Abstract
Study Objectives:
To evaluate the efficacy of a 12-week exercise training program for reducing obstructive sleep apnea (OSA) severity and improving sleep quality, and to explore possible mechanisms by which exercise may reduce OSA severity.
Design:
Randomized controlled trial.
Setting:
Clinical exercise physiology center, sleep laboratory.
Participants:
Forty-three sedentary and overweight/obese adults aged 18-55 years with at least moderate-severity untreated OSA (screening apnea-hypopnea index [AHI] ≥ 15).
Interventions:
Participants randomized to exercise training (n = 27) met 4 times/week for 12 weeks and performed 150 min/week of moderate-intensity aerobic activity, followed by resistance training twice/week. Participants randomized to a stretching control (n = 16) met twice weekly for 12 weeks to perform low-intensity exercises designed to increase whole-body flexibility.
Measurements and Results:
OSA severity was assessed with one night of laboratory polysomnography (PSG) before and following the 12-week intervention. Measures of sleep quality included PSG, actigraphy (7-10 days), and the Pittsburgh Sleep Quality Index. Compared with stretching, exercise resulted in a significant AHI reduction (exercise: 32.2 ± 5.6 to 24.6 ± 4.4, stretching: 24.4 ± 5.6 to 28.9 ± 6.4; P < 0.01) as well as significant changes in oxygen desaturation index (ODI; P = 0.03) and stage N3 sleep (P = 0.03). Reductions in AHI and ODI were achieved without a significant decrease in body weight. Improvements in actigraphic sleep and subjective sleep quality were also noted following exercise compared with stretching.
Conclusions:
Exercise training had moderate treatment efficacy for the reduction of AHI in sedentary overweight/obese adults, which suggests that exercise may be beneficial for the management of OSA beyond simply facilitating weight loss.
Trial Registration:
Clinicaltrials.gov identification number NCT00956423.
Citation:
Kline CE; Crowley EP; Ewing GB; Burch JB; Blair SN; Durstine JL; Davis JM; Youngstedt SD. The effect of exercise training on obstructive sleep apnea and sleep quality:a randomized controlled trial. SLEEP 2011;34(12):1631-1640.
Keywords: Actigraphy, exercise training, obstructive sleep apnea, polysomnography, randomized controlled trial, sleep quality
INTRODUCTION
Obstructive sleep apnea (OSA) is a prevalent sleep disorder, affecting up to 15% of the population.1 If left untreated, OSA has potentially severe health consequences, such as cognitive impairment,2 cardiovascular disease,3 diabetes,4 and early mortality.5
Unfortunately, treatment options for OSA remain limited. Although continuous positive airway pressure (CPAP) is effective when used,6 much of its benefits go unrealized in practice because of low adherence.7 Other treatment options, such as oral appliances and upper airway surgery, only partially reduce OSA severity and produce frequent side effects.8,9 In addition, the effects of these treatments on the health consequences of OSA are not well established.8,9
The possible utility of exercise training in the management of OSA has not been thoroughly investigated despite intriguing preliminary evidence. Epidemiologic research has suggested that individuals who are physically active have a reduced risk of OSA compared to individuals who are less active.10,11 Moreover, small-scale (often uncontrolled) experimental studies have found the apnea-hypopnea index (AHI) to be reduced up to 50% following chronic exercise training.12–15
Although weight loss is the most obvious plausible mediator explaining how exercise may reduce OSA severity, decreases in AHI following exercise training have been found to be independent of changes in body weight in the limited epidemiologic and experimental studies that have examined this hypothesis.10,12,13 Other possible mechanisms of improvement in OSA following exercise training include a general strengthening and fatigue resistance of the ventilatory and upper airway dilator muscles,16 attenuation of respiratory instability from reduced sleep fragmentation,17 decreased nasal resistance,18 and prevention of lower-extremity fluid accumulation.19
Regardless of whether OSA severity is improved, exercise training may improve sleep in this population. There is a consistent epidemiologic association between exercise and sleep quality,20 and experimental studies utilizing individuals without OSA have found that chronic exercise training significantly improves sleep.21 However, experimental research in individuals with OSA has produced conflicting results about changes in objective sleep from exercise training,12–15 and subjective sleep quality has not been evaluated.
The primary purpose of this investigation was to examine the efficacy of a 12-week moderate-intensity exercise training program for reducing the severity of OSA in currently untreated adults. A secondary purpose of the study was to determine whether exercise training improved subjective and objective sleep. Finally, we explored possible mechanisms by which exercise training may reduce OSA severity, namely via changes in body weight, respiratory muscle strength, sleep quality, and lung function.
METHODS
Participants
Adults ages 18-55 years with at least moderate-severity OSA (AHI ≥ 15 at screening) who were overweight/obese (body mass index ≥ 25), sedentary (< 2 exercise sessions/week), at stable (> 3 mo) medication doses (e.g., antihypertensives, antidepressants), not currently being treated for OSA and not actively attempting to lose weight were eligible for participation. Exclusion criteria included known or suspected significant cardiovascular, pulmonary, or metabolic disease; uncontrolled hypertension (> 159/99 mm Hg); pregnancy; and inability to exercise due to orthopedic or musculoskeletal problems.
Recruitment, Screening, and Timeline
Individuals were recruited from local sleep clinics and from the general population via media advertisements. Following an initial phone screen, individuals were mailed additional screening materials, including the Berlin Questionnaire.22 Individuals who were previously diagnosed with OSA or classified as “high risk” for OSA based on the Berlin Questionnaire and otherwise eligible were invited to the laboratory to further review the protocol. At the conclusion of the visit, participants provided written informed consent approved by the Institutional Review Boards of the University of South Carolina and the WJB Dorn VA Medical Center. Participants were then scheduled for one night of laboratory polysomnography (PSG) to further screen for OSA. Individuals with a screening AHI ≥ 15 were enrolled in the study.
Prior to baseline assessment, participants were required to meet with the study team on 2 occasions to become familiar with the research facility, practice study procedures, and view a presentation on OSA (prevalence, pathophysiology, established treatment options). The primary purposes for these “run-in” visits were to educate the participants on OSA and to establish whether the participants were committed to participating in the study.
Individuals who completed the run-in visits were scheduled for 3 baseline assessments, which took place over a 7-10 day period. The assessments were conducted on separate days and consisted of: (1) a single night of laboratory PSG; (2) a laboratory assessment, in which body composition, pulmonary function, and respiratory muscle strength were assessed; (3) a physician-supervised graded exercise test to screen for possible adverse responses to exercise. Throughout this period, participants continuously wore a wrist actigraph to monitor sleep at home.
