammatory cytokines and growth factors, including VEGF, through the Flt-1 receptor, have been shown to induce MMP 2 and 9 expression and activation [72–74]. On the other hand, MMP 2 and 9 have been shown to increase the bioavailability of TGF-β, VEGF and FGF-2 by releasing them from their binding proteins and the ECM where they are sequestered in their latent forms [75–78]. Thus, pro-angiogenic growth factors, inflammatory cytokines and MMPs appear to be able to amplify each other’s effects through positive feedback mechanisms. In addition, MMPs can also generate anti-angiogenic peptides, endostatin and tumstatin by degrading type XVIII and IV collagen in some types of basement membranes [79]. Therefore, MMPs may play a critical role in the regulation of collateral growth not only by removing the physical ECM barrier to allow for efficient migration of endothelial and vascular smooth muscle cells, but perhaps more importantly by regulating the balance of the pro-angiogenic growth factors and the anti-angiogenic peptides generated through MMP-dependent ECM degradation which ultimately determines the extent and duration of collateral growth (Figure 8).
Figure 8.
Proposed interaction between growth factors and MMPs in the coordinated regulation of collateral growth.
Supplementary Material
Highlights.
p38 MAPK is required for MMP 2/9 activity during coronary collateral growth (CG)
MMP 2/9 activity is essential for extracellular matrix (ECM) degradation and CG
Lack of CG in metabolic syndrome correlates with lack of MMP 2/9 activity and reduced ECM degradation
Acknowledgments
Supported by NIH R01 HL093052.
Footnotes
Disclosures: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–9. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- 2.Seiler C. The human coronary collateral circulation. Heart. 2003;89:1352–7. doi: 10.1136/heart.89.11.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554–60. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz MB, Biyikoglu SF, Akin Y, Guray U, Kisacik HL, Korkmaz S. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord. 2003;27:1541–5. doi: 10.1038/sj.ijo.0802474. [DOI] [PubMed] [Google Scholar]
- 5.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol. 2008;28:61–7. doi: 10.1161/ATVBAHA.107.154294. [DOI] [PubMed] [Google Scholar]
- 6.Wilson D, Massaeli H, Russell JC, Pierce GN, Zahradka P. Low matrix metalloproteinase levels precede vascular lesion formation in the JCR:LA-cp rat. Mol Cell Biochem. 2003;249:151–5. [PubMed] [Google Scholar]
- 7.Scholz D, Cai WJ, Schaper W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis. 2001;4:247–57. doi: 10.1023/a:1016094004084. [DOI] [PubMed] [Google Scholar]
- 8.Unthank JL, Nixon JC, Lash JM. Early adaptations in collateral and microvascular resistances after ligation of the rat femoral artery. J Appl Physiol. 1995;79:73–82. doi: 10.1152/jappl.1995.79.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–23. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 11.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 12.Murphy G, Nguyen Q, Cockett MI, Atkinson SJ, Allan JA, Knight CG, et al. Assessment of the role of the fibronectin-like domain of gelatinase A by analysis of a deletion mutant. J Biol Chem. 1994;269:6632–6. [PubMed] [Google Scholar]
- 13.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 14.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 15.Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP) Curr Top Dev Biol. 2003;54:1–74. doi: 10.1016/s0070-2153(03)54004-2. [DOI] [PubMed] [Google Scholar]
- 16.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–72. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 17.Cai WJ, Kocsis E, Wu X, Rodriguez M, Luo X, Schaper W, et al. Remodeling of the vascular tunica media is essential for development of collateral vessels in the canine heart. Mol Cell Biochem. 2004;264:201–10. doi: 10.1023/b:mcbi.0000044389.65590.57. [DOI] [PubMed] [Google Scholar]
