Abstract
The purpose of the study was to investigate whether sleep duration during early childhood was associated with fat mass and bone mineral content (BMC). BMC and fat mass were measured by dual-energy x-ray absorptiometry (DXA) in children (n=336) ages 4–12 years. Sleep was quantified according to parental report of hours slept at night and napping. The relationship between sleep pattern and body composition was tested using ANOVA including confounding factors. Based on the sample distribution, children were grouped into tertiles of sleep duration. BMC was greater in children with longer sleep duration (p=0.02). Age was inversely associated with sleep duration; therefore the sample was analyzed by age category using seven years old as the cut-point. The relationship remained significant only among younger children. Napping was positively associated with BMC (p=0.001). Sleep duration was not associated with fat parameters. Longer sleep duration, may allow for optimal energy resource partitioning in which bone is favored. Sleep duration of less than 8 hours may impair bone mass accrual, particularly during periods of rapid growth.
Keywords: sleep curtailment, bone mineral content, growth, pediatric
INTRODUCTION
There is growing evidence that sleep curtailment is an important risk factor for a number of metabolic diseases (e.g. obesity, diabetes) [1–4], and the impact is apparently greater in children than adults [5]. The myriad of interrelated metabolic changes during childhood and the implication of sleep patterning on not only normal development but also on body tissue compartmentalization (e.g. fat storage vs. bone remodeling) are gaining interest, indicating an intricate balance between metabolic pathways involved [6]. A recent meta-analysis of 17 studies conducted in the pediatric population suggests a dose-dependent response with each additional hour of sleep decreasing obesity risk by nearly 10% in young overweight/obese children, particularly in boys [7]. Among the primary functions of sleep is conservation of energy for growth and restoration/maintenance of tissue structure. It is plausible that sleep deprivation interferes with energy coordination, in which adipogenic pathways are favored over those associated with greater energetic demand (i.e. osteogenesis). Given the high energy cost associated with the linear growth, as well as the interplay between adipogenic and osteogenic pathways [6], the contribution of sleep duration and architecture to these processes is an intriguing area for investigation.
The increased energy requirements of childhood growth and development may necessitate increased amounts of sleep for tissue (re)modeling, repair and other biochemical consequences of waking metabolic activity [8;9]. Although most studies have examined the contribution of sleep to adiposity parameters, little attention has been paid to how sleep relates to bone mass accrual. Skeletal remodeling, an energetically more demanding process relative to fat storage may significantly contribute the association between sleep and energy balance. To that end, the objective of this study was to investigate whether sleep duration patterns during early childhood influence bone mineral content (BMC) and if the effect differs across age or sex groups.
MATERIALS AND METHODS
Participants
Measures on 336 children, aged 4–12 years, were used for current analyses. Participants required two visits. On the first visit anthropometric and body composition assessments were taken. On the second visit, indirect calorimetry was performed. All were pubertal stage ≤3 as assessed by the study’s pediatrician (according to Marshall and Tanner [10], healthy, and not taking medications known to affect body composition. Parents and children provided consent/assent, respectively, after reviewing the protocol with study personnel. The protocol was approved by the Institutional Review Board (IRB) for human participants at the University of Alabama at Birmingham (UAB). All measurements were performed between 2005 and 2010.
Sleep
Night time sleep duration was assessed by survey undertaken by parents asking “what time does our child usually go to bed at night and usually wake up in the morning.” In addition, napping frequency and duration were assessed by asking “how long does your child usually take a nap,” with the response “my child does not take naps” a choice. These items, adapted from the Night Eating Questionnaire, were used to compute average hours of night sleeping and napping, and constituted total sleep. Similar sleep questions have been used with this age group in various studies [3;5;11;12].
Body Composition Assessment
Body composition measures were assessed by dual energy x-ray absorptiometry (DXA) using a GE Lunar Prodigy densitometer (GE Lunar Radiation corp., Madison, WI) with pediatric software (version 1.5e). Participants were scanned in light clothing, lying flat on their back with arms at their sides. Outcome measures included overall fat and lean mass, as well as bone mineral content (BMC) and bone mineral density (BMD). DXA has been reported to be reliable in this population and the coefficient of variation has been reported to be 4% [13].