Once baseline assessments were complete, participants were randomized to either a 12-week exercise training or stretching control treatment. Following completion of the intervention, participants completed the same assessments as at baseline following a day without exercise. Participants were compensated $300 for completion of the study.
Randomization
Following baseline, participants were randomly allocated to exercise and stretching control treatments by a 3:2 ratio, respectively. Randomization was stratified by sex and screening AHI (15-30 or > 30) in blocks of 5, using a computer-generated randomization list (SAS v. 9.2, SAS Institute, Cary, NC). Treatment allocations were prepared by an individual otherwise unaffiliated with the study and placed in sealed opaque envelopes.
Although participants could not be blinded to their treatment, both programs were presented as active treatments. To assess treatment expectancy, participants completed a brief questionnaire following randomization that queried expected changes in OSA severity, sleep quality, daytime sleepiness, mood, and overall health, using 5-point Likert scales (1: much worse; 5: much better). In addition, participants were asked to rate their satisfaction with their randomized treatment (1: very dissatisfied; 5: very satisfied).
Treatments
Exercise training
Individuals assigned to the exercise training treatment met 4 times per week for 12 weeks. All exercise sessions were supervised by staff trained in exercise physiology. To limit the risk of injury, the exercise dose was gradually increased during the initial 4 weeks of training. For weeks 5-12, exercise dose was 150 min/week of aerobic exercise distributed over 4 sessions per week, followed on 2 days per week by resistance exercise consisting of 2 sets of 10-12 repetitions for 8 different exercises. The exercise dose was chosen to comply with public health physical activity recommendations.23 Aerobic training intensity was 60% of heart rate reserve (HRR), considered to be moderate intensity,24 and was continuously monitored with heart rate telemetry (FS2, Polar Electro Oy, Kempele, Finland). Each aerobic exercise session began with a 5-min warm-up and ended with a 5-min cool-down, which was not included in the prescribed duration. Treadmill exercise was the primary aerobic activity, though elliptical or bicycle ergometer exercise was permitted when necessary (e.g., when participants experienced lower leg soreness).
Resistance exercise, performed twice per week on nonconsecutive days, included shoulder press, lat pulldown, leg extension/leg flexion (alternated between sessions), chest press, upright row, leg press, bicep curls/triceps extension (alternated between sessions), and abdominal crunches. Resistance was increased when 12 repetitions could be performed on the second set with proper form.
Stretching control
Participants assigned to the stretching control treatment met 2 times per week for 12 weeks for supervised flexibility training sessions. At each visit, participants performed 2 sets of 12-15 stretches, each held for 15-30 s, which focused on whole body flexibility. Although no change in OSA severity was expected from this intervention, it was chosen to reduce the potential confound of interpersonal interaction on study outcomes.
Sleep Measures
Laboratory polysomnography
Single-night laboratory PSG (Alice 5, Philips Respironics, Murrysville, PA) was performed at screening, baseline, and post-intervention. Participants were prepared for recording with a standard PSG montage25 that included F4/M1, C4/M1, O4/M1 electroencephalograms (EEG), bilateral electroculograms, submentalis electromyogram (EMG), thoracic and abdominal respiratory inductance plethysmography, modified lead II electrocardiogram, body position sensor, and single-leg tibialis anterior EMG. Airflow was monitored with an oronasal thermistor and nasal cannula pressure transducer, and arterial oxyhemoglobin saturation (SpO2) was assessed with finger pulse oximetry (LNOP DCI, Masimo, Irvine, CA). Time in bed was fixed at 8 h and initiated according to the participant's usual bedtime.
Sleep stage scoring was performed according to standard criteria25 by one registered PSG technician blinded to treatment assignment. An apnea was defined as ≥ 90% airflow reduction for ≥ 10 s, and a hypopnea was defined as ≥ 30% reduction in airflow accompanied by ≥ 4% desaturation from baseline. An arousal was defined as ≥ 3-s increase in EEG frequency following ≥ 10 s of stable sleep, accompanied by an increase in submentalis EMG activity for ≥ 1 s during REM sleep. The AHI was calculated as the number of apneas and hypopneas per hour of sleep, and was summarized by body position and sleep stage. The arousal index was calculated as the number of arousals per hour of sleep, and the oxygen desaturation index (ODI) was calculated as the number of SpO2 drops ≥ 4% per hour of sleep.
Objective home sleep
For approximately 7 days at baseline and again at post-intervention, participants wore an Actiwatch Spectrum actigraph (Philips Respironics, Bend, OR) on the non-dominant wrist to monitor home sleep/wake status. Participants wore the same actigraph at baseline and post-intervention. Participants were instructed to press an event marker to denote bedtime and out-of-bed time and at the initiation and end of daytime naps. Following data retrieval, individual records were inspected and edited (e.g., to set event markings as the start and end of rest intervals). Sleep/wake status was estimated with the Actiware software algorithm26 (v. 5.59.0015; Philips Respironics, Bend, OR) set to medium-threshold for wake detection and 5 immobile minutes for sleep onset and end.27 Sleep onset latency (SOL), total sleep time (TST), wakefulness after sleep onset (WASO), and sleep efficiency (SE) were obtained for analysis. In addition, a fragmentation index, a measurement of movement and restlessness, was calculated by the software algorithm. Due to an allergic skin reaction to the Actiwatch for one participant, 42 participants provided data for analysis. Values were averaged across all baseline and post-intervention nights for analyses.
Subjective sleep quality
The Pittsburgh Sleep Quality Index (PSQI)28 was administered at baseline and post-intervention prior to laboratory PSG to assess subjective sleep quality over the previous 2 weeks. Seven component scores were generated: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. A global score between 0-21 was calculated from summing the subscale scores. Global scores > 5 have been considered to be indicative of poor sleep quality.28
Additional Measures
Assessment of lifestyle activity and eating habits
Participants were asked to maintain their normal lifestyle activity patterns and eating habits throughout the study. To monitor unsupervised activity, participants wore a piezoelectric pedometer (NL-1000, New Lifestyles Inc., Lees Summit, MO) from baseline through the end of post-intervention assessment. Participants removed the pedometer during the supervised activity sessions. The NL-1000 recorded daily steps and time spent in moderate- to vigorous-intensity activity (MVPA),29 and data were organized into 2-week bins for analysis.
Dietary habits were evaluated with the Rapid Eating Assessment for Participants-Short Version (REAP-S)30 at baseline and post-intervention. With the 13-item REAP-S, participants were asked to indicate how frequently they skipped breakfast, ate at restaurants, and ate various categories of food (e.g., processed meats, fried foods) using 3 response options (1 = rarely/never, 2 = sometimes, 3 = usually/often). The total score was derived by summing the scores of the 13 items.