- 18.Haas TL, Doyle JL, Distasi MR, Norton LE, Sheridan KM, Unthank JL. Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am J Physiol Heart Circ Physiol. 2007;293:H2429–37. doi: 10.1152/ajpheart.00100.2007. [DOI] [PubMed] [Google Scholar]
- 19.Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol. 2007;213:355–64. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- 20.McCawley LJ, Li S, Wattenberg EV, Hudson LG. Sustained activation of the mitogen-activated protein kinase pathway. A mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem. 1999;274:4347–53. doi: 10.1074/jbc.274.7.4347. [DOI] [PubMed] [Google Scholar]
- 21.Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol. 2000;165:5788–97. doi: 10.4049/jimmunol.165.10.5788. [DOI] [PubMed] [Google Scholar]
- 22.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, et al. Interleukin-1beta induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol. 2007;211:759–70. doi: 10.1002/jcp.20992. [DOI] [PubMed] [Google Scholar]
- 23.Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol. 2007;292:H2729–36. doi: 10.1152/ajpheart.01330.2006. [DOI] [PubMed] [Google Scholar]
- 24.Reed R, Potter B, Smith E, Jadhav R, Villalta P, Jo H, et al. Redox-sensitive Akt and Src regulate coronary collateral growth in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;296:H1811–21. doi: 10.1152/ajpheart.00920.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O’Malley P, et al. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112:2108–13. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 26.Jadhav R, Dodd T, Smith E, Bailey E, Delucia AL, Russell JC, et al. Angiotensin type I receptor blockade in conjunction with enhanced Akt activation restores coronary collateral growth in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2011;300:H1938–49. doi: 10.1152/ajpheart.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monaco S, Sparano V, Gioia M, Sbardella D, Di Pierro D, Marini S, et al. Enzymatic processing of collagen IV by MMP-2 (gelatinase A) affects neutrophil migration and it is modulated by extracatalytic domains. Protein Sci. 2006;15:2805–15. doi: 10.1110/ps.062430706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mainardi CL, Dixit SN, Kang AH. Degradation of type IV (basement membrane) collagen by a proteinase isolated from human polymorphonuclear leukocyte granules. J Biol Chem. 1980;255:5435–41. [PubMed] [Google Scholar]
- 29.Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25:6401–8. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ardini E, Sporchia B, Pollegioni L, Modugno M, Ghirelli C, Castiglioni F, et al. Identification of a novel function for 67-kDa laminin receptor: increase in laminin degradation rate and release of motility fragments. Cancer Res. 2002;62:1321–5. [PubMed] [Google Scholar]
- 31.Sivaprasad S, Chong NV, Bailey TA. Serum elastin-derived peptides in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46:3046–51. doi: 10.1167/iovs.04-1277. [DOI] [PubMed] [Google Scholar]
- 32.Lei H, Kalluri R, Furth EE, Baker AH, Strauss JF., 3rd Rat amnion type IV collagen composition and metabolism: implications for membrane breakdown. Biol Reprod. 1999;60:176–82. doi: 10.1095/biolreprod60.1.176. [DOI] [PubMed] [Google Scholar]
- 33.Yao PL, Lin YC, Richburg JH. TNF alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 2009;80:581–9. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown S, Bernardo MM, Li ZH, Kotra LP, Tanaka Y, Fridman R, et al. Potent and selective mechanism-based inhibition of gelatinases. J Am Chem Soc. 2000;122:6799–800. [Google Scholar]
- 35.Qiu LB, Zhou Y, Wang Q, Yang LL, Liu HQ, Xu SL, et al. Synthetic gelatinases inhibitor attenuates electromagnetic pulse-induced blood-brain barrier disruption by inhibiting gelatinases-mediated ZO-1 degradation in rats. Toxicology. 2011;285:31–8. doi: 10.1016/j.tox.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Cai WJ, Koltai S, Kocsis E, Scholz D, Kostin S, Luo X, et al. Remodeling of the adventitia during coronary arteriogenesis. Am J Physiol Heart Circ Physiol. 2003;284:H31–40. doi: 10.1152/ajpheart.00478.2002. [DOI] [PubMed] [Google Scholar]