Resting Energy Expenditure (REE)
REE was measured in the morning following an overnight fast. A computerized, open-circuit indirect calorimetry system with a ventilated canopy (Delta Trac II; Sensor Medics, Yorba Linda, CA) was used. While lying supine in a quiet, well-ventilated room, the head of the subject was enclosed in a plexiglass canopy. Participants were instructed not to sleep and remain quiet and still, breathing normally. One-minute average intervals of oxygen uptake (VO2) and carbon dioxide production (CO2) were measured continuously for thirty minutes, in which the last 20 minutes were used to calculate REE. Day to day subject variability for the Delta Trac is 5% [14].
Anthropometric Measures
Anthropometric measures were obtained by a registered dietitian. Height (Heightronic 235; Measurement Concepts, Snoqualmie, WA) and weight (Scale-tronix 6702W; Scale-tronix, Carol Stream IL) were obtained in minimal clothing without shoes. BMI percentile was calculated using age- and sex-specific growth charts [15].
Pubertal Status
Pubertal progression is associated with characteristic influences on body composition and energy metabolism. The one-to-five staging of Marshall and Tanner, based on examination of both breast and pubic hair development, was used for pubertal stage assessment, with one composite number representing the higher of the two assigned values [10].
Statistical Methods
The mean and standard error of sample characteristics (age, height, weight, pubertal stage, BMI%, BMI z-score, total fat, percent fat, total BMC, total sleep, night sleep, nap sleep, REE) were determined for the total sample and stratified by age category (4–7y, 8–12y) and sex (Table 1). Sleep duration was analyzed to determine the sample distribution. Subsequently, the group was divided into tertiles based on the observed distribution in the sample. Two sets of multiple linear regression models were analyzed. In the first models, we tested contributions of sleep to BMC with fat as a covariate and sleep to fat parameters (total fat, percent fat, BMI). The models were adjusted for height, age, sex, and race. Both nighttime sleep duration and day time sleep were evaluated as independent variables.
Table 1.
Sample Characteristics (Mean±SE)
| Total Sample N=336 |
Age<7 N=232† |
Age>7 N=104 |
Boys N=168 |
Girls N=168 |
|
|---|---|---|---|---|---|
| Age | 9.0±0.1 | 5.9±0.2 | 9.9±0.1 | 9.1±0.2 | 8.9±0.1 |
| Height | 53.5±0.3 | 46.3±0.6 | 55.5±0.2 | 53.5±0.4 | 53.4±0.4 |
| Weight | 76.5±1.2 | 54.5±1.5 | 82.7±1.2 | 77.2±1.8 | 75.9±1.5 |
| Tanner Stage | 1.44±0.1 | 1.0±0 | 1.6±0.1 | 1.3±0.1a | 1.6±0.1b |
| BMI % | 66.0±1.5 | 75.3±3.1 | 64.6±1.6 | 66.1±2.0 | 65.9±2.1 |
| BMI z-score | 0.1±0.1 | 0.1±0.1 | −0.3±0.1 | 0.1±0.1 | −0.1±0.1 |
| Total Fat | 8.7±0.3 | 6.1±0.3 | 9.4±0.3 | 8.0±0.4a | 9.4±0.4b |
| % Fat | 24.2±0.5 | 24.8±0.9 | 24.1±0.6 | 21.7±0.7a | 26.9±0.6b |
| Total BMC | 1208.6±18.1 | 838.5±23.9 | 1312.9±17.9 | 1230.6±26.4 | 1185.6±24.4 |
| BMD | 0.92±0.01 | 0.87±0.01 | 0.92±0.01 | 0.93±0.01 | 0.91±0.01 |
| Total Sleep | 9.2 | 9.9±0.1 | 9.0±0.1 | 9.1±0.1a | 9.4±0.1b |
| Night Sleep | 9.0±0.1 | 9.4±0.1 | 8.9±0.1 | 8.9±0.1 | 9.1±0.1 |
| Nap Sleep | 0.3±0.1 | 0.5±0.1 | 0.3±0.1 | 0.3±0.1 | 0.4±0.1 |
| REE | 1192.7±13.7 | 1050.3±24.1 | 1215.1±14.9 | 1239.8±19.8 | 1140.9±17.8 |
denotes significant difference for all variables between age categories p<0.001.
superscript indicates significant difference between sex groups.
A second set of multiple regression models were used to evaluate contribution of sleep to BMC and fat parameters stratified by age category using age seven as a cut-point. We used this age to ensure pre-pubertal status. Models stratified by age category were adjusted for height, age, sex, and race.
