Abstract
Magnetic Resonance Imaging (MRI) could potentially be used to non-invasively predict the strength of an ACL graft after ACL reconstruction. We hypothesized that the volume and T2 relaxation parameters of the ACL graft measured with MRI will predict the graft structural properties and anteroposterior (AP) laxity of the reconstructed knee. Nine goats underwent ACL reconstruction using a patellar tendon autograft augmented with a collagen or collagen-platelet composite. After six weeks of healing, the animals were euthanized, and the reconstructed knees were retrieved and imaged on a 3T scanner. AP laxity was measured prior to dissecting out the femur-graft-tibia constructs which were then tested to tensile failure to determine the structural properties. Regression analysis indicated a statistically significant relationship between the graft volume and the failure load (r2=0.502; p=0.049). When graft volume was normalized to the T2 relaxation time, the relationship was even greater (r2=0.687; p=0.011). There was a significant correlation between the graft volume and the linear stiffness (r2=0.847; p<0.001), which remained significant with T2 normalization (r2=0.764; p=0.002). For AP laxity at 30° flexion, there was not a significant correlation with graft volume, but there was a significant correlation with volume normalized by the T2 relaxation time (r2=0.512; p=0.046). These results suggest that MRI volumetric measures combined with graft T2 properties may be useful in predicting the structural properties of ACL grafts.
Keywords: MRI, ACL, ligament, reconstruction, structural properties
1. Introduction
The injured ACL is commonly replaced by a tendon graft during reconstruction surgery. Because ACL reconstruction does not restore normal kinematics or reduce the risk for premature osteoarthritis, there is an ongoing need to improve treatment options and to verify that new treatment options improve healing. Direct biomechanical measurements of the graft structural properties are frequently used to assess graft healing in research studies. However, these destructive measurements require post-mortem testing, do not permit longitudinal assessments within subjects, and are not suitable for clinical trials. A non-invasive method, such as MRI, that predicts the graft structural properties in vivo is needed to longitudinally evaluate ACL graft healing in vivo within a subject.
MRI has been used to assess ACL graft maturation (Howell et al., 1991; Howell et al., 1995; Orrego et al., 2008; Stockle et al., 1998; Weiler et al., 2001). In an effort to establish the relationship to the biomechanical properties of a healing graft, Weiler et al. found a correlation between the MR signal-to-noise quotient to the failure load, linear stiffness and tensile strength of the graft in the sheep (Weiler, 2001). However, evaluation of the signal-to-noise quotient is limited since it is dependent on acquisition and scanner characteristics. Parameters that are independent of the acquisition characteristics are needed. It seems reasonable to assume that geometric MRI measurements, such as ACL graft volume, would reflect the graft structural properties. T2 mapping may also provide insight because T2 relaxation times are affected by proteoglycan, collagen and water content (Li et al., 2011; Mosher et al., 2000; Regatte et al., 2002; Wayne et al., 2003; White et al., 2006), factors associated with ligament/graft healing (Frank et al., 2006).
The objective of this study was to determine if an MRI-based method, which utilizes both graft volume and T2-mapping, is predictive of the structural properties of the graft and AP knee laxity at 30° and 60° knee flexion. We hypothesized that there are significant correlations between graft volume, T2 relaxation time, and graft volume normalized by T2 relaxation time with the structural properties of an ACL autograft and AP knee laxity when measured six weeks after ACL reconstruction in the goat model.
2. Methods
Model of ACL Reconstruction
Nine 4-year old male Nubian-cross goats underwent unilateral ACL reconstruction using a bone-patellar tendon-bone autograft under an approved IACUC protocol as previously reported (Spindler, 2009). Five goats had their graft enhanced with a collagen-platelet composite, while four were enhanced with a collagen sponge only. One surgeon performed the procedures to minimize surgical variations. The differences in structural properties and AP knee laxity between the two treatment groups for these animals has been previously reported (Spindler, 2009). Postoperatively the animals were allowed unrestricted cage activity for 6 weeks, and were euthanized. The ACL reconstructed knees underwent MR scanning immediately after harvest and were then stored at -20°C prior to mechanical testing.