Assessment of potential mediators of exercise training
Changes in body composition, pulmonary function, and respiratory muscle strength were explored as possible mediators between exercise training and improvement in AHI. These measures were taken in the morning following an overnight fast at baseline and within 5 days of completion of the 12-week intervention.
Body composition:
Height and weight were measured to the nearest 0.5 cm and 0.1 kg using a wall-mounted stadiometer and calibrated physician weight scale, respectively. Neck, chest, waist, and hip circumference measurements were obtained using standardized procedures24; the average of 3 measurements was retained.
Total body dual energy x-ray absorptiometry (DXA; Lunar Prodigy, GE Medical Systems, Madison, WI) measured body fat percentage (BF %), lean tissue mass (LTM), and fat mass (FM). Whole-body values were obtained as well as by region (i.e., arms, trunk, legs). A single technician conducted and analyzed all DXA scans. Quality assurance tests and phantom scans were performed prior to all measurement sessions. Because one participant exceeded the weight limit for the DXA, data from 42 participants were included for analysis.
Pulmonary function:
Pulmonary function testing was conducted with a portable spirometer (Wizard, MicroMedical, Kent, UK) using standardized procedures.31 Maximal inspiratory and expiratory maneuvers were performed 3 times; averages of values obtained from the 3 inspiratory and expiratory flow-volume loops were used for analysis. Variables retained for analysis included forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1.0), peak inspiratory flow (PIF), and peak expiratory flow (PEF).
Respiratory muscle strength:
Maximum static inspiratory (MIP) and expiratory (MEP) mouth pressures served as markers of respiratory muscle strength.32 MIP and MEP assessments were performed with respiratory pressure gauges (VacuMed, Ventura, CA) while seated with nasal clips. Assessments of MIP and MEP were obtained using standardized procedures,32 with the highest pressures developed over 3 consecutive efforts recorded. Respiratory muscle strength was calculated as (MIP+MEP)/2. Assessment of respiratory muscle strength was not possible due to equipment malfunction for one participant who subsequently discontinued the study. Data for the remaining 42 participants were utilized for analysis.
Statistical Power
Anticipated changes in AHI, the primary outcome of interest, were based on published research at the time of study design.12,13 Statistical power was 83% to detect a change in AHI from 27.3 to 17.7 following exercise training, assuming a baseline standard deviation of 10 and no AHI change in the control treatment, with a total of 40 participants randomized in a 3:2 fashion. The unbalanced treatment allocation allowed for similar power as a balanced allocation (i.e., n = 20 for each treatment would have provided 84% power), but a more thorough investigation into the possible benefits of exercise training. Assuming an attrition rate of 20%, 48 individuals were targeted for enrollment. With 37 participants completing the study (i.e., 21 exercise, 16 control), we had 80% power to detect the anticipated changes in AHI.
Statistical Analysis
Analyses were based on an intent-to-treat plan. In the case of dropouts, the last observation was carried forward for analysis. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Unless otherwise specified, data are presented as mean ± standard error. All tests were 2-tailed, with statistical significance set at P < 0.05.
Baseline differences between treatments were evaluated with χ2 tests and independent t-tests. Adherence to the intervention was compared between treatments using independent t-tests by the percentage attendance to scheduled sessions. Pedometer-assessed activity was evaluated for between-treatment differences and change over time using SAS PROC MIXED.
The primary outcome of interest, change in AHI, was evaluated by analysis of covariance on post-intervention AHI values with control for baseline AHI values. To assess clinical significance, participants who completed the intervention were categorized as experiencing treatment “success” and/or “response” based on definitions previously used for evaluating the efficacy of surgical treatment for OSA, with between-treatment comparisons made with Fisher exact tests. Treatment success was defined as a post-intervention AHI < 20 and reduction ≥ 50% from baseline, whereas treatment response was defined as ≥ 20% reduction in AHI from baseline.33
Changes in secondary outcomes (e.g., objective sleep variables, PSQI score) and potential mediator variables (e.g., anthropometrics, respiratory muscle strength) were evaluated in an identical fashion to that of AHI. When significant between-group differences were found for any of these variables, paired t-tests examined whether significant changes occurred within the exercise training group. Effect sizes (Hedges' g) were calculated for all variables by dividing the difference between the baseline and post-intervention changes in the treatment and control treatments by the pooled baseline standard deviation.34 By convention, effect sizes of g = 0.2, g = 0.5, and g = 0.8 were considered small, medium, and large in magnitude, respectively. In addition, the change in prevalence of poor subjective sleep (i.e., PSQI global > 5)28 between treatments was assessed with a Fisher exact test.
Variables that could explain the reduction in OSA severity following exercise training were evaluated for mediation using MacKinnon's product of coefficients test.35 Pre-specified potential mediators included stage N3 sleep %, body weight, trunk BF%, trunk total mass, respiratory muscle strength, PEF, and PIF. Using the complete sample (N = 43), each potential mediator was tested in a single mediator model, which included: (1) estimating the effect of the intervention on the potential mediator (i.e., α coefficient) by regressing the potential mediator's post-intervention value on the intervention treatment while controlling for the potential mediator's baseline value; (2) estimating the effect of changes in the potential mediator on changes in AHI (i.e., β coefficient) by regressing post-intervention AHI on the potential mediator's post-intervention value, controlling for treatment, baseline AHI, and the potential mediator's baseline value; and (3) calculating the product of coefficients by multiplying the α and β coefficients. Coefficients were obtained through linear regression models using SAS PROC GLM. Asymmetric confidence limits based on the distribution of the product of the α and β coefficients were created using the PRODCLIN program36; confidence intervals that did not include zero indicated a statistically significant mediation effect.
RESULTS
Participant Characteristics
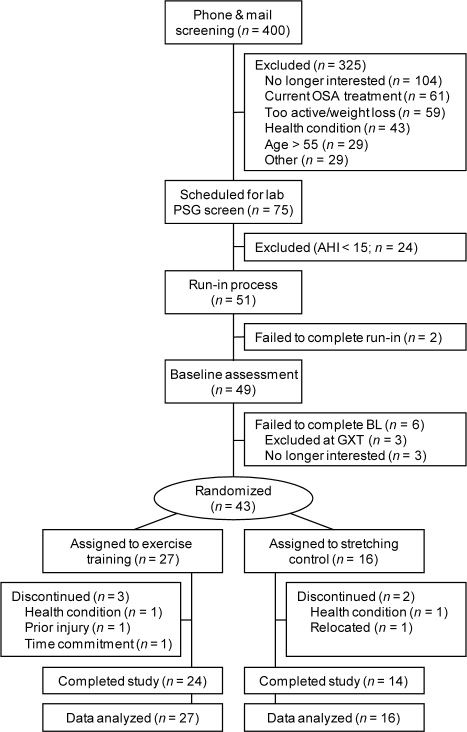
A summary of participant flow is provided in Figure 1. Of the 43 participants randomized to treatment, 5 discontinued participation before completion of the study at an average of 4.6 ± 0.2 weeks into the intervention. Reported reasons for study withdrawal were not directly study-related (e.g., relocation, underlying health condition). No adverse responses to either activity program were reported, though 4 participants in the exercise training group needed to switch from the treadmill to the recumbent bicycle for aerobic training due to the temporary development of shin splints.