Models were also analyzed including BMD rather than BMC as the dependent variable. Similar results, were observed although the associations were not as statistically significant. It is important to note that when estimating measured area expressed as areal bone mineral density (aBMD) it is a composite measure of cortical and cancellous bone is derived utilizing BMC. In addition, DXA measures of aBMD which are influenced by skeletal growth and bone size and because wider bones are also thicker, DXA overestimates substantially BMD of larger bones. Accordingly, only BMC results are reported.
To conform to the assumptions of linear regression, all statistical models were evaluated for residual normality and logarithmic transformations were performed when appropriate. All data were analyzed using SAS 9.1 software (SAS Institute, Cary, NC).
RESULTS
All sample characteristic parameters were significantly different in the younger children compared to the older children (p<0.001; Table 1). Girls were reproductively more mature (p=0.001), had greater adiposity (total and percent fat) and longer total sleep duration (p=0.03).
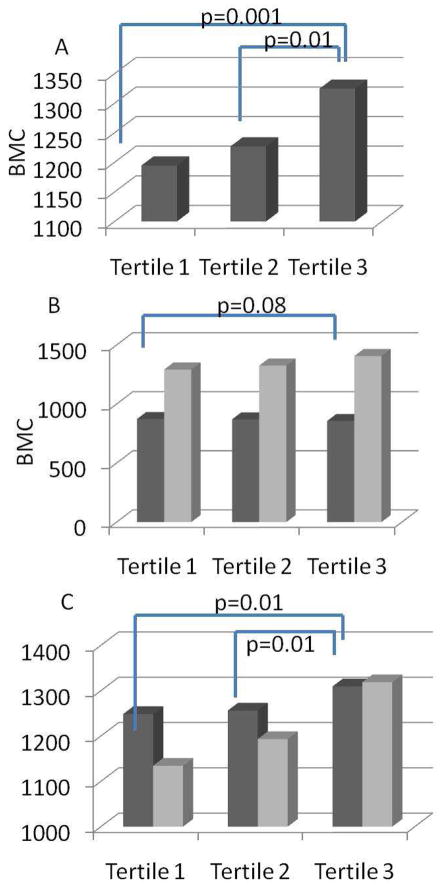
The relationship between sleep duration and BMC for the total sample is depicted in Figure 1A. Individuals in the tertile representing the greatest sleep duration (tertile three) had significantly greater BMC relative to the other groups. There was no difference in BMC between individuals in tertiles one (p=0.001) and two (p=0.01). When stratified by age category, among children seven years and younger, there were no differences in BMC according to sleep duration; however, among older children, those with greater sleep duration had marginally greater BMC (p=0.08). When stratified according to sex, the positive relationship between BMC and sleep duration was only apparent in girls, such that girls in tertile three had significantly greater BMC than tertiles one and two (p<0.01, both).
Figure 1.
Association between tertile sleep duration and bone mineral content (BMC) in A) the total sample B) children <7 (dark gray bars) and >7 (light gray bars) C) boys (dark gray bars) and girls (light gray bars).
Napping was also significant and positively associated with BMC in the total sample (p=0.001; p<0.05), in both age categories (p<0.001young, p<0.05old) and both sexes (p<0.05boys, p<0.01girls).
There were no relationships detected between adiposity parameters (total fat, percent fat or BMI) and sleep duration (Table 2) or nap taking. In addition, no associations were observed between REE and sleep (night or day) duration (data not shown).
Table 2.
Contribution of sleep to adiposity parameters.
| Total Sample | <7 years | >7 years | Boys | Girls | |
|---|---|---|---|---|---|
| Total Body Fat | p=0.88 | p=0.97 | p=0.89 | p=0.81 | p=0.53 |
| Percent Fat | p=0.49 | p=0.69 | p=0.42 | p=0.76 | p=0.75 |
| BMI | p=0.70 | p=0.85 | p=0.41 | p=0.15 | p=0.32 |
DISCUSSION
Because the biology underpinning childhood growth or more specifically body composition patterning is readily influenced by sleep, time spent sleeping has become an important contributor to the foundation of health. Our findings indicate that bone, arguably the most energy-demanding body composition compartment, is impacted by sleep duration in childhood. Further, the differential relationship of sleep and bone across age and sex groups likely reflects the change in metabolic requirements as children approach the pubertal transition, in which an up-regulation occurs for bone (re)modeling. To our knowledge, this is the first study to investigate the contribution of sleep to bone mass accrual, particularly during this critical period of development in which sleep patterns are characteristically dysregulated [8].