MR Imaging
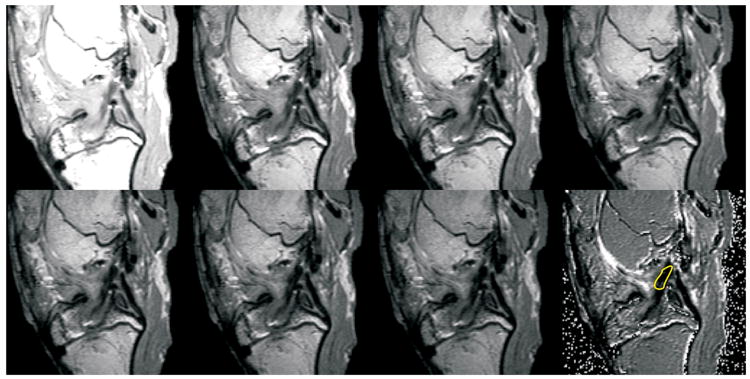
The reconstructed knees were imaged on a 3T MR scanner (Siemens TIMS Trio; Erlangen, Germany) to determine the volume and T2 relaxation time of the graft midsubstance using a T2-relaxometry imaging protocol (TR=2000 ms; TE at 10, 22, 26, 30, 34, 38, and 42 ms, slice thickness = 1mm with 1mm gap). Sagittal oblique images were obtained (Figure 1). The gap of 1 mm between slices was used to avoid artifact due to cross-excitation between adjacent slices. To determine the graft volume, the images were displayed on a graphic workstation (Vitrea workstation; Vital Images, Minnetonka, MN). The outline of the ACL was manually segmented, and the volume computed using the commercial software (Figure 1). Using phantoms, we demonstrated that the volume renderings of the software were accurate to within 1%. T2 maps of the graft midsubstance (Figure 2) were generated from the MR relaxometry data using custom software (IDL; ITT Visual Information Solutions, Boulder, CO) and the mean T2 time of the graft midsubstance was determined.
Figure 1.
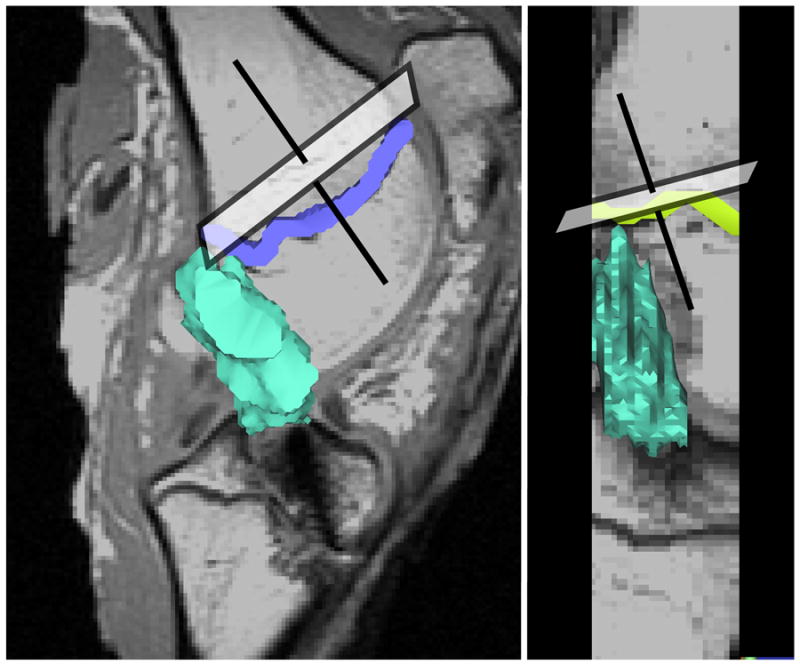
Sagittal oblique images were oriented perpendicular to the distal femoral epiphyseal plate in both the coronal and sagittal planes. The intra-articular volume of the ACL graft (shown in green) was determined by segmenting the ACL within each slice and stacking the slices.