Figure 1.
Summary of participant flow through study. AHI, apnea-hypopnea index; BL, baseline; GXT, graded exercise test; OSA, obstructive sleep apnea; PSG, polysomnography.
Participant characteristics are summarized in Table 1. No between-treatment differences for any of the baseline participant characteristics were observed. Twenty-four of the 43 participants had been previously diagnosed with OSA, and 17 of those 24 had previously been treated but had since voluntarily discontinued treatment. Thirteen had previously discontinued CPAP use, 3 had undergone unsuccessful upper airway surgery, and 1 participant had discontinued oral appliance use.
Table 1.
Baseline participant characteristics
| Variable | All (N = 43) | Exercise Training (n = 27) | Stretching (n = 16) |
|---|---|---|---|
| Sex, n male (% male) | 24 (56) | 15 (56) | 9 (56) |
| Age, y | 46.9 (1.2) | 47.6 (1.3) | 45.9 (2.2) |
| Ethnicity/race, n (%) | |||
| White | 32 (74) | 22 (81) | 10 (63) |
| African American | 8 (19) | 4 (15) | 4 (9) |
| Hispanic/Other | 3 (7) | 1 (4) | 2 (5) |
| Education, y | 15.4 (0.3) | 15.6 (0.3) | 15.1 (0.6) |
| Weight, kg | 103.3 (2.7) | 105.6 (3.0) | 99.3 (5.0) |
| BMI, kg/m2 | 34.8 (0.9) | 35.5 (1.2) | 33.6 (1.4) |
| Total body fat, % | 41.6 (1.4) | 42.1 (1.9) | 40.6 (1.9) |
| Trunk body fat, % | 45.3 (1.2) | 46.3 (1.6) | 43.6 (1.5) |
| Previous OSA dx, n (%) | 24 (56) | 14 (52) | 10 (63) |
| Previous OSA tx, n (%) | 17 (40) | 9 (33) | 8 (50) |
All data are presented as mean (standard error) unless otherwise noted. BMI, body mass index; OSA, obstructive sleep apnea. No between-treatment differences were found for any of the baseline participant characteristics.
Expectations and Adherence
There were no statistically significant differences between treatments regarding expectations for improvement in OSA severity or overall sleep quality. However, participants allocated to the exercise training treatment expressed slightly greater satisfaction with their treatment allocation compared to control (exercise: 4.6 ± 0.2, stretching: 4.0 ± 0.3; t41 = −2.12, P = 0.04).
Exercise training participants attended 87.0% ± 3.7% of the prescribed sessions, completing 81.7% ± 4.3% of the prescribed 12-week aerobic dose at 64.4% ± 2.3% of HRR, and completing 77.5% ± 4.6% of the prescribed 12-week resistance dose. Stretching participants attended 79.7% ± 5.2% of their prescribed sessions. Adherence, defined as rate of attendance, did not differ between the 2 treatments.
Exercise training participants who completed the 12-week intervention (n = 24) attended 93.1% ± 1.7% of the prescribed sessions. They completed 88.6% ± 2.1% of the prescribed aerobic activity dose at 63.3% ± 2.2% of HRR, and completed 84.4% ± 2.9% of the prescribed resistance training dose. Participants (n = 14) who completed the stretching program attended 86.9% ± 2.0% of their prescribed sessions, which was a significantly lower rate of attendance than the exercise training participants who completed treatment (t36 = −2.30, P = 0.03).
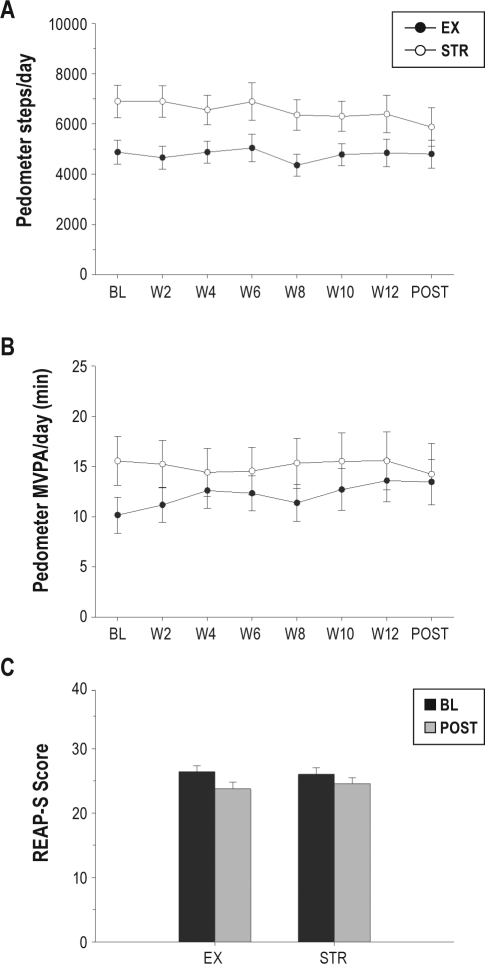
Figure 2 provides a summary of changes in lifestyle activity and dietary habits. Pedometer activity did not change over the course of the 12-week study between treatments, as no significant treatment, time, or treatment × time interaction effects were noted for daily step counts or for daily time spent in MVPA. Dietary habits, as assessed by the REAP-S, did not change between treatments over the course of the study.
Figure 2.
Changes in unsupervised activity and diet during study. (A) Daily steps (averaged into 2-week bins); (B) Minutes of moderate- to vigorous-intensity activity (MVPA; averaged into 2-week bins); (C) Overall REAP-S score at baseline and post-intervention between treatments. Data are presented as mean ± standard error. BL, baseline assessment; EX, exercise training treatment; POST, post-intervention assessment; STR, stretching control treatment.