Though the mechanisms have not been identified, we hypothesize on potential mediators focusing on the relationship between sleep and bone mass accrual may be similar to those relating sleep to adipogenesis, although in the opposing direction. The interplay between fat and bone mass is plausibly divergently impacted by altered reproductive hormone signaling secondary to sleep insufficiency. While the link between energy availability and sex hormone patterns, as well hormones related to growth, appetite and stress (each dynamic during growth and sensitive to sleep time/quality) is well established [3;8;16], the actual physiologic linking signals and the degree of influence by sleep are less clear, warranting further exploration.
Beyond changes in nighttime sleep duration, napping frequency and duration both decline with age. Although evidence suggests that napping doesn’t substitute for nighttime sleep in terms of body composition outcomes [11;17], across both age and sex groups, we observed that napping was associated with greater BMC, likely attributable the positive relationship between contemporaneous total sleep duration and napping.
Our data showed a moderating effect by both age and sex, suggesting the potential for a temporal window of vulnerability of bone acquisition to shortened sleep duration. Older children had shorter sleep duration, despite on-going (and potentially increasing) need. Indeed, sleep duration decrease in adolescence [11] can have profound effects on body composition parameters, particularly bone acquisition. Sleep requirement for bone acquisition may truly differ between girls and boys as our data suggests or may reflect a closer proximity to puberty experienced by girls in our cohort. It is possible that during linear growth with rapid bone modeling sleep is essential, whereas, boys, who mature later than girls, are less impacted at this age. The relatively rapid rate of mineral gain coupled with the puberty-related metabolic perturbations when approaching the pubertal transition offers the possibility of profound interactions between the skeleton and energy homeostasis at this stage of life.
Although our findings are in concordance with some but not all studies, [18–21], [2;3;11;22;23], we did not find a relationship between adiposity and sleep (nighttime or daytime) duration, nor did we observe a sex difference in sleep and adiposity parameters. The disconcordance may be the result of using a relatively lean sample (average BMI percentile), while others are inclusive of a wider range of body habitus. Bayer and colleagues report that the relationship between sleep and adiposity parameters may be greater in those children whole body fat is already elevated [24]. Interestingly, Lytle et al has reported a weaker association between adiposity and sleep in girls relative to boys. They speculate that girls may be evolutionarily more resilient and able to cope with reduced sleep without health compromise [2]. Given that studies in girls included a significantly older cohort (and reproductively more mature) than ours suggest a non-monotonic relationship may exist over the reproductive maturation process and differ in the timing and tempo between boys and girls.
Sleep has also been associated with lower resting energy expenditure (REE), typically the greatest contributor to total energy expenditure in most individuals [19]. Although we did not detect a difference in REE between sleep tertiles, sleep duration alone may not be sufficient to alter homeostatic mechanisms that promote energy imbalance, but subtle differences could result in changes over time.
Although our study yields valuable insight into sleep biology and health, there are some important limitations to this research. We examined the relationship cross-sectionally, but we believe that poor sleep patterns are risk factors for the development of impairments in bone modeling processes. Also in the analysis, we control for relevant covariates rather than study their effects in a causal pathway. Although it is a limitation often inherent in population-based research, sleep duration was obtained from parental interview. The use of actigraphy may improve methodology by incorporating data of an objective nature. Although causation cannot be inferred based on the results of this study, adequate sleep would likely benefit energy-dependent somatic responses throughout adolescence and into adulthood.
The contribution of sleep to energy metabolism is particularly pertinent during childhood, as several growth-related alterations in resource partitioning have been linked to sleep. The relative importance of sleep duration on body tissue compartmentalization, the pathways by which interactions occur, and the moderation on energy utilization mechanisms remain to be fully elucidated. During early childhood and throughout adolescent transitioning, a time when body composition trajectories are established, sleep patterns appear to undergo significant reorganization (e.g., later bedtimes, decreases in non-REM sleep). Sleep duration may represent an underlying pathophysiological change associated with the maturation process, if left unchecked, could certainly influence body composition trajectory and future disease risk. Accordingly, targeting an ‘optimal’ amount of sleep duration during growth and development would enhance capacity for energy homeostasis and optimize resource partitioning.
Acknowledgments
Funding: NIH K99 DK083333; R01-DK067426; P30-DK56336; M01-RR-00032; CA47888; P60-DK079626.