Figure 2.
Seven MR relaxometry images of the same slice, acquired with a fixed repetition time (TR) of 2000 ms, and varying excitation time (TE) of 0, 22, 26, 30, 34, 38 and 42 ms, respectively, followed by the T2 map calculated from the first 7 images are shown. For each voxel in the slice, the T2 is calculated by fitting the signal intensity, S, as a function of TE, according to the equation: S(TE) ∝S0exp(−TE/T2), where S0 is the signal intensity at TE=0. A linear least-squares fit was used to calculate the T2 and S0. The T2 map shown shows the image constructed from the pixel-wise T2 fitting. The region of interest (ROI) used to calculate the mean T2 of the graft is shown in yellow on the T2 map.
Mechanical Testing
The knees were thawed and prepared for laxity and failure testing as previously described (Spindler, 2009). The soft tissues surrounding the tibia and femur were dissected free leaving the joint capsule intact, and the bones were potted in PVC pipes so that they could be mounted on the material testing system.
The AP load-displacement responses of the reconstructed knees were measured using a custom designed fixture with the knee locked at 30° and 60° of flexion (Fleming et al., 2001). Anterior and posterior directed shear loads of ±60 Newtons were applied to the femur with respect to the tibia using an MTS 810 Materials Testing System (MTS, Prairie Eden, MN) while the AP displacement was measured. Tibial axial rotation was locked in the neutral position while the coronal plane translations and varus-valgus angulation were left unconstrained. AP laxity was reported as motion of the tibia with respect to the femur.
After completing the AP laxity tests, the femur-graft-tibia complex was isolated and positioned so that the mechanical axis of the graft was collinear with the loading axis of the material test system (Woo et al., 1991). The knee flexion angle was initially set at 30°. The tibia was mounted to the base of the MTS via a sliding X-Y platform while the femur was unconstrained to rotations. This enabled each specimen to seek its own position so that the tensile load was distributed over the cross-section of the graft. The femur-graft-tibia complex was then loaded to failure at 20mm/min, and the failure load and the linear stiffness values calculated (Fleming et al., 2010).
Statistical Analyses
Regression analyses were performed to determine whether the independent variables of graft volume and T2 relaxation time predicted the dependent variables (the graft structural properties and AP laxity values at the 60° and 30° of flexion) using commercially available software (SigmaStat 3.5; Systat Software, Inc). Regressions were also performed to determine if the volume normalized by the T2 relaxation time was predictive of the same dependent variables. One animal was excluded from analysis because we were unable to obtain a T2 measurement; all calculations were made with data from the remaining eight animals.
3. Results
All graft failures occurred in the midsubstance. There was a significant correlation between graft volume and the failure load (r2=0.502; p=0.049). When the volume was normalized by the T2 relaxation parameter, the variability was reduced and the predictability was improved (r2=0.687; p=0.011; Fig. 1). When the T2 parameter alone was correlated to failure load, the relationship was not significant (r2 = 0.191; p = 0.278).
There was a significant correlation between graft volume and the linear stiffness (r2=0.847; p=0.001). When the volume was normalized by the T2 relaxation parameter, the variability remained essentially unchanged (r2=0.764; p=0.005; Fig. 2). When the T2 parameter alone was correlated to linear stiffness, the relationship was not significant (r2=0.049; p=0.597).
AP laxity at 30° did not correlate with graft volume (r2=0.205; p=0.260). However, when the volume was normalized by the T2 relaxation parameter, a significant correlation was observed (r2=0.512; p=0.046; Fig. 3). When the T2 parameter alone was correlated to AP laxity at 30°, the relationship was not significant (r2=0.471; p=0.060).
Figure 3.