Exercise Training and OSA Severity
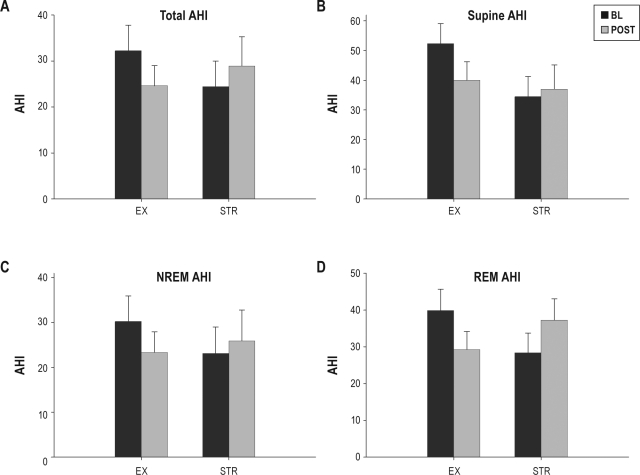
Between-treatment changes in AHI are summarized in Figure 3. Compared with the stretching control treatment, exercise training resulted in a significant reduction in AHI (F1,40 = 9.54, P < 0.01). On average, AHI was reduced by 7.6 ± 2.5 following exercise training, whereas AHI increased 4.5 ± 2.4 following stretching treatment (F1,41 = 10.45, P < 0.01). When restricted to exercise training participants only, the post-training reduction in AHI was found to be statistically significant (t26 = 3.03, P < 0.01). Treatment success (i.e., post-intervention AHI < 20 and reduction ≥ 50% from baseline) was noted in 25% and 7% of exercise training and stretching treatment participants, respectively (Fisher exact test, P = 0.23). Treatment response (≥ 20% AHI reduction) was documented in 63% and 21% of the exercise training and stretching treatment participants, respectively (Fisher exact test, P = 0.02).
Figure 3.
Changes in AHI during study. (A) Total AHI; (B) Supine AHI; (C) NREM AHI; (D) REM AHI. AHI, apnea-hypopnea index; BL, baseline assessment; EX, exercise training treatment; NREM, NREM sleep; POST, post-intervention assessment; REM, REM sleep; STR, stretching control treatment. Data are presented as mean ± standard error.
Significantly greater reductions in AHI were found for both NREM and REM sleep following exercise vs. control (NREM: F1,40 = 5.71, P = 0.02; REM: F1,40 = 8.97, P < 0.01). Although not statistically significant, the magnitude of effect for supine AHI (g = −0.46) was similar to that for total AHI (g = −0.45), and the amount of time spent supine did not change between treatments from pre- to post-intervention assessment. The number of apneas per hour of sleep were significantly reduced following exercise training relative to control (F1,40 = 10.34, P < 0.01), whereas no change between treatments was found in the number of hypopneas per hour of sleep (Table 2).
Table 2.
Polysomnographic sleep variables
| Variable | Exercise Training |
Stretching |
g | ||
|---|---|---|---|---|---|
| BL | POST | BL | POST | ||
| TST, min | 402.2 (6.7) | 406.5 (8.7) | 410.6 (7.7) | 400.3 (13.6) | 0.44 |
| SOL, min | 9.6 (1.7) | 9.8 (1.8) | 12.0 (2.8) | 13.5 (2.9) | −0.14 |
| WASO, min | 68.2 (6.4) | 63.7 (7.7) | 57.4 (6.8) | 66.1 (12.7) | −0.43 |
| SE, % | 83.8 (1.4) | 84.7 (1.8) | 85.5 (1.6) | 83.4 (2.8) | 0.44 |
| Stage N1 sleep (%) | 10.6 (1.0) | 9.6 (1.0) | 13.9 (3.2) | 14.3 (3.2) | −0.16 |
| Stage N2 sleep (%) | 60.2 (1.5) | 59.0 (2.1) | 55.0 (3.3) | 57.8 (2.5) | −0.40 |
| Stage N3 sleep (%) | 12.8 (1.2) | 13.2 (1.1)* | 12.4 (1.7) | 9.2 (1.5) | 0.58 |
| REM sleep (%) | 16.4 (1.1) | 18.3 (1.3) | 18.7 (1.4) | 18.7 (2.2) | 0.32 |
| Arousal index | 36.9 (4.0) | 35.8 (3.5) | 31.2 (4.3) | 32.7 (5.0) | −0.13 |
| Supine sleep, min | 139.0 (21.6) | 138.2 (15.7) | 157.0 (28.4) | 164.0 (23.4) | −0.07 |
| Apnea index | 21.7 (4.8) | 15.0 (3.3)* | 15.1 (4.1) | 20.9 (5.2) | −0.56 |
| Hypopnea index | 10.5 (2.2) | 9.6 (2.0) | 9.3 (2.3) | 8.0 (1.9) | 0.03 |
| Minimum SpO2, % | 79.6 (1.4) | 79.9 (1.5) | 80.4 (1.9) | 78.7 (2.0) | 0.27 |
| ODI | 24.5 (4.2) | 21.5 (3.7)* | 16.8 (4.2) | 23.2 (5.8) | −0.47 |
| SpO2 < 90, % TST | 5.2 (1.2) | 4.7 (1.1) | 3.6 (1.2) | 5.2 (1.4) | −0.37 |
All data are presented as mean (standard error). Figure 3 summarizes change in AHI. N1-N3, NREM sleep stages 1-3, respectively; ODI, oxygen desaturation index; SpO2, arterial oxygen saturation; SE, sleep efficiency; SOL, sleep onset latency; TST, total sleep time; WASO, wakefulness after sleep onset. g indicates Hedges' g effect size measure.
Statistically significant difference between treatments at post-intervention following control for baseline values (P < 0.05).
A summary of changes in other markers of OSA is provided in Table 2. A significant reduction in ODI following exercise training relative to control was discovered (F1,40 = 5.05, P = 0.03), though the ODI improvement was not significant when considering only exercise training participants (t26 = 1.56, P = 0.13). No between-treatment changes were found for minimum SpO2 or the percentage of TST with SpO2 < 90%.
Exercise Training and Objective Sleep
Table 2 provides a summary of the changes in PSG-measured objective sleep. Exercise training produced minimal PSG sleep improvements compared with the stretching control treatment. Small to moderate effects (g = 0.14-0.58) were noted for measures of sleep that were not statistically significant. Only the percentage of TST spent in stage N3 sleep was significantly changed following exercise training relative to control (F1,40 = 5.75, P = 0.02), and this effect was not significant when considering only exercise training participants (t26 = —0.38, P = 0.71).
