Abstract
Introduction
Whether intracellular Ca2+ regulates sinoatrial node cell (SANC) action potential (AP) firing rate on a beat-to-beat basis is controversial.
Objective
To directly test the hypothesis of beat-to-beat intracellular Ca2+ regulation of the rate and rhythm of SANC.
Methods and results
We loaded single isolated SANC with a caged Ca2+ buffer, NP-EGTA, and simultaneously recorded membrane potential and intracellular Ca2+. Prior to introduction of the caged Ca2+ buffer, spontaneous local Ca2+ releases (LCRs) during diastolic depolarization (DD) were tightly coupled to rhythmic APs (r2=0.9). The buffer markedly prolonged the decay time (T50) and moderately reduced the amplitude of the AP-induced Ca2+ transient and partially depleted the SR load, suppressed spontaneous diastolic LCRs and uncoupled them from AP generation, and caused AP firing to become markedly slower and dysrhythmic. When Ca2+ was acutely released from the caged compound by flash photolysis, intracellular Ca2+ dynamics were acutely restored and rhythmic APs resumed immediately at a normal rate. After a few rhythmic cycles, however, these effects of the flash waned as interference with Ca2+ dynamics by the caged buffer was reestablished.
Conclusions
Our results directly support the hypothesis that intracellular Ca2+ regulates normal SANC automaticity on a beat-to-beat basis.
Keywords: pacemaker cell automaticity, Ca2+ cycling, pacemaker Ca2+ clock, Ca2+-excitation contraction coupling, arrhythmia
Introduction
A novel hypothesis for the basis of sinoatrial nodal cell (SANC) normal automaticity, supported by experiments in numerous species generated by many labs (cf [1] for review) postulates that surface membrane electrogenic proteins of SANC, functioning as a voltage oscillator (M clock), and intracellular proteins, generating rhythmic Ca2+ oscillations (Ca2+ clock), interact as a system of symmetrically entrained, inter-dependent oscillators.
The hypothesis embraces several key interactions resulting in clock entrainment. Following an action potential (AP), SERCA pumps Ca2+ from the cytosol into a sarcoplasmic reticulum (SR) that is partially depleted of Ca2+, due to periodic Ca2+ release triggered by the AP. Shortly after the maximum diastolic potential, when sufficient Ca2+ has been pumped to achieve a threshold SR Ca2+ load required for spontaneous ryanodine receptor (RyR) activation, RyRs begin to generate spontaneous local Ca2+ releases (LCRs) beneath the cell surface membrane. LCRs grow in number and size during the diastolic depolarization (DD). Their contribution to the DD, via activation of Na+-Ca2+ exchange (NCX) current, also grows exponentially, and this is a major mechanism that drives the DD to threshold for activation of L-type current, which initiates the next AP. The AP, in turn, by (1) inducing Ca2+ release from SR, synchronizes the SR in a relatively Ca2+ depleted state and (2) by effecting Ca2+ influx, restores SR Ca2+ lost from the cell via NCX. Thus, during each cycle the AP “resets” and “rewinds” the SR function within the coupled-clock system.
The coupled-clock system pacemaker hypothesis challenges a long-standing pacemaker research community tenet that the membrane clock is the sole or dominant mechanism for normal automaticity of SANC [2, 3]. Acute photolysis of intracellular caged Ca2+ compound has provided novel mechanistic insights into beat-to-beat Ca2+ cycling in ventricular myocytes [4–7]. Experiments to determine whether impaired SR Ca2+ cycling dynamics by an intracellular caged Ca2+ buffer, or an acute increase in intracellular Ca2+ via photolysis of the caged-Ca2+ buffer regulates SANC AP firing, would be a stringent test of the hypothesis that a coupled clock system regulates normal automaticity specifically on a beat-to-beat basis.
Methods
We loaded NP-EGTA, a caged Ca2+ buffer, into single, isolated rabbit SANC and simultaneously recorded membrane potential via a perforated patch, and imaged Ca2+ by confocal microscopy to determine the effects on AP firing in SANC, of: (1) Ca2+ buffering by the caged EGTA; and (2) the acute increase in intracellular Ca2+ induced by flash photolysis of the Ca2+ buffer. A detailed expanded methods description is available in the Online Data Supplement.