- 37.Cai W, Vosschulte R, Afsah-Hedjri A, Koltai S, Kocsis E, Scholz D, et al. Altered balance between extracellular proteolysis and antiproteolysis is associated with adaptive coronary arteriogenesis. J Mol Cell Cardiol. 2000;32:997–1011. doi: 10.1006/jmcc.2000.1137. [DOI] [PubMed] [Google Scholar]
- 38.Ries C, Popp T, Egea V, Kehe K, Jochum M. Matrix metalloproteinase-9 expression and release from skin fibroblasts interacting with keratinocytes: Upregulation in response to sulphur mustard. Toxicology. 2009;263:26–31. doi: 10.1016/j.tox.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27:2734–43. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 40.Kusukawa J, Sasaguri Y, Shima I, Kameyama T, Morimatsu M. Expression of matrix metalloproteinase-2 related to lymph node metastasis of oral squamous cell carcinoma. A clinicopathologic study. Am J Clin Pathol. 1993;99:18–23. doi: 10.1093/ajcp/99.1.18. [DOI] [PubMed] [Google Scholar]
- 41.Kossakowska AE, Hinek A, Edwards DR, Lim MS, Zhang CL, Breitman DR, et al. Proteolytic activity of human non-Hodgkin’s lymphomas. Am J Pathol. 1998;152:565–76. [PMC free article] [PubMed] [Google Scholar]
- 42.Mydel P, Shipley JM, Adair-Kirk TL, Kelley DG, Broekelmann TJ, Mecham RP, et al. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J Biol Chem. 2008;283:9513–22. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 45.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–27. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 46.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996;270:F123–30. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 47.Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–95. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 48.Pap T, Pap G, Hummel KM, Franz JK, Jeisy E, Sainsbury I, et al. Membrane-type-1 matrix metalloproteinase is abundantly expressed in fibroblasts and osteoclasts at the bone-implant interface of aseptically loosened joint arthroplasties in situ. J Rheumatol. 1999;26:166–9. [PubMed] [Google Scholar]
- 49.Lagente V, Boichot E. Role of matrix metalloproteinases in the inflammatory process of respiratory diseases. J Mol Cell Cardiol. 2010;48:440–4. doi: 10.1016/j.yjmcc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Kumar B, Koul S, Petersen J, Khandrika L, Hwa JS, Meacham RB, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res. 2010;70:832–41. doi: 10.1158/0008-5472.CAN-09-2918. [DOI] [PubMed] [Google Scholar]
- 51.Keshet Y, Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- 52.Schieven GL. The biology of p38 kinase: a central role in inflammation. Curr Top Med Chem. 2005;5:921–8. doi: 10.2174/1568026054985902. [DOI] [PubMed] [Google Scholar]
- 53.Rousseau S, Houle F, Huot J. Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc Med. 2000;10:321–7. doi: 10.1016/s1050-1738(01)00072-x. [DOI] [PubMed] [Google Scholar]
- 54.Yang S, Xin X, Zlot C, Ingle G, Fuh G, Li B, et al. Vascular endothelial cell growth factor-driven endothelial tube formation is mediated by vascular endothelial cell growth factor receptor-2, a kinase insert domain-containing receptor. Arterioscler Thromb Vasc Biol. 2001;21:1934–40. doi: 10.1161/hq1201.099432. [DOI] [PubMed] [Google Scholar]
- 55.Issbrucker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, et al. p38 MAP kinase--a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J. 2003;17:262–4. doi: 10.1096/fj.02-0329fje. [DOI] [PubMed] [Google Scholar]
- 56.Boyd PJ, Doyle J, Gee E, Pallan S, Haas TL. MAPK signaling regulates endothelial cell assembly into networks and expression of MT1-MMP and MMP-2. Am J Physiol Cell Physiol. 2005;288:C659–68. doi: 10.1152/ajpcell.00211.2004. [DOI] [PubMed] [Google Scholar]