Changes in actigraphic sleep are summarized in Table 3. Compared with the stretching control treatment, exercise training resulted in significant improvements in SOL (F1,39 = 10.70, P < 0.01), SE (F1,39 = 5.08, P = 0.03), and the fragmentation index (F1,39 = 4.41, P = 0.04). When considering only exercise training participants, significant improvements were noted for SOL (t26 = 2.21, P = 0.04) and SE (t26 = −2.19, P = 0.04), but not for the fragmentation index (t26 = 2.22, P = 0.34).
Table 3.
Actigraphic and subjective sleep quality variables
| Wrist Actigraphy | Exercise Training |
Stretching |
g | ||
|---|---|---|---|---|---|
| BL | POST | BL | POST | ||
| TST, min | 398.3 (10.4) | 390.2 (9.3) | 388.3 (17.9) | 385.3 (45.9) | −0.25 |
| SOL, min | 8.2 (1.4) | 5.2 (0.7)* | 10.0 (3.2) | 11.9 (12.7) | −0.52 |
| WASO, min | 57.4 (4.9) | 54.9 (4.8) | 63.5 (10.4) | 63.5 (39.3) | −0.08 |
| SE, % | 85.8 (1.2) | 86.7 (1.1)* | 84.1 (2.0) | 83.8 (7.1) | 0.17 |
| Napping, min | 29.0 (4.3) | 26.7 (5.2) | 30.7 (6.9) | 31.2 (32.6) | −0.12 |
| Fragmentation index | 42.4 (3.4) | 41.1 (2.9)* | 53.4 (9.0) | 55.1 (35.2) | −0.12 |
| Pittsburgh Sleep Quality Index | |||||
| Poor sleep quality, prevalence | 48% | 29% | 75% | 75% | |
| Global score, 0-21 | 6.2 (0.6) | 4.7 (0.5)* | 8.1 (1.0) | 8.0 (1.0) | −0.43 |
| Sleep quality, 0-3 | 1.6 (0.1) | 1.0 (0.2)* | 1.6 (0.2) | 1.4 (0.2) | −0.60 |
| Sleep latency, 0-3 | 0.7 (0.2) | 0.5 (0.1)* | 1.3 (0.3) | 1.4 (0.3) | −0.32 |
| Sleep duration, 0-3 | 0.5 (0.1) | 0.4 (0.1) | 1.1 (0.3) | 1.0 (0.2) | 0.07 |
| Sleep efficiency, 0-3 | 0.2 (0.1) | 0.2 (0.1) | 0.8 (0.3) | 0.6 (0.3) | 0.26 |
| Sleep disturbances, 0-3 | 1.6 (0.1) | 1.4 (0.1)* | 1.6 (0.2) | 1.8 (0.1) | −0.62 |
| Sleep medications, 0-3 | 0.4 (0.2) | 0.3 (0.2) | 0.4 (0.3) | 0.6 (0.3) | −0.24 |
| Daytime dysfunction, 0-3 | 1.3 (0.1) | 0.9 (0.1) | 1.3 (0.2) | 1.3 (0.2) | −0.48 |
All data are presented as mean (standard error) unless otherwise noted. A PSQI global score > 5 was considered to be poor sleep quality.28 SE, sleep efficiency; SOL, sleep onset latency; TST, total sleep time; WASO, wakefulness after sleep onset.
Statistically significant difference between treatments at post-intervention following control for baseline values (P < 0.05).
Exercise Training and Subjective Sleep Quality
Table 3 provides a summary of changes in subjective sleep quality. Of the participants with poor sleep at baseline, a greater proportion of exercise training participants were found to have good sleep quality at post-intervention compared to control (Fisher exact test, P = 0.02). Significantly greater reductions in PSQI global score (F1,40 = 8.38, P = 0.01), as well as subscale scores of sleep quality (F1,40 = 4.42, P = 0.04), sleep latency (F1,40 = 6.04, P = 0.02), and sleep disturbances (F1,40 = 7.83, P < 0.01), were found following exercise training compared to the control treatment. When considering only exercise training participants, significant improvements were noted for the PSQI global score (t26 = 2.68, P = 0.01) and sleep quality subscale (t26 = 3.86, P < 0.01), but not for the sleep latency (t26 = 1.15, P = 0.26) or sleep disturbance subscales (t26 = 1.80, P = 0.08).
Mediators of OSA Improvements by Exercise Training
A summary of the changes in the proposed mediators by which exercise training could reduce AHI is provided in Table 4. Exercise training resulted in no significant change in body weight (exercise change: −0.9 ± 0.6 kg; stretching change: −0.6 ± 0.5 kg) or circumference measures, but a significant reduction in total body fat percentage relative to control was noted (F1,39 = 8.85, P < 0.01). Exercise training resulted in a 1.1% ± 0.3% reduction in body fat, whereas the stretching intervention resulted in a 0.2% ± 0.3% gain in body fat. Likewise, exercise training failed to significantly improve parameters of spirometric lung volumes or respiratory muscle strength, though global respiratory strength showed marginal improvement following exercise training relative to the stretching control treatment (F1,39 = 3.79, P = 0.06).
Table 4.
Changes in potential mediators
| Measure | Exercise Training |
Stretching |
g | ||
|---|---|---|---|---|---|
| BL | POST | BL | POST | ||
| Anthropometry | |||||
| Total body weight, kg | 105.6 (3.0) | 104.7 (3.1) | 99.3 (5.1) | 98.7 (5.0) | −0.02 |
| Total body fat, % | 42.1 (1.9) | 41.0 (1.9)* | 40.6 (1.9) | 40.8 (1.9) | −0.14 |
| Trunk body fat, % | 46.3 (1.6) | 44.9 (1.6) | 43.6 (1.5) | 43.6 (1.4) | −0.19 |
| Neck circumference, cm | 40.7 (0.6) | 40.9 (0.6) | 39.5 (0.9) | 39.8 (1.0) | −0.01 |
| Chest circumference, cm | 109.9 (1.8) | 109.3 (1.6) | 106.9 (3.7) | 105.9 (3.6) | 0.04 |
| Waist circumference, cm | 110.8 (2.3) | 110.2 (2.3) | 104.4 (3.8) | 105.8 (3.9) | −0.16 |
| Hip circumference, cm | 120.1 (2.8) | 120.0 (2.8) | 114.8 (2.9) | 115.2 (3.1) | −0.03 |
| Spirometry | |||||
| FVC, L | 3.7 (0.2) | 3.6 (0.2) | 3.5 (0.3) | 3.6 (0.3) | −0.23 |
| FEV1.0, L | 3.2 (0.2) | 3.1 (0.2) | 3.1 (0.3) | 3.1 (0.3) | −0.11 |
| PIF, L/min | 203.6 (18.8) | 209.3 (20.7) | 223.0 (23.1) | 255.6 (26.6) | −0.28 |
| PEF, L/min | 400.1 (25.7) | 448.1 (29.5) | 408.7 (36.0) | 429.1 (51.7) | 0.20 |
| Respiratory muscle strength | |||||
| MIP, cm H2O | 94.2 (7.6) | 114.4 (6.6) | 106.8 (8.5) | 111.1 (7.4) | 0.43 |
| MEP, cm H2O | 119.8 (8.9) | 139.0 (8.7) | 115.3 (12.7) | 124.7 (14.7) | 0.21 |
| Global strength, cm H2O | 107.0 (7.6) | 126.7 (6.9) | 111.0 (9.2) | 117.9 (10.1) | 0.34 |
All data are presented as mean (standard error). FEV1.0, forced expiratory volume in 1 s; FVC, forced vital capacity; MEP, maximum expiratory pressure; MIP, maximum inspiratory pressure; PEF, peak expiratory flow; PIF, peak inspiratory flow.