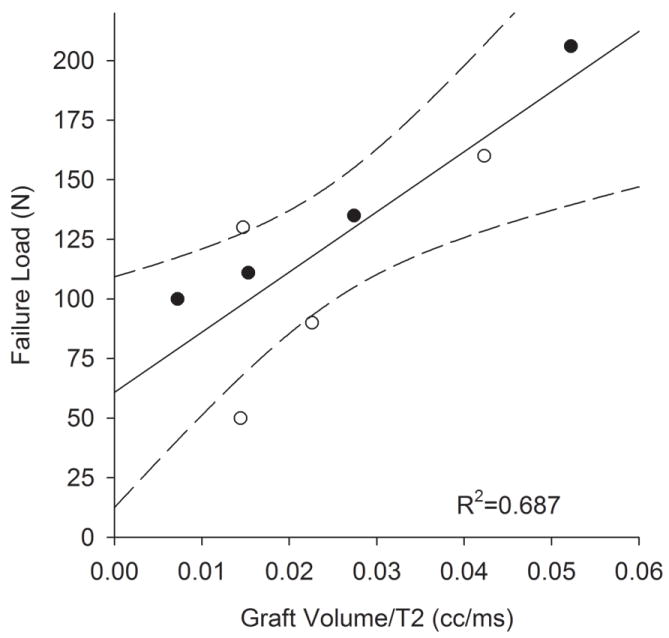
Graft failure load after 6 weeks of healing significantly correlated with graft volume when normalized by the T2 relaxation signal. Solid line represents the linear regression while dashed lines represent 95% confidence intervals.
AP laxity at 60° was not significantly correlated with graft volume (r2=0.182; p=0.291). When the volume was normalized by the T2 relaxation parameter, variability was reduced but remained insignificant (r2=0.415; p=0.085). When the T2 parameter alone was correlated to AP laxity at 60°, the relationship was also not significant (r2=0.288; p=0.170).
4. Discussion
This study demonstrates significant correlations between ACL graft volume via MRI and the structural properties of the ACL autograft and AP knee laxity after 6 weeks of healing. T2 relaxation time alone was not significant. T2 relaxation time is affected by several compositional factors, including water, proteoglycan, and collagen content and collagen orientation, the interactions of which may increase variability and reduce the correlation coefficient. However, it was interesting to note that normalizing the volume by the T2 relaxation time of the graft, as compared to volume alone, improved the failure load and AP laxity at 30° predictions (r2=0.49 to r2=0.69).
Investigators have studied MR characteristics in both human and animal ACLs to gain insight into the biology of graft healing (Anderson et al., 2001; Arai et al., 2008; Howell et al., 1991; Howell, 1995; Murray et al., 2007; Ntoulia et al., 2011; Orrego, 2008; Radice et al., 2010; Stockle, 1998; Weiler, 2001). Many of these studies have focused on signal intensity of T2-weighted MR images, a measure of water content, which has been linked to vascularity and graft tensile strength (McFarland et al., 1986). Using the rabbit model, Anderson et al noted that graft signal intensity may be related to the tensile strength (Anderson, 2001). Weiler et al reported significant correlations between the signal to noise quotient and biomechanical strength (Weiler, 2001). Building on their study, we found that a significant correlation between the volume normalized by the T2 relaxation time on the failure load and stiffness of the healing ACL graft. Because T2 relaxometry is not dependent on the scanner and acquisition parameters, it may provide a better indicator of graft healing when used in conjunction with graft volume.
There are several study limitations that should be considered. Four of the animals received autograft ACL reconstruction using a collagen-platelet composite while the remaining four received the collagen sponge only. It is possible that the two treatments could have independently affected the MRI properties. However, this concern may be minor given that the regression lines for each treatment subgroup were nearly parallel to that when the data were pooled, and that there was no visual evidence of the CPC or sponge in the joint at the time of harvest. All MRI measurements were taken 6 weeks after ACL reconstruction. Future studies will be performed to determine how these predictions may change with time and whether one time point is prediction of another. The volume measurement accuracy was determined using phantoms and not ACL tissue. While the same degree of accuracy cannot be expected due to the heterogeneous nature of the graft and surrounding tissue, it is reasonable to expect a fairly accurate ACL volume using this methodology. Other limitations include the imaging of post-mortem samples. Nonetheless, the proof of principle demonstrated here suggests that further research in this area should be pursued.