This research was supported by NIH K99 DK083333 (KC); R01-DK067426 (JRF, KC, LJH); P30-DK56336 (UAB, Nutrition Obesity Research Center); M01-RR-00032 (UAB, Clinical Research Unit); CA47888 (LJH); P60-DK079626 (UAB Diabetes Research Training Center).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008 May 1;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lytle LA, Pasch KE, Farbakhsh K. The Relationship Between Sleep and Weight in a Sample of Adolescents. Obesity (Silver Spring) 2010 Oct 14; doi: 10.1038/oby.2010.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padez C, Mourao I, Moreira P, Rosado V. Long sleep duration and childhood overweight/obesity and body fat. Am J Hum Biol. 2009;21:371–376. doi: 10.1002/ajhb.20884. [DOI] [PubMed] [Google Scholar]
- 4.Touchette E, Petit D, Tremblay RE, Boivin M, Falissard B, Genolini C, Montplaisir JY. Associations between sleep duration patterns and overweight/obesity at age 6. Sleep. 2008 Nov 1;31:1507–1514. doi: 10.1093/sleep/31.11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Paula FJ, Horowitz MC, Rosen CJ. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res Rev. 2010;26:622–630. doi: 10.1002/dmrr.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 8.Siegel JM. Functional implications of sleep development. PLoS Biol. 2005;3:e178. doi: 10.1371/journal.pbio.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005 Oct 27;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 11.Rutters F, Gerver WJ, Nieuwenhuizen AG, Verhoef SP, Westerterp-Plantenga MS. Sleep duration and body-weight development during puberty in a Dutch children cohort. Int J Obes (Lond) 2010;34:1508–1514. doi: 10.1038/ijo.2010.161. [DOI] [PubMed] [Google Scholar]
- 12.Werner H, Lebourgeois MK, Geiger A, Jenni OG. Assessment of chronotype in four- to eleven-year- old children: reliability and validity of the Children’s Chronotype Questionnaire (CCTQ) Chronobiol Int. 2009;26:992–1014. doi: 10.1080/07420520903044505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huffman DM, Landy NM, Potter E, Nagy TR, Gower BA. Comparison of the Lunar DPX-L and Prodigy dual-energy X-ray absorptimeters for assessing total and regional body composition. International Journal of Body Composition Research. 2005;3:25–30. Ref Type: Generic. [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper JA, Watras AC, O’Brien MJ, Luke A, Earthman CP, Schoeller DA. Assessing validity and reliability of Resting Metabolic Rate in six gas analysis systems. Journal of the American Dietetic Association. 2009;109:128–132. doi: 10.1016/j.jada.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. usa.gov. Aug 4, 2009. CDC Growth Charts. 8-4-0009. Ref Type: Electronic Citation. [Google Scholar]
- 16.Rogol AD. Sex steroids, growth hormone, leptin and the pubertal growth spurt. Endocr Dev. 2010;17:77–85. doi: 10.1159/000262530. [DOI] [PubMed] [Google Scholar]
- 17.Bell JF, Zimmerman FJ. Shortened nighttime sleep duration in early life and subsequent childhood obesity. Arch Pediatr Adolesc Med. 2010;164:840–845. doi: 10.1001/archpediatrics.2010.143. [DOI] [PubMed] [Google Scholar]
- 18.Eisenmann JC, Ekkekakis P, Holmes M. Sleep duration and overweight among Australian children and adolescents. Acta Paediatr. 2006;95:956–963. doi: 10.1080/08035250600731965. [DOI] [PubMed] [Google Scholar]
- 19.Hitze B, Bosy-Westphal A, Bielfeldt F, Settler U, Plachta-Danielzik S, Pfeuffer M, Schrezenmeir J, Monig H, Muller MJ. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. Eur J Clin Nutr. 2009;63:739–746. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- 20.Knutson KL, Spiegel K, Penev P, Van CE. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78:309–323. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfield RL, Bordini B. Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity. Brain Res. 2010 Dec 10;1364:186–197. doi: 10.1016/j.brainres.2010.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touchette E, Mongrain V, Petit D, Tremblay RE, Montplaisir JY. Development of sleep-wake schedules during childhood and relationship with sleep duration. Arch Pediatr Adolesc Med. 2008;162:343–349. doi: 10.1001/archpedi.162.4.343. [DOI] [PubMed] [Google Scholar]
- 24.Bayer O, Rosario AS, Wabitsch M, von KR. Sleep duration and obesity in children: is the association dependent on age and choice of the outcome parameter? Sleep. 2009 Sep 1;32:1183–1189. doi: 10.1093/sleep/32.9.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]