Results and Discussion
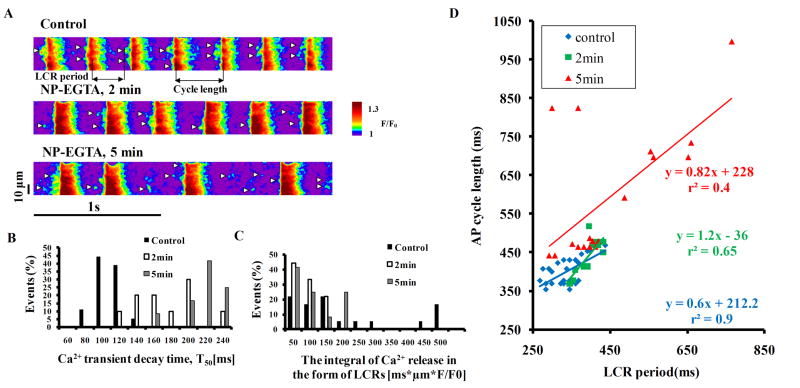
Prior to Ca2+ buffer loading, SANC generate spontaneous rhythmic diastolic LCRs that are highly coupled to the subsequent AP occurrence (Fig. 1A). In response to loading of the Ca2+ buffer, the kinetics of Ca2+ removal from the cytosol are markedly slowed (Fig. 1B and Table S1). The integral of Ca2+ release in the form of LCRs (detected by the Ca2+ indicator) becomes substantially reduced (Fig. 1C). The LCR period, defined as the time from the post AP-induced Ca2+ transient peak to LCR occurrence, becomes prolonged and dysrhythmic. As a result, LCRs become uncoupled from AP generation, which becomes markedly slowed and then dysrhythmic (Fig. 1D). Coefficient of variation (SD/mean) of AP cycle length (CL) prior to NP-EGTA loading was 7±1.5% and increased at 2 min and 5 min following NP-EGTA loading to 9±2% and 20±5%, respectively (n=7 cells). Note that the LCR period closely predicted each AP CL prior to introduction of Ca2+ buffer (r2=0.9, p<0.01), but not after (r2=0.4) (Fig. 1D). Average data on AP induced transients, LCR characteristics and AP CL (n=7 cells) before and after NP-EGTA application are provided in the online supplement Table S1.
Figure 1.
(A) Confocal line-scan images of Ca2+ (expressed as a peak value (F) normalized to minimal fluorescence (F0)) of a representative SANC before and following exposure to 15μM NP-EGTA (small white arrow heads marks LCRs). Histograms of (B) T50 of the AP-induced Ca2+ transient, and (C) the integral of Ca2+ release in the form of LCRs (detected by the Ca2+ indicator). (D) Prior to introduction of the Ca2+ buffer, rhythmic LCR periods were tightly linked to each AP occurrence; but in the presence of NP-EGTA, the LCR periods become dysrhythmic and uncoupled from the AP cycle length. Regression lines are shown with their respective equations.
Figure 2 illustrates the effect of photolysis to release Ca2+ in a cell that had been preloaded with the caged buffer. Prior to flash photolysis, AP firing rate is slow and dysrhythmic and LCRs are small and partially uncoupled from AP generation (Fig.2A). The SR Ca2+ load was reduced by NP-EGTA (the amplitude of the Ca2+ transient induced by a rapid application of caffeine onto the cells decreased from 1.6±0.1 to 1.3±0.1 F/F0, n=9 in each group). Four 50 ms flashes were applied within a time interval of about 150 ms (black arrows in Fig. 2B). Although small in magnitude (Fig. S1), the photo-released Ca2+ dramatically affects SANC function. The AP-induced Ca2+ transient, LCR characteristics/pattern, the DD slope, and the pattern of AP firing are affected (Fig. 2C-D). The initial effect of the flashes to markedly accelerate DD and acutely reduce CL by ~50% occurs after the termination of the last flash (between beats 3 and 4 in Figure 2B and shown in a slower time base in Fig.2F) and corresponds in time to the emergence of augmented and synchronous LCRs, the ensemble of which produces substantial Late Diastolic Ca2+ Elevation (“LDCaE” in Fig. 2E-F) (reaching the red color of F/F0 in the second cycle). This strongly suggests that the marked increase in DD slope, and reduction in CL induced by the flash, do not result directly from Ca2+ release during the flash, but rather require SR pumping of Ca2+ released by the flash and subsequent SR Ca2+ release, resulting in the emergence of large, abundant LCRs. In other words, the acute, transient, marked reduction in CL caused by the flash was likely mediated by its effects to markedly increase LCRs, which accelerate the DD via Ca2+-dependent effects on sarcolemmal molecules. It cannot be directly predicted, however, to what extent the reduction in CL is attributable to augmentation in LCRs that resulted from SR Ca2+ cycling resulting from the flash, or to a direct effect of flash-released Ca2+ on sarcolemmal proteins.
Figure 2.
Photo-released caged Ca2+ acutely re-establishes coupling of Ca2+ clock and M clocks. (A) Prior to the flash, the Ca2+ buffer uncoupled LCRs (line scan image) from APs (brown lines) resulting in a slow, dysrhythmic AP firing rate. (B) Following the last of four 50 ms UV flashes (black arrows), large, synchronous LCRs emerge (small white arrows) and are coupled to the AP occurrence via an acute increase in DD rate, resulting in acute increase in AP firing rate (C). The concomitant acute increase of integral of Ca2+ release in the form of LCRs (detected by the Ca2+ indicator) (LDCaE) is shown in panel D. (Note that APs continued to be recorded for a number of beats beyond the Ca2+ recording). (E, F) The absence of LDCaE pre-flash and its appearance following flash. (G) The acute coupling of the clocks post flash induces a shift in the Ca2+-membrane potential phase-plane diagram relates to AP cycles in E and F.