Figure 4.
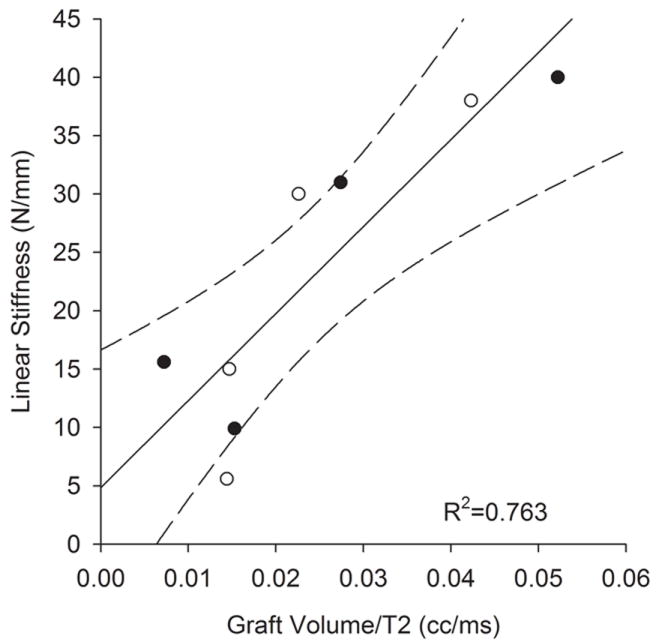
Graft linear stiffness after 6-weeks of graft healing was proportional to the graft volume when normalized by the T2 relaxation signal. Solid line represents the linear regression while dashed lines represent 95% confidence intervals.
Figure 5.
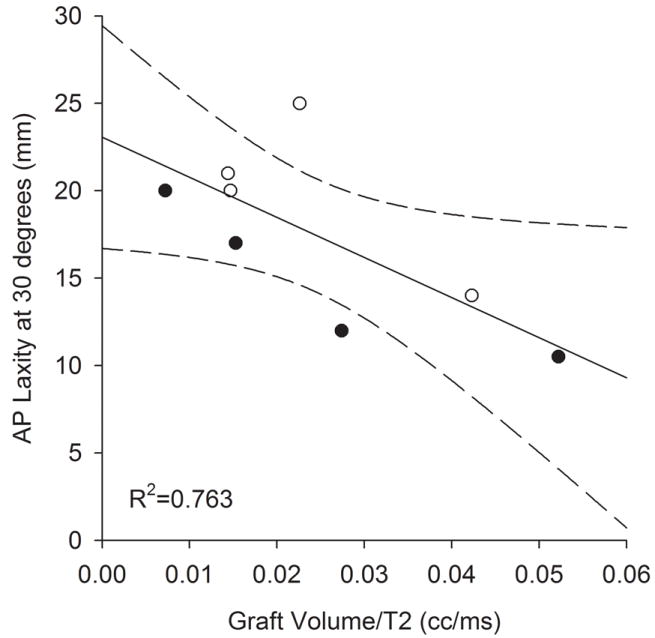
AP knee laxity at 30° flexion after 6-weeks of healing correlated with graft volume normalized by the T2 relaxation signal. Solid line represents the linear regression while dashed lines represent 95% confidence intervals.
Acknowledgments
Funding for this study was received from NIH; RO1-AR049199 (BCF) and RO1-AR052772 (MMM), the Vanderbilt’s Kenneth D. Schermerhorn Endowed Professorship and the RIH Orthopaedic Foundation. The authors would like to thank Alison Biercevicz for her help with the creation of the figures.
Footnotes
Conflict of Interest The authors have no financial or personal relationships that could bias this work.