Statistically significant difference between treatments at post-intervention following control for baseline values (P < 0.05).
Table 5 provides a summary of the mediation pathway analyses. None of the proposed variables were successful at explaining how exercise training may reduce AHI.
Table 5.
Mediation pathway analysis results
| Potential Mediators | α coefficient (intervention effect on mediator) | β coefficient (mediator effect on outcome) | Asymmetric confidence limits |
|---|---|---|---|
| Stage N3 sleep % | −3.800 (1.585)* | −0.021 (0.356) | −2.708, 2.920 |
| Total body weight | 0.247 (0.915) | 0.784 (0.601) | −1.257, 1.885 |
| Neck circumference | 0.024 (0.454) | −0.673 (1.186) | −0.698, 0.643 |
| Trunk BF % | 1.294 (0.706) | −0.365 (0.826) | −2.984, 1.615 |
| Trunk body weight | −0.478 (0.688) | 0.575 (0.837) | −1.671, 0.707 |
| PEF | 28.463 (28.137) | 0.009 (0.020) | −0.887, 1.764 |
| PIF | −28.777 (20.321) | 0.030 (0.028) | −3.337, 0.681 |
| Respiratory muscle strength | −12.145 (6.241) | −0.058 (0.090) | −1.371, 3.369 |
Data are expressed as coefficient (standard error). All mediation analyses were performed using the complete sample (N = 43). N3, NREM sleep stage 3; PEF, peak expiratory flow; PIF, peak inspiratory flow.
Statistically significant effect (P < 0.05).
DISCUSSION
The primary purpose of this study was to examine the efficacy of exercise training for reducing the severity of OSA. Compared to stretching control, exercise training resulted in moderate improvements in AHI and ODI. The improvement in AHI was attained despite a lack of change in body weight and was not found to be significantly mediated by exercise-induced changes in stage N3 sleep, respiratory muscle strength, or lung volumes. Improvements in actigraphic and subjective sleep parameters were likewise noted following exercise training.
Previous research that documented the potential efficacy of exercise training for improving OSA had been limited by small sample sizes (N ≤ 20)12–15,37 and/or uncontrolled research designs.12,13,37 The current study, which is the largest randomized trial to date on this topic, documented a reduction in AHI that was similar to other studies in which minimal weight loss occurred.12,14 Although other studies have also documented similar or superior reductions of AHI following intensive lifestyle interventions which included exercise training,12,38,39 the dietary interventions (and subsequent greater weight loss) in those trials made it difficult to separate the effects of diet-induced weight loss from exercise training per se. The lack of robust reduction in other markers of OSA severity noted in this study is also consistent with previous research, as studies have rarely reported significant improvements in SpO2 measures following exercise training.12–14,37–39
While only 25% of individuals who completed the exercise training intervention experienced treatment success (i.e., post-intervention AHI < 20 and reduction ≥ 50%), 63% experienced an AHI reduction ≥ 20%. Thus, when evaluated as a stand-alone treatment for OSA, the efficacy of exercise training seems to be lower than oral appliance use or multilevel surgery, but similar to other surgical treatments40,41 and approximately equivalent to a 10% reduction in body weight.42 However, it is worth noting that the present data suggest a chronic effect of exercise training, with the post-intervention AHI reduction occurring following a day of non-exercise. In contrast, even one night of non-use of CPAP and oral appliances results in a return of AHI to near pre-treatment levels.7,40
None of the variables that were explored to explain how exercise training may reduce AHI were found to be significant mediators. It is possible that there was insufficient statistical power to detect significant mediation or that the mediators chosen were either incorrect or lacked sensitivity. To determine how exercise training reduces AHI, it will likely be necessary to include more specific mechanistic assessments, such as measurement of upper airway size and collapsibility,43,44 measurement of nasal resistance,45 assessment of changes in upper airway dilator activity46 and strength/fatigability,47 and evaluation of lung volume alterations during sleep.48
The improvements in PSG sleep that were observed in the present study are generally consistent with previous research, as significant improvements in PSG-measured sleep have rarely been noted following exercise training in populations with or without OSA.49 Although robust improvements in PSG-measured TST, SE, and arousals have been noted following exercise training in one study of adults with OSA,13 others have found no change in PSG sleep12,14 or only selected improvements.37 To our knowledge, the improvements in actigraphic and subjective sleep, which in some respects are preferred because they estimate sleep in the home setting over multiple nights, have not been previously documented following exercise in this population. However, these improvements have not been uncommon following exercise training in individuals with sleep complaints unrelated to OSA.49
One of the main limitations of the study was the single night of laboratory PSG performed at pre- and post-intervention, which likely introduced additional variability in measures of OSA severity.50 A lack of snoring measurement was a further limitation. Snoring has been associated with adverse cardiovascular health outcomes independent of OSA,51 and anecdotally, bed partners of many participants reported robust decreases in snoring following exercise training.
Like most adults treated for OSA, many participants in the present study had either failed to adhere to traditional treatments or experienced treatment failure. Therefore, even moderately efficacious effects, such as what was found for exercise training, would be more beneficial than lack of treatment. When considering that exercise training may also alleviate many of the health consequences of OSA, a case can be made for the potential utility of exercise in the management of OSA. Additionally, these findings provide evidence that exercise training may be a desirable first trial of treatment before progression to more invasive measures.
It has been suggested that individuals with OSA may have a difficult time adhering to an exercise training program. Although the excessive sleepiness associated with OSA has been hypothesized to contribute to the disinclination to exercise,52 CPAP therapy has not been shown to increase activity patterns, even after sleepiness has been reduced.53 We found that, using a carefully progressed supervised exercise intervention, participant adherence was excellent, and most participants reported intentions to continue exercising following completion of the supervised intervention. However, the participants were self-selected and thus may have had higher motivation to exercise than may be expected in the general population. Future research should evaluate whether exercise under less controlled conditions still produces a significant reduction in OSA severity.