- 57.Curry JM, Eubank TD, Roberts RD, Wang Y, Pore N, Maity A, et al. M-CSF signals through the MAPK/ERK pathway via Sp1 to induce VEGF production and induces angiogenesis in vivo. PLoS One. 2008;3:e3405. doi: 10.1371/journal.pone.0003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whyte J, Bergin O, Bianchi A, McNally S, Martin F. Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 2009;11:209. doi: 10.1186/bcr2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swarnakar S, Paul S, Singh LP, Reiter RJ. Matrix metalloproteinases in health and disease: regulation by melatonin. J Pineal Res. 2011;50:8–20. doi: 10.1111/j.1600-079X.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 60.Touyz RM. Mitochondrial redox control of matrix metalloproteinase signaling in resistance arteries. Arterioscler Thromb Vasc Biol. 2006;26:685–8. doi: 10.1161/01.ATV.0000216428.90962.60. [DOI] [PubMed] [Google Scholar]
- 61.Wei S, Rothstein EC, Fliegel L, Dell’Italia LJ, Lucchesi PA. Differential MAP kinase activation and Na(+)/H(+) exchanger phosphorylation by H(2)O(2) in rat cardiac myocytes. Am J Physiol Cell Physiol. 2001;281:C1542–50. doi: 10.1152/ajpcell.2001.281.5.C1542. [DOI] [PubMed] [Google Scholar]
- 62.Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, et al. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C494–9. doi: 10.1152/ajpcell.00439.2003. [DOI] [PubMed] [Google Scholar]
- 63.Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85:413–23. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- 64.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–75. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Cook R, Wu CC, Kang YJ, Han J. The role of the p38 pathway in adaptive immunity. Cell Mol Immunol. 2007;4:253–9. [PubMed] [Google Scholar]
- 66.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 67.Luangdilok S, Box C, Harrington K, Rhys-Evans P, Eccles S. MAPK and PI3K signalling differentially regulate angiogenic and lymphangiogenic cytokine secretion in squamous cell carcinoma of the head and neck. Eur J Cancer. 2011;47:520–9. doi: 10.1016/j.ejca.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Spallarossa P, Altieri P, Garibaldi S, Ghigliotti G, Barisione C, Manca V, et al. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: The role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res. 2006;69:736–45. doi: 10.1016/j.cardiores.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Meisner JK, Price RJ. Spatial and temporal coordination of bone marrow-derived cell activity during arteriogenesis: regulation of the endogenous response and therapeutic implications. Microcirculation. 2010;17:583–99. doi: 10.1111/j.1549-8719.2010.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28:2108–14. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 71.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, et al. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid Redox Signal. 2009;11:1961–74. doi: 10.1089/ars.2009.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaneko H, Anzai T, Takahashi T, Kohno T, Shimoda M, Sasaki A, et al. Role of vascular endothelial growth factor-A in development of abdominal aortic aneurysm. Cardiovasc Res. 2011;91:358–67. doi: 10.1093/cvr/cvr080. [DOI] [PubMed] [Google Scholar]
- 73.Ghosh S, Maity P. VEGF antibody plus cisplatin reduces angiogenesis and tumor growth in a xenograft model of ovarian cancer. J Environ Pathol Toxicol Oncol. 2010;29:17–30. doi: 10.1615/jenvironpatholtoxicoloncol.v29.i1.50. [DOI] [PubMed] [Google Scholar]
- 74.Funahashi Y, Shawber CJ, Sharma A, Kanamaru E, Choi YK, Kitajewski J. Notch modulates VEGF action in endothelial cells by inducing Matrix Metalloprotease activity. Vasc Cell. 2011;3:2–22. doi: 10.1186/2045-824X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–77. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 77.Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072–84. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suhr F, Brixius K, Bloch W. Angiogenic and vascular modulation by extracellular matrix cleavage products. Curr Pharm Des. 2009;15:389–410. doi: 10.2174/138161209787315756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.