The phase-plane diagram of the respective duty cycles of the Ca2+ signal and the membrane potential (Fig. 2G) illustrates that photo-released Ca2+ produces a profound change in the coupling of Ca2+ and M-clocks. Prior to the flash, the Ca2+-membrane-voltage loop cycles counterclockwise throughout the cycle, but following the flash the loop cycles half clockwise/half counterclockwise (Fig. 2G). The flash-induced clockwise cycling at the beginning of the loop at low diastolic potentials reflects the emergence of the strong diastolic Ca2+ signal that accelerates the DD. Similar trends were found in nine of the SANC. After three rhythmic APs shown in Fig.2B (AP recording), the effects of the flash waned, likely due to reestablishment of altered/impaired SR Ca2+ cycling dynamics by Ca2+ by the caged buffer, and SANC AP firing rate again becomes slower and dysrythmic on a beat-to-beat basis.
Numerous prior studies, in a variety of species have demonstrated that intracellular Ca2+ buffering by BAPTA-AM or EGTA-AM markedly slows the spontaneous AP firing rate, but intracellular Ca2+ was not measured in these cells (review [1]). However, by using caged Ca2+ buffer and measuring intracellular Ca2+ we examined not only the link between impaired Ca2+ cycling dynamics and disturbed AP firing affected by intracellular Ca2+ buffering, but also the beat-to-beat relationship between intracellular Ca2+ cycling and AP generation by an acute increase in intracellular Ca2+ accomplished by flash photolysis.
Of note, recent reports challenge the existence or symmetry of the coupled-clock system [2, 3]. Introduction of BAPTA into guinea pig SANC via acute rupture of a surface membrane patch failed (in the absence of intracellular Ca2+ measurements) to detect any acute reduction in AP firing rate [3]. The null result, which conflicts with the present and numerous other studies (cf [1] for review) can be explained on the basis of the method to apply BAPTA via a pipette; i.e. a small, artifactual rupture-induced patch seal leak current that shifts the current balance of tiny DD currents offsetting the true BAPTA effect that would, in the absence of the artifact, suppress the DD and prolong the cycle length [8] as demonstrated by numerical modeling of the null results [8].
In summary, our results provide direct evidence to support the hypothesis that a system of an intracellular Ca2+ clock, coupled to a surface membrane clock [1], rather than a dominant surface membrane ion channel clock [2, 3], regulates SANC normal automaticity on a beat-to-beat basis.
Supplementary Material
Acknowledgments
Sources of Funding
The work was supported, in part, by the Intramural Research Program of the National Institute on Aging, National Institute of Health and NIH R01-HL-084487 (ALE).
Glossary
- AP
action potential
- DD
diastolic depolarization
- LCR
local Ca2+ releases
- LDCAE
late diastolic Ca2+ elevation
- NCX
Na+-Ca2+ exchanger
- RyR
ryanodine receptor
- SANC
sinoatrial nodal cells
- SERCA
Ca2+ ATPase sarcoplasmic reticulum pump
- SR
sarcoplasmic reticulum
Footnotes
Disclosures
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res. 2010;106(4):659–73. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noble D, Noble PJ, Fink M. Competing oscillators in cardiac pacemaking: historical background. Circ Res. 2010;106(12):1791–7. doi: 10.1161/CIRCRESAHA.110.218875. [DOI] [PubMed] [Google Scholar]
- 3.Himeno Y, Toyoda F, Satoh H, Amano A, Cha CY, Matsuura H, et al. Minor contribution of cytosolic Ca2+ transients to the pacemaker rhythm in guinea pig sinoatrial node cells. Am J Physiol Heart Circ Physiol. 2010;300(1):H251–61. doi: 10.1152/ajpheart.00764.2010. [DOI] [PubMed] [Google Scholar]
- 4.Sobie EA, Kao JP, Lederer WJ. Novel approach to real-time flash photolysis and confocal [Ca2+] imaging. Pflugers Arch. 2007;454(4):663–73. doi: 10.1007/s00424-007-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz ME, Trafford AW, Eisner DA. The effects of exogenous calcium buffers on the systolic calcium transient in rat ventricular myocytes. Biophys J. 2001;80(4):1915–25. doi: 10.1016/S0006-3495(01)76161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escobar AL, Velez P, Kim AM, Cifuentes F, Fill M, Vergara JL. Kinetic properties of DM-nitrophen and calcium indicators: rapid transient response to flash photolysis. Pflugers Arch. 1997;434(5):615–31. doi: 10.1007/s004240050444. [DOI] [PubMed] [Google Scholar]
- 7.Momotake A, Lindegger N, Niggli E, Barsotti RJ, Ellis-Davies GC. The nitrodibenzofuran chromophore: a new caging group for ultra-efficient photolysis in living cells. Nat Methods. 2006;3(1):35–40. doi: 10.1038/nmeth821. [DOI] [PubMed] [Google Scholar]
- 8.Maltsev VA, Vinogradova TM, Stern MD, Lakatta EG. Letter to the editor: “Validating the requirement for beat-to-beat coupling of the Ca2+ clock and M clock in pacemaker cell normal automaticity”. Am J Physiol Heart Circ Physiol. 2011;300(6):H2323–4. doi: 10.1152/ajpheart.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.