References
- Anderson K, Seneviratne AM, Izawa K, Atkinson BL, Potter HG, Rodeo SA. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001;29:689–698. doi: 10.1177/03635465010290060301. [DOI] [PubMed] [Google Scholar]
- Arai Y, Hara K, Takahashi T, Urade H, Minami G, Takamiya H, Kubo T. Evaluation of the vascular status of autogenous hamstring tendon grafts after anterior cruciate ligament reconstruction in humans using magnetic resonance angiography. Knee Surg Sports Traumatol Arthrosc. 2008;16:342–347. doi: 10.1007/s00167-007-0478-6. [DOI] [PubMed] [Google Scholar]
- Fleming BC, Abate JA, Peura GD, Beynnon BD. The relationship between graft tensioning and the anterior-posterior laxity in the anterior cruciate ligament reconstructed goat knee. J Orthop Res. 2001;19:841–844. doi: 10.1016/S0736-0266(01)00020-1. [DOI] [PubMed] [Google Scholar]
- Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28:703–709. doi: 10.1002/jor.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SM, Berns GS, Farley TE. Unimpinged and impinged anterior cruciate ligament grafts: MR signal intensity measurements. Radiol. 1991;179(3):639–643. doi: 10.1148/radiology.179.3.2027966. [DOI] [PubMed] [Google Scholar]
- Howell SM, Clark JA, Blasier RD. Serial magnetic resonance imaging of hamstring anterior cruciate ligament autografts during the first year of implantation. A preliminary study. Am J Sports Med. 1991;19:42–47. doi: 10.1177/036354659101900107. [DOI] [PubMed] [Google Scholar]
- Howell SM, Knox KE, Farley TE, Taylor MA. Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am J Sports Med. 1995;23:42–49. doi: 10.1177/036354659502300107. [DOI] [PubMed] [Google Scholar]
- Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, Ma CB, Majumdar S. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiol. 2011;258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland EG, Morrey BF, An KN, Wood MB. The relationship of vascularity and water content to tensile strength in a patellar tendon replacement of the anterior cruciate in dogs. Am J Sports Med. 1986;14:436–448. doi: 10.1177/036354658601400602. [DOI] [PubMed] [Google Scholar]
- Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: Influence of aging and early symptomatic degeneration on the spatial variation of T2-Preliminary findings at 3 T1. Radiol. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- Ntoulia A, Papadopoulou F, Ristanis S, Argyropoulou M, Georgoulis AD. Revascularization Process of the Bone-Patellar Tendon-Bone Autograft Evaluated by Contrast-Enhanced Magnetic Resonance Imaging 6 and 12 Months After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2011;39:1478–1486. doi: 10.1177/0363546511398039. [DOI] [PubMed] [Google Scholar]
- Orrego M, Larrain C, Rosales J, Valenzuela L, Matas J, Durruty J, Sudy H, Mardones R. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy. 2008;24:1373–1380. doi: 10.1016/j.arthro.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Radice F, Yanez R, Gutierrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010;26:50–57. doi: 10.1016/j.arthro.2009.06.030. [DOI] [PubMed] [Google Scholar]
- Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- Spindler KP, Murray MM, Carey JL, Zurakowski D, Fleming BC. The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res. 2009;27:631–638. doi: 10.1002/jor.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockle U, Hoffmann R, Schwedke J, Lubrich J, Vogl T, Sudkamp NP, Haas N. Anterior cruciate ligament reconstruction: the diagnostic value of MRI. Int Orthop. 1998;22:288–292. doi: 10.1007/s002640050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne JS, Kraft KA, Shields KJ, Yin C, Owen JR, Disler DG. MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiol. 2003;228:493–499. doi: 10.1148/radiol.2282012012. [DOI] [PubMed] [Google Scholar]
- Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging - A two-year study in sheep. Am J Sports Med. 2001;29:751–761. doi: 10.1177/03635465010290061401. [DOI] [PubMed] [Google Scholar]
- White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiol. 2006;241:407–414. doi: 10.1148/radiol.2412051750. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effect of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]