In conclusion, this study found that exercise training resulted in a modest reduction in AHI despite minimal change in body weight. Moderate improvements in objective and subjective sleep quality also occurred following exercise training. The results suggest that exercise training exerts a significant effect on OSA severity and sleep quality, and that exercise training may provide benefit for the management of OSA beyond that of solely facilitating weight loss.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Blair receives book royalties from Human Kinetics, and has received honoraria for service on the scientific/medical advisory boards for Alere, Technogym, Santec, Clarity, and Jenny Craig. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by Public Health Dissertation Grant 1R36CD000695-1 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. Additional funding support for this work was provided by NHLBI T32 HL082610-04.
The authors gratefully acknowledge the assistance of SleepMed of South Carolina and the WJB Dorn VA Sleep Laboratory for their assistance with recruitment and data collection, respectively. The authors also are indebted to Colin Kane and DeAnna Milton for their assistance with data collection, and to Dr. Xuemei Sui and Dr. Meghan Baruth for their help with statistical analyses. The work was performed in Dr. Youngstedt's Chronobiology Laboratory, the Clinical Exercise Research Center at the University of South Carolina, and the WJB Dorn VA Medical Center Sleep Laboratory.
Footnotes
A commentary on this article appears in this issue on page 1621.
REFERENCES
- 1.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 2.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 4.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62:354–9. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 8.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006:CD004435. doi: 10.1002/14651858.CD004435. [DOI] [PubMed] [Google Scholar]
- 9.Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea--a systematic review. Sleep. 2009;32:27–36. [PMC free article] [PubMed] [Google Scholar]
- 10.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–4. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 11.Quan SF, O'Connor GT, Quan JS, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11:149–57. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 12.Giebelhaus V, Strohl KP, Lormes W, Lehmann M, Netzer N. Physical exercise as an adjunct therapy in sleep apnea--an open trial. Sleep Breath. 2000;4:173–6. doi: 10.1007/s11325-000-0173-z. [DOI] [PubMed] [Google Scholar]
- 13.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- 14.Sengul YS, Ozalevli S, Oztura I, Itil O, Baklan B. The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep Breath. 2011;15:49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]
- 15.Ueno LM, Drager LF, Rodrigues AC, et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32:637–47. doi: 10.1093/sleep/32.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent HK, Shanely RA, Stewart DJ, et al. Adaptation of upper airway muscles to chronic endurance exercise. Am J Respir Crit Care Med. 2002;166:287–93. doi: 10.1164/rccm.2104120. [DOI] [PubMed] [Google Scholar]
- 17.Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150:481–5. doi: 10.1164/ajrccm.150.2.8049833. [DOI] [PubMed] [Google Scholar]
- 18.Olson LG, Strohl KP. The response of the nasal airway to exercise. Am Rev Respir Dis. 1987;135:356–9. doi: 10.1164/arrd.1987.135.2.356. [DOI] [PubMed] [Google Scholar]
- 19.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–6. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 20.Youngstedt SD, Kline CE. Epidemiology of exercise and sleep. Sleep Biol Rhythms. 2006;4:215–21. doi: 10.1111/j.1479-8425.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24:355–65. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. [DOI] [PubMed] [Google Scholar]
- 25.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 26.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 27.Chae KY, Kripke DF, Poceta JS, et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–5. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.McMinn D, Rowe DA, Stark M, Nicol L. Validity of the New Lifestyles NL-1000 accelerometer for measuring time spent in moderate-to-vigorous physical activity in school settings. Meas Phys Educ Exerc Sci. 2010;14:67–78. [Google Scholar]
- 30.Segal-Isaacson CJ, Wylie-Rosett J, Gans KM. Validation of a short dietary assessment questionnaire: the Rapid Eating and Activity Assessment for Participants short version (REAP-S) Diabetes Educ. 2004;30:774, 776, 778. doi: 10.1177/014572170403000512. [DOI] [PubMed] [Google Scholar]
- 31.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 32.American Thoracic Society; European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 33.Richard W, Kox D, den Herder C, van Tinteren H, de Vries N. One stage multilevel surgery (uvulopalatopharyngoplasty, hyoid suspension, radiofrequent ablation of the tongue base with/without genioglossus advancement), in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2007;264:439–44. doi: 10.1007/s00405-006-0182-z. [DOI] [PubMed] [Google Scholar]
- 34.Hedges LV, Olkin I. Statistical methods for meta-analysis. New York: Academic Press; 1985. [Google Scholar]
- 35.Mackinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods. 2007;39:384–9. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes M, Goldsworthy UR, Cary BA, Hill CJ. A diet and exercise program to improve clinical outcomes in patients with obstructive sleep apnea—a feasibility study. J Clin Sleep Med. 2009;5:409–15. [PMC free article] [PubMed] [Google Scholar]
- 38.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–7. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 39.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 41.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 43.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 44.Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- 45.Li HY, Engleman H, Hsu CY, et al. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep. 2005;28:1554–9. doi: 10.1093/sleep/28.12.1554. [DOI] [PubMed] [Google Scholar]
- 46.Pierce R, White D, Malhotra A, et al. Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur Respir J. 2007;30:345–53. doi: 10.1183/09031936.00063406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatigability: relationship to apnoea/hypopnoea index and age. J Sleep Res. 2000;9:389–93. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 48.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61:435–9. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King AC, Pruitt LA, Woo S, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A Biol Sci Med Sci. 2008;63:997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levendowski DJ, Zack N, Rao S, et al. Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath. 2009;13:163–7. doi: 10.1007/s11325-008-0214-6. [DOI] [PubMed] [Google Scholar]
- 51.Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207–13. [PMC free article] [PubMed] [Google Scholar]
- 52.Aguillard RN, Riedel BW, Lichstein KL, Grieve FG, Johnson CT, Noe SL. Daytime functioning in obstructive sleep apnea patients: exercise tolerance, subjective fatigue, and sleepiness. Appl Psychophysiol Biofeedback. 1998;23:207–17. doi: 10.1023/a:1022257514209. [DOI] [PubMed] [Google Scholar]
- 53.West SD, Kohler M, Nicoll DJ, Stradling JR. The effect of continuous positive airway pressure treatment on physical activity in patients with obstructive sleep apnoea: a randomised controlled trial. Sleep Med. 2009;10:1056–8. doi: 10.1016/j.sleep.2008.11.007. [DOI] [PubMed] [Google Scholar]